Introduction
According to the American Cancer Society, ~81,190
cases of bladder cancer (62,380 men and 18,810 women) will be
diagnosed in the USA in 2018 (1).
During the same year, ~17,240 bladder cancer-related deaths are
expected to occur (12,520 men and 4,720 women) (1). Bladder cancer is the fourth most common
cancer in men, but it is less common in women. In general,
urothelial carcinoma (transitional cell carcinoma) accounts for
>90% of all bladder cancers, whereas squamous cell carcinoma
accounts for 3–8% and adenocarcinoma for 1–2% of all cases.
Approximately 75% of patients are diagnosed with
non-muscle-invasive bladder cancer (NMIBC) (2), 50–70% of whom develop disease recurrence
and 10–15% develop disease progression. Urinary tumor biomarkers,
including BTA®, NMP22®, UroVysion®
FISH and ImmunoCyt™, have been investigated for the purposes of
improving the diagnosis, surveillance and staging of bladder cancer
(3). However, their low specificity
has limited the use of these biomarkers. Currently, standard
guidelines recommend the use of these biomarkers as an adjunctive
surveillance strategy, to reduce the need for invasive cystoscopy
(3). Cxbladder is another urinary
biomarker that measures the mRNA expression profile of five genes
(IGF, HOXA, MDK, CDC and IL8R), with a sensitivity of 93% and
specificity of 85%. It has been used to classify high- and low-risk
subgroups of patients presenting with hematuria, to assess whether
invasive cystoscopy is required (4).
Development of bladder cancer occurs via
accumulation of molecular events. Genetic alterations may result in
uncontrolled cellular proliferation, inhibition of differentiation
and invasiveness of tumor cells. These changes determine tumor
growth, relapse, progression and metastasis. It is necessary to
identify reliable bladder cancer progression-associated genes in
order to improve current methods of detecting and monitoring cancer
recurrence and metastatic invasion.
Abnormal spindle-like microcephaly-associated
(ASPM) gene, also referred to as abnormal spindle
microtubule assembly, is located on chromosome 1q31 and encodes the
ASPM protein. Expression of ASPM is essential for normal mitotic
spindle function in embryonic neuroblasts and regulation of
neurogenesis (5). Defects in the
ASPM gene are associated with autosomal recessive primary
microcephaly (6,7). Neuronal depletion is associated with
ASPM mutations and predominantly affects the anterior cortex
during development (8,9). An expression study revealed that
ASPM mRNA levels are higher in fetal tissues, but are very
low in adult tissue (10). It has
been reported that the ASPM gene is overexpressed in
glioblastoma and malignant glioma compared with normal brain tissue
(11–13). Knockdown of ASPM by small
interfering RNA was shown to reduce tumor cell and neural stem cell
proliferation (12). ASPM
expression is also associated with ependymoma recurrence in
children (14). Abnormalities in
ASPM expression are associated with numerous cancer types.
ASPM mRNA is overexpressed in hepatocellular carcinoma
(10,15). Upregulation is also observed in SV40
immortalized cells and non-small cell lung cancer tissues (16). It has also been proposed that
upregulation of ASPM expression increases the invasive
capacity of melanoma cells (17).
Studies in patients have revealed that ASPM is significantly
associated with poor outcomes in hepatocellular carcinoma (10), ovarian cancer (18), pancreatic cancer (19) and prostate cancer (20). However, to date, the clinical
relevance and prognostic significance of ASPM in bladder
cancer remains unknown.
In the present study, the clinical relevance and
prognostic significance of ASPM were evaluated based on six
bladder cancer gene expression datasets. Stratification and
multivariate analyses were conducted to reduce confounding effects.
Gene set enrichment analysis (GSEA) was performed to identify
cancer-associated gene signatures of ASPM in bladder cancer.
ASPM co-expression proteins were also analyzed using the
STRING database, and the prognostic relevance of the corresponding
genes was evaluated. Based on these findings, the prognostic
significance of ASPM in bladder cancer was evaluated.
Materials and methods
Analysis of human bladder tissues by
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR)
Ten paired bladder cancer tissue samples (6 male and
4 female, age from 57 to 84) were collected from the Affiliated
Dongyan People's Hospital, Wenzhou Medical University (Dongyang,
China), according to a protocol approved by the Institutional
Review Board. Informed consent was obtained from each participant.
Total RNA from formalin-fixed paraffin-embedded tissue samples was
extracted using an RN30-EASYspin kit (Aidab Biotechnologies Co.,
Ltd., Beijing, China) in order to evaluate ASPM mRNA expression.
cDNA was produced from 1 µg total RNA using the Promega M-MLV
Reverse Transcriptase (Promega Corporation, Madison, WI, USA) and
oligo dT primers according to the standard protocol. The ASPM
primers for qPCR were as follows: Forward,
5′-AGCATTCCTTTTATCCCAGAAACACCTG-3′ and reverse,
5′-GCTTGCAGGGGATTTGTGATTTCTTCC-3′. Actin was used as a loading
control. The human actin primers were as follows: Forward,
5′-CCCCAACTTGAGATGTATGAAGGCT-3′ and reverse,
5′-TCTCAAGTCAGTGTACAGGTAAGCC3′. RT-qPCR was performed using 1 µl
cDNA in a 50-µl reaction volume. The thermocycling conditions were
as follows: 95°C for 10 min, followed by 40 cycles at 95°C for 10
sec and at 60°C for 1 min. The experiment was performed in
triplicate.
