Introduction
China has a high incidence of esophageal cancer, as
this malignancy had the fourth highest mortality rate and the third
highest incidence rate of all malignancies in 2015 (1). Esophageal squamous cell carcinoma (ESCC)
is the major histological type of esophageal cancer in China,
accounting for >90% of all esophageal cancer cases (2). Although the application of combination
treatment strategies that consist of surgery, chemotherapy and
radiotherapy has improved the prognosis of ESCC, the 5-year overall
survival (OS) rate remains low at 20–30% (3). Therefore, there is an urgent requirement
to develop more effective therapeutic and prognostic
strategies.
In previous years, notable therapeutic results have
been achieved in advanced stages of cancer by blocking immune
checkpoint molecules; of which programmed death-1 (PD)-1 and its
ligand (PD-L1) have attracted substantial attention (4). Upon binding to PD-1, PD-L1 expressed on
tumor cells and tumor-infiltrating lymphocytes (TILs), transduces
inhibitory signals to regulate T-lymphocyte activation, tolerance
and immune-mediated tissue damage, and promote the immune escape of
tumor cells (5–7). Abnormal PD-L1 expression has been
associated with the prognosis and therapeutic response in a number
of malignancies, including esophageal cancer (8–10). Several
studies revealed that PD-L1 positivity was significantly associated
with disease progression, response to chemotherapy or radiotherapy,
or disease control in ESCC (8–10).
Notably, PD-L1 expression has been suggested to have positive
(8) and negative prediction values in
ESCC (9); therefore, the data on the
prognostic value of PD-L1 expression in ESCC that is currently
available remains limited and conflicting.
The present study aimed to determine the association
between PD-L1 expression status and prognosis in an overall
population of patients with ESCC and in subgroups of patients with
ESCC who received chemotherapy or radiotherapy. The expression of
PD-L1 in surgical ESCC specimens from 246 patients was examined,
including those who underwent chemotherapy and/or radiotherapy
following surgery. The association of PD-L1 expression with
clinicopathological characteristics and survival was
retrospectively analyzed with the aim of clarifying whether PD-L1
expression status was associated with a favorable or poor prognosis
in patients with ESCC, including those undergoing different
postoperative treatments.
Patients and methods
Patients
A total of 246 patients with resectable locally
advanced ESCC, who underwent surgical treatment at the Zhejiang
Cancer Hospital (Zhejiang, China) between January 2007 and December
2012, were included in the present study. All tissue specimens were
obtained from the tissue bank of Zhejiang Cancer Hospital, and all
patients provided written informed consent prior to surgery. The
Institutional Review Board of Zhejiang Cancer Hospital provided
ethical approval. Of the 246 patients, 222 (90.2%) were male and 24
(9.2%) were female. The median age of these patients at diagnosis
was 58.0 years old (range, 37–80 years). The patients had not
received preoperative chemotherapy, radiotherapy, targeted therapy
or immune therapy. The surgical tumor specimens collected from
these patients were used for immunohistochemical staining. Patient
characteristics, including age, sex, history of smoking, alcohol
drinking, Charlson comorbidity index (CCI) (11) and vessel invasion, and tumor
characteristics [size, differentiation, resection margin and TNM
stage according to the 7th American Joint Committee on Cancer
guidelines (12)] were recorded.
Follow-ups were performed using clinical records every three months
following patient discharge until mortality or the follow-up
deadline (August 2016).
Immunohistochemical staining
Immunohistochemical staining for PD-L1 was manually
performed. ESCC specimens obtained from primary tumors were fixed
with 10% formalin at 65°C, embedded in paraffin and cut into 4-µm
sections. Tissues were immersed for 10 min in serial dilutions of
xylene for de-waxing (dilution: 100, 90 and 80%), and then immersed
in a graded alcohol series (dilution: 100, 90 and 80%) for 10 min
to eliminate the xylene. Then, antigen retrieval was performed
using PT-Link (Dako; Agilent Technologies, Inc., Santa Clara, CA,
USA) subsequent to de-waxing at 95°C. Following immersion in 3%
hydrogen peroxide to block endogenous peroxidase at room
temperature for 30 min, the sections were incubated with an
anti-PD-L1 rabbit monoclonal antibody (1:200; cat. no. 13684;
E1L3N; Cell Signaling Technology, Inc., Danvers, MA, USA) at 4°C
overnight, followed by incubation with a horseradish
peroxidase-conjugated anti-rabbit secondary antibody (ready to use;
cat. no. SM802; Envision FLEX HRP; Dako; Agilent Technologies,
Inc., Santa Clara, CA, USA) for 30 min at room temperature.
Subsequently, horseradish peroxidase activity was detected using
3,3′-diaminobenzidine at room temperature. The sections were
counterstained using hematoxylin and examined under a light
microscope (magnification, ×10 and ×40).
Evaluation of immunohistochemical
staining
Immunoreactivity for PD-L1 was evaluated
semi-quantitatively using the H-score, which combines the staining
intensity and the percentage of stained tumor cells (13). The staining intensity was graded on a
four-point scale: 0, negative; 1, weak; 2, moderate and 3, strong.
The percentage of stained tumor cells was scored between 0 and 100,
increasing by 5% increments. Therefore, the final H-score values
obtained ranged from 0 to 300. The H-scores were separated into low
and high categories using the X-tile program (Olympus Corporation,
Tokyo, Japan), with the mean H-score as the cutoff value (14). Two experienced pathologists
independently calculated the percentage of stained tumor cells and
the staining intensity and discussed to reach a consensus in cases
where there were disagreements.
Statistical analysis
All statistical analyses were performed using IBM
SPSS software (version 22; IBM Corp., Armonk, NY, USA).
Associations between clinicopathological variables and PD-L1
expression were analyzed using a Pearson's χ2 test. The
Kaplan-Meier method and log rank test were used to calculate the
rate of OS and to compare differences between the survival curves.
