Introduction
Epithelial ovarian cancer (EOC) is among the most
common gynecological malignancies. Despite substantial improvements
in surgery and chemotherapy, the survival rate of patients with EOC
remains low, since the majority of patients with ovarian cancer
relapse and/or become drug-resistant following a period of
chemotherapy (1). Thus, identifying
the potential biomarkers of chemoresistance is of great
significance for EOC.
Nitric oxide (NO) is a lipophilic, highly diffusible
and short-lived physiological messenger (2). Endogenous NO may be produced by
different isoforms of NO synthase (NOS), including NOS1, 2 and 3,
during arginine metabolism. Among the three types of NOS; NOS1 and
3, are constitutively expressed in cells and thus termed as cNOS,
may produce a low level of NO transiently in a
Ca2+-dependent manner (3).
Meanwhile, NOS2, which is induced by inflammatory factors and
cytokines in a Ca2+-independent manner, generates high
and sustained concentrations of NO (4). At high concentrations (>500 nM), NO
induces an inflammatory reaction, apoptosis and other process that
inhibit tumor biological functions, whereas NO at lower, more
physiological levels (<100 nM) typically exhibits the opposite
effects, promoting tumor behaviors including metastasis,
angiogenesis and chemoresistance (5,6).
Previously, chemoresistant phenotypes of colon cancer, head and
neck cancer and human breast carcinoma cells have been reported to
be associated with different NOS isoforms (5,7,8). NOS2-derived NO may induce apoptosis in
cancer cells, while the lower concentration of NO typically
generated by NOS1 may inhibit apoptosis and promote chemoresistance
(9). However, the mechanism
underlying NOS1 associated chemoresistance is unclear.
ATP-binding cassette, subfamily G, member 2 (ABCG2),
also known as breast cancer resistance protein, was originally
cloned from multidrug-resistant breast cancer cells, and its
upregulation has been linked to chemoresistance in various cancer
cells, including ovarian cancer (10). ABCG2 extrudes xenobiotics and certain
drugs from cells, thereby mediating drug resistance and affecting
the pharmacological behavior of many compounds (11). Additionally, ABCG2 has been revealed
to be a transporter of glutathione, which is vital for the
maintenance of cellular redox balance (12). It was also been documented that human
ABCG2 could limit the uptake of pheophorbide-A and many porphyrin
derivatives, both of which can produce singlet oxygen, which causes
DNA fragmentation and caspase-3 dependent apoptosis (12). Therefore, ABCG2 may play an important
role in chemotherapy resistance through its redox related
function.
It has been reported that NO endogenously produced
by NOS enzymes promotes the transcriptional activity of
antioxidant-related transcription factors, including
hypoxia-inducible factor (HIF)-1α and nuclear factor erythroid
2-related factor 2 (Nrf2); these then induce transcription of
antioxidant factors including superoxide dismutase and thioredoxin
reductase 1, thereby increasing the ability of cancer cells to
resist chemotherapy-induced reactive oxygen species stress, and
thus decreasing sensitivity to chemotherapy-induced apoptosis
(13–16). Cis-diamminedichloroplatinum
(cisplatin/DDP), a chemotherapy drug, destroyed tumor cells by
binding to DNA strands, interfering with DNA replication. DDP has
been one of first lines of defense against tumors, especially those
of ovary, lung and testes (17).
However to date, it has remained to be investigated whether
NOS1/N-related chemotherapy resistance is associated with the
multidrug resistance-associated gene ABCG2 in the regulation of
redox balance.
In the present study, the plausible mechanisms of
NOS1 contribution to DDP resistance via ABCG2 in EOC were
investigated. In order to detect the expression changes of NOS1 and
ABCG2 prior to and following DDP incubation, western blot analysis
was performed to detect the protein expression level. Additionally,
the levels of NOS1 and ABCG2 in chemoresistant and chemosensitive
ovarian cancer profiles were detected by GEO database analysis. In
addition, protein levels of ABCG2 were detected by western blot and
immunohistochemistry (IHC) staining, in order to investigate the
impact of NOS1 on ABCG2, MTT and flow cytometry analysis were used
to detect cell viability and apoptosis. The present results may
provide a basis for chemotherapeutic improvement via NOS1
inhibition.
Materials and methods
Reagents and plasmids
The following chemicals and plasmids were used:
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
(MTT), dimethyl sulfoxide (DMSO), ABCG2 inhibitor verapamil
hydrochloride (20 µM; used in the MTT assay), specific NOS1
inhibitor Nω-propyl-L-arginine hydrochloride
(N-PLA), specific NOS2 inhibitor 1400W dihydrochloride and general
NOS inhibitor Nω-nitro-L-arginine methyl
ester hydrochloride (L-NAME) (all Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany); DETA-NONOate was purchased from Cayman
Chemical Company (Ann Arbor, MI, USA); FastQuant RT kit and
SYBR-Green PCR kit (Takara Bio, Inc., Otsu, Japan); rabbit
monoclonal anti-NOS1 antibody (ab76067) and mouse monoclonal
anti-ABCG2 antibody (ab3380; Abcam, Cambridge, MA, USA); mouse
monoclonal anti-GAPDH antibody (ZSGB-BIO, Beijing, China); a
fluorescein isothiocyanate-Annexin V apoptosis detection kit (BD
Biosciences, San Jose, CA, USA); TRIzol reagent (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA); lentiviral plasmid
GV375-NOS1, negative control GV375-NC, enhanced infection solution
and polybrene (Shanghai GeneChem Co., Ltd., Shanghai, China); small
interfering (si)RNAs for NOS1 and negative control siRNA (Guangzhou
Ribobio Co., Ltd., Guangzhou, China); Lipofectamine 2000 reagent
(Thermo Fisher Scientific, Inc.); and an IHC universal SP test kit
(ZSGB-BIO).
GEO database assays analysis
Gene expression profiles from the Gene Expression
Omnibus database (www.ncbi.nlm.nih.gov/geo; GSE26712 and GSE51373) were
downloaded. The data in GSE26712 included 185 advanced ovarian
cancer cases, and in GSE51373 were 28 high-grade serous ovarian
cancer cases. Serous ovarian carcinoma is divided into high-grade
serous (HGSOC) and low-grade serous ovarian carcinoma (LGSOC). The
two-tier system based on nuclear atypia was introduced in 2004 by
the University of Texas M.D. Anderson Cancer Center (MDACC)
(18). Despite large amount of gene
expression data in the assays, we only analyzed the expression data
of ABCG2 and NOS1. The correlation of expression of NOS1 and ABCG2
was analyzed by Pearson correlation coefficient analysis. In
addition, the expression differences of NOS1 and ABCG2 were
detected in DDP-sensitive and DDP-resistant patients.
