Introduction
Breast cancer is the most common tumor among women
and the fifth leading cause of cancer-related death worldwide
confirmed by high rates of incidence and mortality (1,2). Breast
tumors are very heterogeneous with multiple histological and
molecular subtypes and features, and thus, one of the major
challenges for clinical prediction and therapy response assessment
(3,4).
Therefore, the understanding of the molecular characteristics of
tumors has contributed to a better prognosis and development of new
therapeutic strategies (5–8).
Currently, the classification of breast cancer is
based on estrogen receptor (ER), progesterone receptor (PR) and
growth factor receptor epidermal 2 (HER-2/neu). The status of these
receptors is used within the clinical routine and allows
establishment of tumor subtypes with distinct clinical behavior and
individualized treatment (9).
Approximately 60 to 75% of breast cancers express ER-a and/or PR,
which are markers and determinants for the use of endocrine
therapies (10,11). Conversely, triple negative breast
cancer (TNBC) is a heterogeneous subgroup of breast cancer
characterized by the absence of expression of ER, PR and HER-2/neu.
TNBC represents approximately 15–20% of all breast cancer cases and
is generally considered as the most severe subgroup of breast
cancer associated with the occurrence of metastases and poor
survival of patients (12–14).
In recent decades, it has become clear that this
type of cancer can no longer be considered as a single disease
entity. It is essential to know about the tumor microenvironment
and adaptations of the cells beyond the analysis of molecular
characteristics of breast cancer. During the tumorigenesis, cancer
cells adapt their metabolic pathways to meet the high-energy
demands required for their accelerated growth and proliferation and
the associated metabolic stresses (15). Once the tumor size reaches more than
1–2 mm of diameter size and the growth outpaces that of adequate
vasculature, oxygen and nutrient delivery become insufficient. This
dynamic interplay between the normal stroma and the malignant cells
coupled with inevitable hypoxia is common in any solid tumor
microenvironment (16).
The environmental stress of hypoxia triggers
molecular changes especially the suppression of the degradation of
hypoxia-inducible factors (HIFs) that facilitate metabolic
adaptations in order to maintain the tumor growth (17–20). One
important cell feature capable of modulating the HIFs expression is
the activation of glucose transporter (GLUT)-1 which facilitates
the glucose transportation into the cells which is essential for
the malignant cells to adapt their metabolism under hypoxia
conditions (17,21). Instead the normal cells, the tumor
cells show anaerobic glycolysis as predominant metabolic activity
even in presence of oxygen a process called Warburg effect
(22,23). Once that the cells are on anaerobic
glycolysis mode, the levels of lactate and hydrogen increase
forcing them to export it out and hence decreasing the pH and
acidifying the extracellular matrix (19,24,25). The
acid effect is able to induce apoptosis in healthy cells but the
malignant cells have adaptive feature which avoid this mechanism
enhancing the advantages on the proliferation and progression
(25).
Based on those prior features, the hypoxia and
acidification in the tumor microenvironment are key factors that
give advantages to the malignancy of the tumor cells (25–28).
Therefore, the development of models that mimic the tumor
microenvironment heterogeneity has become important to find new
therapeutic targets to eliminate different populations of tumor
cells. In this context, we highlight melatonin, an indole hormone,
mainly produced and secreted by the pineal gland and also can be
found in all biological fluids including cerebrospinal fluid,
saliva, bile, synovial fluid, amniotic fluid, and breast milk
(29,30). The melatonin's actions involved in
cancer are varied, including: Antioxidant effects, regulation of
the ER expression and transactivation, modulation of the enzymes
involved in the local synthesis of estrogens, modulation of cell
cycle and induction of apoptosis, inhibition of telomerase
activity, inhibition of metastasis, prevention of circadian
disruption, antiangiogenics, epigenetic effects, stimulation of
cell differentiation and activation of the immune system (31,32).
Researchers have focused in the role of melatonin in metastasis and
proliferation providing results that show inhibition in the
proliferation as well as invasion/migration of human cells
disrupting the tube formation and counteracted the VEGF-stimulated
tubular network formation in vitro (33). Another work has found that melatonin
(both endogenous and exogenous) significantly represses this
invasive/metastatic phenotype through a mechanism that involves the
suppression of EMT, either by promoting mesenchymal-to-epithelial
transition, and/or by inhibiting key signaling pathways involved in
later stages of metastasis (34).
However, the comprehension melatonin function on low PH
microenvironment is fundamental to verify its use as adjuvant
treatment in breast cancer.
The aim of this study was therefore to determine the
capability of melatonin on the modulation of proliferation and
apoptosis in acid microenvironment of ER-positive tumor cell line
MCF-7 and triple-negative tumor cell line MDA-MB-231 through the
expression of proteins involved in the tumorigenic process.
Materials and methods
Cell culture
This study was performed using human breast cancer
cell lines MCF-7 [American Type Culture Collection, (ATCC),
Manassas, VA, USA] and MDA-MB-231 (ATCC). Both cell lines were
grown in 75 cm2 flasks (Sarstedt, Nümbrecht, Germany)
with DMEM (Thermo Fisher Scientific, Inc., Waltham, MA, USA) and
RPMI-1640 medium (Thermo Fisher Scientific, Inc.), respectively and
supplemented with 10% fetal bovine serum (FBS; Cultilab, Campinas,
SP, Brazil), penicillin (100 U/ml) and streptomycin (100 mg/ml)
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). Both cell lines
were cultured in a humidified chamber with 5% CO2 and at
37°C.
Experimental conditions
The MCF-7 and MDA-MB-231 cells were seeded in
complete medium with initial number of 2.1×106 cells.
Six experimental conditions were performed. Group I: Control group,
cells grown in complete medium, maintained at pH 7.4; group II:
Cells grown in culture medium (pH 7.4) and treated with vehicle
(ethanol 100%: PBS) for 24 h; group III: Cells grown in culture
medium (pH 7.4) and treated with melatonin (1 mM) (Sigma-Aldrich;
Merck KGaA) for 24 h (35); group IV:
Cells grown for 24 h in complete medium with MES, pH adjusted to
6.7 (36); group V: Cells cultured in
low pH medium with MES reagent (4-Morpholineethanesulfonic acid
monohydrate; Sigma-Aldrich; Merck KGaA) and treated with melatonin
(1 mM) for 24 h; group VI: Cells cultured in low pH medium with MES
for 12 h, and then treated with melatonin (1 mM) for an additional
12 h in the same medium. It should be emphasized here that the
concentration of 1 mM melatonin used for the treatment of the cells
was defined according to the literature. This is the
pharmacological concentration used in several studies about the
effects of melatonin in breast cancer (35,37,38). For
an induction of the acute acidosis condition, the growth medium was
replaced for a medium supplemented with 25 mM buffer
2-(N-Morpholino) ethanesulfonic acid (MES; Sigma-Aldrich; Merck
KGaA) and the pH adjusted to 6.7 and maintained for 24 h (39).
Cell viability
MCF-7 and MDA-MB-231 cells were grown on a 96 well
plate (Sarstedt, Nümbrecht, Germany) with 100 µl of medium
containing 0.05×106 cells/well. The cells were incubated
under the different experimental conditions described above. Then
the cells were washed and pulsed with 10 µl of MTT at 0.5 mg/ml
[3-(4,5-Dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide;
Thermo Fisher Scientific, Inc.] to each well and the plate was
incubated at 37°C for 4 h. The solubilization of the MTT formazan
crystals was made adding 10 mM SDS-HCl (Thermo Fisher Scientific,
Inc.) for 4 h at 37°C. Measurement of the absorbance was carried
out on ELISA reader (Thermo Fisher Scientific, Inc.) at 570 nm and
the results were expressed as percentage of viable cells compared
to the control group. All treatments were performed in
triplicate.
