Introduction
Hepatocellular carcinoma (HCC) is one of the
relatively common gastrointestinal malignant tumors. Its early
onset symptoms are not obvious, and the onset is rapid. Patients
are already in the advanced stage when definitely diagnosed with
HCC. HCC is also the third most common cause of cancer death
worldwide (1). The biological
activity involved in the occurrence and development processes of
the tumor is a hotspot and focus of research. A study has shown
that abnormal activities of tumor suppressor genes and oncogenes
play key roles in these processes (2). Fragile histidine triad (FHIT) is a novel
tumor suppressor gene, which exists in the vast majority of normal
organ tissues (3). It has been
confirmed that FHIT can induce cell apoptosis and arrest the cell
growth cycle, thereby inhibiting tumor proliferation (4). p16 is a multi-tumor suppressor known for
its expression products, namely, approximately 16-kDa protein
molecules. The active function of cyclin-dependent kinase (CDK) is
a prerequisite for all cells to enter the growth cycle. One of the
functions of p16 is to inhibit the biological activity of CDK and
block the growth cycle, thereby playing a role in inhibiting the
growth (5). In this study, 148
patients with primary HCC undergoing interventional embolotherapy
were examined by immunohistochemistry, so as to investigate the
expression of FHIT and p16 in tissues and the correlation between
the expression of the two, which is of important significance for
clinical diagnosis.
Patients and methods
General data
A total of 148 patients with primary HCC who
underwent interventional embolotherapy in the Department of
Gastroenterology in Qingdao Central Hospital (Qingdao, China) from
March 2014 to March 2016 were selected. Among them, 122 were males
and 26 females, aged 28–75 years. All patients were definitely
diagnosed with primary HCC by clinical, imaging and α-fetoprotein
tests or needle biopsy, and patients with lung and bone metastases
were excluded. The tumor-node-metastasis (TNM) staging was
conducted based on the criteria set by the Union for International
Cancer Control (UICC). The study was approved by the Ethics
Committee of Qingdao Central Hospital and informed consents were
signed by the patients or the guardians.
Interventional embolotherapy
Interventional embolotherapy was performed for all
patients using Seldinger technique. Conventional indirect arterial
angiography for superior mesenteric arteries and celiac arterial
angiography were conducted, followed by selective and
superselective intubations in tumor feeding arteries, and then
chemotherapeutics adriamycin, 5-fluorouracil, oxaliplatin and
leucovorin were reperfused. All patients were treated with iodized
oil for embolization, and the dosage used in each patient varied.
The dosage of iodized oil was adjusted at any time according to the
size of HCC lesions and the patient's tolerance during the
interventional embolotherapy.
Immumohistochemical staining
Cancer-adjacent and HCC tissues were taken from
patients with primary HCC before and after treatment, and were
fixed with 10% paraffin at 20°C for 16 h. Then, the tissues were
cut into 5 µm slices using paraffin, followed by dewaxing,
hydration and rinsing with phosphate-buffered saline (PBS). The
non-specific background was sealed with 10% serum at room
temperature for 15 min, followed by the addition of mouse
anti-human primary FHIT and p16 monoclonal antibodies (1:300; cat.
nos. sc-390481 and sc-377412; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) for incubation in a refrigerator at 4°C overnight.
The tissues were used after rinsing with PBS. After that,
biotin-labeled goat anti-mouse secondary polyclonal antibody
(1:800; cat. no. SA00004-1; ProteinTech Group, Inc.; Wuhan Sanying
Biotechnology, Wuhan, China) were added for incubation at room
temperature for 30 min, followed by rinsing with PBS. Streptomyces
antibiotic protein-peroxidase solution was added for incubation at
room temperature for 30 min, followed by rinsing with PBS and
3,3′-diaminobenzidine (DAB) color development. Then the tissues
were washed using tap water, re-stained with hematoxylin, sealed
with neutral gum, and observed via a light microscope (Olympus
Corp., Tokyo, Japan).