Worldwide microarray gene expression
datasets
A total of six independent worldwide bladder cancer
microarray datasets were used in the present study. Four Gene
Expression Omnibus (GEO) bladder cancer gene expression datasets
were downloaded from www.ebi.ac.uk/arrayexpress; these datasets included
GSE13507 (21), GSE31684 (22), E-MTAB-1803 (23) and E-MTAB-4321 (24). Two bladder cancer gene expression
datasets, TCGA set1 and TCGA set2, were obtained from The Cancer
Genomic Atlas (TCGA) research network: Cancergenome.nih.gov. All datasets contained clinical
and follow-up annotations. Datasets without prognostic outcome
information were excluded from the study. A total of 1,355
assessable bladder cancer cases with recurrence information and
overall survival (OS) data were collected. Detailed information
regarding the downloaded datasets is presented in Table I. Additional gene expression datasets
of prostate cancer (PCa) (n=496) and renal cell carcinoma (RCC)
(n=532) with clinical and outcome information were downloaded for
further validating clinical meaning of ASPM.
 | Table I.Summary of downloaded gene expression
data sets of bladder cancer. |
Table I.
Summary of downloaded gene expression
data sets of bladder cancer.
|
| GEO | TCGA |
|---|
|
|
|
|
|---|
| Accession no. | GSE13507 | GSE31684 | E-MTAB-1803 | E-MTAB-4321 | TCGA set 1 | TCGA set 2 |
|---|
| No. of
patients | 256 | 93 | 170 | 476 | 407 | 131 |
| Assessable
casesa | 165 | 93 | 85 | 476 | 407 | 129 |
| Date of study | NA | 1993–2004 | NA | NA | NA | NA |
|
Platformsa | GPL6102 | GPL570 | NA | NA | NA | NA |
| Country | South Korea | USA | NA | NA | NA | NA |
| Sex | Y | Y | Y | Y | NA | Y |
| Age at
diagnosis | 66 (24–88) | 69 (41–91) | 69 (44–89) | 69 (24–95) | 68 (34–90) | 69 (34–88) |
| Grade | Y | Y | Y | Y | Y | Y |
| Tumor size | NA | NA | NA | Y | NA | NA |
| TNM stage | Y | NA | NA | NA | Y | Y |
| Tumor stage | Y | Y | Y | Y | Y | Y |
| Lymph node | Y | Y | Y | NA | Y | Y |
| Metastasis | Y | Y | Y | NA | Y | Y |
| Tobacco smoking
history | NA | Y | NA | NA | NA | Y |
| OS months
(range) | 1.03–136.97 | 0.39–175.5 | 0–132 | NA | 0–165.9 | 0–140.7 |
| Recurrence months
(range) | NA | 0.39–175.5 | NA | NA | NA | 2.76–43.97 |
| Progression months
(range) | NA | NA | NA | 0–74.9 | 0–163.2 | NA |
Bioinformatics analysis
The detailed GSEA protocol can be obtained from the
Broad Institute Gene Set Enrichment Analysis website (http://software.broadinstitute.org/gsea/index.jsp)
(25). GSEA software v3.0 was used on
a JAVA 8.0 platform. All dataset (.gct) and phenotype label (.cls)
files were created and loaded into GSEA software, and gene sets
were downloaded from the Broad Institute website. The number of
permutations was set to 1,000. A ranked-list metric was generated
by calculating the signal-to-noise ratio, which is based on the
difference of means scaled according to the standard deviation.
Data management
Each database was downloaded, converted, constructed
and managed using Microsoft Excel (version 2007, Microsoft
Corporation, Redmond, WA, USA) and Python software (www.python.org). For pooled analysis, the expression
levels of genes were normalized prior to merging. ASPM and
related genes were re-stratified into four grades (Q1, Q2, Q3 and
Q4), based on the percentiles of each independently downloaded
dataset. The Q1 subgroup (<25% percentile) was considered as a
reference for statistical analysis. Subgroups Q2, Q3 and Q4 were
≥25 to 50%, >50 to <75%, and ≥75% respectively. In addition,
expression levels less than the median value were designated
‘ASPM-low’, and levels greater than or equal to the median
value were designated ‘ASPM-high’.
Statistical analysis
R software (www.r-project.org) and JMP 10.0 software (SAS
Institute, Cary, NC, USA) were used for general statistical
analysis. Group comparisons of continuous data were performed using
paired t-tests for independent means or one-way analyses of
variance with Tukey's post-hoc test for multiple groups.
Categorical variables were compared using χ2 analysis,
Fisher's exact test or the binomial test of proportions.
Kaplan-Meier analysis and Cox proportional hazard models were used
to analyze OS and progression-free survival (PFS). In PFS analysis,
patients with distant metastatic bladder cancer not completely
resected were excluded. Multivariate Cox analysis was applied to
adjust for covariate effects, and stratification analysis was used
to reduce the confounding effect of the calculated hazard ratios
(HRs). Missing data were coded and excluded from the analysis.
Results
Association of ASPM mRNA expression
with bladder cancer invasiveness
The mRNA expression levels of ASPM in normal
bladder mucosae and bladder cancer tissue samples were determined
by RT-qPCR. In the 10 paired samples, the mRNA expression of
ASPM was significantly increased in primary bladder cancer
compared with adjacent normal bladder mucosae (P=0.004; Fig. 1A). Further bioinformatics data from
the GSE13507 dataset supported this finding. As indicated in
Fig. 1B, ASPM mRNA levels in
primary bladder cancer and recurrent NMIBC were significantly
higher compared with normal bladder, as well as normal bladder
mucosae adjacent to cancer tissue (P<0.01).