Immunocytochemistry
The immunocytochemical (ICC) technique was performed
to evaluate the expression of the protein transporter of GLUT-1
(1:1,200; Abcam, Cambridge, UK), Ki-67 (1:200; BioCare, Concord,
CA, USA) and cleaved Caspase-3 (1:100; BioCare) (Table I). Initially, 0.05×106
cells were attached to 8-well chamber slides (Sarstedt, Newtoon,
NC, USA) and maintained at 37°C and 5% CO2. After
cellular adherence, cells were incubated at different experimental
conditions for 24 h. Subsequently, the chamber slides were removed
and the slides were washed with PBS and immediately fixed in 4%
paraformaldehyde for 24 h. After this period, the cells were washed
with 1 ml of PBS, blocked with 10% PBS+BSA (Sigma-Aldrich; Merck
KGaA) for 30 min and with the primary antibody at 4°C for 12 h. In
sequence, the slides were washed in PBS and incubated with Reveal
Biotin-Free Polyvalent DAB (Spring Bioscience, Pleasanton, CA, USA)
containing the secondary antibody (Biotinylated antimouse, rabbit,
goat immunoglobulins) for one hour. Positive staining was detected
using 3,3′-diaminobenzidine (DAB) as a substrate (Thermo Fisher
Scientific, Inc.) and counterstained with hematoxylin for 40 sec.
The slides were mounted with medium Erv-Mount (EasyPath, Erviegas,
SP, Brazil). All ICC reactions were performed with a positive
control for the tested antibody and a negative control was obtained
by omitting the primary antibody. For densitometric analyses, three
slides from each experimental group were used, and 20 points were
analyzed in three fields to obtain an average related to the
intensity of immunoreactivity. At the end of the procedure, the
expression of the antibodies was quantified by densitometry
technique with the Nikon Eclipse E200 microscope (Nikon
Corporation, Tokyo, Japan). The image analyzer and values obtained
as arbitrary units (a.u.) were performed using the software
HistoQuant.
 | Table I.Antibodies used in
immunocytochemistry. |
Table I.
Antibodies used in
immunocytochemistry.
| Antibody | Supplier | Code | Dilution |
|---|
| GLUT-1 | Abcam | AB 652 |
1:1,200 |
| Ki-67 | BioCare
medical | CRM 325 | 1:200 |
| Caspase-3
(cleaved) | BioCare
medical | CP 229 | 1:100 |
| Secondary
antibodya | Spring
biosciences | SPD 015 | Ready to use |
Statistical analysis
All data were expressed as mean ± standard error
comparison between treatment with melatonin and the vehicle group
using the Student t-test and analysis of variance (ANOVA), followed
by test Bonferroni with GraphPad Prism 5.0 software (GraphPad
Software, Inc., La Jolla, CA, USA). All assays were performed in
three independent experiments done in triplicate and P<0.05 was
considered to indicate a statistically significant difference.
Ethical considerations
The study was discharged from the ethics committee
of the Faculty of Medicine of São José do Rio Preto, Sao Paulo,
Brazil because it was performed with breast cancer cell lines.
Results
Effects of low pH microenvironment and
melatonin treatment on the cell viability of MCF-7 and MDA-MB-231
cells
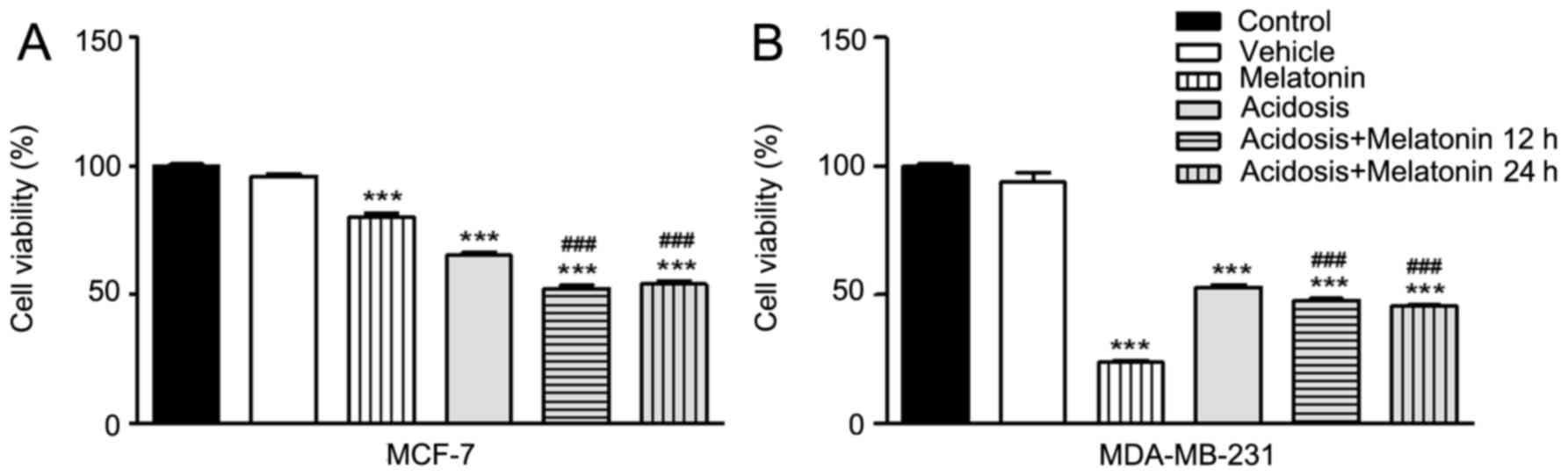
The cell viability was investigated under the
different experimental conditions via MTT assay. The assay was
performed on MCF-7 and MDA-MB-231 cell lines under normal
conditions (pH 7.4), under acidosis (pH 6.7) and with or without
treatment with melatonin. The results showed decrease of cell
viability of both cells lines under acidosis condition compared to
the control group (P<0.001; Fig.
1). The treatment with melatonin in complete medium with pH 7.4
significantly decreased cell viability compared with the control
group (P<0.001; Fig. 1). The same
effect was observed under acute acidosis when the cells were
treated with melatonin for 24 h or after 12 h of acidosis induction
(P<0.001; Fig. 1). However, the
results revealed no significant differences in cell viability
between 12 or 24 h of melatonin treatment under acidosis (Fig. 1).