Result assessment
A total of 100 cells were randomly selected in the
field of view to be observed, and the average number of cells in
the field was calculated as the number of positive cells of the
expressed proteins in the tissues. Score of staining depth: 0–2
points represented no staining, weak staining and strong staining,
respectively. Score of the positive rate of stained cells: 1–4
points represented the percentage of positive cells, namely [1,
25], [26, 50], [51, 75] and [76, 100], respectively. The product of
the above two scores: ≤2 points for negative (−), 3–4 points for
weakly positive (+), 5–8 points for moderately positive (++), and
≥9 points for strongly positive (+++).
Detection of the expression of FHIT
and p16 genes via reverse transcription-quantitative polymerase
chain reaction (RT-qPCR)
The total ribonucleic acids (RNAs) were extracted
from HCC and cancer-adjacent tissues according to the instructions
of TRIGene kit (Geneaid Biotech, Ltd., New Taipei, Taiwan). The
concentration and purity of the two kinds of total RNAs were
determined via a spectrophotometer (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA), and the mean A260/A280 value was 1.8–2.0.
According to the instructions of the RT kit [RevertAid First Strand
cDNA Synthesis kit (K1622; Thermo Fisher Scientific, Inc., Waltham,
MA, USA)], the primer sequences were synthesized by Shanghai Jiran
Biotechnology Co., Ltd. (Shanghai, China) (Table I). A total volume of 20 µl reaction
system was reversely transcribed into cDNA on an RT-PCR machine.
The experimental results were analyzed using the 2−ΔΔCq
method (6).
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Gene | Sequence |
|---|
| FHIT | F:
5′-AAGAGGAAAACTGAGCCATCTG-3′ |
|
| R:
5′-CGGCTAACATCCCACTGATAAT-3′ |
| p16 | F:
5′-TGGTTAGAGGCTGCCTGTG-3′ |
|
| R:
5′-TGGACAAGACCCTGAAGACA-3′ |
| β-actin | F:
5′-CAGGAAGGAAGGCTGGAAG-3′ |
|
| R:
5′-CGGGAAATCGTGCCTGAC-3′ |
According to the instructions of the real-time
fluorescence quantitative PCR kit (2X RealStar Green Power Mixture,
A311; GenStar BioSolutions Co., Ltd., Beijing, China), the reaction
system was 25 µl and the reaction conditions were as follows: 95°C
for 10 min, 95°C for 30 sec, 59.4°C for 30 sec, 40 cycles, and 95°C
for 15 sec, followed by cooling to 65°C. Fluorescence values were
read, and β-actin was used as an internal reference. The relative
expression of FHIT and p16 messenger RNAs (mRNAs) were calculated
via RT-qPCR.
Detection of the expression of FHIT
and p16 proteins by western blotting
According to the instructions of the total protein
extraction kit, the total proteins of HCC and cancer-adjacent
tissues were separately extracted using ProteoPrep®
Total Extraction Sample kit (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany), they were stored at −70°C for standby application, and
the concentration of the extracted proteins was determined by a
spectrophotometer (Bio-Rad, Hercules, CA, USA). Gels (15%) were
prepared for sodium dodecyl sulfate (SDS)-polyacrylamide gel
electrophoresis. A 15-µl solution was added per lane. Based on the
marker band, the locations of the two proteins in the gel were
selected. After membranes were transferred to PVDF membrane,
proteins were transferred for staining for 35 min, 5% skim milk was
added for sealing at 37°C for 90 min, followed by the addition of
primary mouse anti-human primary FHIT and p16 monoclonal antibodies
(1:400; cat. nos. sc-390481 and sc-377412; Santa Cruz
Biotechnology, Inc.) for incubation at 4°C overnight. After that,
Tris-buffered saline with Tween-20 (TBST) was added, and then the
proteins were placed in a shaker for vibration and washing 3 times
at 15 min each time. Afterwards, secondary goat anti-mouse
polyclonal antibody (1:2,000; cat. no. sc-2005; Santa Cruz
Biotechnology, Inc.) was added for incubation at 37°C for 1 h, TBST
was added, and the proteins were placed in a shaker for vibration
and washing for 15 min, which was repeated 3 times. Then, the
electrochemical luminescence (ECL; EMD Millipore, Burlington, MA,
USA) was added in a dark room for coloration, followed by exposure,
color development and fixation. Finally, the images were scanned
using the ChemiDoc™ MP Imaging System (Bio-Rad), they were analyzed
via ImageJ professional image analysis software (National
Institutes of Health, Bethesda, MD, USA), and the optical density
value was recorded.