Cell culture and transfection
The human ovarian cancer lines OVCAR-3 and SKOV-3,
purchased from the Chinese Academy of Sciences (Shanghai, China),
were grown in Dulbecco's modified Eagle's medium (Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum
(BI, Salt Lake City, UT, USA; www.bioind.com/worldwide/), 100 U/ml penicillin and
100 µg/ml streptomycin, and incubated at 37°C in a humidified
chamber supplemented with 5% CO2.
The lentiviral plasmid GV375-NOS1 and negative
control GV375-NC were individually transfected into OVCAR-3 cells,
and the enhanced infection solution and polybrene were used to
concentrate the lentiviral supernatant. Briefly, 3×104
OVCAR-3 cells were plated in 24-well culture plates. When the
confluence reached 20–30%, lentiviral supernatant was added to the
wells (5 µl/well, 1×108 transducing units/ml). Puromycin
(5 µg/ml) was used to select for positive clones. The transfected
OVCAR-3 cells (hereafter termed GV375-NOS1 ov-3 and GV375-NC ov-3)
were used in further experiments.
The sequence of the NOS1 siRNA was: Forward,
5′-GGUCUAUCCAAUGUCCACAdTdT-3′ and reverse,
3′-dTdTCCAGAUAGGUUACAGGUGU-5′. The target sequence was:
GGTCTATCCAATGTCCACA. The siRNAs for NOS1 and the negative control
(si-NC) were designed and synthesized by Guangzhou Ribobio Co.,
Ltd. The NOS1-specific and negative control siRNAs (100 nM,
Transfection efficiency: >80%) were transfected into SKOV-3
cells using Lipofectamine 2000 reagent according to the
manufacturer's protocol. Cells were then incubated at 37°C for
48–72 h and collected to assess transfection efficiency by western
blotting and real time PCR, and were then used in further
experiments.
Flow cytometry analysis
Cultured cells were trypsinized and resuspended in
phosphate-buffered saline (PBS). To examine whether NOS is involved
in promoting platinum-based chemotherapy resistance, apoptosis
induction was detected by flow cytometric analysis using a FACS
Aria II (BD Biosciences). For this, a fluorescein
isothiocyanate-Annexin V apoptosis detection kit was used according
to the manufacturer's instructions. Cells were incubated with 2 µM
DDP only or 2 µM DDP plus either 1 mM L-NAME, 100 µM N-PLA, 100 µM
1400W or 20 µM DETA-NONOate at 37°C for 48 h without changing the
medium. The ratios of apoptotic cells in the treated and untreated
groups were analyzed with FlowJo software (version 7.6.1; Tree
Star, Inc., Ashland, OR, USA).
Assessment of cell viability
OVCAR-3 and SKOV-3 cell viabilities were determined
by MTT assay following transfection or treatment with different
drugs (2 µM DDP, 20 µM verapamil, 1 mM L-NAME, 100 µM N-PLA, 100 µM
1400W or 20 µM DETA-NONOate) for 48 h. Briefly, cells were seeded
in 96-well tissue culture plates (100 µl/well) at a density of
5×103 cells/well. After the cell monolayer reached
50–60% confluence, the culture medium of each well was refreshed
with 100 µl medium combined with drugs as described above. After 48
h, the cells were incubated with MTT solution (2 mg/ml) for 4 h.
Subsequently, the MTT solution was removed and DMSO (150 µl/well)
was added. A Multiskan Spectrum spectrophotometer (Thermo Fisher
Scientific, Inc.) was used for measuring absorbance at 490 nm. The
half maximal inhibitory concentration (IC50) was calculated using
the following equation: IC50=(mean OD of specific treatment
group)/(mean OD of negative control group)%.
RNA extraction and real-time PCR
Total RNA from L-NAME-treated and untreated OVCAR-3
cells was extracted with TRIzol Reagent following the
manufacturer's protocol. First strand cDNA synthesis and
amplification were performed using a FastQuant RT kit. The cDNA was
used to amplify ABCG2 and endogenous control GAPDH via PCR.
SYBR-Green was used for quantitative PCR. The PCR cycles were as
follows: 95°C for 15 min, followed by 40 cycles of 95°C for 10 sec
and 60°C for 32 sec, performed in a Mx3005 Sequence Detection
system (Stratagene, La Jolla, CA, USA). The comparative
Cq (ΔΔCq) method (19) was used to determine the expression
fold change. The primer sequences used were as follows: For GAPDH
forward, 5′-GAAGGTGAAGGTCGGAGTC-3′ and reverse,
5′-GAAGATGGTGATGGGATTTC-3′; and for ABCG2, forward,
5′-ACGAACGGATTAACAGGGTCA-3′ and reverse,
5′-CTCCAGACACACCACGGAT-3′.
Western blot analysis
OVCAR-3 cells following transfection or treatment
with different drugs (DDP, L-NAME, N-PLA or 1400W) for 48 h and
negative control cells were lysed in a radioimmunoprecipitation
assay lysis buffer (Thermo Fisher Scientific, Inc.) containing 1 mM
phenylmethylsulfonyl fluoride. The proteins were separated by 10%
SDS denaturing polyacrylamide gel and then transferred onto
polyvinylidene difluoride membranes. The membranes were blocked in
5% bovine serum albumin (BI, Salt Lake City, UT, USA) at room
temperature for 2 h, and blotted with the mouse antihuman antibody
against ABCG2 and the rabbit anti-human antibody against NOS1 at
their respective recommended dilution (1:1,000) overnight at 4°C,
and then incubated with the appropriate secondary antibody (FD0128;
FD0142; 1:5,000; Fude Biological Technology Co. Ltd. China) at room
temperature for 1 h. Following a wash with tris-buffered saline
with Tween-20, visualization of the secondary antibody was
performed using a chemiluminescence detection assay (ChemiDoc XRS+
Imaging System; Bio-Rad Laboratories, Inc., Hercules, CA, USA)
according to the manufacturer's protocol. Image Lab software
(version 3.0.1 Bio-Rad Laboratories, Inc.) was used to quantify
band intensities. GAPDH (1:1,000) was used as a loading
control.