Expression of GLUT-1 in MCF-7 and
MDA-MB-231 cells
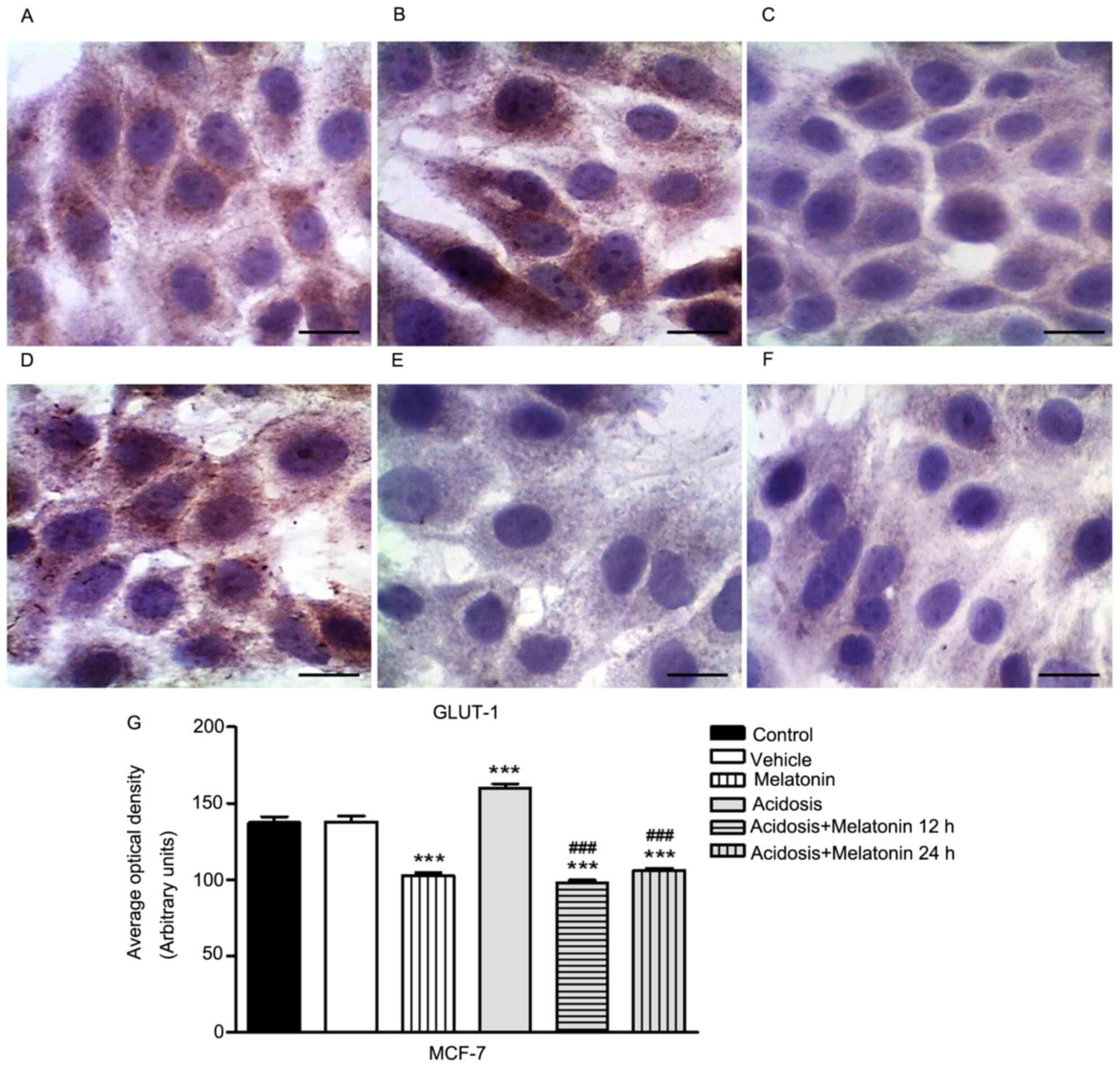
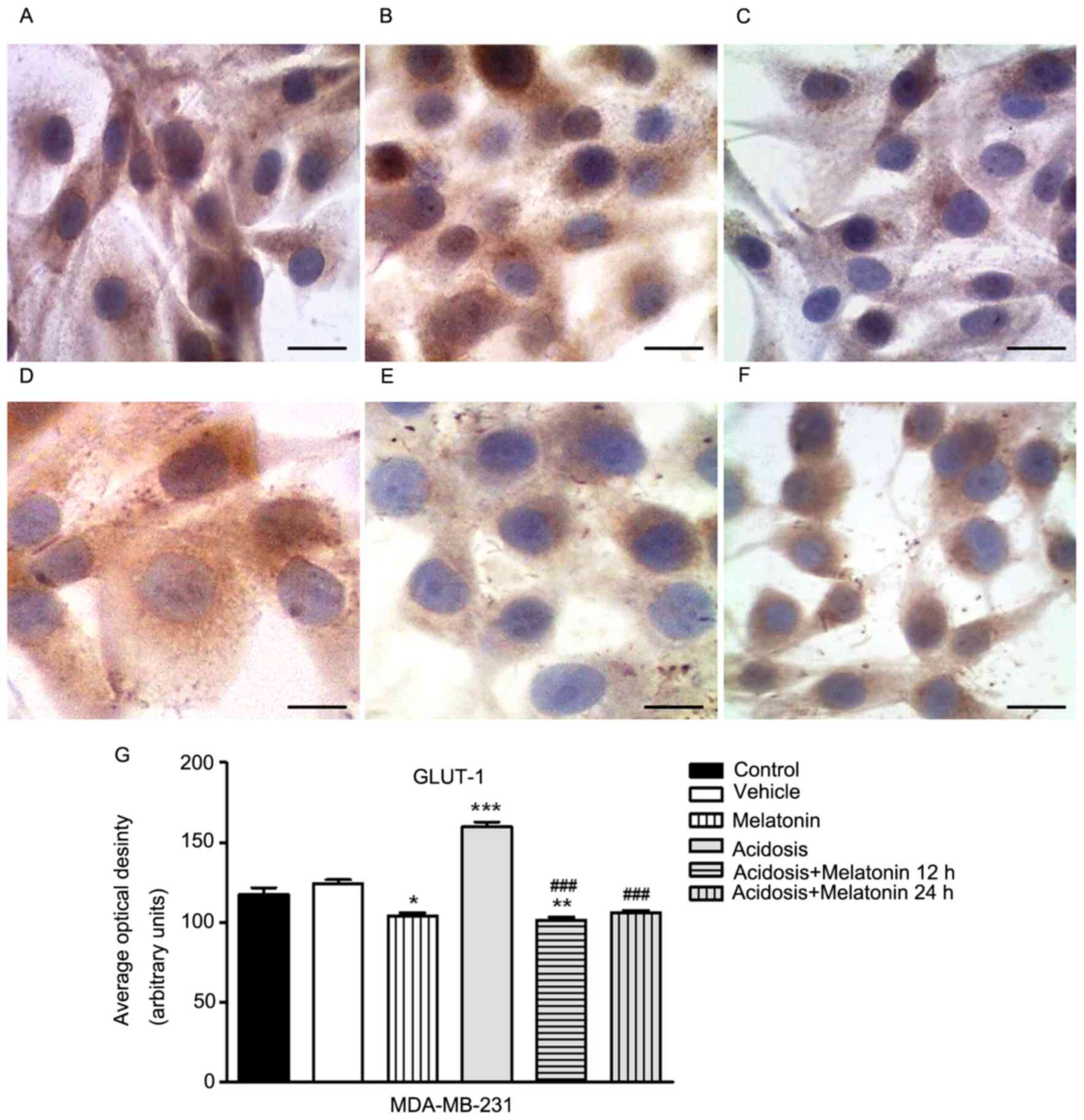
The immunocytochemistry assay to evaluate the
expression of GLUT-1 was conducted in MCF-7 (Fig. 2) and MDA-MB-231 cells (Fig. 3) control, melatonin (1 mM), acidosis
and acidosis/melatonin at 12 and 24 h. There was no significant
difference between cells treated with vehicle (Figs. 2B and 3B) compared to control group (Figs. 2A and 3A). The treatment with melatonin for 24 h
efficiently reduced the expression of GLUT-1 (Figs. 2C and 3C) compared to control cells (Figs. 2A and 3A). Acute exposure to acidic medium
increased the expression of GLUT-1 (Figs.
2D and 3D) and decreased after
treatment with melatonin for 12 h (Figs.