Statistical analysis
In this study, the professional statistical
software, Statistical Product and Service Solutions (SPSS) 17.0
(Beijing Xinmeijiahong Technology Co., Ltd. Beijing, China), was
applied for the data analysis. The data are expressed as mean ±
standard deviation (SD). Comparison of the positive rate and the
expression levels of FHIT and p16 with clinicopathological
parameters was made using the Chi-square test. Spearman's
correlation analysis was used to detect the correlation between
FHIT and p16 expression levels. α=0.05 was taken as the test
statistical standard. P<0.05 was considered to indicate a
statistically significant difference.
Results
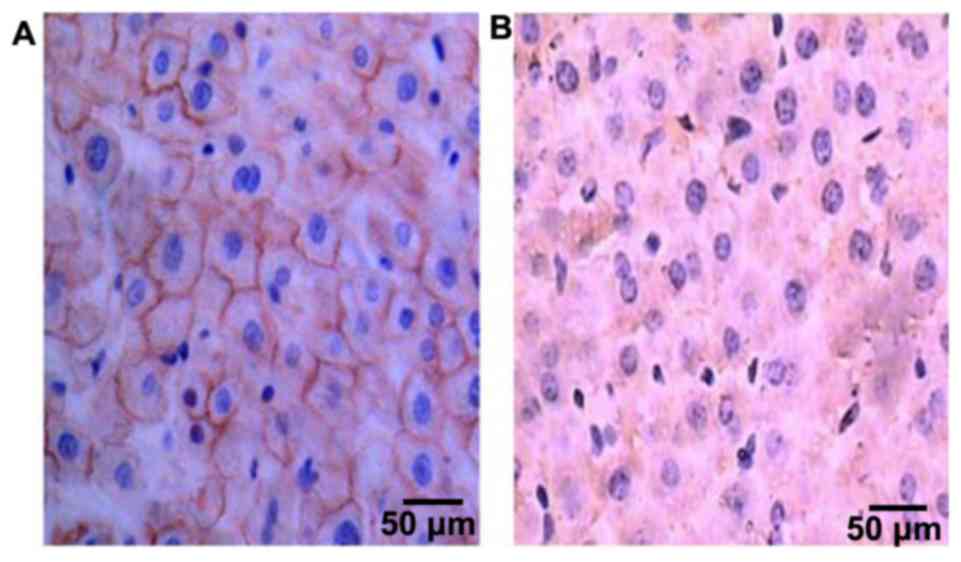
The expression of FHIT protein in
tissues
FHIT protein was mainly expressed in the cytoplasm,
and became yellow or pale yellow after immunohistochemistry
(Fig. 1). The positive expression
rates of FHIT protein were 47.97% (71/148) and 92.57% (137/148), in
HCC and cancer-adjacent tissues, respectively, and the difference
in comparison was statistically significant (P<0.05) (Table II).
 | Table II.FHIT protein expression in HCC and
cancer-adjacent tissues. |
Table II.
FHIT protein expression in HCC and
cancer-adjacent tissues.
