Introduction
There are more than 500,000 new cases and 275,000
deaths of cervical cancer every year, and the morbidity and
mortality rates of cervical cancer rank 3rd and 4th in the cancer
in women around the world (1,2). Cervical cancer is mainly caused by the
infection of oncogenic human papilloma virus (HPV). Compared with
other cancers, HPV-based detection is the most effective screening
means for cervical cancer (3).
However, conventional operation and chemoradiotherapy are still
dominated in the treatment of cervical cancer. Investigating the
molecular mechanisms of occurrence and progression of cervical
cancer and searching new targets are still research hotspots.
Nitric oxide (NO), as one of the smallest bioactive
products known in mammalian cells, can be produced by almost all
cells (4). NO is produced by
inducible and endothelial NO synthase (NOS) in the NOS family
(5). Studies have found that the NOS
expression is generally enhanced in a variety of tumor tissues, and
three kinds of different NOS subtypes, namely neuronal (N)-type,
inducible (I)-type and endothelial (E)-type, have been identified
currently. These synthases possess different positions, regulation
abilities, catalytic properties and sensitivity to inhibitor, which
can promote tumor proliferation, metastasis and resistance to
chemotherapy drugs (6,7). NOS1, the N-type NOS, can secrete NO in a
lower concentration, promoting tumor growth and angiogenesis
(8). NOS2 mainly exists in
mastocytes, macrophages and neutrophils, which, after being
activated by external stimuli, can produce NO in a high
concentration, thus inducing inflammation-related reactions and
promoting or affecting tumor growth (9). NO plays an important role as a
biological medium in vascular biology and inflammation. Moreover,
studies have demonstrated that the low-concentration NO promotes
tumor growth, while the high-concentration NO is the free radical
that inhibits cell proliferation and induces apoptosis, with a
biological effect of suppressing tumor growth (10). Currently, however, the expression
level and biological effect of NOS, an important catalytic enzyme
for NO production, in cervical cancer have not been fully clarified
yet.
Adenosine triphosphate (ATP)-binding cassette
sub-family G member 2 (ABCG2), also known as breast cancer
resistance protein, is correlated with the drug resistance in a
variety of tumors, which is usually deleted or expressed at a low
level in the pancreas. According to recent studies, ABCG2 is highly
expressed in pancreatic cancer cells and promotes cell survival via
changing the cell epigenetic program (11). Moreover, the ABCG2 expression is
significantly increased in side population cells in gastric cancer
and other tumors (12). However, the
ABCG2 expression in cervical cancer is not clear, and the mechanism
of normally regulating ABCG2 expression is poorly understood.
In this study, NOS1 and ABCG2 messenger ribonucleic
acid (mRNA) levels were detected in 40 cases of human cervical
cancer tissues and 20 cases of benign cervical tissues, and the
correlation between NOS1 and ABCG2 mRNA levels was analyzed.
Besides, lentivirus transfection technique was used to
interfere in the expression of NOS1 and ABCG2 in cervical cancer
cells, so as to investigate its effects on the proliferation and
apoptosis of cervical cancer cells and the specific regulatory
mechanism.
Materials and methods
Clinical specimens
A total of 40 patients with cervical cancer who had
not received any treatment prior to the study in the Department of
Gynaecology of Affiliated Hospital of Taishan Medical University
(Taian, China) from September 2015 to March 2017 were randomly
collected. The enrolled patients were aged 37–63 years with an
average of 42.4 years. Another 20 paraffin-embedded specimens of
normal cervical tissues were collected from the Pathology
Department of Affiliated Hospital of Taishan Medical University.
This study was approved by the Ethics Committee of the hospital,
and all patients enrolled in the study signed the informed
consent.
Detection of NOS1 and ABCG2 mRNA levels via reverse
transcription-quantitative polymerase chain reaction (RT-qPCR). The
total RNA was extracted from cells using the TRIzol method, and
reverse transcribed into complementary deoxyribonucleic acid (cDNA)
using the reverse transcription kit (Gene Copoeia, Guangzhou,
China). The mRNA expression levels of SYBR-Green (Thermo Fisher
Scientific, Inc., Waltham, MA, USA) NOS1 and ABCG2 in cells were
detected via RT-qPCR, and glyceraldehyde-3-phosphate dehydrogenase
(GAPDH) was used as the internal reference. Reaction conditions
were as follows: pre-degeneration at 95°C for 15 min, 95°C for 5
sec, annealing at 64°C for 30 sec, a total of 40 cycles. The
primers which used were: NOS1 sense, 5′-GAATACCAGCCTGATCCCTGGAA-3′
and anti-sense, 5′-TCCAGGAGGGTGTCCACAGCGTG-3′ (599 bp in length of
product). ABCG2 sense, 5′-AATACATCAGCGGATACTACAGAG-3′ and
anti-sense, 5′-AGCCACCATCATAAAGGGGTAAACAT-3′ (472 bp in length of
product). GAPDH sense, 5′-CGGAGTCAACGGATTTGGTCGTAT-3′ and
anti-sense, 5′-AGCCTTCTCCATGGTGGTGAAGAC-3′. The relative expression
level of mRNA in each index was calculated using the
2−ΔCq method [ΔCq = Cq (target gene) - Cq (GAPDH)]
(13).