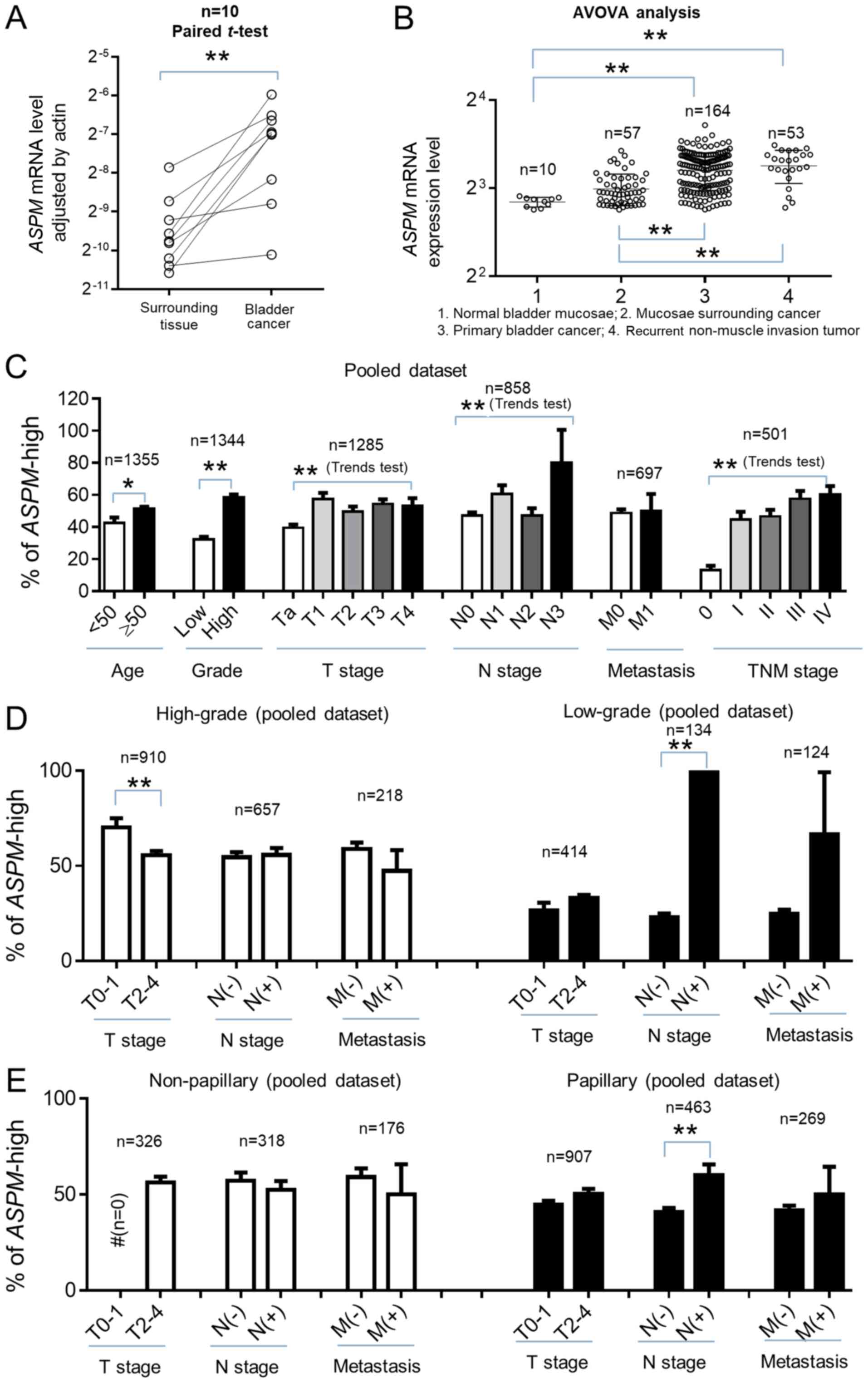 | Figure 1.ASPM mRNA expression and
clinical characteristics of bladder carcinoma. The mRNA expression
of ASPM in bladder cancer tissue and adjacent bladder mucosae was
determined by RT-qPCR analysis. The difference in mRNA levels
between normal and bladder cancer tissues was consistent with the
GSE13507 dataset. In the pooled dataset, bladder cancer cases were
designated as either ASPM-high or ASPM-low according
to the median expression value in each dataset. (A) Relative
ASPM mRNA expression in primary bladder cancer and adjacent
normal bladder mucosae. Actin was used as an internal reference.
(B) Comparison of ASPM mRNA expression in normal bladder
mucosae, mucosae adjacent to bladder cancer, primary bladder cancer
and recurrent non-muscle invasive bladder cancer in the GSE13507
dataset. (C) Distribution of ASPM mRNA expression according
to clinical characteristics, including age, grade, tumor stage,
lymph node involvement, distant metastasis and TNM stage. (D)
ASPM distribution and TNM stage in high- and low-grade
bladder cancers. (E) Clinical relevance of ASPM in papillary
and non-papillary bladder cancer histological subtypes. #,
statistical test could not be performed due to there being no T0-1
stage in non-papillary bladder cancer. ASPM, abnormal spindle-like
microcephaly-associated protein; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction. *P<0.05;
**P<0.01. |
The clinical relevance of ASPM was further
investigated in six gene expression datasets, comprising a total of
1,355 bladder cancer cases. The median ASPM expression level
was used as a cut-off point for each dataset. All participants were
stratified as ASPM-high or ASPM-low and then merged.
As indicated in Table II, analysis
revealed that the percentage of ASPM-high was significantly
and positively associated with high-grade bladder cancer, lymph
node involvement and smoking history in the pooled GEO and TCGA
datasets (P<0.05). ASPM expression was also significantly
associated with tumor stage and TNM stage in the pooled GEO dataset
(P<0.05) but not in the TCGA dataset (P>0.05). ASPM
expression was significantly associated with grade, tumor stage and
lymph node involvement in the overall pooled dataset (GEO + TCGA;
Fig. 1C). In addition, analysis
indicated that ASPM expression was more likely to be
significantly associated with lymph node involvement and metastasis
in low-grade (Fig. 1D) and papillary
(Fig. 1E) bladder cancer. T0-1 stage
bladder cancer was considered to be NMIBC, and T2-4 stage was
considered to be muscle-invasive bladder cancer (MIBC). ASPM
expression was weakly associated with MIBC (T2-4 stage) in
low-grade and papillary bladder cancer, but this result was not
identified as statistically significant. However, ASPM
expression was significantly increased in high-grade NMIBC
(P<0.001). Since there is no T0-1 stage non-papillary bladder
cancer, the association between ASPM and T stage could not
be evaluated in this subgroup. Further stratification analysis
indicated that ASPM was also significantly associated with
high grade in both NMIBC and MIBC (P<0.001). An association
between ASPM and aggressive characteristics was observed in
both male and female bladder cancer patients. Overall, gene
expression data suggested that ASPM was significantly
associated with invasive pathological characteristics in bladder
cancer, particularly in low-grade and papillary bladder cancer
subtypes.
 | Table II.Demographic distribution of ASPM in
bladder cancer. |
Table II.