|
|
| FHIT protein |
|
|
|
|---|
|
|
|
|
|
|
|
|---|
| Tissues | n | − | + to +++ | Positive rate
(%) | χ2 | P-value |
|---|
| HCC | 148 | 77 | 71 | 47.97 | 39.02 | <0.001 |
| Cancer-adjacent | 148 | 11 | 137 | 92.57 |
|
|
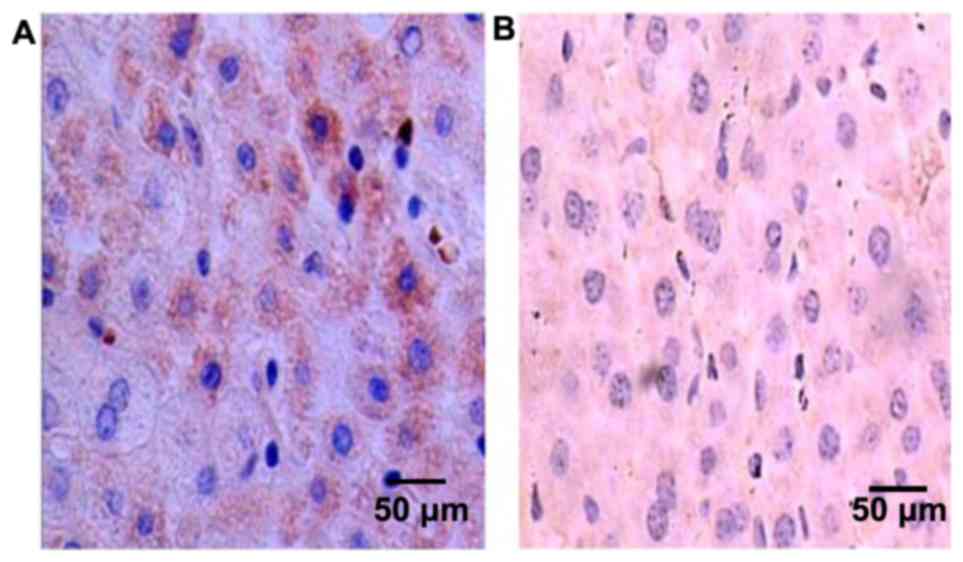
The expression of p16 protein in
tissues
p16 protein was mostly expressed in the cytoplasm
and the nucleus, and became yellow or pale yellow after
immunohistochemistry (Fig. 2). The
positive expression rates of p16 protein were 60.14% (89/148) and
95.95% (142/148), in HCC and cancer-adjacent tissues, respectively,
and the difference in comparison was statistically significant
(P<0.05) (Table III).
 | Table III.p16 protein expression in HCC and
cancer-adjacent tissues. |
Table III.
p16 protein expression in HCC and
cancer-adjacent tissues.
|
|
| p16 protein |
|
|
|
|---|
|
|
|
|
|
|
|
|---|
| Tissues | n | − | + to +++ | Positive rate
(%) | χ2 | P-value |
|---|
| HCC | 148 | 59 | 89 | 60.14 | 33.27 | 0.001 |
| Cancer-adjacent | 148 | 6 | 142 | 95.95 |
|
|
Correlation between FHIT and p16
protein expression levels in HCC tissues
In 148 patients with primary HCC, there were 71
patients with positive expression of FHIT protein, in which 55
patients had positive expression of p16 protein simultaneously,
with the proportion of 77.46%. The statistical analysis revealed
that the two proteins showed a positive correlation (Spearman's
correlation coefficient, r=0.308; P=0.025) (Table IV).
 | Table IV.Correlation between FHIT and p16
protein expression levels in HCC tissues after treatment. |
Table IV.
Correlation between FHIT and p16
protein expression levels in HCC tissues after treatment.
|
| FHIT |
|
|
|
|---|
|
|
|
|
|
|
|---|
| Items | + | − | Total | r (Spearman) | P-value |
|---|
| p16 |
| + | 55 | 34 | 89 | 0.308 | 0.025 |
| − | 16 | 43 | 59 |
|
|
| Total | 71 | 77 | 148 |
|
|
Correlation of the positive expression
of FHIT and p16 proteins in HCC tissues with clinicopathological
indicators
The analysis results demonstrated that the positive
expression of FHIT was not related to age, sex and tumor size
(P>0.05 in all comparisons), but was associated with HCC TNM
staging, the differentiation degree in Edmondson-Steiner grading,
lymph node metastasis and portal vein thrombosis (P<0.05 in all
comparisons). p16 protein was not related to age, sex, TNM staging,
lymph node metastasis and portal vein thrombosis (P>0.05 in all
comparisons), but was correlated with tumor size and the
differentiation degree in Edmondson-Steiner grading (P<0.05 in
all comparisons) (Table V).
 | Table V.Correlation of the positive expression
levels of FHIT and p16 proteins in HCC tissues with
clinicopathological indicators. |
Table V.