Cell culture
Human cervical cancer cell lines (CaSki, HeLa, HCE1
and C-33A) were purchased from the Cell Bank of the Chinese Academy
of Sciences (Wuhan, China). Cells were cultured in the high-glucose
Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal
bovine serum, and added with 100 µg/ml streptomycin and 100 IU/ml
penicillin. The medium was placed in an incubator with 5%
CO2 and humidity of 95% at 37°C.
Western blotting
The protein was extracted from the 6-well plate,
with protein extraction kit (Invent Biotechnologies, Inc.,
Plymouth, MA, USA) and the protein concentration was detected using
the bicinchoninic acid (BCA) protein concentration assay kit
(Beyotime Institute of Biotechnology, Haimen, China). Then 40 µg
total proteins were separated via 10% sodium dodecyl
sulphate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred
onto a polyvinylidene fluoride (PVDF) membrane, and blocked in 5%
skim milk at 20°C for 1 h. The mouse anti-human NOS1 or ABCG2
(1:2,000; R&D Systems, MN, USA; article no: 85327, FAB995P) and
GAPDH primary monoclonal antibodies (1:1,000; cat. no. ab8245;
Abcam, Cambridge, UK) were incubated at 4°C. The protein band was
incubated with the corresponding horseradish peroxi-dase-labeled
secondary antibody (1:2,000; cat. no. A0216; Beyotime Institute of
Biotechnology, Guangzhou, China) at room temperature for 1 h. The
membrane was visualized using the enhanced chemiluminescence (ECL)
detection system (Bio-Rad Laboratories, Inc., Hercules, CA, USA),
and the gray scale was analyzed using a gel analyzer. (Bio-Rad
Laboratories, Inc.).
Lentivirus transfection
One pair of specific short hairpin RNA (shRNA)
target sequences for human NOS1 and ABCG2 genes, and one negative
control shRNA sequence not interfering in any human gene expression
were designed and synthesized by Shanghai GenePharma Co., Ltd.,
Shanghai, China. Sequences were constructed into the
pHBLV-U6-ZsGreen-Puro lentiviral vector, followed by transfection
using the lentiviral plasmid system. HeLa and C-33A cells were
inoculated into a 6-well plate at a density of
5×105/well. When 50–60% cells were fused, the
lentivirus was added for infection. The original medium was
replaced with the puromycin-containing medium once every 24 h for
screening. Then cells were digested with 0.25% trypsin, followed by
passage once every 2 days. Finally, cells were collected after
passaged 3–4 times. NOS1-targeted shRNA sequences: sense,
5′-GGCCAUACUCUUAGUTT-3′ and antisense, 5′-AUUUGAGUAUUUCAGCUCCTT-3′.
The shRNA sequence targeting ABCG2: sense strand
5′-TGTCAGACUCCAGAGUTA-3′; antisense strand
5′-GATTAUAUCCTGTAGGAG-3′. Negative control (sh-vector) sequences:
sense, 5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense,
5′-ACGUGACACGUUCGGAGAATT-3′.
Cell proliferation and apoptosis
detection
Colony-forming assay: HeLa and C-33A cells in good
growth status were inoculated into the 6-well plate at a density of
5×102/well, and cultured in the incubator with 5%
CO2 at 37°C until there were visible cell colonies. Then
cells were fixed with 4% paraformaldehyde for 30 min, stained with
crystal violet for 15 min, and photographed under a microscope
(Nikon Instrument, NY, USA) and the number of cell colonies was
counted.
Detection of apoptosis via flow cytometry: apoptosis
was detected using the apoptosis kit (BD Science, Harlingen,
Chicago, USA). At 2 days after transfection, cells were digested
and centrifuged at 3,000 × g for 8 min at 4°C, washed twice with
iced phosphate-buffered saline (PBS), resuspended in 100 µl IX Bing
buffer, and added with 5 µl propidium iodide (PI) and Annexin V,
respectively, followed by incubation at room temperature in the
dark for 15 min. Then the mixture was sent to the Scientific
Research Center of Affiliated Hospital of Taishan Medical
University for detection on the machine within 1 h. Apoptotic rate
= early apoptotic rate + late apoptotic rate.
Statistical analysis. Statistical Product and
Service Solutions (SPSS) 19.00 software (IBM Corp., Armonk, NY,
USA) was used for the analysis of the data in this study.
Measurement data were presented as mean ± standard deviation, the
differences in indexes between the two groups were compared and
analyzed using t-test, and the correlation between NOS1 and ABCG2
mRNA expression levels in cervical cancer tissues was analyzed via
Pearsons correlation analysis. P<0.05 was considered to indicate
a statistically significant difference.