Cox's proportional hazard regression model was utilized to compare
categorical variables using backward elimination with a stay level
of 0.10. Logistic regression was performed to investigate the
association between PD-L1 expression status and clinicopathological
factors using a backward regression procedure that included
variables where P<0.1 in the univariate analysis. All P-values
were two-tailed, and P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression of PD-L1 in ESCC
tissues
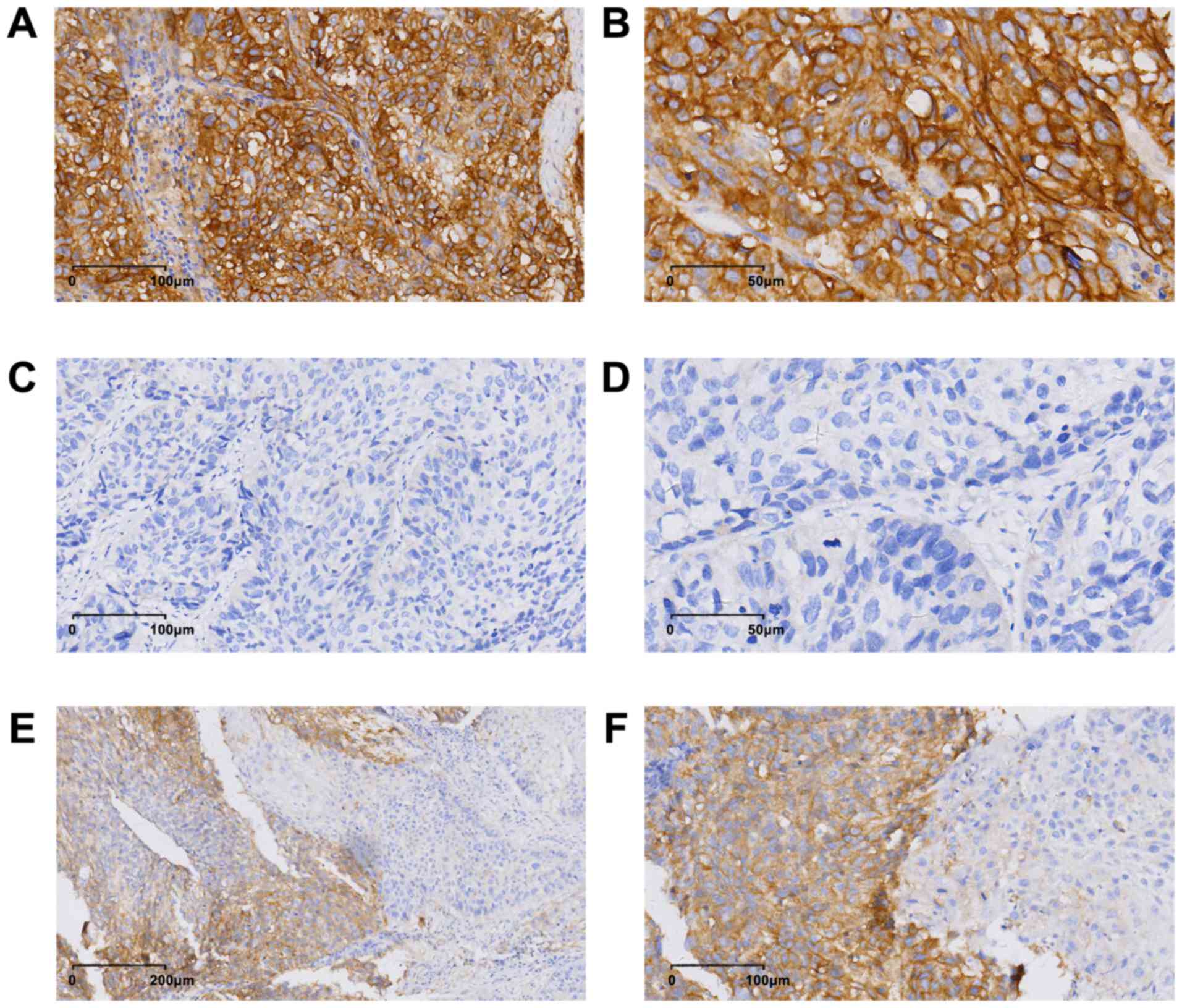
PD-L1 was expressed on the membrane and in the
cytoplasm, and PD-L1 expression exhibited heterogeneity in the ESCC
specimens (Fig. 1). The mean H-score
for PD-L1 expression in the patient population was 16 (range,
0–240). According to a cut-off value of 15 obtained using X-tile
software analysis, 60 (24.4%, 60/246) patients were considered
positive for PD-L1.
Association between PD-L1 expression
status and clinicopathological factors in ESCC patients
The clinicopathological characteristics of patients
are summarized in Table I. Among
these patients, 222 (90.2%, 222/246) were male and 24 (9.8%,
24/246) were female. The median age of these patients was 58.0
years old (range, 37–80 years old) at diagnosis, and the median OS
time was 53.0 months [95% confidence interval (CI), 41.4–64.6
months]. The χ2 test revealed a significant association
between positive PD-L1 expression and tumor node metastasis (TNM)
stage (P=0.022). However, there was no association between positive
PD-L1 expression and factors, including age, sex, CCI, smoking
history, alcohol drinking, vessel invasion, tumor differentiation,
tumor location, T stage and N stage. Multivariate logistic
regression analysis also indicated that PD-L1 expression was
significantly associated with TNM stage in patients with ESCC
(P=0.009; Table II).
 | Table I.Association between PD-L1 expression
and clinicopathological factors in patients with esophageal
squamous cell carcinoma. |
Table I.
Association between PD-L1 expression
and clinicopathological factors in patients with esophageal
squamous cell carcinoma.
|
| PD-L1 |
|
|---|
|
|
|
|
|---|
| Variable | + | − | P-value |
|---|
| Age, years |
|
| 0.312 |
|
<65 | 48 | 159 |
|
|
≥65 | 12 | 27 |
|
| Sex, n |
|
| 0.942 |
|
Female | 6 | 18 |
|
|
Male | 54 | 168 |
|
| CCI |
|
| 0.600 |
|
Low | 8 | 19 |
|
|
Moderate | 51 | 160 |
|
|
High | 1 | 7 |
|
| Vessel
invasion |
|
| 0.137 |
|
Yes | 13 | 59 |
|
| No | 47 | 127 |
|
|
Differentiation |
|
| 0.542 |
|
Well | 17 | 67 |
|
|
Moderate | 36 | 101 |
|
|
Poor | 7 | 18 |
|
| Tumor location |
|
| 0.209 |
|
Upper | 21 | 77 |
|
|
Middle | 39 | 103 |
|
|
Lower | 0 | 6 |
|
| T stage |
|
| 0.109 |
|
1–2 | 10 | 136 |
|
|
3–4 | 50 | 50 |
|
| N stage |
|
| 0.213 |
|
0–1 | 33 | 119 |
|
|
2–3 | 27 | 67 |
|
| TMN
stagea |
|
| 0.022 |
| II | 27 | 132 |
|
|
III | 33 | 54 |
|
| Smoking
history |
|
| 0.837 |
|
Yes | 47 | 148 |
|
| No | 13 | 38 |
|
| Alcohol
consumption |
|
| 0.892 |
|
Yes | 46 | 141 |
|
| No | 14 | 45 |
|
 | Table II.Multivariate logistic regression
analysis of risk factors for programmed death-ligand 1 expression
in patients with esophageal squamous cell carcinoma. |
Table II.