Animal experiments
Animal experiments were approved by the Ethical
Committee of Southern Medical University (Guangzhou, China). A
total of 12 mice were randomly assigned to two groups (n=6/group)
of BALB/c-nu female mice (4–6 weeks old; 18–20 g) were housed in
specific pathogen-free facilities under 12-h light/dark cycles. The
feeding environment temperature was 18–29°C and the humidity was
40–70%. Animals' foods were sterile and highly nutritional and
water was clean filtered, access to food and water was ad
libitum. A total of 5×106 GV375-NOS1 ov-3 or
GV375-NC ov-3 cells were pelleted (450 × g for 5 min at room
temperature), resuspended in 100 ml Matrigel (BD Biosciences) and
injected into the right flank of nude mice. The tumor volumes were
calculated with the formula: Volume (mm3)=width × width
× length ×0.5. Mice were sacrificed when tumors reached 50
mm3, and the tumor tissues were excised and weighed.
IHC staining
The tumor tissues were fixed by 10% neutral buffered
formalin (Wexis, Guangzhou, China) at room temperature for 24 h.
The slides (5 mm sections) of xenografted tumors were
deparaffinized using xylene and dehydrated using ethanol, then
washed in PBS. For antigen unmasking, slides were placed in a
container, covered with citrate buffer (pH 6.0) and heated in a
steamer for 5 min at 100°C. The slides were then washed and blocked
with 5% normal goat serum (BioLab, Beijing, China; http://www.hbzhan.com/st161595/erlist_692382.html) for
30 min, then incubated with primary antibody (anti-ABCG2, 1:100)
overnight at 4°C, and subsequently stained with horseradish
peroxidase-coupled secondary antibodies (FD0128; 1:200; FD Science,
Hangzhou, China) at room temperature for 30 min. The slides were
then visualized using an upright light microscope (Nikon
Corporation, Tokyo, Japan).
Statistical analysis
Statistical analyses were performed in SPSS version
19.0 software for Windows (IBM, Corp., Armonk, NY, USA). All
experiments were performed at least in triplicate. The expression
levels of NOS1 and ABCG2 were compared between two groups by
Student t-tests. Multiple comparisons were performed by a one-way
analysis of variance followed by a least significant difference
post-hoc test. Pearson's correlation analysis was used to assess
the association between the mRNA levels of NOS1 and ABCG2. Data are
presented as the mean ± standard deviation. Significance was
determined at P<0.05.
Results
Expression of NOS1 and ABCG2 is
increased following treatment with DDP
To determine the association between the expression
of NOS1 and ABCG2 and the chemoresistant phenotype in EOC, the mRNA
levels of NOS1 and ABCG2 and their potential correlation was
investigated in gene expression profiles from the Gene Expression
Omnibus database (www.ncbi.nlm.nih.gov/geo; GSE26712 and GSE51373;
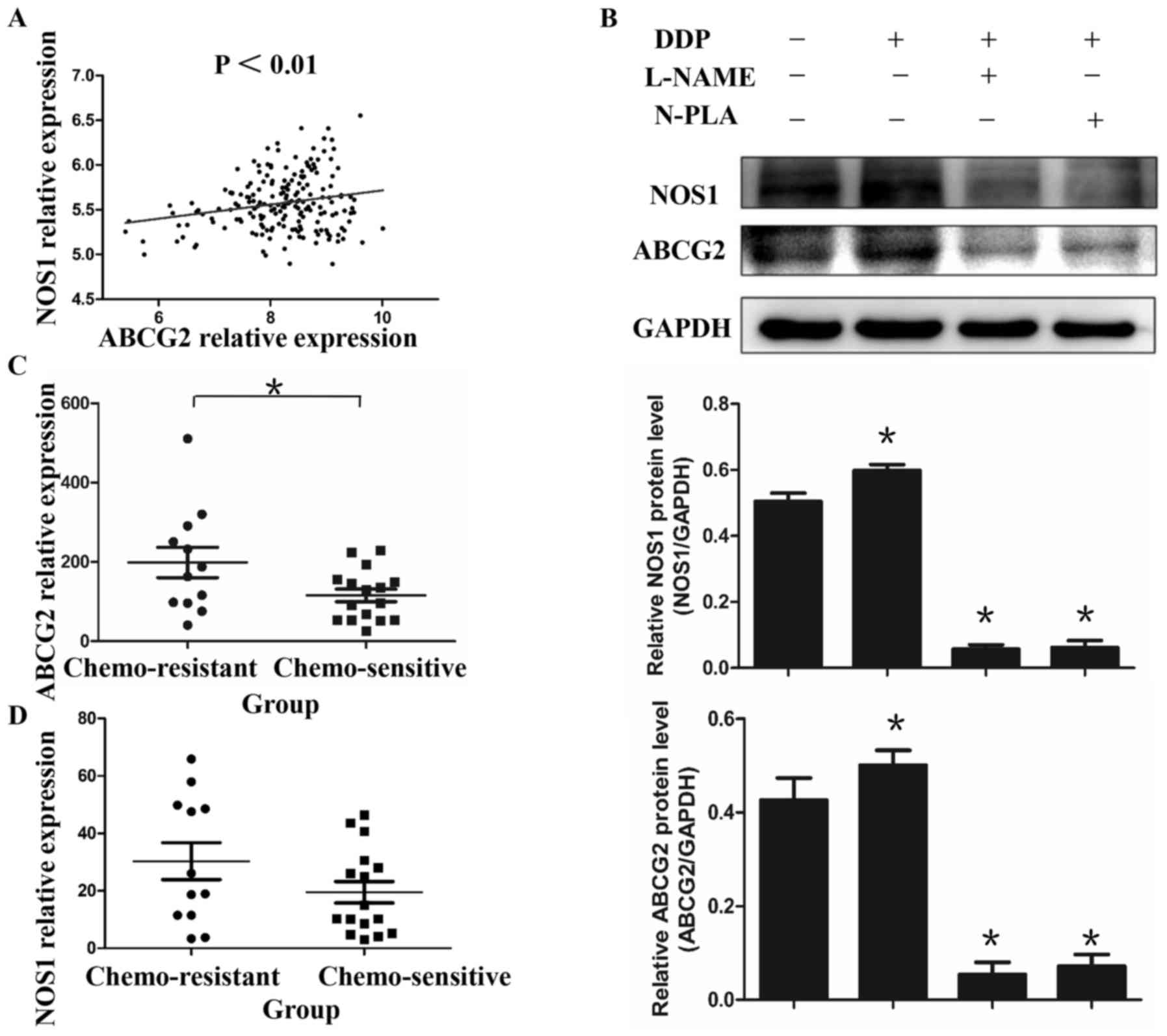
Fig. 1). It was identified that the
mRNA levels of NOS1 and ABCG2 were significantly correlated in EOC
(Fig. 1A; r=0.2115; P<0.01).