2E and 3E) and 24 h (Figs. 2F and 3F). The densitometric analysis confirmed the
reduction of GLUT-1 in cells treated with melatonin after 24 h. A
similar effect was observed in the group after acidosis conditions
and treatment with melatonin for 12 and 24 h. On the other hand,
low pH environment conditions increased the expression of GLUT-1
(P<0.05; Figs. 2G and 3G).
Expression of cell proliferation
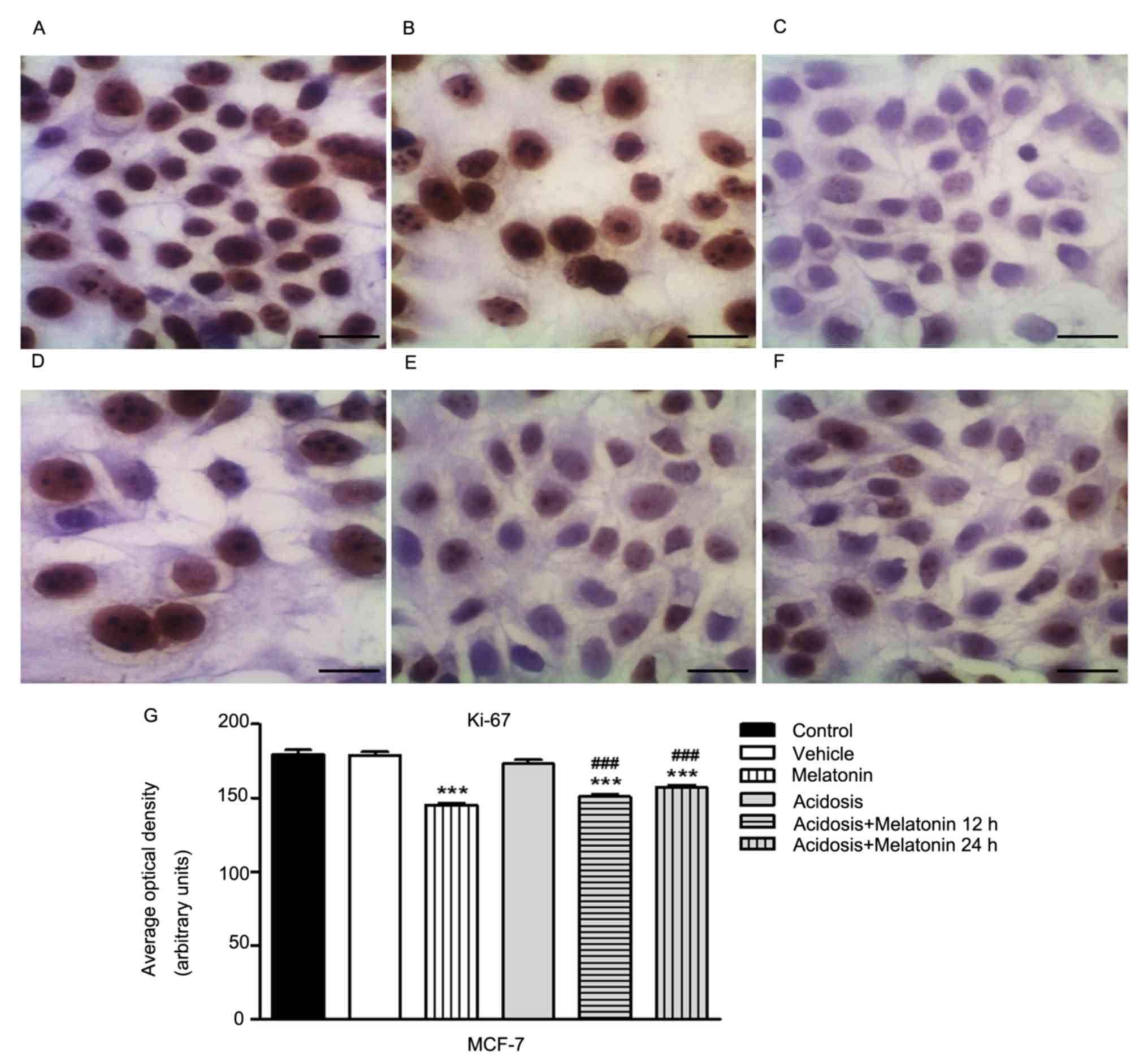
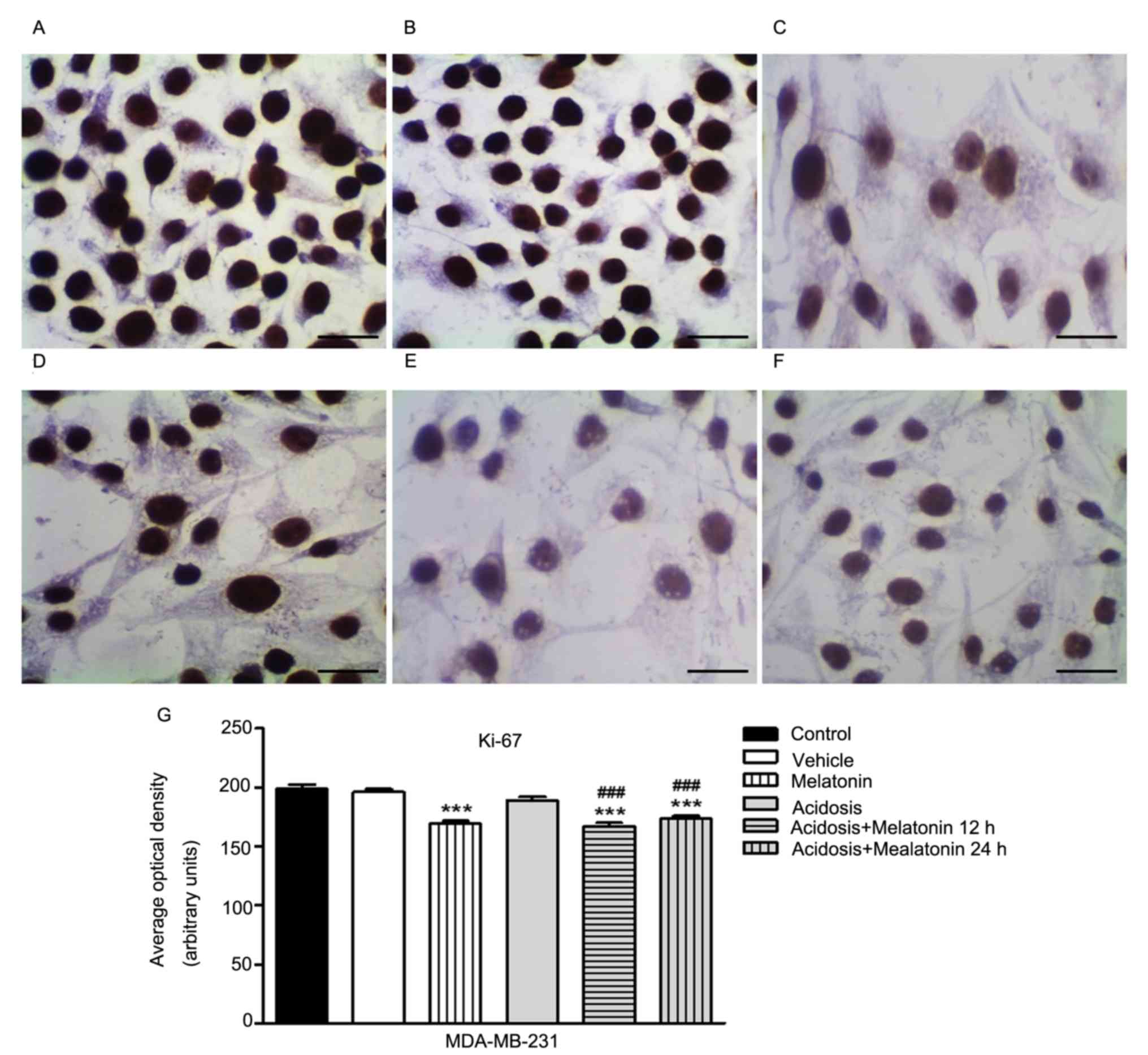
marker Ki-67 in MCF-7 and MDA-MB-231 cells
The expression of Ki-67 was analyzed in MCF-7
(Fig. 4) and MDA-MB-231 cells
(Fig. 5) control, melatonin (1 mM),
acidosis and acidosis/melatonin at 12 and 24 h. There was no
significant difference when compared cells treated with vehicle
(Figs. 4B and 5B) with the control cells (Figs. 4A and 5A). We observed reduced expression of Ki-67
after treatment with melatonin for 24 h (Figs. 4C and 5C) compared to control cells (Figs. 4A and 5A). After acute acidosis (Figs. 4D and 5D) an increase in Ki-67 was observed and the
expression decreased after treatment with melatonin for 12 h
(Figs. 4F and 5F) and 24 h (Figs.