Multivariate logistic regression
analysis of risk factors for programmed death-ligand 1 expression
in patients with esophageal squamous cell carcinoma.
| Variable | OR | 95% CI | P-value |
|---|
| Sex | 1.01 | 0.34–3.01 | 0.993 |
| Age | 1.33 | 0.61–2.91 | 0.473 |
| Smoking
history | 1.25 | 0.60–2.61 | 0.553 |
| Alcohol
consumption | 1.02 | 0.45–2.33 | 0.953 |
| Vessel
invasion | 2.04 | 1.00–4.17 | 0.051 |
| T stage | 1.33 | 0.59–3.01 | 0.494 |
| N stage | 1.82 | 0.57–5.82 | 0.311 |
| TNM stage | 2.30 | 1.23–4.29 | 0.009 |
Association between PD-L1 expression
and survival in patients with ESCC
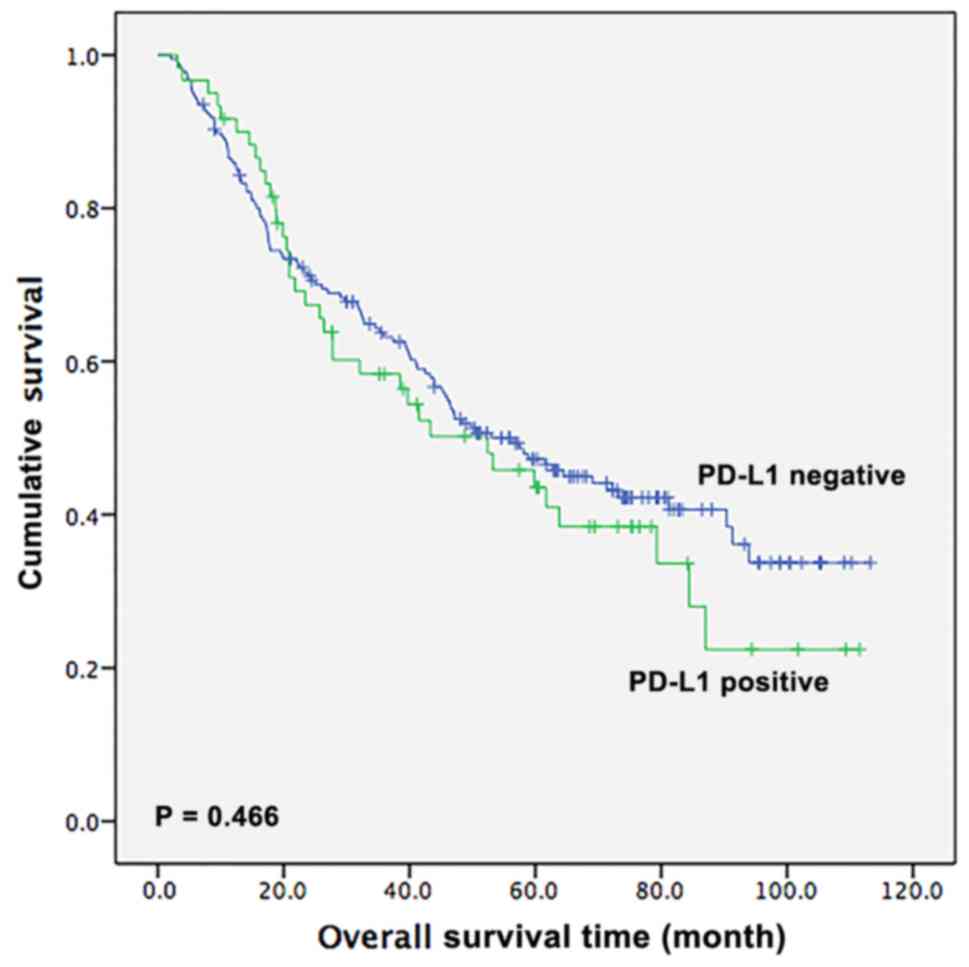
Next, the survival outcomes of patients with ESCC
based on PD-L1 expression status were assessed. During the
follow-up period, 139 (56.5%, 139/246) patients succumbed to
mortality. The median OS time of the 246 patients was 53.0 months
(95% CI, 41.4–64.6 months). For patients with positive PD-L1
expression, the OS time was 52.4 months (95% CI, 30.3–74.5 months),
whereas for patients with negative PD-L1 expression, the OS time
was 56.4 months (95% CI, 43.0–69.8 months). However, the difference
between the OS time of patients who were positive and those who
were negative for PD-L1 was not statistically significant, as
presented by the Kaplan-Meier analysis (P=0.466; Fig. 2).