Results
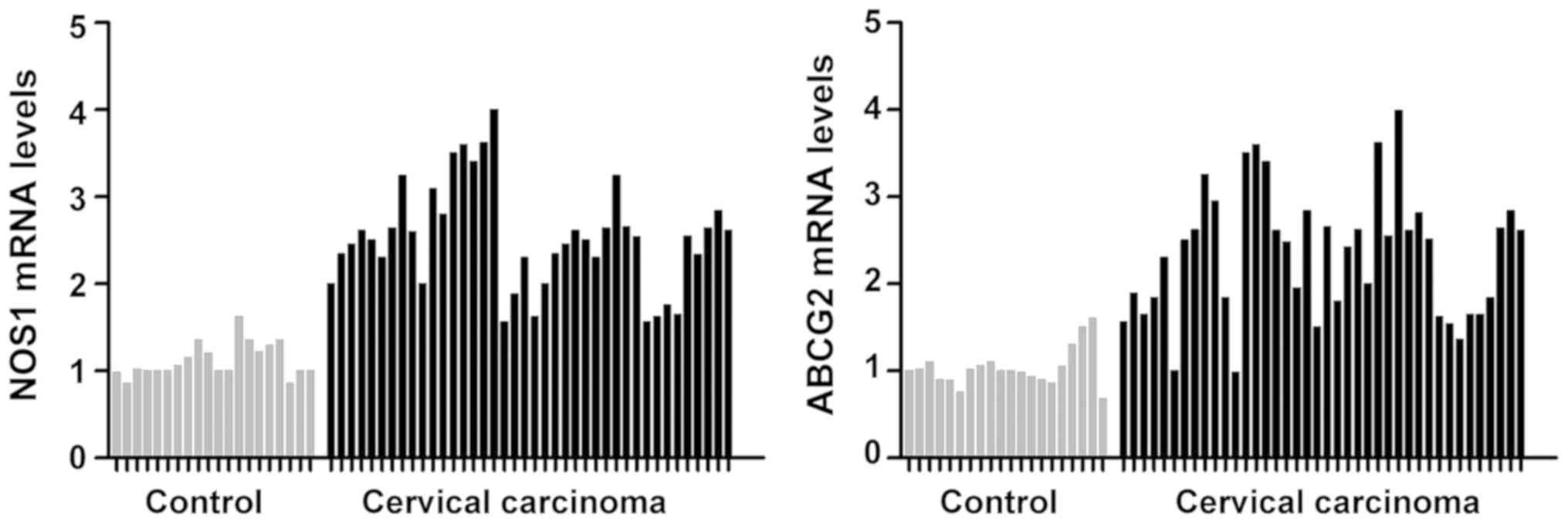
Detection of NOS1 and ABCG2 mRNA
levels in normal cervical tissues and cervical cancer tissues via
RT-qPCR
The mRNA levels of NOS1 and ABCG2 in 20 cases of
normal cervical tissues and 40 cases of cervical cancer tissues
were quantitatively detected via RT-qPCR. It was found that the
mRNA levels of NOS1 and ABCG2 in cervical cancer group were
significantly increased compared with those in the normal cervical
control group, and the mean differences were 2.63 and 2.02 times,
respectively (P<0.05) (Fig.
1).
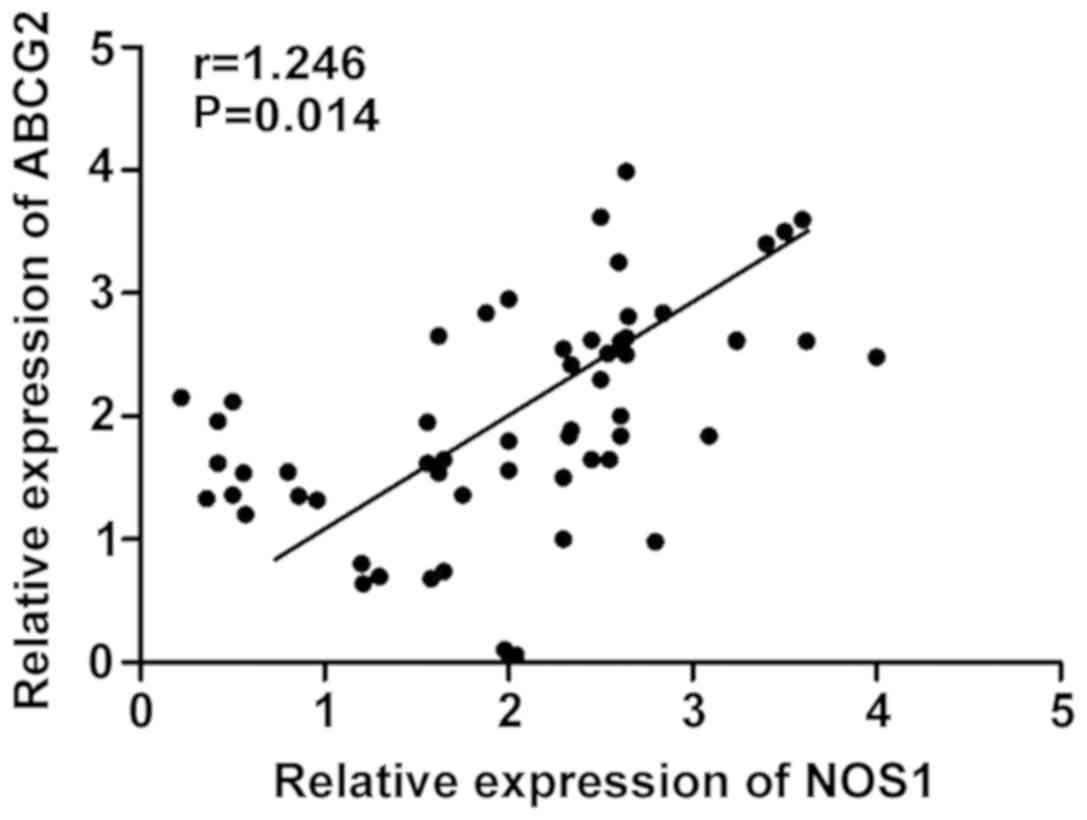
Detection of correlation between NOS1
and ABCG2 mRNA levels in cervical cancer tissues
The mRNA expression levels of NOS1 and ABCG2 in 40
cases of cervical cancer tissues were analyzed. Pearsons
correlation analysis revealed that there was a positive correlation
between NOS1 and ABCG2 mRNA expression levels in cervical cancer
tissues (r=1.246, P=0.014) (Fig.