Demographic distribution of ASPM in
bladder cancer.
|
| GEO | TCGA |
|---|
|
|
|
|
|---|
|
Characteristics | High (%) | Low (%) |
P-valuesa | High (%) | Low (%) |
P-valuesa |
|---|
| Sex |
|
Male | 319 (49.8) | 321 (50.2) |
| 48 (49.5) | 49 (50.5) |
|
|
Female | 92 (51.4) | 87 (48.6) | 0.713 | 17(53.1) | 15 (46.9) | 0.721 |
| Age |
| <55
yrs. | 40 (40.8) | 58 (59.2) |
| 25(45.5) | 30 (54.5) |
|
| ≥55
yrs. | 371 (51.5) | 350 (48.5) | 0.048 | 245(50.9) | 236 (49.1) | 0.441 |
| Tumor size |
| <3
cm | 125 (44.2) | 158 (55.8) |
|
|
|
|
| ≥3
cm | 60 (69.0) | 27 (31.0) | <0.001 |
|
|
|
| Grade |
|
Low | 276 (65.1) | 148 (34.9) |
| 1 (3.70) | 26 (96.3) |
|
|
High | 133 (34.3) | 255 (65.7) | <0.001 | 267 (52.9) | 238 (47.1) | <0.001 |
| TNM stage |
| 0 | 3 (13.0) | 20 (87.0) |
|
|
|
|
| I | 35 (43.8) | 45 (56.2) |
| 2 (66.7) | 1 (33.3) |
|
| II | 16 (61.5) | 10 (38.5) |
| 47 (43.1) | 62 (56.9) |
|
|
III | 15 (75.0) | 5 (25.0) |
| 64 (54.7) | 53 (45.3) |
|
| IV | 13 (81.2) | 3 (18.8) | <0.001 | 61 (57.0) | 46 (43.0) | 0.163 |
| Subtype |
|
NMIBC | 266 (45.9) | 313 (54.1) |
| 1 (100.0) | 0 (0.00) |
|
|
MIBC | 145 (60.4) | 95 (39.6) | <0.001 | 262 (50.9) | 253 (49.1) | 0.326 |
| Tumor stage |
| Ta | 148 (39.5) | 226 (60.4) |
|
|
|
|
| T1 | 115 (56.9) | 87 (43.1) |
| 3 (75.0) | 1 (25.0) |
|
| T2 | 46 (58.2) | 33 (41.8) |
| 67 (45.0) | 82 (55.0) |
|
| T3 | 54 (56.2) | 42 (43.8) |
| 138 (53.7) | 119 (46.3) |
|
| T4 | 31 (62.3) | 18 (36.7) | <0.001 | 35 (46.7) | 40 (53.3) | 0.236 |
| Node stage |
| N0 | 110 (45.5) | 132 (54.5) |
| 150 (48.4) | 160 (51.6) |
|
| NX | 10 (58.8) | 7 (41.2) |
| 25 (54.4) | 21 (45.6) |
|
| N1 | 38 (58.5) | 27 (41.5) |
| 36 (63.2) | 21 (36.8) |
|
| N2 | 6 (100.0) | 0 (0.0) |
| 44 (44.0) | 56 (56.0) |
|
| N3 | 1 (100.0) | 0 (0.0) | 0.023 | 11 (78.6) | 3 (21.4) | 0.032 |
| Metastasis |
| M0 | 78 (49.4) | 80 (50.6) |
| 131 (48.3) | 140 (51.7) |
|
| M1 | 4 (57.1) | 3 (42.9) | 0.687 | 7 (46.7) | 8 (53.3) | 0.568 |
| Smoking status |
|
Never | 6 (33.3) | 12 (66.7) |
| 55 (39.0) | 86 (61.0) |
|
|
Yes | 90 (53.6) | 78 (46.4) | 0.084 | 203 (53.7) | 175 (46.3) | 0.003 |
Prognostic significance of ASPM in
bladder cancer
The aforementioned findings suggested that
ASPM may be associated with poor survival in bladder cancer.