Furthermore, the expression of the ABCG2 gene was higher in
chemoresistant EOC tissues compared with that in chemosensitive
tissues represented in GSE26712 and GSE51373, but no significant
difference was observed in the mRNA levels of NOS1 (Fig. 1C and D). This phenomenon was verified
in vitro by evaluating alteration of the protein levels of
NOS1 and ABCG2 in EOC cell lines following DDP treatment. It was
observed that the treatment with DDP (2 µM, 48 h) increased the
protein levels of ABCG2 and NOS1 when assessed by immunoblotting.
Notably, this enhanced expression of both genes by DDP treatment
could be reduced by the non-selective NOS inhibitor L-NAME or the
NOS1 selective inhibitor N-PLA (Fig.
1B; P<0.05), suggesting that NOS1 may regulate the
expression of ABCG2.
NOS1 upregulates the expression of
ABCG2
It has been demonstrated that NOS1 may promote the
transcription of numerous genes by activating transcription factors
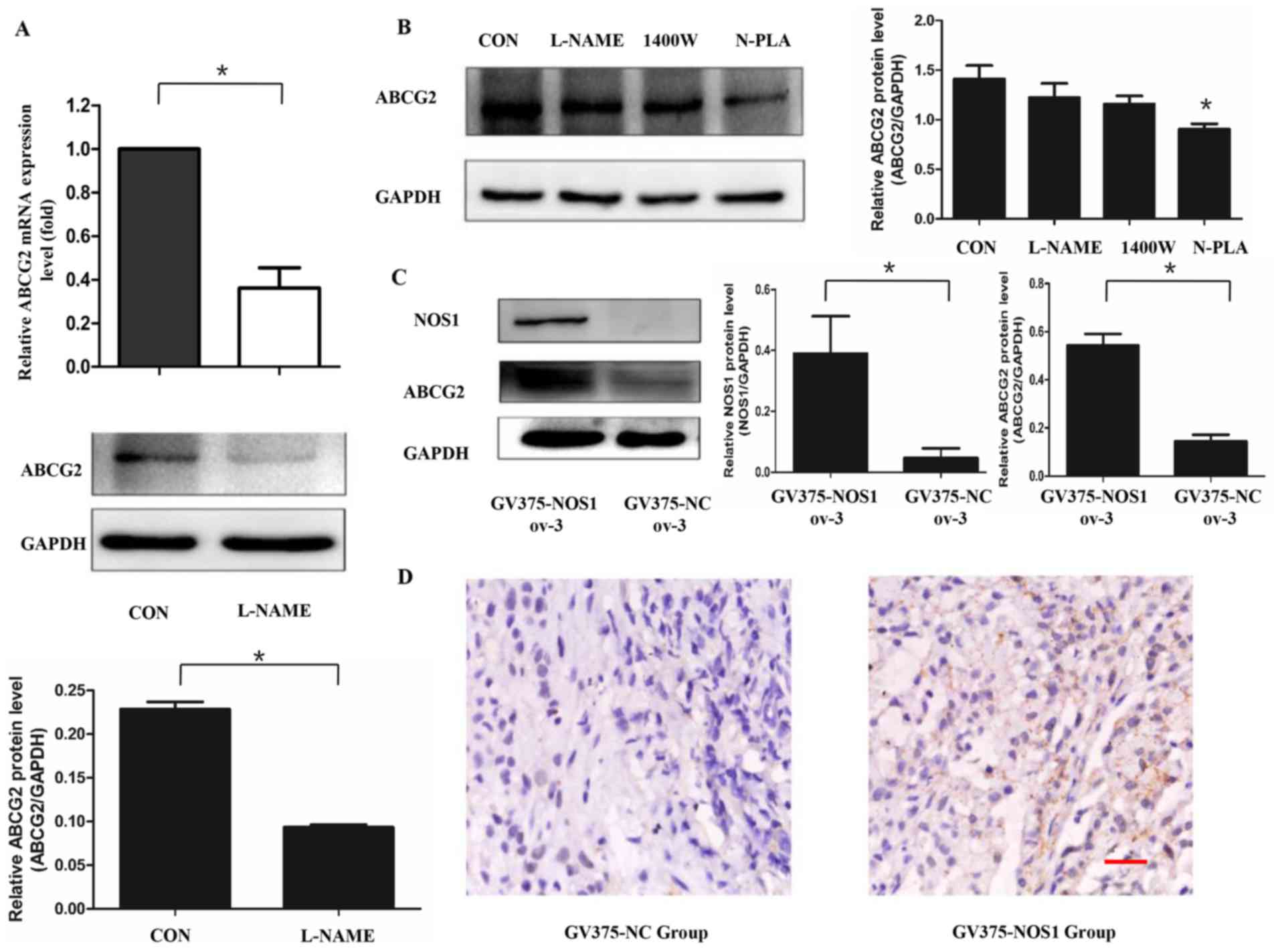
(15,20). The change of ABCG2 expression in
OVCAR-3 cells was analyzed in response to functional modulation of
NOS by L-NAME treatment. As depicted in Fig. 2A, the mRNA and protein levels of ABCG2
were decreased following L-NAME treatment detected by real-time PCR
(0.36-fold; P<0.05) and western blot analysis (t=44.43,
P<0.05). The experiments were repeated 3 times. Subsequently,
the different contributions of NOS isoforms to ABCG2 regulation was
analyzed using NOS selective inhibitors. The expression of ABCG2
was downregulated by N-PLA in comparison with that in control cells
(Fig. 2B, P<0.05). By contrast,
L-NAME and the NOS2 specific inhibitor 1400W did not induce a
change in ABCG2 expression (P>0.05). The impact of NOS1 gene
overexpression on the expression of ABCG2 was next investigated by
comparing the expression levels of ABCG2 in OVCAR-3 cells
constitutively overexpressing NOS1 (GV375-NOS1 ov-3) and the
negative controls (GV375-NC ov-3). It was observed that the
overexpression of NOS1 upregulated ABCG2 expression when compared
with in the negative control group (Fig.