2).
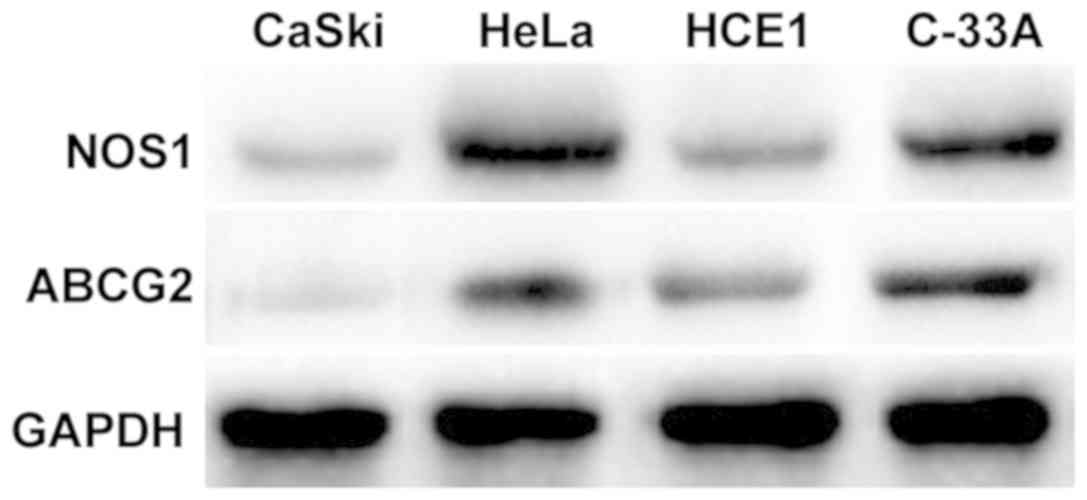
Expression of NOS1 and ABCG2 protein
in cervical cancer cell lines
Results of western blotting showed that NOS1 was
expressed in CaSki, HeLa, HCE1 and C-33A cells in cervical cancer,
among which the expression levels are relatively higher in HeLa and
C-33A cell lines (Fig. 3).
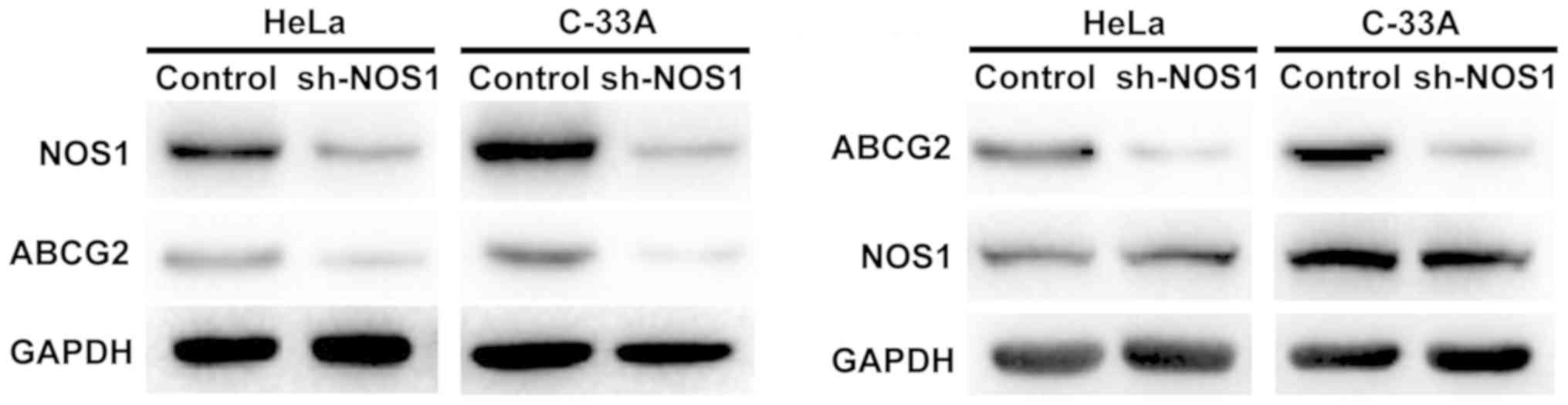
Detection of regulatory relationship
between NOS1 and ABCG2 via western blotting
HeLa and C-33A cell lines with relatively high
expression of NOS1 and ABCG2 were selected, and the regulatory
relationship between NOS1 and ABCG2 in cervical cancer cells was
detected using the lentiviral plasmid transfection technique.