4E and 5E). The densitometric
analysis showed significant decrease in Ki-67 expression in cells
treated with melatonin after 24 h (P<0.05). A similar effect was
observed in both cells lines culture in pH 6.7 and treated with
melatonin for 12 and 24 h. However, cells cultured transiently at
low pH increased the expression of Ki-67 (P<0.05; Figs. 4G and 5G).
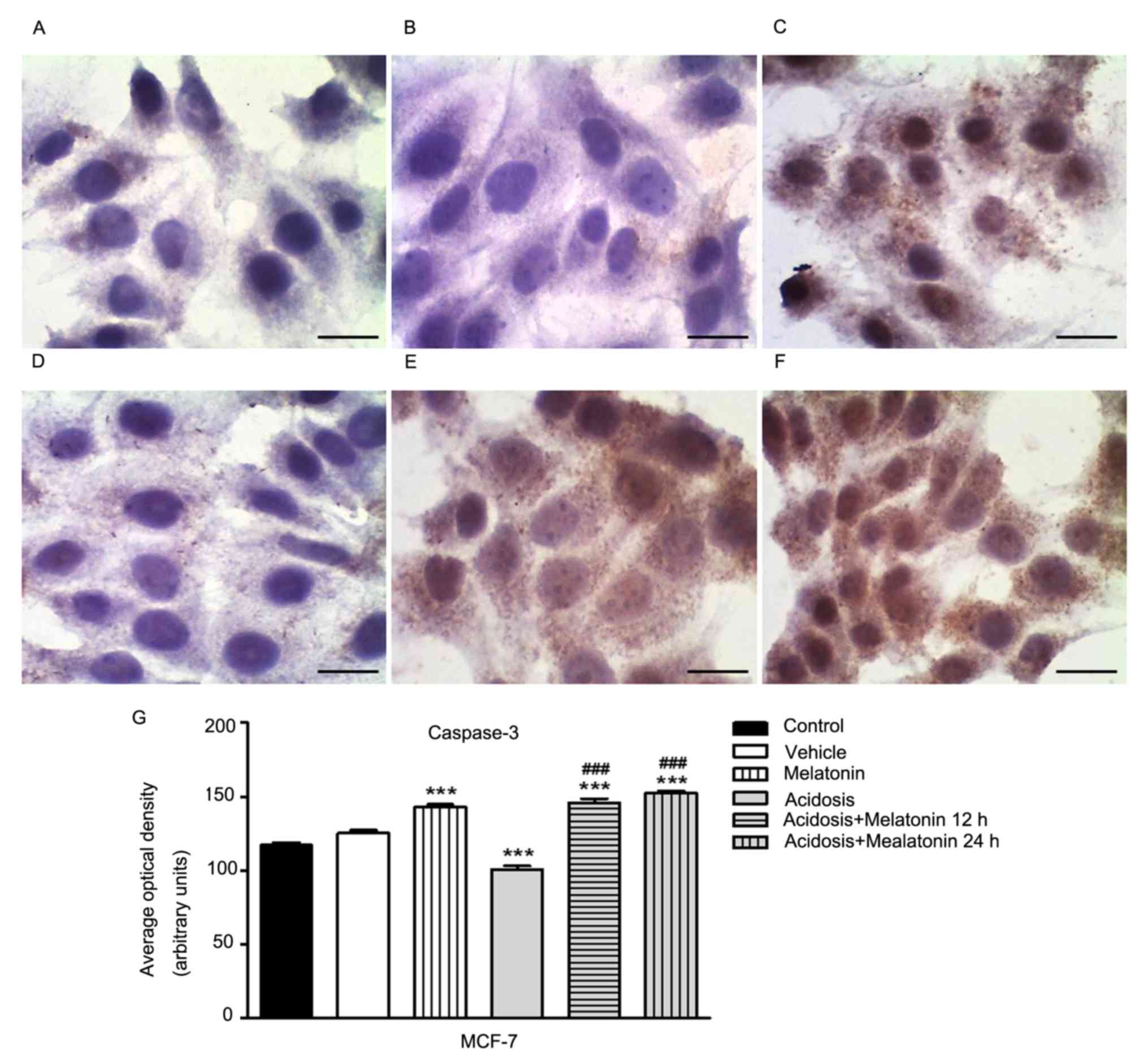
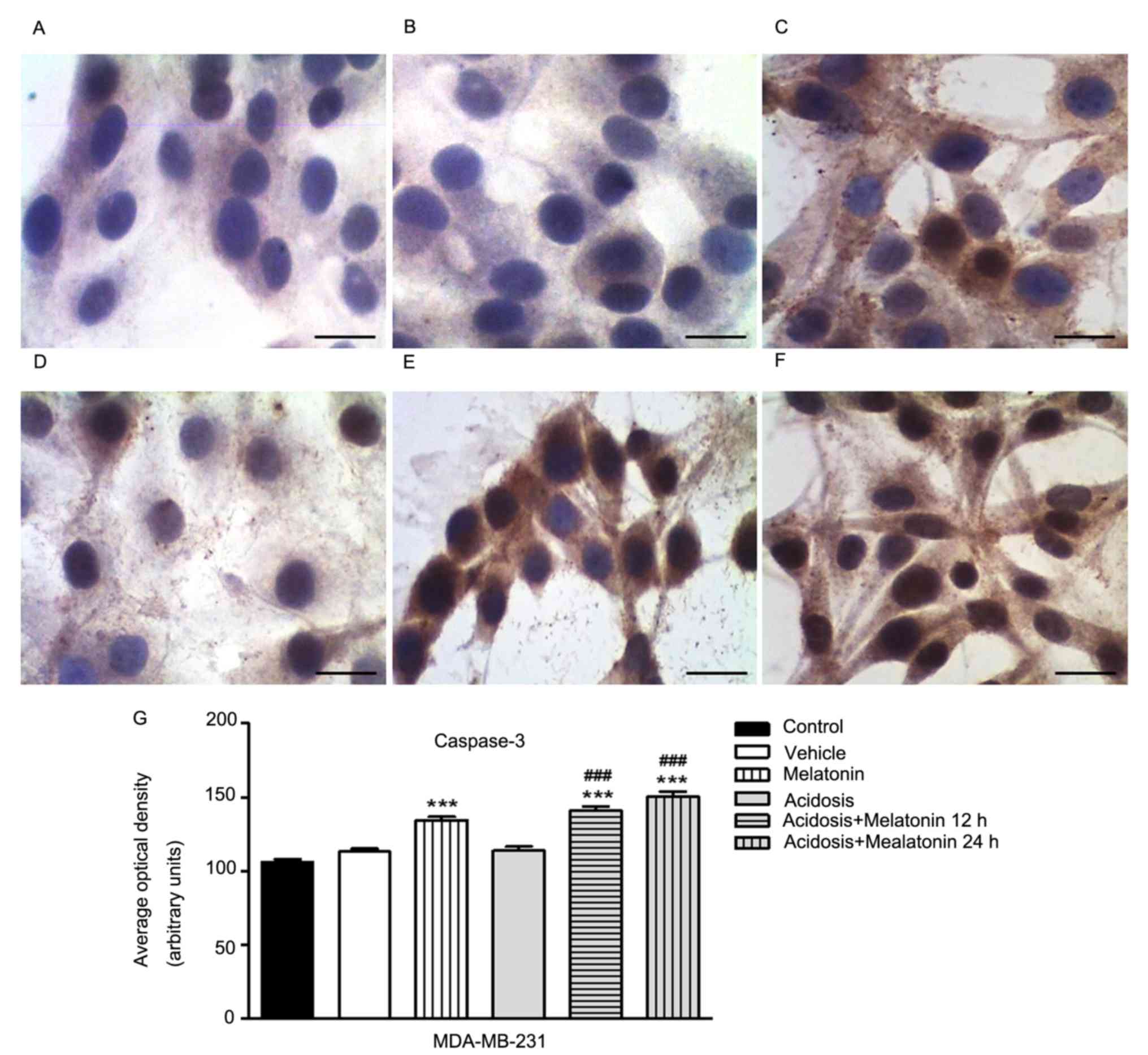
Expression of cell death marker
Caspase-3 (cleaved) in MCF-7 and MDA-MB-231 cells
The immunocytochemistry analysis to evaluate the
expression of cleaved Caspase-3 was conducted in MCF-7 (Fig. 6) and MDA-MB-231 cells (Fig. 7) under the different experimental
conditions. There was no significant difference when the cells were
compared under normal growth conditions (Figs. 6A and 7A) with cells treated with vehicle (Figs. 6B and 7B). The treatment with melatonin for 24 h
increases the expression of Caspase-3 (Figs. 6C and 7C) compared to control group (Figs. 6A and 7A). We showed a decrease in Caspase-3
expression in both cells lines grown in low pH media for 24 h
(Figs. 6D and 7D) and increased after treatment with
melatonin for 12 h (Figs. 6F and
7F) and 24 h (Figs. 6E and 7E). Similarly, densitometry analysis
confirmed these observations (P<0.05; Figs. 6G and 7G).
Discussion
In this study the effects of melatonin were
investigated on the ER-positive tumor cell line MCF-7 and
triple-negative tumor cell line MDA-MB-231 under acute acidosis. We
showed that low pH conditions increased the levels of poor
prognostic markers in tumor cells and that melatonin can reverse
this by decreasing the cell viability, expression of GLUT-1 and
Ki-67 and by increasing the expression of Caspase-3.
The inhibitory effect of melatonin on cell viability
in MCF-7 and MDA-MB-231 was achieved with the concentration of 1 mM
in both low pH and normal pH (7.4) medium conditions. In our study,
MTT assay also showed that at low pH conditions the cell viability
of MCF-7 and MDA-MB-231 cell lines were reduced compared with
control group. Another study also noted that acute exposure to
acidic medium reduced the proliferation of MDA-MB-231 cells after
72 h and that large percentage of these cells were in G1 phase at
24 and 72 h. In addition, both cells lines were grown in pH 6.7
medium for 3 months, under chronic acidosis conditions and had
similar growth rates to non-adapted cells using the autophagy
mechanism to survive (39).
After investigation of the effect of melatonin on
cell viability, we evaluated the expression of GLUT-1 by
immunocytochemistry in MCF-7 and MDA-MB-231 cell lines under normal
conditions and acidosis. Densitometric analysis showed an increase
in expression of GLUT-1 under acidosis conditions and a decrease in
expression after treatment with melatonin in both cell lines. The
upregulation of GLUT-1 was observed in a variety of tumors and it
is clearly associated with tumor progression (40). Although the metabolic consequences of
increased glucose transport are not understood, GLUT-1 expression
seems to have significant clinical function in several tumors
(41). The fast tumor growth induces
metabolic changes in tumor cells to increase the consumption of
glucose and supply the large energetic cost required by the intense
activity of cell proliferation and accelerated metabolism. To