2C; NOS1:t=5.006, P<0.05; ABCG2:t=17.89, P<0.05). The
experiments were repeated 3 times.
Furthermore, GV375-NOS1 ov-3 and GV375-NC ov-3 cells
were injected into the backs of nude mice. The expression of ABCG2
was detected in the xenograft cell model by IHC staining, and it
was found that the overexpression of NOS1 increased the expression
of ABCG2 in the resulting tumor tissues of nude mice (Fig. 2D). These results suggested that NOS1
expressed by ovarian cancer cells could increase the expression of
ABCG2 in vitro and in vivo.
NOS1 contributes to DDP resistance in
ovarian cancer cell lines
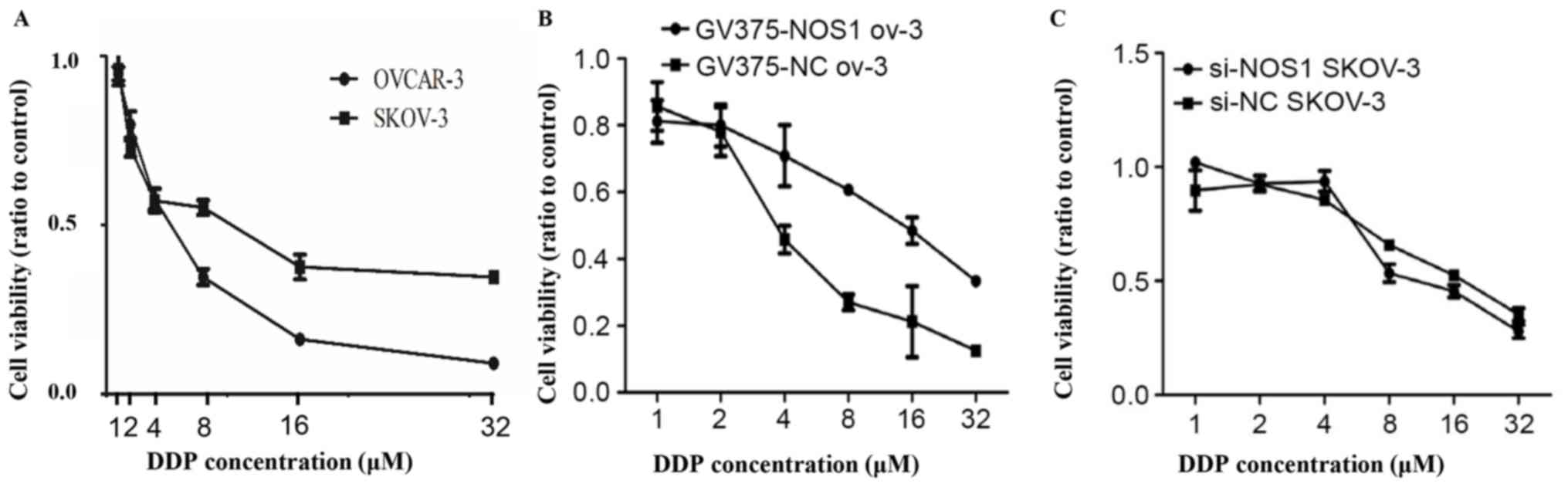
Two ovarian cancer cell lines with differing DDP
resistance, OVCAR-3 and SKOV-3, were examined by MTT assay to
investigate whether NOS1 expression was associated with DDP
resistance. Consistent with published findings that SKOV-3 is more
chemoresistant than OVCAR-3 (21),
the present study observed that the IC50 of SKOV-3 and OVCAR-3
cells for cisplatin was 11.3193±0.192 and 6.5093±1.902 µM,
respectively (Fig. 3A). Subsequently,
NOS1 was overexpressed in OVCAR-3 cells and it was revealed that
DDP resistance was enhanced in the cells following the
overexpression, compared with controls (Fig. 3B). Meanwhile, knockdown of NOS1 by
using RNA interference in SKOV-3 cells somewhat reduced the DDP
resistance at higher concentrations (8–32 µM) of DPP (Fig. 3C). These results indicated that NOS1
expression may partially promote DDP resistance in ovarian
carcinoma.
NOS1-induced DDP resistance is
reversed by ABCG2 inhibition
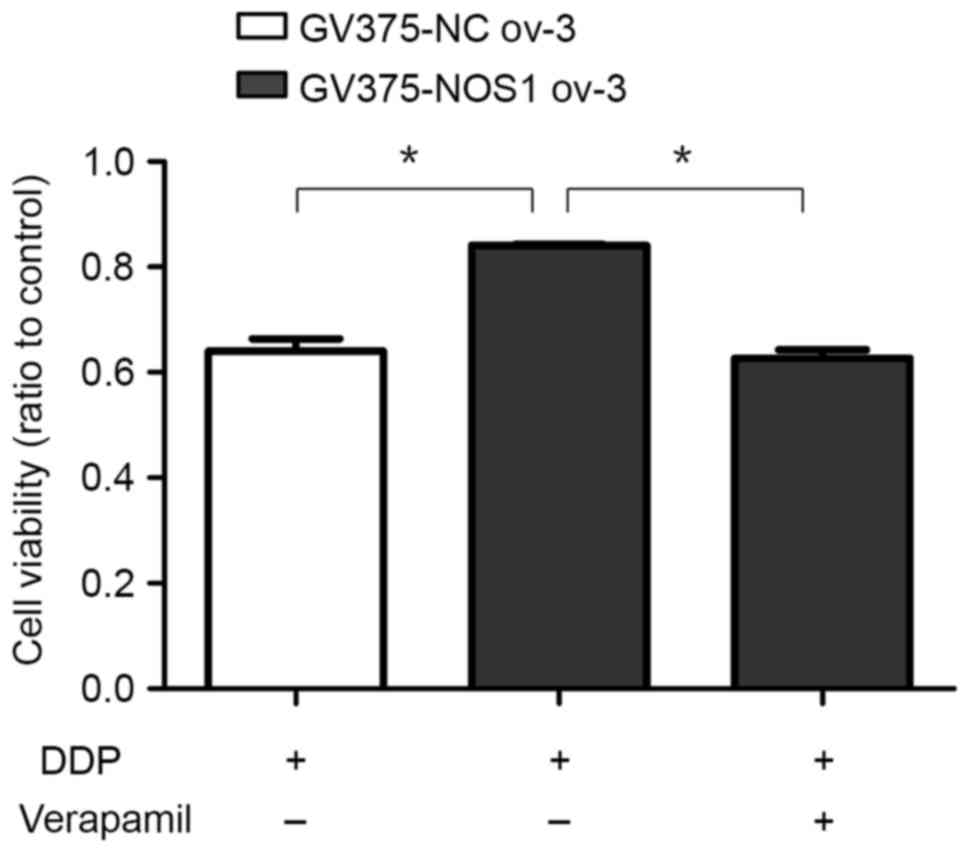
In order to detect whether ABCG2 serves a role in
NOS1-induced chemoresistance, the ABCG2 inhibitor verapamil was
used. GV375-NOS1 ov-3 and negative control cells were incubated
with DDP only or DDP plus verapamil, a calcium antagonist used as
an ABCG2 inhibitor, for 48 h and the ratios of viable cells were
measured via MTT assay. The cell viability of GV375-NOS1 ov-3 cells
was higher (0.841±0.038) than that of the negative controls
(0.641±0.021; P<0.05), and this effect was significantly
reversed by verapamil (0.639±0.025; P<0.05; Fig. 4). These results indicated that the
function of ABCG2 contributed to NOS1-related chemoresistance.