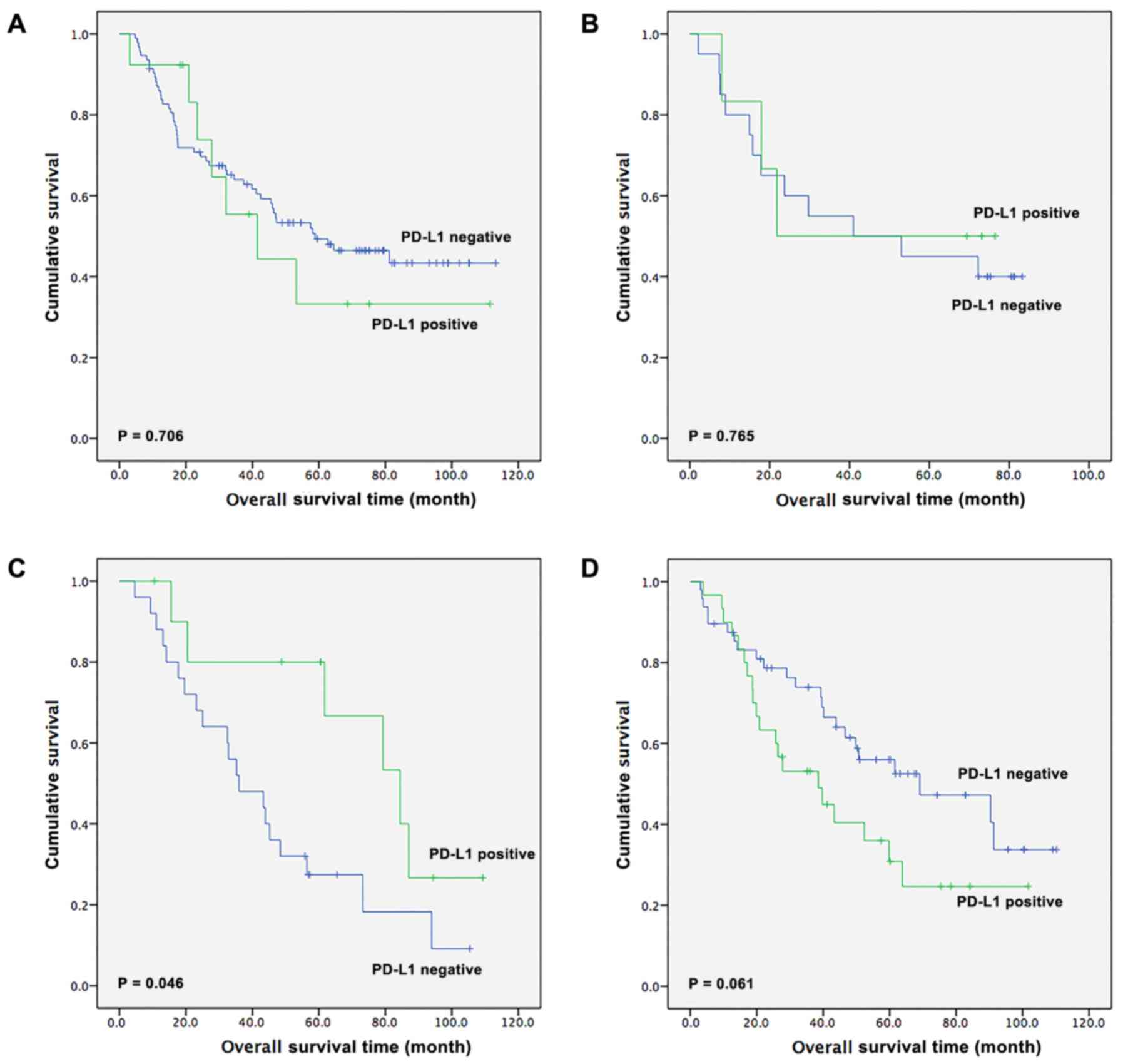
The survival outcomes in patients receiving
different treatments were also assessed (Fig. 3). Among the 246 patients with ESCC,
106 patients received surgical resection alone, 26 patients
received surgery in addition to adjuvant chemotherapy, 36 patients
received surgery with adjuvant radiotherapy and 78 patients
underwent surgery in addition to adjuvant chemoradiotherapy. In the
surgery-alone group, there was no significant difference in OS time
between PD-L1-positive and -negative patients [41.5 months (95% CI,
15.7–67.4 months) vs. 58.9 months (95% CI, 29.2–88.7 months),
respectively; P=0.706; Fig. 3A]. In
the surgery with adjuvant chemotherapy subgroup, there was also no
statistical difference in OS time between PD-L1-positive and
-negative patients (21.8 vs. 41.0 months, respectively; P=0.765;
Fig. 3B). Notably, in the surgery
with adjuvant radiotherapy subgroup, there was a statistical
significant difference between patients with positive and negative
PD-L1 expression [84.4 months (95% CI, 55.2–113.6 months) vs. 36.0
months (95% CI, 18.9–53.1 months), respectively; P=0.046; Fig. 3C]. Furthermore, in the surgery with
adjuvant chemoradiotherapy subgroup, there was a tendency for
patients with ESCC that had positive PD-L1 expression to have a
shorter OS compared with patients with negative PD-L1 expression
[38.5 months (95% CI, 17.8–59.2 months) vs. 69.1 months (95% CI,
25.2–113.0)]; however, this difference was not significant
(P=0.061; Fig. 3D).
Univariate and multivariate analyses
of factors affecting survival in patients with ESCC
As presented in Table
III, univariate Cox regression analysis revealed that lymph
node metastasis and TNM stage were significant prognostic factors
for OS in patients with ESCC [hazard ratio (HR)=1.61, 95% CI,
1.15–2.26, P=0.005; HR, 1.62, 95% CI, 1.15–2.29, P=0.006,
respectively]. Vessel invasion showed a tendency to be a prognostic
factor, but the P-value was not statistically significant (HR=1.47;
95% CI, 0.99–2.17; P=0.058). Unexpectedly, PD-L1 expression status
was not a significant prognostic factor for OS in patients with
ESCC (HR, 1.15, 95% CI, 0.79–1.68, P=0.467). Multivariate Cox
regression hazards analysis revealed that lymph node metastasis
(P=0.002), vessel invasion (P=0.011) and smoking history (P=0.049)
were independent prognostic factors in patients with ESCC. Patients
with advanced N stage, vessel invasion or smoking history had
poorer survival than patients with early N stage, non-vessel
invasion of non-smoking history (Table
III). Similarly, multivariate analysis indicated that PD-L1
expression status was not a significant independent prognostic
factor (HR, 1.05; 95% CI, 0.70–1.59; P=0.804).
 | Table III.Univariate and multivariate Cox
regression analyses of factors affecting the overall survival time
of patients with esophageal squamous cell carcinoma (n=246). |
Table III.
Univariate and multivariate Cox
regression analyses of factors affecting the overall survival time
of patients with esophageal squamous cell carcinoma (n=246).
| A, Univariate
analysis |
|---|
|
|---|
| Variable | HR | 95% CI | P-value |
|---|
| PD-L1 |
|
|
|
|
Positive vs. negative | 1.152 | 0.787–1.684 | 0.467 |
| Sex |
|
|
|
| Male
vs. female | 1.106 | 0.624–1.961 | 0.730 |
| Age (years) |
|
|
|
| ≥65 vs.