First, after interference in the NOS1 expression in HeLa and C-33A
cells with sh-NOS1, it was found that the protein expression of
ABCG2 was also decreased. Furthermore, the protein expression level
of NOS1 remained unchanged after interference in the ABCG2
expression, suggesting that NOS1 may be an upstream regulatory
molecule of ABCG2 in cervical cancer cells (Fig. 4).
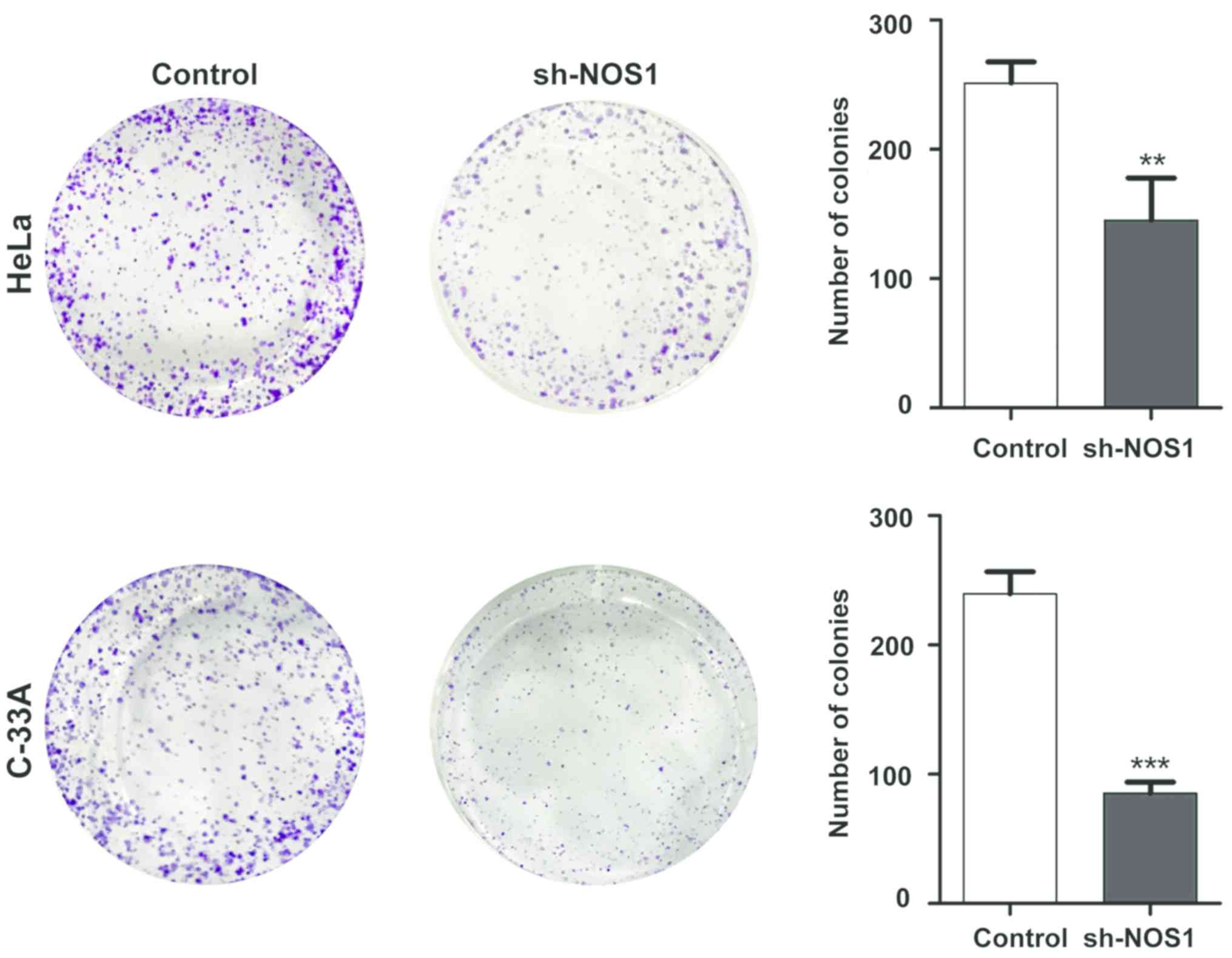
Detection of NOS1 silencing effect on
cell proliferation capacity via colony-forming assay
After cell lines with stable NOS1 interference were
established, the effect of NOS1 on proliferation of cervical cancer
cells was detected via colony-forming assay. Results demonstrated
that compared with those in the control group, the number of
proliferating HeLa cells and C-33A cells in the sh-NOS1 group was
significantly decreased, and there were statistically significant
differences (P<0.05) (Fig. 5).