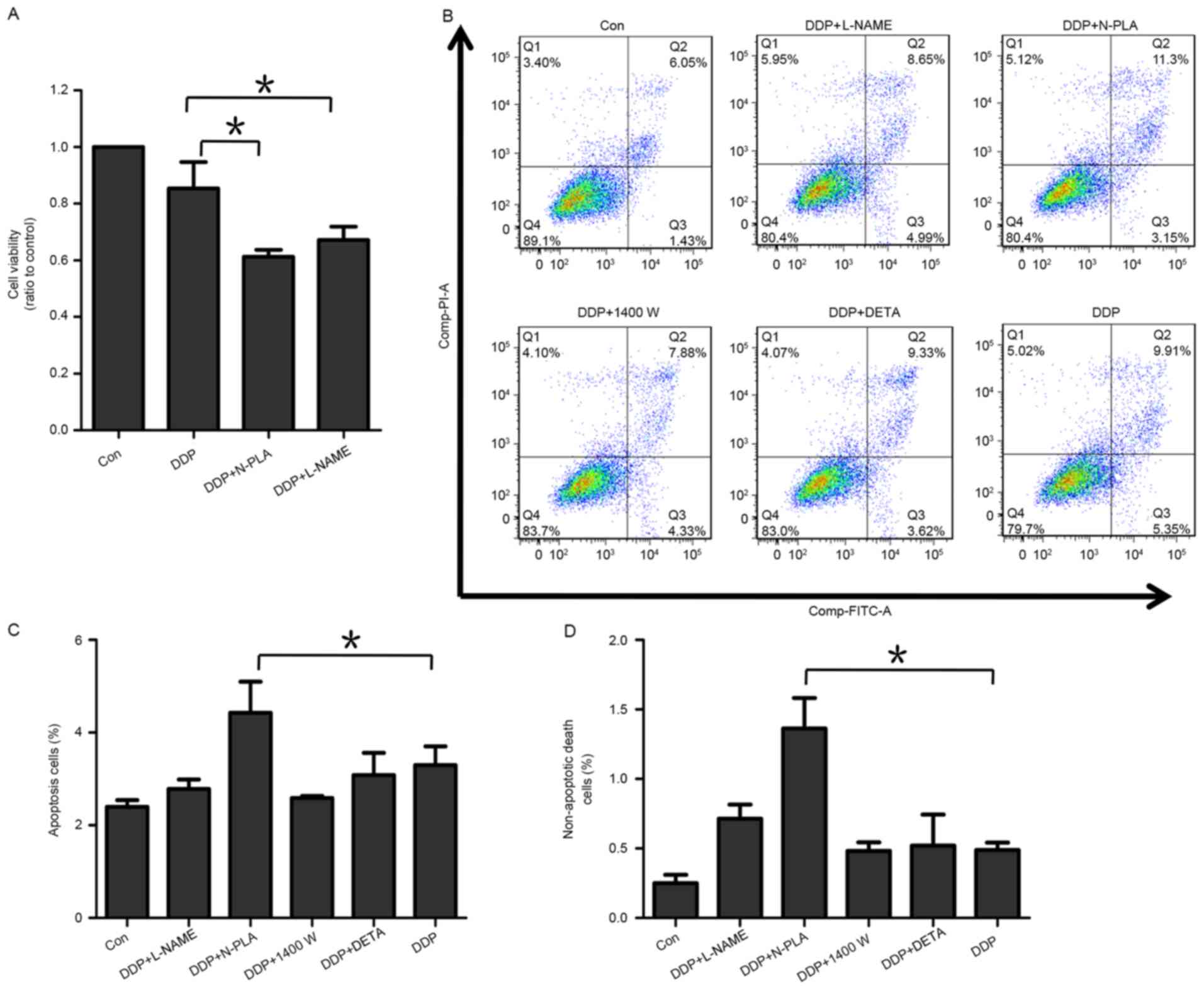
NOS1 inhibitor increases DDP-induced
apoptosis and cell death
As an effect of NOS1 on DDP-resistance was observed
in ovarian cancer cells, it was next investigated whether NOS1
inhibition could enhance the apoptosis and cell death induced by
DDP treatment. For this, an Annexin-V/PI apoptosis assay was
performed to investigate the levels of non-apoptotic cell death and
apoptosis in OVCAR-3 cells following DPP exposure (Fig. 5). In this experiment, 5 treatment
groups were investigated: DDP alone or with either L-NAME, N-PLA,
1400W or DETA. Fig. 5B presents the
flow cytometry scatter plots of the 5 treatment groups and negative
control. In all scatter plots, the X axis presented fluorescence
intensity of FITC Annexin-V, meanwhile Y axis presented
fluorescence intensity of PI. In each single plot, cells in the
first quadrant (Q1) were FITC-/PI+. Cells in the second quadrant
(Q2) were FITC+/PI+(non-apoptotic death cells). Cells in the third
quadrant (Q3) were FITC+/PI-(apoptotic cells). Cells in the fourth
quadrant (Q4) were FITC-/PI-(viable cells). As depicted in Fig. 5C and D, addition of N-PLA, the NOS1
selective inhibitor, significantly increased the levels of
apoptosis and non-apoptotic cell death induced by DDP in OVCAR-3
cells as compared with DDP alone (P<0.05). By contrast,
combination of DPP with the NOS2 selective inhibitor 1400W appeared
to somewhat decrease the DDP-induced apoptosis of cells (Fig. 5C), while the change of non-apoptotic
cell death was not apparent (Fig.