<65 | 1.080 | 0.700–1.665 | 0.729 |
| Smoking
history |
|
|
|
| Yes vs.
no | 1.388 | 0.894–2.156 | 0.144 |
| Alcohol
drinking |
|
|
|
| Yes vs.
no | 1.100 | 0.746–1.621 | 0.631 |
| CCI |
|
|
|
| Low vs.
moderate vs. high | 1.176 | 0.741–1.866 | 0.491 |
| Vessel
invasion |
|
|
|
| Yes vs.
no | 1.466 | 0.987–2.174 | 0.058 |
| Tumor
differentiation |
|
|
|
| Well
vs. moderate vs. poor | 1.055 | 0.814–1.366 | 0.688 |
| Tumor location |
|
|
|
| Lower
vs. middle vs. upper | 1.242 | 0.907–1.704 | 0.177 |
| T stage |
|
|
|
| T1-2
vs. T3-4 | 1.100 | 0.741–1.631 | 0.637 |
| N stage |
|
|
|
| N0-1
vs. N2-3 | 1.613 | 1.152–2.257 | 0.005 |
| TNM
stagea |
|
|
|
| II vs.
III | 1.623 | 1.151–2.288 | 0.006 |
| Chemotherapy |
|
|
|
| Yes vs.
no | 1.038 | 0.741–1.456 | 0.827 |
| Radiotherapy |
|
|
|
| Yes vs.
no | 1.168 | 0.838–1.631 | 0.359 |
|
| B, Multivariate
analysis |
|
|
Variable | HR | 95% CI | P-value |
|
| PD-L1 |
|
|
|
|
Positive vs. negative | 1.053 | 0.698–1.589 | 0.804 |
| Sex |
|
|
|
| Male
vs. female | 1.051 | 0.545–2.028 | 0.882 |
| Age, years |
|
|
|
| ≥65 vs.
<65 | 1.049 | 0.652–1.690 | 0.843 |
| Smoking
history |
|
|
|
| Yes vs.
no | 1.658 | 1.002–2.740 | 0.049 |
| Alcohol
drinking |
|
|
|
| Yes vs.
no | 1.454 | 0.935–2.262 | 0.097 |
| CCI |
|
|
|
| Low vs.
moderate vs. high | 1.053 | 0.664–1.669 | 0.826 |
| Vessel
invasion |
|
|
|
| Yes vs.
no | 1.685 | 1.128–2.518 | 0.011 |
| Tumor
differentiation |
|
|
|
| Well
vs. moderate vs. poor | 1.104 | 0.838–1.456 | 0.483 |
| Tumor location |
|
|
|
| Lower
vs. middle vs. upper | 1.248 | 0.908–1.718 | 0.172 |
| T stage |
|
|
|
| T1-2
vs. T3-4 | 1.068 | 0.665–1.717 | 0.785 |
| N stage |
|
|
|
| N0-1
vs. N2-3 | 1.709 | 1.215–2.404 | 0.002 |
| TNM
stagea |
|
|
|
| II vs.
III | 1.350 | 0.777–2.347 | 0.287 |
| Chemotherapy |
|
|
|
| Yes vs.
no | 1.092 | 0.740–1.611 | 0.659 |
| Radiotherapy |
|
|
|
| Yes vs.
no | 1.183 | 0.838–1.672 | 0.339 |
Discussion
The present study investigated the expression of
PD-L1 and its association with the clinicopathological factors and
clinical outcomes of different treatments in patients with
resectable locally advanced ESCC. It was detected that PD-L1
expression was significantly associated with TNM stage in ESCC.
High PD-L1 expression is positively associated with advanced TNM
stage. In addition, it was revealed that PD-L1 expression was a
potential predictor of a favorable response to radiotherapy in
patients with ESCC, although median OS time did not differ
significantly between patients with positive and negative PD-L1
expression in the overall population.
Although one previous study did not reveal any
significant associations between PD-L1 expression and
clinicopathological factors in 177 patients with ESCC (15), other studies obtained different
results. A study conducted by Chen et al (16) on ESCC revealed that PD-L1 expression
associated with factors, including upper esophageal location,
well-differentiated tumor type, an absence of lymph node metastasis
and an early stage of disease suggesting that PD-L1 expression is
an indicator of less aggressive tumor types. By contrast, a number
of other studies have indicated that basal high PD-L1 expression is
positively associated with advanced T stage, lymph node metastasis
and loco-regional failure in ESCC (17–19). In
the present study, it was revealed that patients with ESCC with an
advanced TNM stage had a significant association with positive
PD-L1 expression, and these results suggest that positive PD-L1
expression may be associated with malignant biological behavior in
ESCC. However, whether PD-L1 expression results in an advanced TNM
stage or whether an advanced TNM stage promotes PD-L1 expression
remains unknown. Further studies are required to investigate
this.
The present study also revealed that patients with
positive and negative PD-L1 expression had similar median OS time
(52.4 months vs. 56.4 months, P=0.466), which is inconsistent with
a majority of previous studies. For instance, three meta-analyses
indicated that PD-L1 expression was associated with a poorer OS
time in esophageal cancer (20–22).
However, one study revealed that a high PD-L1 expression predicted
a favorable prognosis in patients with ESCC (8). The discrepancies among different studies
in terms of the association of PD-L1 expression status with
clinicopathological factors and survival in ESCC may be due to a
combination of several reasons as follows: Different sensitivities
of antibodies used; different cut-off values for positive PD-L1
staining; non-uniform PD-L1 expression (as detected in Fig. 1E and F in the present study) and
different sampling timing and location (23). In the present study, no association
was detected between PD-L1 expression and prognosis in ESC. Future
studies should carefully address these issues in order to
standardize the immunohistochemical staining procedure and
interpretation of immunohistochemical results, enabling the
comparison of results obtained from different studies.