Correlation of the positive expression
levels of FHIT and p16 proteins in HCC tissues with
clinicopathological indicators.
|
|
| FHIT | p16 |
|---|
|
|
|
|
|
|---|
| Clinicopathological
indicators | n | Positive | χ2 | P-value | Positive | χ2 | P-value |
|---|
| Age (years) |
|
<40 | 44 | 16 | 0.068 | 0.914 | 12 | 0.045 | 0.861 |
|
≥40 | 104 | 38 |
|
| 44 |
|
|
| Sex |
|
Male | 102 | 34 | 0.917 | 0.626 | 42 | 1.375 | 0.472 |
|
Female | 46 | 12 |
|
| 18 |
|
|
| Tumor size
(cm) |
|
<5 | 96 | 64 | 1.385 | 0.744 | 54 | 5.026 | 0.035 |
| ≥5 | 52 | 24 |
|
| 30 |
|
|
| TNM staging |
| Stage
I–II | 88 | 58 | 8.494 | 0.025 | 40 | 2.195 | 0.218 |
| Stage
III–IV | 60 | 18 |
|
| 18 |
|
|
| Edmondson-Steiner
grading |
| High
differentiation | 30 | 26 | 15.027 | 0.001 | 22 | 12.941 | 0.003 |
|
Moderate differentiation | 64 | 30 |
|
| 26 |
|
|
| Low
differentiation | 54 | 14 |
|
| 16 |
|
|
| Lymph node
metastasis |
|
Yes | 46 | 18 | 7.492 | 0.024 | 24 | 1.492 | 0.174 |
| No | 102 | 54 |
|
| 46 |
|
|
| Portal vein
thrombosis |
|
Yes | 38 | 10 | 9.621 | 0.004 | 18 | 3.285 | 0.376 |
| No | 110 | 62 |
|
| 60 |
|
|
Expression of FHIT and p16 mRNAs in
tissues
The expression levels of FHIT gene mRNA in HCC and
cancer-adjacent tissues were 1.31±0.15 and 8.34±1.48, respectively,
showing a statistically significant difference in comparison
(P<0.05). The expression levels of p16 gene mRNA were 6.37±1.05
and 13.44±2.86, respectively, and the difference in comparison was
statistically significant (P<0.05) (Table VI).
 | Table VI.Relative expression levels of FHIT
and p16 mRNAs in tissues. |
Table VI.
Relative expression levels of FHIT
and p16 mRNAs in tissues.
| Tissues | FHIT | p16 |
|---|
| HCC |
1.31±0.15a |
6.37±1.05a |
|
Cancer-adjacent | 8.34±1.48 | 13.44±2.86 |
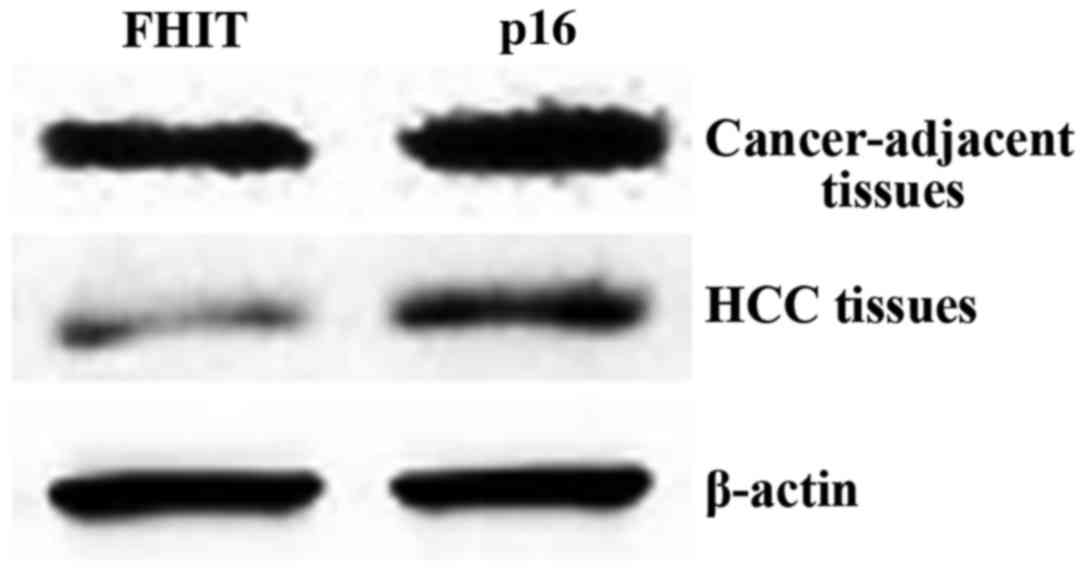
Expression of FHIT and p16 proteins in
tissues
The expression levels of FHIT protein in HCC and
cancer-adjacent tissues were 7.05±0.92 and 19.37±3.48,
respectively, manifesting a statistically significant difference in
comparison (P<0.05). The expression levels of p16 protein were
13.99±3.46 and 50.31±3.87, and the difference in comparison was
statistically significant (P<0.05) (Table VII; Fig.
3).
 | Table VII.Expression of FHIT and p16 proteins
in tissues. |
Table VII.
Expression of FHIT and p16 proteins
in tissues.
| Tissues | FHIT | p16 |
|---|
| HCC |
7.05±0.92a |
13.99±3.46a |