Cox proportional hazard and Kaplan-Meier analyses were performed to
evaluate the prognostic significance of ASPM in bladder
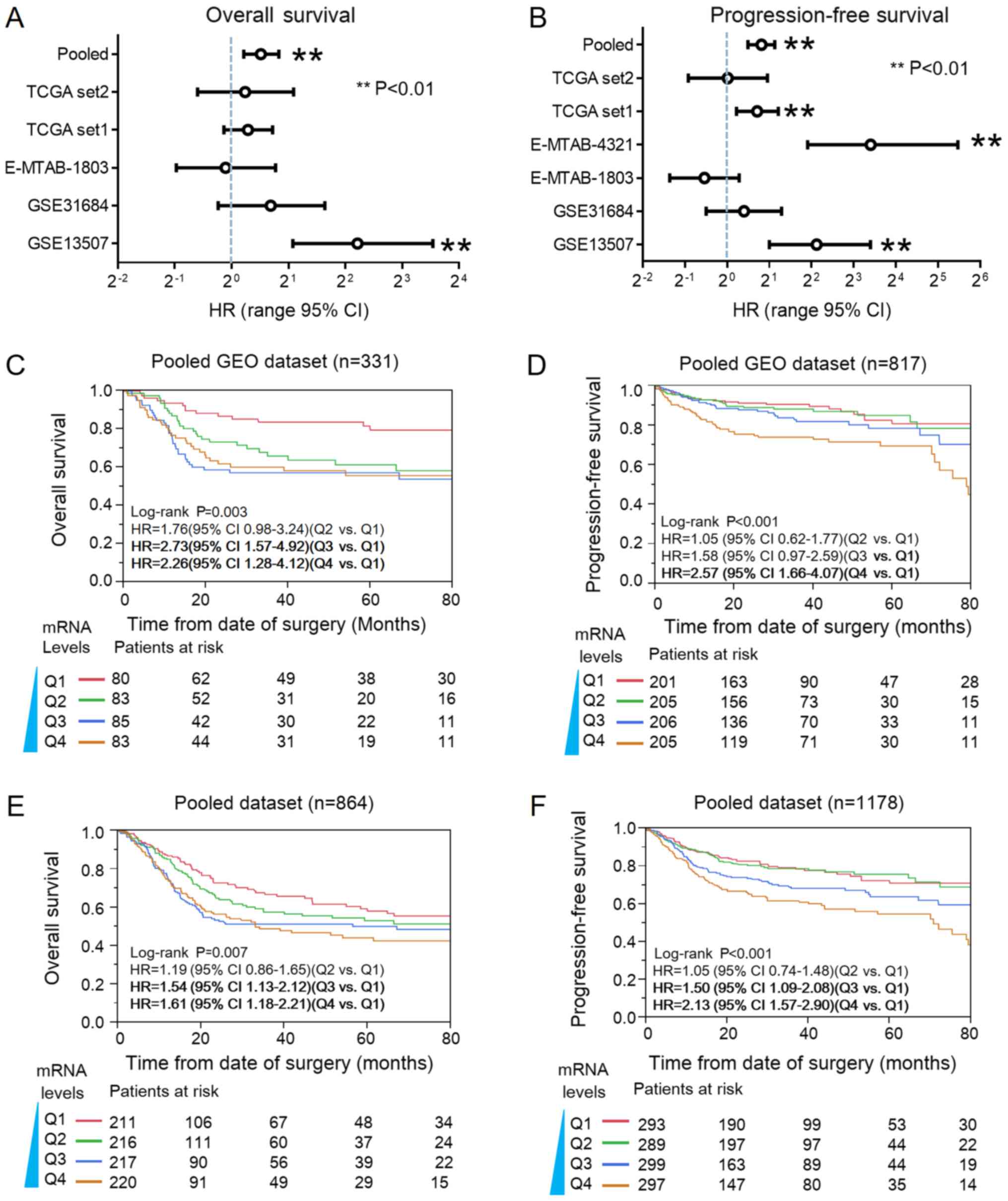
cancer. As indicated in Fig. 2A, Cox
analysis revealed ASPM-high was significantly associated
with relative risk of death from bladder cancer in the GSE13507
dataset (P<0.01). In pooled (GEO+TCGA) analysis, ASPM was
significantly associated with poor OS. The HR was 1.46 (95% CI:
1.14–1.78; P<0.01). In the TCGA dataset1, E-MTAB-4321 datasets
and GSE13507 datasets, ASPM expression was significantly
associated with progression of bladder cancer (P<0.01). The
pooled HR of ASPM for PFS was 1.76 (95% CI: 1.41–2.19;
P<0.01; Fig. 2B). Furthermore,
Kaplan-Meier plots revealed that ASPM was significantly
associated with poor OS and PFS in the pooled GEO dataset (Fig. 2C and D) and the overall pooled dataset
(Fig. 2E and F).
Prognostic significance of ASPM
expression in bladder cancer subtypes
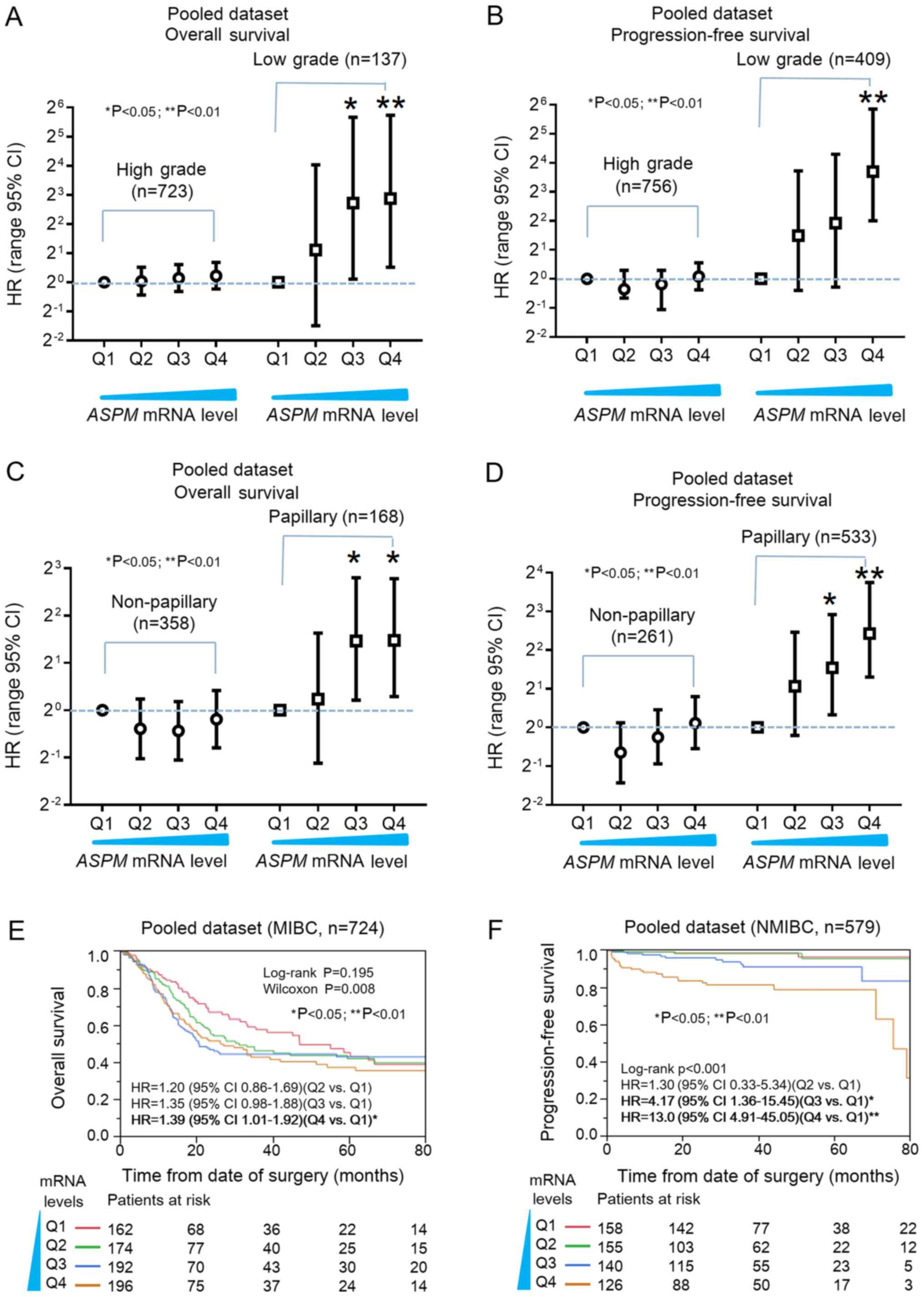
To reduce potential confounding effects, further
stratification and multivariate analysis were conducted to
investigate the prognostic significance of ASPM expression
in bladder cancer subtypes. Aforementioned findings indicated that
ASPM was associated with invasive characteristics in