Notably, the results of the present study indicated
that in patients treated with adjuvant radiotherapy, the prognosis
was significantly improved in patients with positive PD-L1
expression compared with those with negative PD-L1 expression
(median OS time, 84.4 months vs. 36.0 months; P=0.046). In patients
treated with adjuvant chemotherapy, median OS was poorer in
PD-L1-positive patients compared with PD-L1-negative patients,
although there was no significant statistical difference (21.8
months vs. 41.0 months, P=0.765). In agreement with this finding, a
number of previous studies have indicated that there is an
association between PD-L1 expression and the outcomes of different
types of clinical management. In a study of 177 Japanese patients
with ESCC who underwent an esophagectomy without preoperative
therapy, PD-L1 expression was significantly associated with an
improved prognosis in patients who underwent surgery alone, but not
in patients treated with surgery plus postoperative adjuvant
chemotherapy (15). Another study of
180 Japanese patients with ESCC who were treated by radical
resection with or without preoperative neoadjuvant chemotherapy
revealed that PD-L1 expression correlated with a poorer OS time in
patients treated with preoperative neoadjuvant chemotherapy, but
not in those without preoperative neoadjuvant chemotherapy
(24). Theoretically, ESCC is
moderately sensitive to chemotherapy, but the role of postoperative
chemotherapy against ESCC has been debated. The National
Comprehensive Cancer Network only recommends postoperative
chemotherapy for patients with ESCC with R1 (cancer cells present
by microscopy at the resection) or R2 resections (tumor tissue
present by naked eyes at the resection margin) (25). The present study revealed that
PD-L1-positive patients with ESCC do not benefit from postoperative
adjuvant chemotherapy, which indicates that chemotherapy not only
has a direct cytotoxic effect on tumor cells, but may also affect
the tumor immune system (15).
Radiotherapy is one of the main types of treatment
for ESCC (25). Radiation increases
the leakage of tumor antigens by killing tumor cells and promotes
the activation of tumor-specific T cells by increasing the ability
of antigen-presenting cells to display antigens on their surface
(26). Previous studies have
documented the synergism between several types of immunotherapy and
radiotherapy (27). Previous
preclinical studies have revealed that PD-L1 expression may be
upregulated in tumor cells by radiation therapy (28,29). In
addition, anti-PD-L1 therapy combined with radiotherapy improves
survival compared with radiotherapy alone in mice (30).
PD-L1 expression on tumor cells is generally
considered to be one of the mechanisms for immune evasion via
downregulating the function of TILs (CD8+ T-lymphocytes
and CD4+ T-lymphocytes) or activating the epidermal
growth factor receptor signaling pathway (31–33).
However, in the present study, positive PD-L1 expression was
associated with a favorable survival in patients who underwent
radiotherapy. It was hypothesized that patients with PD-L1
expression had highly immunogenic tumors prior to radiation
therapy, which indicates a strong adaptive immune response. In
patients with PD-L1 expression, there was an increased infiltration
of other subpopulations of TILs, which has been reported to be a
strong predictor of good prognosis for patients with ESCC (34). A number of studies reported that high
PD-L1 expression on tumor cells was associated with increased
intraepithelial CD3+ T-lymphocytes, and both factors
were associated with a favorable prognosis in ESCC (8,35).
Autophagy is an evolutionarily conserved process for
the clearance of damaged or superfluous intracellular products via
the lysosomal-mediated pathway (36).
Autophagy is associated with a decrease in radiosensitivity.
Autophagy inhibitor combined with radiotherapy resulted in enhanced
cytotoxicity of radiotherapy in ESCC cells by decreasing the
expression of autophagy-associated gene Beclin1 and arresting the
cell cycle at the G2/M phase (37,38).
Tumor-intrinsic PD-L1 signals promote mammalian target of rapamycin
(MTORC)1 and inhibit MTORC2, therefore inhibiting autophagy and
enhancing the sensitivity to growth suppression by autophagy
inhibitors (39). However, a number
of studies reported that PD-L1 promoted immune evasion via
activation of the protein kinase B/mTOR oncogenic pathway (40,41). Other
studies reported that PD-L1 expression was associated with
resistance to anticancer therapies, including radiotherapy and
chemotherapy (19,42).
The mechanisms responsible for the survival benefit
of PD-L1 in patients with ESCC receiving radiotherapy remain to be
further investigated. The present study was based on surgical
samples obtained from patients with ESCC who did not undergo
preoperative chemotherapy, radiotherapy or immunotherapy,
suggesting that basal PD-L1 expression status may serve as a
potential predictive factor of the effects of adjuvant
radiotherapy. Additional research should be performed to
investigate the potential molecular mechanism of these associations
for ESCC. Studies with larger sample sizes are required to clarify
this issue.
The present study was subjected to certain
limitations. First, although the present study had a larger sample
size compared with other studies (16,43), there
was still bias due to its retrospective design. Secondly, only
24.4% of these patients were regarded as PD-L1-positive, while a
meta-analysis demonstrated that nearly 50% of patients with
gastrointestinal cancer were positive for PD-L1 expression,
regardless of the evaluation method used (20). The standard used for the positive
expression of PD-L1 in the present study was inconsistent with
several previous studies (20–22), and
there was no unified standard for the positive expression of PD-L1.
Whether the threshold selected to determine PD-L1 expression was
positive or negative may be arbitrary. In the present study, PD-L1
expression in a number of ESCC tumor tissues appeared to be
heterogeneous (Fig. 1E and F).
Consistent with this observation, previous studies have revealed
that PD-L1 expression was heterogeneous in other malignant tumors
(44–46). Therefore, heterogeneous expression may
be another reason why the PD-L1 positive rate in the present study
was lower compared with those in other studies (20–22).
Finally, as no standardized PD-L1 immunohistochemistry assay is
currently available, caution should be taken in the interpretation
of these results.
In conclusion, the present study demonstrates, to
the best of our knowledge, for the first time that high PD-L1
expression in associated with a favorable prognosis in patients
with ESCC undergoing postoperative adjuvant radiotherapy. However,
this association was not consistent in the overall ESCC population.
Therefore, patients with positive PD-L1 expression may benefit from
postoperative adjuvant radiotherapy. However, further studies are
required in order to confirm these results.
Acknowledgements
The present study was presented as a conference
abstract (Oral no. 117) on 2–5 September, 2017 at the 14th World
Conference Global Perspectives in Esophageal Diseases in Geneva,
Switzerland.
Funding
Not applicable.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YX, JS and GZ contributed to the conception of the
study and method selection. CJ, YZ, ST and QL made substantial
contribution to the acquisition and arrangement of data. CJ, YZ, ZG
and QL analyzed the data. CJ, YZ and ST drafted the manuscript. All
authors agreed to be accountable for all aspects of the work and to
ensure that questions related to the accuracy and integrity of any
part of the work are appropriately investigated and resolved. All
authors gave final approval of the version to be published.