|
Cancer-adjacent | 19.37±3.48 | 50.31±3.87 |
Discussion
Primary HCC is the third most common cause of cancer
death in the world at present, and the current treatments for this
disease are not satisfactory. Its 5-year survival rate is only 10%,
and the liver transplantation is considered as the only radical
method for primary HCC. Patients with advanced HCC and unresectable
liver constitute the vast majority (over 80%) (7,8). The
latest developed interventional embolotherapy has become the main
method for primary HCC. Although the occurrence and development
processes of HCC are not yet fully clear, it is generally accepted
by academic circles that the occurrence of HCC is a multi-factor
and multi-step process affected by cross-over factors, including
the overexpression of oncogenes and the expression deficiency of
tumor suppressor genes (9).
FHIT is a tumor suppressor gene, and the expressed
protein is located in the cytoplasm. A study has manifested that
FHIT protein mainly acts on the specific structure of mRNA and
interferes with the normal translation function of mRNA, thus
resulting in the deficiency of the expression function of the
target gene. Additionally, the accumulation of these deficient
functions may induce, to a certain extent, tumor occurrence and
development (10). Fassan et
al (11) have reported on the
presence of abnormalities in FHIT gene transcription in more than
half of gastric cancer patients and the expression deficiency of
FHIT gene in nearly 70% of patients. Lim et al (12) have found the abnormal transcription of
FHIT gene in the tissues of 87% of gastric cancer patients. Also,
Czarnecka et al (13) have
detected the abnormal transcriptional expression of FHIT gene in
gastric and colorectal cancer via PCR, suggesting the normal
expression deficiency of FHIT gene in tumors of the digestive
system such as gastric cancer. A previous study has evidenced that
in 40% of tumor cells, FHIT gene expression is decreased, FHIT
protein is exhausted, and lymph node metastasis occurs in most
patients (14), indicating that the
downregulated expression of FHIT protein may be associated with
lymph node metastasis and poor prognosis of tumor patients. In the
present study, FHIT was abnormally expressed in primary HCC
tissues, and the expression level in primary HCC tissues was
remarkably lower than that in cancer-adjacent tissues (P<0.05).