low-grade rather than high-grade bladder cancer (Fig. 1D). Stratification analysis indicated
that increased ASPM expression significantly promoted
relative progression in low-grade bladder cancer, and was directly
correlated with the risk of death (Fig.
3A and B). Notably, the number of cases in the high-grade
subtype was notably higher compared with the low-grade subtype in
both the OS and PFS survival analyses, which rules out the
possibility that any statistical significance could be caused by a
larger sample size. Cox analysis revealed that ASPM
expression was associated with OS and PFS in the papillary
histological subtype, but not in the non-papillary subtype
(Fig 3C and D), which was consistent
with the aforementioned findings (Fig.
1E). The prognostic significance of ASPM in MIBC and
NMBC was also determined (Fig. 3E and
F). Kaplan-Meier plots revealed that higher ASPM
expression was associated with poor OS in MIBC (Wilcoxon P=0.008;
Fig. 3E), and ASPM was
significantly associated with poor PFS in NMIBC (log-rank
P<0.01; Fig. 3F). These findings
suggest that ASPM may serve as a prognostic biomarker for
low-grade or papillary bladder cancer.
ASPM-associated genes and their
prognostic significance in bladder cancer
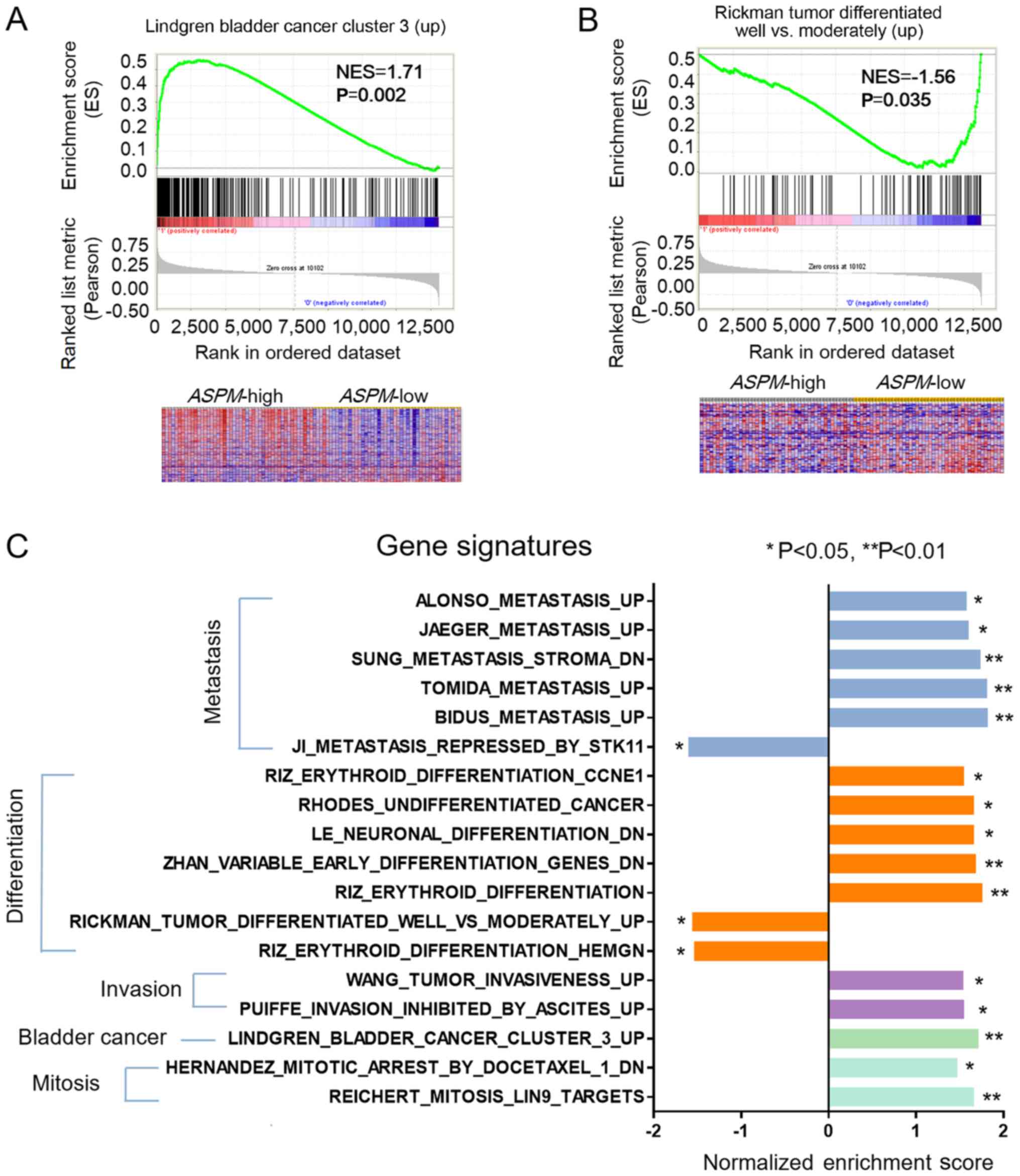
GSEA was performed to investigate the association
between ASPM gene expression and cancer-related gene
signatures (Fig. 4). A heat map
revealed that the majority of genes in the Lindgren bladder cancer
cluster 3 (up) signature were upregulated (labeled red) in the
ASPM-high subgroup (Fig. 4A),
whereas genes in the Rickman tumor differentiated well vs.
moderately (up) signature were enriched in the ASPM-low
subgroup (Fig. 4B). The gene
signatures enriched by ASPM are presented in Fig. 4C. ASPM-high enriched signatures
included: Metastasis [BIDUS Metastasis (UP) and TOMIDA Metastasis
(UP)], differentiation [RHODES Undifferentiation Cancer and LE
Neuronal Differentiation (DN)], invasion [WANG Tumor Invasiveness
(UP)], mitosis (REICHERT Mitosis) and bladder cancer (Fig. 4C). By contrast, RIZ Erythroid
Differentiation HEMGN and JI Metastasis Repressed by STK11 were
enriched in the ASPM-low subgroup (Fig. 4C).
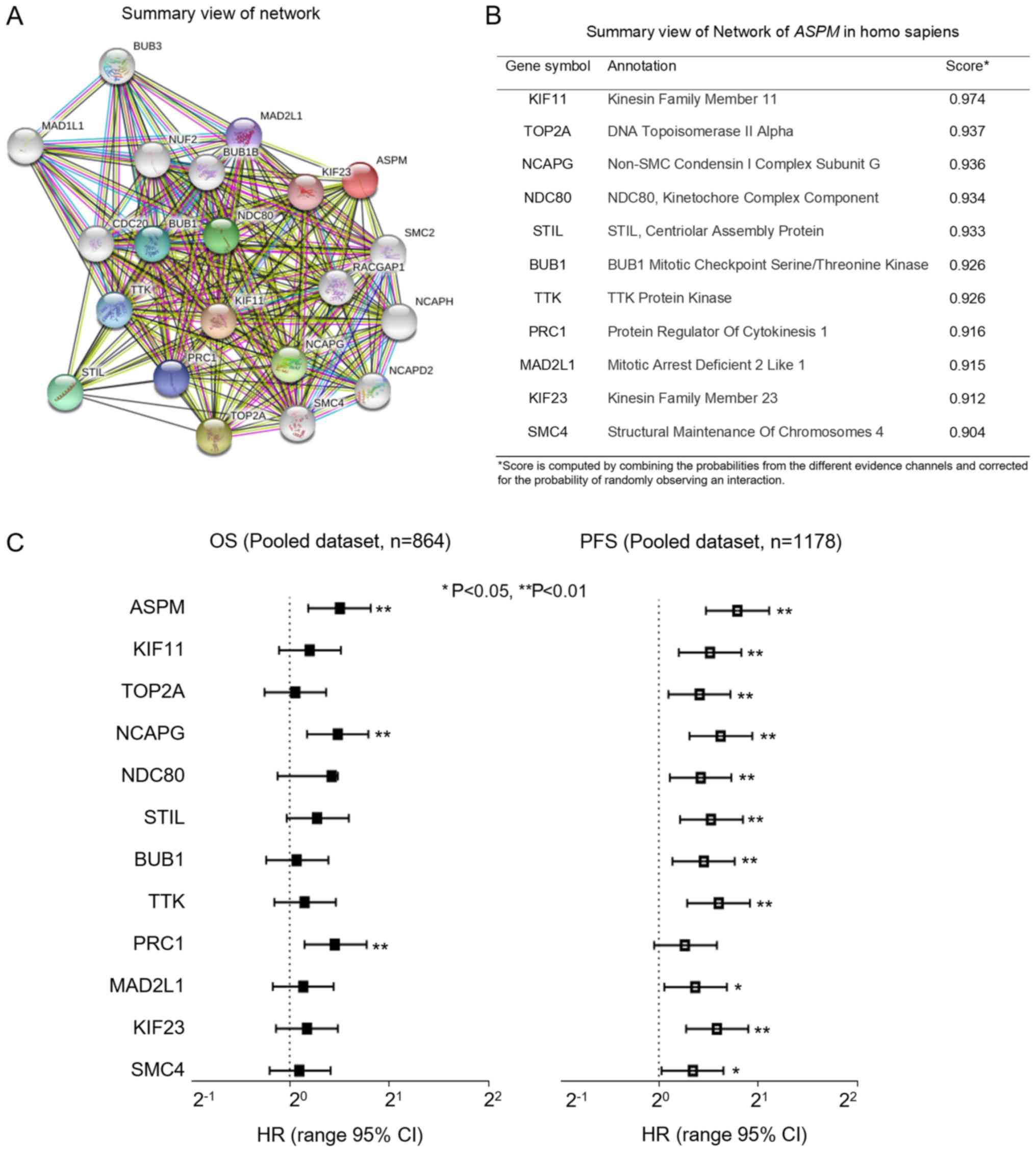
ASPM network proteins were identified by
STRING (string-db.org) and are presented in
Fig. 5A. The top 11 proteins and
corresponding gene names, annotations and scores are listed in
Fig. 5B. These genes included: KIF11,
TOP2A, NCAPG, NDC80, STIL, BUB1, TTK, PRC1, MAD2L1, KIF23 and SMC4.