5D). L-NAME, as a general inhibitor of all NOS isoforms,
exhibited a mixed effect through NOS1 and 2 inhibition (Fig. 5C and D). In general, these data appear
to suggest that NOS1 promoted the survival of ovarian cancer
cells.
The NO donor DETA-NONOate may mimic paradoxical
functions of the three types of NOS by releasing different
concentrations of NO (22,23). In the current experiments, a low
concentration of NO was maintained by the addition of a low dosage
of DETA-NONOate (20 µM) in order to simulate the effect of NOS1. It
was observed that the low dose of DETA-NONOate had a mildly
reducing effect on DDP-induced apoptosis, but no effect on
DDP-induced non-apoptosis cell death (Fig. 5C and D). These data indicated that the
low dosage of DETA-NONOate may have simulated a NOS1 effect in
protecting ovarian cancer cells from DDP-mediated cytotoxicity.
To investigate whether NOS1 inhibition could improve
sensitivity to DDP chemotherapy, OVCAR-3 cells were incubated with
DDP only or DDP plus L-NAME or N-PLA for 48 h, and the percentage
of viable cells was assessed by MTT assay. The cells incubated with
L-NAME or N-PLA exhibited enhanced sensitivity to DDP (Fig. 5A; P<0.05).
Collectively, these data indicated that NOS1 had a
function of reducing the rate of cell apoptosis and cell death
under DDP treatment, thereby enhancing chemoresistance to DDP in
ovarian cancer cells.
Discussion
The impact of NO on malignant biological properties
is complex, and NO production depends partly on the NOS isoform
(6). NOS1 and NOS3 are constitutively
expressed in cells, and both produce low levels of NO. In contrast,
NOS2 becomes increasingly expressed in response to inflammation and
produces high levels of NO in a Ca2+-independent manner
(3). Enhanced expression of NOS
isoforms has been determined in a range of cancers (24). The 3 isoforms of NOS produce
endogenous NO during arginine metabolism. The protumor effects of
NO occur at low concentrations, while at higher concentrations
anti-tumor effects are typically induced (4). Previously, it has been reported that
NOS1 could produce transient, low levels of NO, compared with the
higher and more sustained concentrations of NO generated by NOS2
(25). A previous study has revealed
that the 3 types of NOS were differentially regulated in
chemosensitive and chemoresistant cells, and DDP resistance was
associated with low NOS2 content and a high level of NOS1 in
DDP-unresponsive cells (9). Thus,
NOS1 appears to have a tendency to promote tumor chemoresistance.
The present findings suggested that NOS1 promotes DDP
chemotherapeutic resistance in ovarian cancer cell lines. By using
NOS1-selective inhibitor, NOS1 was indicated to increase
DDP-induced cell apoptosis and non-apoptotic cell death. To
investigate the mechanism of NOS1 in promoting DDP resistance, the
expression of the multidrug resistance associated gene ABCG2 was
detected, and it was confirmed that suppression of ABCG2 function
could reverse DDP-resistance induced by NOS1 expression.
However, the exact mechanism underlying the
upregulation of ABCG2 expression via NOS1 remains unclear. Several
transcription factors, including nuclear factor-κB (NF-κB), HIFs
and Nrf2 have been demonstrated to bind to their response elements
in the promoter/enhancer region to activate the transcription of
ABCG2 (26–28). Disruption of Nrf2 expression in lung
cancer and prostate cancer cells by small hairpin RNA attenuated
the expression of the ABCG2 transcript and protein in Nrf2-depleted
cancer cells (27). Furthermore,
depleted levels of ABCG2 in these Nrf2-knockdown cells caused
sensitization to mitoxantrone and topotecan, two chemotherapy drugs
detoxified mainly by ABCG2. In addition, it has been reported that
ABCG2 expression may be activated by NF-κB through direct DNA
binding and downregulated by wt-p53 through a decrease in NF-κB
activity in MCF-7 cells (26).
Another study has revealed a cytoprotective functional role of
ABCG2 in response to oxidative stress, occurring downstream of
HIF-2α. It also found a dose-dependent activation of ABCG2
expression by HIF-2α (28). As
mentioned, NOS1, which produces low levels of NO, may act as an
environmental stimulus to activate oxidative stress-related
transcription factors. Thus, it may be assumed that this is one
potential mechanism by which NOS1 upregulates ABCG2 expression.
This is a current area of focus and the intended subject of
prospective studies by our group. Overall, it may be concluded that
the NOS1-induced enhancement of DDP chemotherapeutic resistance in
ovarian cancer cells is mediated by increased expression of
ABCG2.
Among the greatest issues compromising the
successful treatment of ovarian cancer is chemoresistance (29). It has been reported that NOS and its
product NO serves roles in chemoresistance (7,30,31). However, understanding of the potential
mechanisms involved is still limited. The present study
demonstrated that NOS1 regulated the expression level of ABCG2 and
contributed to DDP-induced chemoresistance in an ABCG2-dependent
manner. In summary, the current data support NOS1 as a valid tumor
therapeutic target and suggest that NOS1 inhibitor used in
conjunction with first-line chemotherapy may be a useful
therapeutic strategy in the treatment of patients with EOC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Fund for
Technological Innovation Projects at the Department of Education of
Guangdong, China (grant no. 2013KJCX0042), the Specialized Research
Fund for the Doctoral Program of Higher Education by the Ministry
of Education of the People's Republic of China (grant no.
20134433110009) and the Special Clinical Scientific Research Fund
of Wu Jieping Medical Foundation (grant no. 320.6755.15010).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XL performed all experiments and ZZ assisted
performing cells cultures and western blotting. QL, YW and XL
designed the study. JT assisted in performing IHC staining. YZ
assisted in RNA extraction. YLi and YLu analyzed experimental data.
YW reviewed and edited the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The animal experiments were approved by the Ethics
Committee of Southern Medical University (2015-FCK-002).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sundar S, Neal RD and Kehoe S: Diagnosis
of ovarian cancer. BMJ. 351:h44432015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Burke AJ, Sullivan FJ, Giles FJ and Glynn
SA: The yin and yang of nitric oxide in cancer progression.