maintain homeostasis of the tumor environment, tumor cells undergo
anaerobic glycolysis, which leads to a high consumption of glucose;
this activity is dependent on the GLUT-1 transporter (42). The treatment with melatonin (1 mM)
reduces the GLUT-1 expression in normal conditions and under
acidosis. The effects of melatonin in GLUTs expression are not well
established. Hevia et al (43)
showed that melatonin competes with glucose to bind the GLUT-1
receptor decreasing the glucose uptake and GLUT-1 expression in
prostate cancer cells. The authors suggested that melatonin can be
transported by GLUT-1, increasing its intracellular levels
(43).
A similar effect was observed for Ki-67. Analysis of
Ki-67 protein expression by immunocytochemistry showed that
melatonin decreases Ki-67 protein levels under acidosis conditions
and it also decrease after melatonin treatment in both cell lines.
Hill and Black (1988) showed that melatonin inhibits MCF-7 cell
proliferation (44). It is true that
melatonin has many anti-tumor properties, including
anti-proliferative and pro-apoptotic actions, broadly investigated
in many tumor types especially in breast cancer (45,46).
However it is poorly understood how these effects correlate with
breast cancer proliferation under acidosis conditions particularly
with the Ki-67 protein expression, a known cell proliferation
biomarker used for breast cancer prognosis (47). Based on this, the Ki-67 expression was
evaluated in cell lines under acidosis treated with melatonin and
the results showed a decrease in Ki-67 expression after 12 and 24 h
of treatment. These data corroborate with other studies that showed
a significant decrease in Ki-67 expression in MCF-7 cells after
treatment with melatonin (46,48). Lower
expression of Ki-67 was also showed in a previous study using nude
mice with breast cancer treated with 40 mg/kg of melatonin for 21
days (35).
Danielczyk and Dziegiel (49) examined the relationship between
expression of melatonin MT1 receptors and the level of Ki-67 in
dermal melanoma (primary tumours and metastatic lymph nodes). In
both, primary and metastasis, a very weak correlation was disclosed
between MT1 and Ki-67 expression. Taken together these observations
published so far suggest a variable relation between the expression
of Ki-67 and melatonin. This relationship depends on the type of
cell and tumor involved, as suggested by the authors (49).
The melatonin effect on GLUT-1 and Ki-67 protein
expression have already been described only in melanoma (50) and prostate cancer (43). Studies that analyze the induction of
melatonin apoptosis are based on its kinetic suppression effect on
cell growth and on its metabolic activity in breast cancer. Thus,
studies involving molecular mechanisms of this action are still
scarce. So, the molecular pathways of melatonin on the expression
of GLUT-1 and Ki-67 still unclear. Moreover, further experiments
should be done in order to investigate the possible mechanisms that
melatonin is involved.
Differently of the regulation of melatonin in the
expression of GLUT-1 and Ki-67, the expression of Capase-3 after
treatment increases its protein levels under normal conditions and
acidosis in both tumor cell lines. The modulation of Caspases by
melatonin was previous showed only in leukemia cells, but not in
breast cancer cell lines (51).