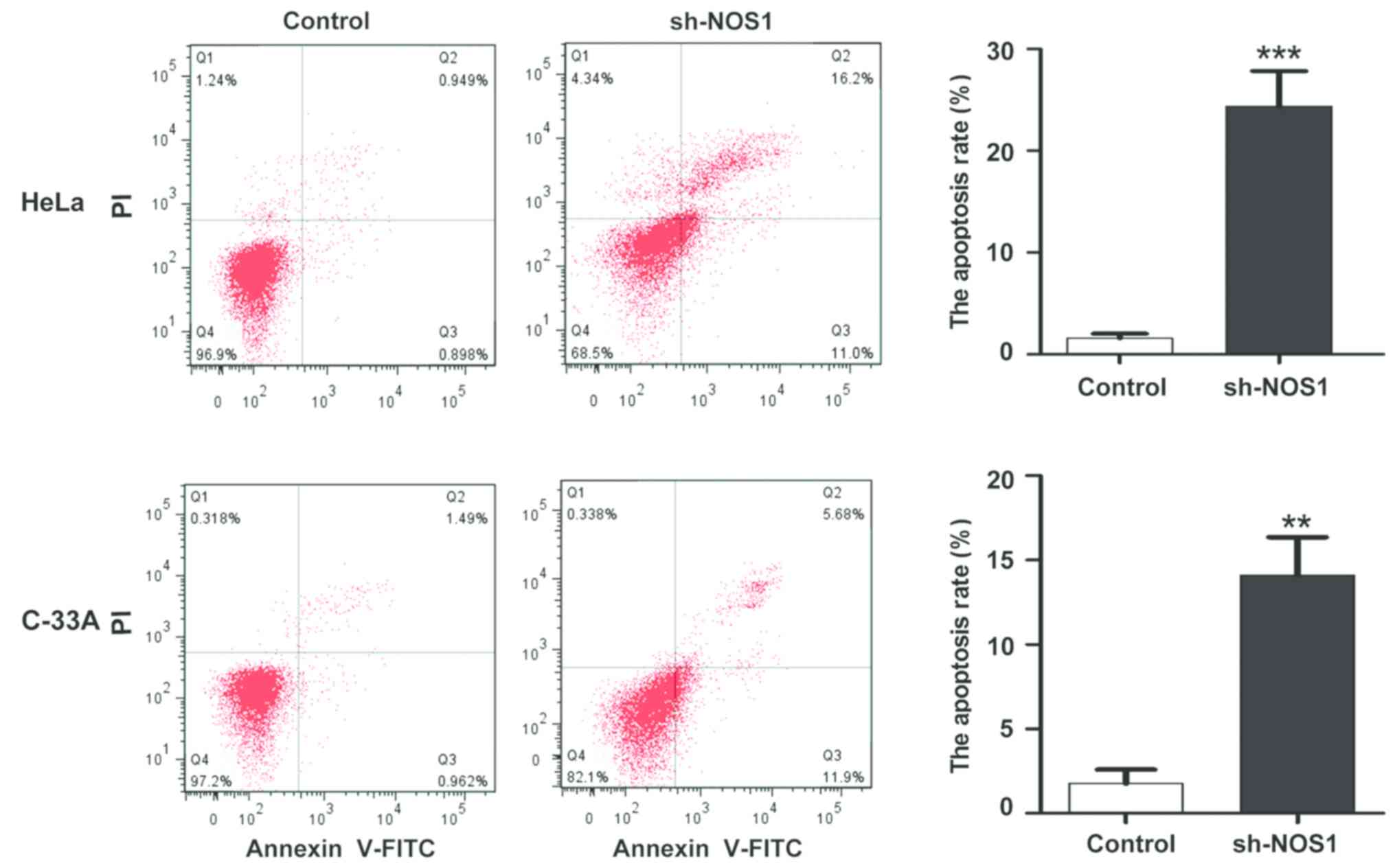
Detection of changes in apoptosis
level after NOS1 silencing via flow cytometry
After the NOS1 expression silencing in HeLa and
C-33A cells, changes in the apoptosis level were detected via flow
cytometry. Compared with those in the control group, the apoptosis
levels were obviously increased after NOS1 silencing (HeLa,
1.45±0.72 vs. 24.75±6.27%; C-33A, 2.11±1.16 vs. 17.24±7.82%),
displaying statistically significant differences (P<0.05)
(Fig. 6).
Discussion
In recent years, studies have demonstrated that NO,
as a kind of gas signal molecule, affects the biological functions
of a variety of malignant tumors. Fionda et al found that NO
can lead to DNA damage, cause mutation and induce genetic lesions
(14). Fahey and Girotti found that
NO promotes tumor progression through mediating key processes, such
as angiogenesis, tumor cell growth and invasion (15). However, NO in a low concentration has
a protective effect on mouse colon cancer in some tumor immune
responses (16). NOS is an enzyme
that catalyzes the NO biosynthesis. Due to the short half-life of
NO, it exerts the biological effect entirely depending on NOS. It
has already been verified that the main effect form of NOS1 is the
transient synthesis of NO in a lower concentration, while that of
NOS2 is the catalyzed synthesis of persistent NO in a sustainably
high concentration (17).
The related gene to the risk of prostate cancer NOS1
was also reported in a small case-control study involving 125 cases
(5). It has been proved that the
expression of NOS1 is remarkably increased in several kinds of
human cancer specimens and precancerous chronic inflammation
specimens (18). Recently, a global
genetic screening experiment revealed that NOS2 and NOS3 gene
polymorphisms are correlated with the risk of prostate cancer,
especially invasive cancer, in Caucasians and African-Americans
(19). Wang et al studied the
correlations of NOS1 repeat polymorphism with high histological
grading of prostate cancer and shedding of circulating tumor cells
in the blood. These findings are supported by evidence, in other
words, NOS1 promotes tumor growth rate, vascular density and
invasiveness, and maintains the blood supply of prostate tumor
(20). Moreover, there are studies
showing that in p53-mutated tumor cells, NOS increases the
expression of vascular endothelial growth factor and promotes tumor
growth, indicating that the tumor-killing activity of NO depends on
the state of p53 (21).