Ethics approval and consent to
participate
All tissue specimens were obtained from the tissue
bank of Zhejiang Cancer Hospital, and all patients provided written
informed consent prior to surgery. The present study was approved
by the Medical Ethics Committee of Zhejiang Cancer Hospital
ethically (Ethics Approval document IRB-2017-195).
Patient consent for publication
The patients of our study provided written informed
consent for the publication of any associated data and accompanying
images.
Competing interests
The authors declare that there are no competing
interests.
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tepper J, Krasna MJ, Niedzwiecki D, Hollis
D, Reed CE, Goldberg R, Kiel K, Willett C, Sugarbaker D and Mayer
R: Phase III trial of trimodality therapy with cisplatin,
fluorouracil, radiotherapy, and surgery compared with surgery alone
for esophageal cancer: CALGB 9781. J Clin Oncol. 26:1086–1092.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nishino M, Ramaiya NH, Hatabu H and Hodi
FS: Monitoring immune-checkpoint blockade: Response evaluation and
biomarker development. Nat Rev Clin Oncol. 14:655–688. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sharpe AH and Freeman GJ: The B7-CD28
superfamily. Nat Rev Immunol. 2:116–126. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Francisco LM, Sage PT and Sharpe AH: The
PD-1 pathway in tolerance and autoimmunity. Immunol Rev.
236:219–242. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ceeraz S, Nowak EC and Noelle RJ: B7
family checkpoint regulators in immune regulation and disease.
Trends Immunol. 34:556–563. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jesinghaus M, Steiger K, Slotta-Huspenina
J, Drecoll E, Pfarr N, Meyer P, Konukiewitz B, Bettstetter M,
Wieczorek K, Ott K, et al: Increased intraepithelial CD3+
T-lymphocytes and high PD-L1 expression on tumor cells are
associated with a favorable prognosis in esophageal squamous cell
carcinoma and allow prognostic immunogenic subgrouping. Oncotarget.
8:46756–46768. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jiang Y, Lo AWI, Wong A, Chen W, Wang Y,
Lin L and Xu J: Prognostic significance of tumor-infiltrating
immune cells and PD-L1 expression in esophageal squamous cell
carcinoma. Oncotarget. 8:30175–30189. 2017.PubMed/NCBI
|
|
10
|
Zhang W, Pang Q, Zhang X, Yan C, Wang Q,
Yang J, Yu S, Liu X, Pan Y, Yuan Z, et al: Programmed death-ligand
1 is prognostic factor in esophageal squamous cell carcinoma and is
associated with epidermal growth factor receptor. Cancer Sci.
108:590–597. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yamashita K, Watanabe M, Mine S, Fukudome
I, Okamura A, Yuda M, Hayami M and Imamura Y: The impact of the
Charlson comorbidity index on the prognosis of esophageal cancer
patients who underwent esophagectomy with curative intent. Surg
Today. 48:632–639. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen LJ, Sun J, Wu HY, Zhou SM, Tan Y, Tan
M, Shan BE, Lu BF and Zhang XG: B7-H4 expression associates with
cancer progression and predicts patient's survival in human
esophageal squamous cell carcinoma. Cancer Immunol Immunother.
60:1047–1055. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Camp RL, Dolled-Filhart M and Rimm DL:
X-tile: A new bio-informatics tool for biomarker assessment and
outcome-based cut-point optimization. Clin Cancer Res.
10:7252–7259. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wakita A, Motoyama S, Nanjo H, Sato Y,
Yoshino K, Sasaki T, Kawakita Y, Liu J, Imai K, Saito H and
Minamiya Y: PD-L1 expression is a prognostic factor in patients
with thoracic esophageal cancer treated without adjuvant
chemotherapy. Anticancer Res. 37:1433–1441. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen K, Cheng G, Zhang F, Zhang N, Li D,
Jin J, Wu J, Ying L, Mao W and Su D: Prognostic significance of
programmed death-1 and programmed death-ligand 1 expression in
patients with esophageal squamous cell carcinoma. Oncotarget.
7:30772–30780. 2016.PubMed/NCBI
|
|
17
|
Loos M, Langer R, Schuster T, Gertler R,
Walch A, Rauser S, Friess H and Feith M: Clinical significance of
the costimulatory molecule B7-H1 in Barrett carcinoma. Ann Thorac
Surg. 91:1025–1031. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen L, Deng H, Lu M, Xu B, Wang Q, Jiang
J and Wu C: B7-H1 expression associates with tumor invasion and
predicts patient's survival in human esophageal cancer. Int J Clin
Exp Pathol. 7:6015–6023. 2014.PubMed/NCBI
|
|
19
|
Chen MF, Chen PT, Chen WC, Lu MS, Lin PY
and Lee KD: The role of PD-L1 in the radiation response and
prognosis for esophageal squamous cell carcinoma related to IL-6
and T-cell immunosuppression. Oncotarget. 7:7913–7924.
2016.PubMed/NCBI
|
|
20
|
Huang B, Chen L, Bao C, Sun C, Li J, Wang
L and Zhang X: The expression status and prognostic significance of
programmed cell death 1 ligand 1 in gastrointestinal tract cancer:
A systematic review and meta-analysis. Onco Targets Ther.
8:2617–2625. 2015.PubMed/NCBI
|
|
21
|
Wu P, Wu D, Li L, Chai Y and Huang J:
PD-L1 and survival in solid tumors: A meta-analysis. PLoS One.
10:e01314032015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pyo JS, Kang G and Kim JY: Prognostic role
of PD-L1 in malignant solid tumors: A meta-analysis. Int J Biol
Markers. 32:e68–e74. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang X, Teng F, Kong L and Yu J: PD-L1
expression in human cancers and its association with clinical
outcomes. Onco Targets Ther. 9:5023–5039. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tanaka K, Miyata H, Sugimura K, Kanemura
T, Hamada-Uematsu M, Mizote Y, Yamasaki M, Wada H, Nakajima K,
Takiguchi S, et al: Negative influence of programmed
death-1-ligands on the survival of esophageal cancer patients
treated with chemotherapy. Cancer Sci. 107:726–733. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
National Comprehensive Cancer Network
(NCCN). Clinical Practice Guidelines in Oncology. Esophageal and
Esophagogastric Junction Cancers. Version 2.2017. 2017.
|
|
26
|
Lim SH, Hong M, Ahn S, Choi YL, Kim KM, Oh
D, Ahn YC, Jung SH, Ahn MJ, Park K, et al: Changes in tumour
expression of programmed death-ligand 1 after neoadjuvant
concurrent chemoradiotherapy in patients with squamous oesophageal
cancer. Eur J Cancer. 52:1–9. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Formenti SC and Demaria S: Combining
radiotherapy and cancer immunotherapy: A paradigm shift. J Natl
Cancer Inst. 105:256–265. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dovedi SJ, Adlard AL, Lipowska-Bhalla G,
McKenna C, Jones S, Cheadle EJ, Stratford IJ, Poon E, Morrow M,
Stewart R, et al: Acquired resistance to fractionated radiotherapy
can be overcome by concurrent PD-L1 blockade. Cancer Res.