The positive expression of FHIT was not related to age, sex or
tumor size (P>0.05 in all comparisons), but correlated with TNM
staging, the differentiation degree in Edmondson-Steiner grading,
lymph node metastasis and portal vein thrombosis (P<0.05 in all
comparisons). Therefore, it is speculated that the detection of
FHIT gene expression can be used as a reference for the diagnosis,
treatment and metastasis of primary HCC, which has important
clinical significance.
p16 gene is a multi-tumor suppressor gene that acts
primarily on the cell cycle anti-oncogene (15). The study demonstrated that p16 protein
mainly competes with cyclin D1 for binding to CDK4/CDK6 and
promotes cell arrest in G1 phase, which ultimately plays a negative
regulatory role in cell proliferation (16,17). Kumar
et al (18) have discovered
that p16 protein can inhibit tumor cell proliferation and
metastasis. Seiwert (19) has found
that the methylation level of p16 gene in serum of patients with
gastric cancer after operation is significantly decreased, while
the level of products normally expressed by p16 is significantly
increased. The results of the present study revealed that p16
protein is abnormally expressed in the tissues of patients with
primary HCC. The expression level in primary HCC tissues was
significantly lower than that in cancer-adjacent normal tissues
(P<0.05) and the positive expression of p16 was correlated with
tumor size and the differentiation degree in Edmondson-Steiner
grading (P<0.05 in all comparisons).
A previous study has revealed that deficiencies
exist concerning the expression of FHIT and p16 in the occurrence
and development processes of lung cancer, and the deficiency of
FHIT gene is a high-frequency event in the early stage (20). Mai et al (21) have confirmed that the expression
deficiency of p16 gene appears at a later stage after the
occurrence of the tumor, and the prognosis of patients will be
worse when deficiencies exist in both FHIT and p16. It was also
found in the present study that there was an obvious positive
correlation between the expression of FHIT and p16 proteins in HCC
tissues, indicating that the expression deficiencies of both are
involved in the occurrence process of HCC, and jointly play a
suppressive role. However, the specific mechanism of the
synergistic inhibitory effect is not yet understood and needs to be
further studied via follow-up experiments.
In conclusion, FHIT and p16 genes, as tumor
suppressor genes, inhibit the proliferation of HCC, and there is a
positive correlation between the two. The proteins of both can be
used as new indicators for clinical examination, thus providing a
new method for clinical diagnosis.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
WX wrote the manuscript and was in charge of the
interventional embolotherapy. XQ was responsible for the
immumohistochemical staining. XW and JS performed RT-qPCR. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Qingdao Central Hospital (Qingdao, China) and informed consents
were signed by the patients or the guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rasheed MA, Tariq F, Afzal S and Mannanv
S: In silico analysis of fragile histidine triad involved in
regression of carcinoma. J Pak Med Assoc. 67:616–621.
2017.PubMed/NCBI
|
|
2
|
Sun G, Zhang C, Feng M, Liu W, Xie H, Qin
Q, Zhao E and Wan L: Methylation analysis of p16, SLIT2, SCARA5,
and Runx3 genes in hepatocellular carcinoma. Medicine (Baltimore).
96:e82792017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zekri AR, Bahnasy AA, Shoeab FE, Mohamed
WS, El-Dahshan DH, Ali FT, Sabry GM, Dasgupta N and Daoud SS:
Methylation of multiple genes in hepatitis C virus associated
hepatocellular carcinoma. J Adv Res. 5:27–40. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cai JP, Wang YD, Zhang X and Xue HZ:
Expression of P16 and survivin in liver cancer and their clinical
significance. Zhonghua Gan Zang Bing Za Zhi. 25:778–780. 2017.(In
Chinese). PubMed/NCBI
|
|
5
|
Zhang C, Ye L, Guan S, Jin S, Wang W, Sun
S, Lee KH, Wei J and Liu B: Autoantibodies against p16
protein-derived peptides may be a potential biomarker for non-small
cell lung cancer. Tumour Biol. 35:2047–2051. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zaki SM, Abdel-Azeez HA, El Nagar MR,
Metwally KA and S Ahmed MM: Analysis of FHIT gene methylation in
egyptian breast cancer women: Association with clinicopathological
features. Asian Pac J Cancer Prev. 16:1235–1239. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lassen P, Primdahl H, Johansen J,
Kristensen CA, Andersen E, Andersen LJ, Evensen JF, Eriksen JG and
Overgaard J; Danish Head and Neck Cancer Group (DAHANCA), . Impact
of HPV-associated p16-expression on radiotherapy outcome in
advanced oropharynx and non-oropharynx cancer. Radiother Oncol.