Overexpression of ABCG2 promotes migration and
invasion of a variety of tumors. Previous studies manifested that
AGBC2 may be regulated by DNA methylation, 5′-untranslated region
mutation, histone modifications, cytokines and growth factors, such
as TGF-β, TNF-α and IL1-β2 (21).
According to recent studies, in addition to affecting the
chemotherapy sensitivity of various malignant tumors, ABCG2, as a
transporter of glutathione, plays an important role in
intracellular redox balance (22).
Moreover, it was found that ABCG2 can limit the intake of
chlorophyllin A and porphyrins, thereby affecting the singlet
oxygen production in cells, and leading to DNA hydrolysis and
apoptosis (23). Studies have
confirmed that NOS enhances the activity of antioxidation-related
transcription factors (HIF-1α and Nrf-2), and upregulates the
transcription of antioxidant substance SOD through its product NO,
thus enhancing the tolerance of tumor cells to the hypoxia
environment after chemotherapy, and reducing tumor cell apoptosis
(24). However, there has been no
report on whether NOS1 and multidrug resistance gene ABCG2 are
involved in the proliferation and apoptosis of cervical cancer
cells.
In this study, the mRNA levels of NOS1 and ABCG2 in
20 cases of benign cervical tissues and 40 cases of human cervical
cancer tissues were studied. It was found that the mRNA levels of
NOS1 and ABCG2 in the cervical cancer group were significantly
increased compared with those in the normal cervical control group,
and the mean differences were 2.63 and 2.02 times, respectively.
Pearsons correlation analysis revealed that there was a positive
correlation between NOS1 and ABCG2 mRNA expression levels in
cervical cancer tissues (r=1.246, P=0.014). To investigate the
interaction between NOS1 and ABCG2 in cervical cancer, western
blotting was performed for cervical cancer cells (CaSki, HeLa, HCE1
and C-33A), and results manifested that NOS1 and ABCG2 were
expressed in the 4 kinds of cell lines. HeLa and C-33A cell lines
with relatively high expression of NOS1 and ABCG2 were selected for
the in vitro study. After interference in the NOS1
expression in HeLa and C-33A cells with sh-NOS1, the protein
expression of ABCG2 was also decreased. Interestingly, the protein
expression level of NOS1 remained unchanged after interference in
the ABCG2 expression. According to the above results, it is
speculated that NOS1 may be an upstream regulatory molecule of
ABCG2 in cervical cancer cells. The proliferation capacities of
HeLa and C-33A cells were obviously decreased, but the apoptosis
levels were significantly increased after interference in the NOS1
expression, which were consistent with the expected results.
However, the specific regulatory molecular mechanisms of NOS1 and
ABCG2 still need further study.
In conclusion, the overexpression of NOS1 and ABCG2
was found for the first time in clinical specimens of cervical
cancer, and the in vitro experiments showed that the
activity of NOS1, as the upstream regulatory molecule of ABCG2, had
a significantly negative correlation with tumor cell proliferation.
NOS1 and ABCG2 are expected to be new targets for the treatment of
cervical cancer, providing new strategies for the clinical
treatment of cervical cancer patients.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
MD and HZ were responsible for treating patients,
RT-qPCR and western blotting. LL helped with the collection of
clinical specimens. LL and RL contributed to cell culture and
lentivirus transfection. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Affiliated Hospital of Taishan Medical University (Taian, China)
and informed consents were signed by the patients or the
guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Vaccarella S, Franceschi S, Engholm G,
Lönnberg S, Khan S and Bray F: 50 years of screening in the Nordic
countries: Quantifying the effects on cervical cancer incidence. Br
J Cancer. 111:965–969. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pfaendler KS and Tewari KS: Changing
paradigms in the systemic treatment of advanced cervical cancer. Am
J Obstet Gynecol. 214:22–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ronco G, Dillner J, Elfström KM, Tunesi S,
Snijders PJ, Arbyn M, Kitchener H, Segnan N, Gilham C, Giorgi-Rossi
P, et al: International HPV screening working group: Efficacy of
HPV-based screening for prevention of invasive cervical cancer:
Follow-up of four European randomised controlled trials. Lancet.
383:524–532. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lo Faro ML, Fox B, Whatmore JL, Winyard PG
and Whiteman M: Hydrogen sulfide and nitric oxide interactions in
inflammation. Nitric Oxide. 41:38–47. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bogdan C: Nitric oxide synthase in innate
and adaptive immunity: An update. Trends Immunol. 36:161–178. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
King AL, Polhemus DJ, Bhushan S, Otsuka H,
Kondo K, Nicholson CK, Bradley JM, Islam KN, Calvert JW, Tao YX, et
al: Hydrogen sulfide cytoprotective signaling is endothelial nitric
oxide synthase-nitric oxide dependent. Proc Natl Acad Sci USA.