74:5458–5468. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kelly RJ, Zaidi AH, Smith MA, Omstead AN,
Kosovec JE, Matsui D, Martin SA, DiCarlo C, Werts ED, Silverman JF,
et al: The dynamic and transient immune microenvironment in locally
advanced esophageal adenocarcinoma post chemoradiation. Ann Surg.
2017.PubMed/NCBI
|
|
30
|
Zeng J, See AP, Phallen J, Jackson CM,
Belcaid Z, Ruzevick J, Durham N, Meyer C, Harris TJ, Albesiano E,
et al: Anti-PD-1 blockade and stereotactic radiation produce
long-term survival in mice with intracranial gliomas. Int J Radiat
Oncol Biol Phys. 86:343–349. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Leng C, Li Y, Qin J, Ma J, Liu X, Cui Y,
Sun H, Wang Z, Hua X, Yu Y, et al: Relationship between expression
of PD-L1 and PD-L2 on esophageal squamous cell carcinoma and the
antitumor effects of CD8+ T cells. Oncol Rep.
35:699–708. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen K, Zhu Z, Zhang N, Cheng G, Zhang F,
Jin J, Wu J, Ying L, Mao W and Su D: Tumor-infiltrating CD4+
lymphocytes predict a favorable survival in patients with operable
esophageal squamous cell carcinoma. Med Sci Monit. 23:4619–4632.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang W, Pang Q, Yan C, Wang Q, Yang J, Yu
S, Liu X, Yuan Z, Wang P and Xiao Z: Induction of PD-L1 expression
by epidermal growth factor receptor-mediated signaling in
esophageal squamous cell carcinoma. Onco Targets Ther. 10:763–771.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sudo T, Nishida R, Kawahara A, Saisho K,
Mimori K, Yamada A, Mizoguchi A, Kadoya K, Matono S, Mori N, et al:
Clinical impact of tumor-infiltrating lymphocytes in esophageal
squamous cell carcinoma. Ann Surg Oncol. 24:3763–3770. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li J, Tang Y, Huang L, Yu Q, Hu G, Zou Y
and Yuan X: A high number of stromal tumor-infiltrating lymphocytes
is a favorable independent prognostic factor in M0 (stages I–III)
esophageal squamous cell carcinoma. Dis Esophagus. 30:1–7. 2016.
View Article : Google Scholar
|
|
36
|
Chiu B, Jantuan E, Shen F, Chiu B and
Sergi C: Autophagy-inflammasome interplay in heart failure: A
systematic review on basics, pathways, and therapeutic
perspectives. Ann Clin Lab Sci. 47:243–252. 2017.PubMed/NCBI
|
|
37
|
Apel A, Herr I, Schwarz H, Rodemann HP and
Mayer A: Blocked autophagy sensitizes resistant carcinoma cells to
radiation therapy. Cancer Res. 68:1485–1494. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen Y, Song H, Lu Y, Li X, Chen T, Zhang
Y, Xue JX, Liu H, Kan B, Yang G and Fu T: Autophagy inhibition
contributes to radiation sensitization of esophageal squamous
carcinoma cells. Dis Esophagus. 24:437–443. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Clark CA, Gupta HB and Curiel TJ: Tumor
cell-intrinsic CD274/PD-L1: A novel metabolic balancing act with
clinical potential. Autophagy. 13:987–988. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Parsa AT, Waldron JS, Panner A, Crane CA,
Parney IF, Barry JJ, Cachola KE, Murray JC, Tihan T, Jensen MC, et
al: Loss of tumor suppressor PTEN function increases B7-H1
expression and immunoresistance in glioma. Nat Med. 13:84–88. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Dong L, Lv H, Li W, Song Z, Li L, Zhou S,
Qiu L, Qian Z, Liu X, Feng L, et al: Co-expression of PD-L1 and
p-AKT is associated with poor prognosis in diffuse large B-cell
lymphoma via PD-1/PD-L1 axis activating intracellular AKT/mTOR
pathway in tumor cells. Oncotarget. 7:33350–33362. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Afreen S and Dermime S: The
immunoinhibitory B7-H1 molecule as a potential target in cancer:
Killing many birds with one stone. Hematol Oncol Stem Cell Ther.
7:1–17. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hatogai K, Fujii S, Kojima T, Daiko H,
Nomura S, Doi T, Kitano S, Ohtsu A, Takiguchi Y, Yoshino T and
Ochiai A: Large-scale comprehensive immunohistochemical biomarker
analyses in esophageal squamous cell carcinoma. J Cancer Res Clin
Oncol. 143:2351–2361. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Madore J, Vilain RE, Menzies AM, Kakavand
H, Wilmott JS, Hyman J, Yearley JH, Kefford RF, Thompson JF, Long
GV, et al: PD-L1 expression in melanoma shows marked heterogeneity
within and between patients: Implications for anti-PD-1/PD-L1
clinical trials. Pigment Cell Melanoma Res. 28:245–253. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
McLaughlin J, Han G, Schalper KA,
Carvajal-Hausdorf D, Pelekanou V, Rehman J, Velcheti V, Herbst R,
LoRusso P and Rimm DL: Quantitative assessment of the heterogeneity
of PD-L1 expression in non-small-cell lung cancer. JAMA Oncol.
2:46–54. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Rehman JA, Han G, Carvajal-Hausdorf DE,
Wasserman BE, Pelekanou V, Mani NL, McLaughlin J, Schalper KA and
Rimm DL: Quantitative and pathologist-read comparison of the
heterogeneity of programmed death-ligand 1 (PD-L1) expression in
non-small cell lung cancer. Modern Pathol. 30:340–349. 2017.
View Article : Google Scholar
|