113:310–316. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shu R, He J, Wu C and Gao J: The
association between RARβ and FHIT promoter methylation and the
carcinogenesis of patients with cervical carcinoma: A
meta-analysis. Tumour Biol. 39:10104283177091262017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yu GR, Qin WW, Li JP, Hua W, Meng YL, Chen
R, Yan B, Wang L, Zhang X, Jia LT, et al: HIV-TAT-fused FHIT
protein functions as a potential pro-apoptotic molecule in
hepatocellular carcinoma cells. Biosci Rep. 32:271–279. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fassan M, Rusev B, Corbo V, Gasparini P,
Luchini C, Vicentini C, Mafficini A, Paiella S, Salvia R, Cataldo
I, et al: Fhit down-regulation is an early event in pancreatic
carcinogenesis. Virchows Arch. 470:647–653. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lim HK, Park JM, Chi KC, Lee EJ and Jeong
EM: Disappearance of serum methylated p16 indicates longer survival
in patients with gastric cancer. J Gastric Cancer. 13:157–163.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Czarnecka KH, Migdalska-Sęk M, Domańska D,
Pastuszak-Lewandoska D, Dutkowska A, Kordiak J, Nawrot E,
Kiszałkiewicz J, Antczak A and Brzeziańska-Lasota E: FHIT promoter
methylation status, low protein and high mRNA levels in patients
with non-small cell lung cancer. Int J Oncol. 49:1175–1184. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shan M, Zhang X, Liu X, Qin Y, Liu T, Liu
Y, Wang J, Zhong Z, Zhang Y, Geng J, et al: p16 and p53 play
distinct roles in different subtypes of breast cancer. PLoS One.
8:e764082013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tamoto A, Yashima K, Hosoda K, Yamamoto S,
Kawata S, Ikebuchi Y, Matsumoto K, Kawaguchi K, Harada K, Murawaki
Y, et al: Protein expression of fragile histidine triad and
cyclooxgenase-2 in serrated neoplasia of the colorectum. Oncol
Lett. 14:3683–3688. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang JX, Han YP, Bai C and Li Q: Notch1/3
and p53/p21 are a potential therapeutic target for APS-induced
apoptosis in non-small cell lung carcinoma cell lines. Int J Clin
Exp Med. 8:12539–12547. 2015.PubMed/NCBI
|
|
17
|
Lv X, Ye G, Zhang X and Huang T: p16
Methylation was associated with the development, age, hepatic
viruses infection of hepatocellular carcinoma, and p16 expression
had a poor survival: A systematic meta-analysis (PRISMA). Medicine
(Baltimore). 96:e81062017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kumar R, Ghosh SK, Verma AK, Talukdar A,
Deka MK, Wagh M, Bahar HM, Tapkire R, Chakraborty KP and Kannan RR:
p16 expression as a surrogate marker for HPV infection in
esophageal squamous cell carcinoma can predict response to
neo-adjuvant chemotherapy. Asian Pac J Cancer Prev. 16:7161–7165.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Seiwert TY: Ties that bind: p16 as a
prognostic biomarker and the need for high-accuracy human
papillomavirus testing. J Clin Oncol. 32:3914–3916. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu X, Wu G, Yao X, Hou G and Jiang F: The
clinicopathological significance and ethnic difference of FHIT
hypermethylation in non-small-cell lung carcinoma: A meta-analysis
and literature review. Drug Des Devel Ther. 10:699–709.
2016.PubMed/NCBI
|
|
21
|
Mai S, Welzel G, Ottstadt M, Lohr F,
Severa S, Prigge ES, Wentzensen N, Trunk MJ, Wenz F, von
Knebel-Doeberitz M, et al: Prognostic relevance of HPV infection
and p16 overexpression in squamous cell anal cancer. Int J Radiat
Oncol Biol Phys. 93:819–827. 2015. View Article : Google Scholar : PubMed/NCBI
|