Carcinogenesis. 34:503–512. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lala PK and Chakraborty C: Role of nitric
oxide in carcinogenesis and tumour progression. Lancet Oncol.
3:149–156. 2001. View Article : Google Scholar
|
|
4
|
Fukumura D, Kashiwagi S and Jain RK: The
role of nitric oxide in tumour progression. Nat Rev Cancer.
6:521–534. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rao CV: Nitric oxide signaling in colon
cancer chemoprevention. Mutat Res. 555:107–119. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ridnour LA, Thomas DD, Switzer C,
Flores-Santana W, Isenberg JS, Ambs S, Roberts DD and Wink DA:
Molecular mechanisms for discrete nitric oxide levels in cancer.
Nitric Oxide. 19:73–76. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fetz V, Bier C, Habtemichael N, Schuon R,
Schweitzer A, Kunkel M, Engels K, Kovacs AF, Schneider S, Mann W,
et al: Inducible NO synthase confers chemoresistance in head and
neck cancer by modulating survivin. Int J Cancer. 124:2033–2041.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Matthews NE, Adams MA, Maxwell LR, Gofton
TE and Graham CH: Nitric oxide-mediated regulation of
chemosensitivity in cancer cells. J Natl Cancer Inst. 93:1879–1885.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Leung EL, Fraser M, Fiscus RR and Tsang
BK: Cisplatin alters nitric oxide synthase levels in human ovarian
cancer cells: Involvement in p53 regulation and cisplatin
resistance. Br J Cancer. 98:1803–1809. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Robey RW, To KK, Polgar O, Dohse M, Fetsch
P, Dean M and Bates SE: ABCG2: A perspective. Adv Drug Deliv Rev.
61:3–13. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nakanishi T and Ross DD: Breast cancer
resistance protein (BCRP/ABCG2): Its role in multidrug resistance
and regulation of its gene expression. Chin J Cancer. 31:73–99.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Brechbuhl HM, Gould N, Kachadourian R,
Riekhof WR, Voelker DR and Day BJ: Glutathione transport is a
unique function of the ATP-binding cassette protein ABCG2. J Biol
Chem. 285:16582–16587. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Valko M, Leibfritz D, Moncol J, Cronin MT,
Mazur M and Telser J: Free radicals and antioxidants in normal
physiological functions and human disease. Int J Biochem Cell Biol.
39:44–84. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim BR, Seo SH, Park MS, Lee SH, Kwon Y
and Rho SB: sMEK1 inhibits endothelial cell proliferation by
attenuating VEGFR-2-dependent-Akt/eNOS/HIF-1α signaling pathways.
Oncotarget. 6:31830–31843. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gangula P, Ravella K, Chukkapalli S,
Rivera M, Srinivasan S, Hale A, Channon K, Southerland J and
Kesavalu L: Polybacterial periodontal pathogens alter vascular and
gut BH4/nNOS/NRF2-Phase II enzyme expression. PLoS One.
10:e01298852015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Castaldo SA, Freitas JR, Conchinha NV and
Madureira PA: The tumorigenic roles of the cellular REDOX
regulatory systems. Oxid Med Cell Longev. 2016:84130322016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dasari S and Tchounwou PB: Cisplatin in
cancer therapy: Molecular mechanisms of action. Eur J Pharmacol.
740:364–378. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Malpica A, Deavers MT, Lu K, Bodurka DC,
Atkinson EN, Gershenson DM and Silva EG: Grading ovarian serous
carcinoma using a two-tier system. Am J Surg Pathol. 28:496–504.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Baig MS, Zaichick SV, Mao M, de Abreu AL,
Bakhshi FR, Hart PC, Saqib U, Deng J, Chatterjee S, Block ML, et
al: NOS1-derived nitric oxide promotes NF-kB transcriptional
activity through inhibition of suppressor of cytokine signaling-1.
J Exp Med. 212:1725–1738. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Penzvalto Z, Lanczky A, Lénárt J,
Meggyesházi N, Krenács T, Szoboszlai N, Denkert C, Pete I and
Győrffy B: MEK1 is associated with carboplatin resistance and is a
prognostic biomarker in epithelial ovarian cancer. BMC Cancer.
14:8372014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Thomas DD, Ridnour LA, Isenberg JS,
Flores-Santana W, Switzer CH, Donzelli S, Hussain P, Vecoli C,
Paolocci N, Ambs S, et al: The chemical biology of nitric oxide:
Implications in cellular signaling. Free Radic Biol Med. 45:18–31.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu W, Liu LZ, Loizidou M, Ahmed M and
Charles IG: The role of nitric oxide in cancer. Cell Res.
12:311–320. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vannini F, Kashfi K and Nath N: The dual
role of iNOS in cancer. Redox Biol. 6:334–343. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nathan C and Xie QW: Nitric oxide
synthases: Roles, tolls, and controls. Cell. 78:915–918. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang X, Wu X, Wang C, Zhang W, Ouyang Y,
Yu Y and He Z: Transcriptional suppression of breast cancer
resistance protein (BCRP) by wild-type p53 through the NF-kappaB
pathway in MCF-7 cells. FEBS Lett. 584:3392–3397. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Singh A, Wu H, Zhang P, Happel C, Ma J and
Biswal S: Expression of ABCG2 (BCRP) is regulated by Nrf2 in cancer
cells that confers side population and chemoresistance phenotype.
Mol Cancer Ther. 9:2365–2376. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Martin CM, Ferdous A, Gallardo T,
Humphries C, Sadek H, Caprioli A, Garcia JA, Szweda LI, Garry MG
and Garry DJ: Hypoxia-inducible factor-2alpha transactivates Abcg2
and promotes cytoprotection in cardiac side population cells. Circ
Res. 102:1075–1081. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bast RC Jr, Hennessy B and Mills GB: The
biology of ovarian cancer: New opportunities for translation. Nat
Rev Cancer. 9:415–428. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Esteban FJ, Horcajadas A, El-Rubaidi O,
Luque-Barona R, Ibáñez G, Garcia-Carriazo A, Segovia M and del
Moral-Leal ML: Nitric oxide in malignant astrocytes. Rev Neurol.
40:437–440. 2005.(In Spanish). PubMed/NCBI
|
|
31
|
Yang DI, Yin JH, Mishra S, Mishra R and
Hsu CY: NO-mediated chemoresistance in C6 glioma cells. Ann N Y
Acad Sci. 962:8–17. 2002. View Article : Google Scholar : PubMed/NCBI
|