111:3182–3187. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Marigo I, Zilio S, Desantis G, Mlecnik B,
Agnellini AHR, Ugel S, Sasso MS, Qualls JE, Kratochvill F,
Zanovello P, et al: T cell cancer therapy requires CD40-CD40L
activation of tumor necrosis factor and inducible
nitric-oxide-synthase-producing dendritic cells. Cancer Cell.
30:377–390. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Guo FQ and Crawford NM: Arabidopsis nitric
oxide synthase1 is targeted to mitochondria and protects against
oxidative damage and dark-induced senescence. Plant Cell.
17:3436–3450. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ambs S, Merriam WG, Bennett WP,
Felley-Bosco E, Ogunfusika MO, Oser SM, Klein S, Shields PG,
Billiar TR and Harris CC: Frequent nitric oxide synthase-2
expression in human colon adenomas: Implication for tumor
angiogenesis and colon cancer progression. Cancer Res. 58:334–341.
1998.PubMed/NCBI
|
|
10
|
Xu W, Liu LZ, Loizidou M, Ahmed M and
Charles IG: The role of nitric oxide in cancer. Cell Res.
12:311–320. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang F, Xue X, Wei J, An Y, Yao J, Cai H,
Wu J, Dai C, Qian Z, Xu Z, et al: hsa-miR-520h downregulates ABCG2
in pancreatic cancer cells to inhibit migration, invasion, and side
populations. Br J Cancer. 103:567–574. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jiang Y, He Y, Li H, Li HN, Zhang L, Hu W,
Sun YM, Chen FL and Jin XM: Expressions of putative cancer stem
cell markers ABCB1, ABCG2, and CD133 are correlated with the degree
of differentiation of gastric cancer. Gastric Cancer. 15:440–450.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fionda C, Abruzzese MP, Zingoni A, Soriani
A, Ricci B, Molfetta R, Paolini R, Santoni A and Cippitelli M:
Nitric oxide donors increase PVR/CD155 DNAM-1 ligand expression in
multiple myeloma cells: Role of DNA damage response activation. BMC
Cancer. 15:172015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fahey JM and Girotti AW: Accelerated
migration and invasion of prostate cancer cells after a
photodynamic therapy-like challenge: Role of nitric oxide. Nitric
Oxide. 49:47–55. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Puglisi MA, Cenciarelli C, Tesori V,
Cappellari M, Martini M, Di Francesco AM, Giorda E, Carsetti R,
Ricci-Vitiani L and Gasbarrini A: High nitric oxide production,
secondary to inducible nitric oxide synthase expression, is
essential for regulation of the tumour-initiating properties of
colon cancer stem cells. J Pathol. 236:479–490. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang X, Chandrashekar K, Wang L, Lai EY,
Wei J, Zhang G, Wang S, Zhang J, Juncos LA and Liu R: Inhibition of
nitric oxide synthase 1 induces salt-sensitive hypertension in
nitric oxide synthase 1α knockout and wild-type mice. Hypertension.
67:792–799. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rabender CS, Alam A, Sundaresan G,
Cardnell RJ, Yakovlev VA, Mukhopadhyay ND, Graves P, Zweit J and
Mikkelsen RB: The role of nitric oxide synthase uncoupling in tumor
progression. Mol Cancer Res. 13:1034–1043. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang J, He P, Gaida M, Yang S, Schetter
AJ, Gaedcke J, Ghadimi BM, Ried T, Yfantis H, Lee D, et al:
Inducible nitric oxide synthase enhances disease aggressiveness in
pancreatic cancer. Oncotarget. 7:52993–53004. 2016.PubMed/NCBI
|
|
20
|
Wang J, Yang S, He P, Schetter AJ, Gaedcke
J, Ghadimi BM, Ried T, Yfantis HG, Lee DH, Gaida MM, et al:
Endothelial nitric oxide synthase traffic inducer (NOSTRIN) is a
negative regulator of disease aggressiveness in pancreatic cancer.
Clin Cancer Res. 22:5992–6001. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Basudhar D, Somasundaram V, de Oliveira
GA, Kesarwala A, Heinecke JL, Cheng RY, Glynn SA, Ambs S, Wink DA
and Ridnour LA: Nitric oxide synthase-2-derived nitric oxide drives
multiple pathways of breast cancer progression. Antioxid Redox
Signal. 26:1044–1058. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tang Y, Hou J, Li G, Song Z, Li X, Yang C,
Liu W, Hu Y and Xu Y: ABCG2 regulates the pattern of self-renewing
divisions in cisplatin-resistant non-small cell lung cancer cell
lines. Oncol Rep. 32:2168–2174. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Noguchi K, Katayama K and Sugimoto Y:
Human ABC transporter ABCG2/BCRP expression in chemoresistance:
Basic and clinical perspectives for molecular cancer therapeutics.
Pharm Genomics Pers Med. 7:53–64. 2014.
|
|
24
|
Lagas JS, van Waterschoot RA, Sparidans
RW, Wagenaar E, Beijnen JH and Schinkel AH: Breast cancer
resistance protein and P-glycoprotein limit sorafenib brain
accumulation. Mol Cancer Ther. 9:319–326. 2010. View Article : Google Scholar : PubMed/NCBI
|