Regarding the pro-apoptotic effect, studies have shown that
melatonin is able to induce apoptosis activating the Caspase-3
pathway. This enzyme acts as an executor of apoptosis so, once it
is activated, the programmed cell death begins irreversibly
(52). In normal cells, there are two
apoptosis-related pathways: The intrinsic and extrinsic pathway,
the intrinsic pathway implicates the participation of the
mitochondria. Studies suggest that melatonin causes translocation
of the Cytochrome c from inside the mitochondria to the
extracellular matrix through unspecific pores triggering the
Caspase-9 and subsequent the Caspase-3 activation (52). Therefore this mechanism may explain
our data where melatonin administration in both cell lines has
increased the Caspase-3 levels with subsequent cell death. An
additional method to assess apoptosis is the TUNEL assay (Terminal
deoxyribonucleotidyl transferase (TDT)-mediated dUTP-digoxigenin
nick end labeling), which detects DNA fragments in situ.
However, DNA fragmentation is common to different types of cell
death and its in situ detection should not be considered as
a specific marker of apoptosis (53).
Models demonstrate that different evolutionary
trajectories may occur in adaptation to hypoxia and acidosis, but
will generally converge to a final phenotype with constitutive
upregulation of glycolysis and resistance to acid-induced toxicity
(26). Taken together, our results
show that melatonin treatment decreases the cell proliferation and
GLUT-1 protein expression and increase the apoptosis under acute
acidosis in breast cancer cell lines. The analysis of the
expression of the caspase-3, Ki-67 and GLUT-1 markers have been
poorly studied using in vitro assays with breast cancer
cells, especially in MDA-MB-231 and MCF-7, highlighting that our
results present different data from the current literature. In
conclusion, we have proposed in this report that melatonin has the
ability to prevent the aggressive phenotype cell shifting of the
mammary tumor cell lines under acidosis condition. It is noteworthy
that several evidences showed that melatonin is a potential
adjuvant therapeutic target for breast cancer but is required
further investigations for a deeper understanding of the regulating
mechanism of breast tumor under acidosis environment.
Acknowledgements
The authors would like to thank Conselho Nacional de
Desenvolvimento Científico e Tecnológico - CNPq (grant no.
138094/2014-4) and Fundação de Amparo à Pesquisa do Estado de São
Paulo - FAPESP (grant no. 13194-0/2014) for the scholarship. In
addition, the authors would like to thank the Laboratorio de
Investigaçao Molecular do Cancer (LIMC) from Faculdade de Medicina
de Sao Jose do Rio Preto (FAMERP) for providing the materials and
facilities to carry out this study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Parkin DM and Steliarova-Foucher
E: Estimates of cancer incidence and mortality in Europe in 2008.
Eur J Cancer. 46:765–781. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stevens RG, Brainard GC, Blask DE, Lockley
SW and Motta ME: Breast cancer and circadian disruption from
electric lighting in the modern world. CA Cancer J Clin.
64:207–218. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Prat A and Perou CM: Deconstructing the
molecular portraits of breast cancer. Mol Oncol. 5:5–23. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Engelhardt EG, Garvelink MM, de Haes JH,
van der Hoeven JJ, Smets EM, Pieterse AH and Stiggelbout AM:
Predicting and communicating the risk of recurrence and death in
women with early-stage breast cancer: A systematic review of risk
prediction models. J Clin Oncol. 32:238–250. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gonzalez-Angulo AM, Morales-Vasquez F and
Hortobagyi GN: Overview of resistance to systemic therapy in
patients with breast cancer. Adv Exp Med Biol. 608:1–22. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hicks DG and Kulkarni S: Trastuzumab as
adjuvant therapy for early breast cancer: The importance of
accurate human epidermal growth factor receptor 2 testing. Arch
Pathol Lab Med. 132:1008–1015. 2008.PubMed/NCBI
|
|
7
|
Gralow J, Ozols RF, Bajorin DF, Cheson BD,
Sandler HM, Winer EP, Bonner J, Demetri GD, Curran W Jr, Ganz PA,
et al: Clinical cancer advances 2007: Major research advances in
cancer treatment, prevention, and screening-a report from the
American Society of Clinical Oncology. J Clin Oncol. 26:313–325.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Duffy MJ, O'Donovan N and Crown J: Use of
molecular markers for predicting therapy response in cancer
patients. Cancer Treat Rev. 37:151–159. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hsiao YH, Chou MC, Fowler C, Mason JT and
Man YG: Breast cancer heterogeneity: Mechanisms, proofs, and
implications. J Cancer. 1:6–13. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lewis-Wambi JS and Jordan VC: Treatment of
postmenopausal breast cancer with Selective Estrogen Receptor
Modulators (SERMs). Breast Dis. 24:93–105. 2005-2006. View Article : Google Scholar
|
|
11
|
Dauchy RT, Xiang S, Mao L, Brimer S, Wren
MA, Yuan L, Anbalagan M, Hauch A, Frasch T, Rowan BG, et al:
Circadian and melatonin disruption by exposure to light at night
drives intrinsic resistance to tamoxifen therapy in breast cancer.
Cancer Res. 74:4099–4110. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fernandes RC, Bevilacqua JL, Soares IC,
Siqueira SA, Pires L, Hegg R and Carvalho FM: Coordinated
expression of ER, PR and HER2 define different prognostic subtypes
among poorly differentiated breast carcinomas. Histopathology.
55:346–352. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cao MD, Lamichhane S, Lundgren S, Bofin A,
Fjøsne H, Giskeødegård GF and Bathen TF: Metabolic characterization
of triple negative breast cancer. BMC Cancer. 14:9412014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kaplan HG and Malmgren JA: Impact of
triple negative phenotype on breast cancer prognosis. Breast J.
14:456–463. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Qiu F, Chen YR, Liu X, Chu CY, Shen LJ, Xu
J, Gaur S, Forman HJ, Zhang H, Zheng S, et al: Arginine starvation
impairs mitochondrial respiratory function in ASS1-deficient breast
cancer cells. Sci Signal. 7:ra312014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Justus CR, Sanderlin EJ and Yang LV:
Molecular connections between cancer cell metabolism and the tumor
microenvironment. Int J Mol Sci. 16:11055–11086. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rundqvist H and Johnson RS: Tumour
oxygenation: Implications for breast cancer prognosis. J Intern
Med. 274:105–112. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mekhail K, Gunaratnam L, Bonicalzi ME and
Lee S: HIF activation by pH-dependent nucleolar sequestration of
VHL. Nat Cell Biol. 6:642–647. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Swietach P, Vaughan-Jones RD and Harris
AL: Regulation of tumor pH and the role of carbonic anhydrase 9.
Cancer Metastasis Rev. 26:299–310. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Maschio LB, Madallozo BB, Capellasso BA,
Jardim BV, Moschetta MG, Jampietro J, Soares FA and Zuccari DA:
Immunohistochemical investigation of the angiogenic proteins VEGF,
HIF-1α and CD34 in invasive ductal carcinoma of the breast. Acta
Histochem. 116:148–157. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Harris AL: Hypoxia-A key regulatory factor
in tumour growth. Nat Rev Cancer. 2:38–47. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Warburg O: On the origin of cancer cells.
Science. 123:309–314. 1956. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yu L, Chen X, Wang L and Chen S: The sweet
trap in tumors: Aerobic glycolysis and potential targets for
therapy. Oncotarget. 7:38908–38926. 2016.PubMed/NCBI
|
|
24
|
Wu H, Ding Z, Hu D, Sun F, Dai C, Xie J
and Hu X: Central role of lactic acidosis in cancer cell resistance
to glucose deprivation-induced cell death. J Pathol. 227:189–199.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xu K, Mao X, Mehta M, Cui J, Zhang C, Mao
F and Xu Y: Elucidation of how cancer cells avoid acidosis through
comparative transcriptomic data analysis. PLoS One. 8:e711772013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gatenby RA, Gawlinski ET, Gmitro AF,
Kaylor B and Gillies RJ: Acid-mediated tumor invasion: A
multidisciplinary study. Cancer Res. 66:5216–5223. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
De Milito A, Canese R, Marino ML, Borghi
M, Iero M, Villa A, Venturi G, Lozupone F, Iessi E, Logozzi M, et
al: pH-dependent antitumor activity of proton pump inhibitors
against human melanoma is mediated by inhibition of tumor acidity.
Int J Cancer. 127:207–219. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Stüwe L, Müller M, Fabian A, Waning J,
Mally S, Noël J, Schwab A and Stock C: pH dependence of melanoma
cell migration: Protons extruded by NHE1 dominate protons of the
bulk solution. J Physiol. 585:351–360. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hill SM, Belancio VP, Dauchy RT, Xiang S,
Brimer S, Mao L, Hauch A, Lundberg PW, Summers W, Yuan L, et al:
Melatonin: An inhibitor of breast cancer. Endocr Relat Cancer.
22:R183–R204. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Coto-Montes A, Boga JA, Tan DX and Reiter
RJ: Melatonin as a potential agent in the treatment of sarcopenia.
Int J Mol Sci. 17(pii): E17712016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mediavilla MD, Sanchez-Barcelo EJ, Tan DX,
Manchester L and Reiter RJ: Basic mechanisms involved in the
anti-cancer effects of melatonin. Curr Med Chem. 17:4462–4481.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nooshinfar E, Safaroghli-Azar A, Bashash D
and Akbari ME: Melatonin, an inhibitory agent in breast cancer.
Breast Cancer. 24:42–51. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Alvarez-García V, González A,
Alonso-González C, Martínez-Campa C and Cos S: Regulation of
vascular endothelial growth factor by melatonin in human breast
cancer cells. J Pineal Res. 54:373–380. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mao L, Summers W, Xiang S, Yuan L, Dauchy
RT, Reynolds A, Wren-Dail MA, Pointer D, Frasch T, Blask DE and
Hill SM: Melatonin represses metastasis in Her2-postive human
breast cancer cells by suppressing RSK2 expression. Mol Cancer Res.
14:1159–1169. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jardim-Perassi BV, Arbab AS, Ferreira LC,
Borin TF, Varma NR, Iskander AS, Shankar A, Ali MM and de Campos
Zuccari DA: Effect of melatonin on tumor growth and angiogenesis in
xenograft model of breast cancer. PLoS One. 9:e853112014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gatenby RA, Smallbone K, Maini PK, Rose F,
Averill J, Nagle RB, Worrall L and Gillies RJ: Cellular adaptations
to hypoxia and acidosis during somatic evolution of breast cancer.
Br J Cancer. 97:646–653. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Borin TF, Arbab AS, Gelaleti GB, Ferreira
LC, Moschetta MG, Jardim-Perassi BV, Iskander AS, Varma NR, Shankar
A, Coimbra VB, et al: Melatonin decreases breast cancer metastasis
by modulating Rho-associated kinase protein-1 expression. J Pineal
Res. 60:3–15. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gonçalves Ndo N, Colombo J, Lopes JR,
Gelaleti GB, Moschetta MG, Sonehara NM, Hellmén E, Zanon Cde F,
Oliani SM and Zuccari DA: Effect of melatonin in epithelial
mesenchymal transition markers and invasive properties of breast
cancer stem cells of canine and human cell lines. PLoS One.
11:e01504072016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wojtkowiak JW, Rothberg JM, Kumar V,
Schramm KJ, Haller E, Proemsey JB, Lloyd MC, Sloane BF and Gillies
RJ: Chronic autophagy is a cellular adaptation to tumor acidic pH
microenvironments. Cancer Res. 72:3938–3947. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Macheda ML, Rogers S and Best JD:
Molecular and cellular regulation of glucose transporter (GLUT)
proteins in cancer. J Cell Physiol. 202:654–662. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Oliver RJ, Woodwards RT, Sloan P, Thakker
NS, Stratford IJ and Airley RE: Prognostic value of facilitative
glucose transporter Glut-1 in oral squamous cell carcinomas treated
by surgical resection; Results of EORTC Translational Research Fund
studies. Eur J Cancer. 40:503–507. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Suganuma N, Segade F, Matsuzu K and Bowden
DW: Differential expression of facilitative glucose transporters in
normal and tumour kidney tissues. BJU Int. 99:1143–1149. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hevia D, González-Menéndez P,
Quiros-González I, Miar A, Rodríguez-García A, Tan DX, Reiter RJ,
Mayo JC and Sainz RM: Melatonin uptake through glucose
transporters: A new target for melatonin inhibition of cancer. J
Pineal Res. 58:234–250. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hill SM and Blask DE: Effects of the
pineal hormone melatonin on the proliferation and morphological
characteristics of human breast cancer cells (MCF-7) in culture.
Cancer Res. 48:6121–6126. 1988.PubMed/NCBI
|
|
45
|
Cos S and Sánchez-Barceló EJ: Melatonin
and mammary pathological growth. Front Neuroendocrinol. 21:133–170.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chottanapund S, Van Duursen MB, Navasumrit
P, Hunsonti P, Timtavorn S, Ruchirawat M and Van den Berg M:
Anti-aromatase effect of resveratrol and melatonin on hormonal
positive breast cancer cells co-cultured with breast adipose
fibroblasts. Toxicol In Vitro. 28:1215–1221. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Liu YX, Wang KR, Xing H, Zhai XJ, Wang LP
and Wang W: Attempt towards a novel classification of
triple-negative breast cancer using immunohistochemical markers.
Oncol Lett. 12:1240–1256. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Czeczuga-Semeniuk, Wołczyński S, Anchim T,
Dziecioł J, Dabrowska M and Pietruczuk M: Effect of melatonin and
all-trans retinoic acid on the proliferation and induction of the
apoptotic pathway in the culture of human breast cancer cell line
MCF-7. Pol J Pathol. 53:59–65. 2002.PubMed/NCBI
|
|
49
|
Danielczyk K and Dziegiel P: The
expression of MT1 melatonin receptor and Ki-67 antigen in melanoma
malignum. Anticancer Res. 29:3887–3895. 2009.PubMed/NCBI
|
|
50
|
Danielczyk K and Dziegiel P: MT1 melatonin
receptors and their role in the oncostatic action of melatonin.
Postepy Hig Med Dosw (Online). 63:425–434. 2009.(In Polish).
PubMed/NCBI
|
|
51
|
Perdomo J, Cabrera J, Estévez F, Loro J,
Reiter RJ and Quintana J: Melatonin induces apoptosis through a
caspase-dependent but reactive oxygen species-independent mechanism
in human leukemia Molt-3 cells. J Pineal Res. 55:195–206. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Chen X, Duan N, Zhang C and Zhang W:
Survivin and tumorigenesis: Molecular mechanisms and therapeutic
strategies. J Cancer. 7:314–323. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Grasl-Kraupp B, Ruttkay-Nedecky B,
Koudelka H, Bukowska K, Bursch W and Schulte-Hermann R: In situ
detection of fragmented DNA (TUNEL assay) fails to discriminate
among apoptosis, necrosis, and autolytic cell death: A cautionary
note. Hepatology. 21:1465–1468. 1995. View Article : Google Scholar : PubMed/NCBI
|