These genes are related to cell mitosis, DNA replication and cell
cycle checkpoint. This was consistent with the findings from GSEA.
Subsequently, the prognostic significance of the mRNA expression
levels was analyzed. As indicated in Fig.
5C, Cox analysis indicated that the majority of these genes had
HR values >1. In addition, PFS analysis revealed that all the
genes except PRC1 were significantly associated with progression of
bladder cancer (P<0.05). Overall, these findings indicate that
ASPM is involved with bladder cancer invasiveness through
interactions with several genes related to cell proliferation,
undifferentiation and the metastasis signaling pathway.
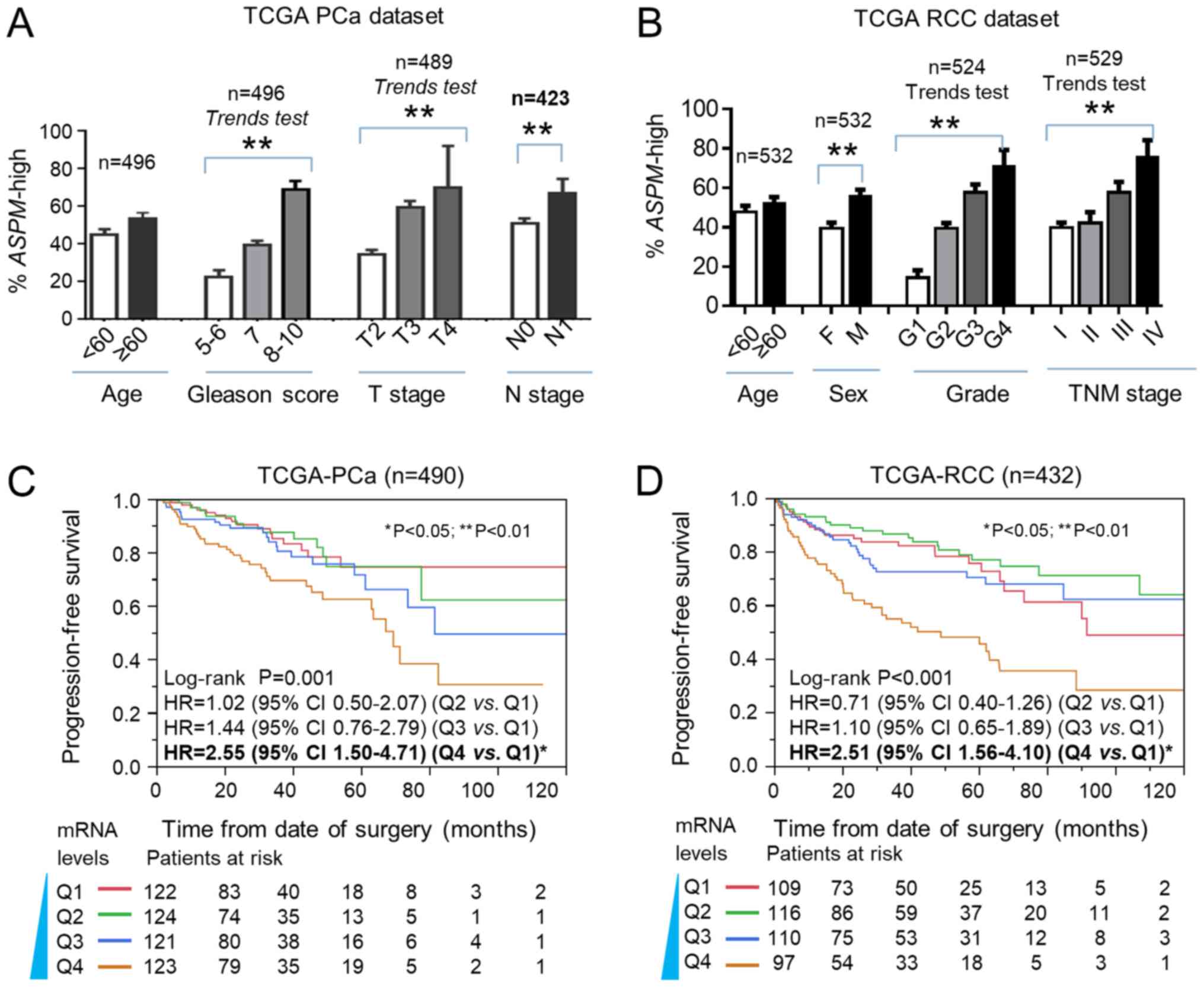
The clinical significance of ASPM in
prostate cancer and renal cell carcinoma
The clinical relevance and prognostic significance
of ASPM was also validated in urologic cancers, including
prostate cancer (PCa) and renal cell carcinoma (RCC) (Fig. 6). The expression of ASPM was found to
be associated with Gleason score, T stage and N stage of PCa
(P<0.05; Fig. 6A). ASPM expression
was also significantly associated with older age and later TNM
stage in RCC (Fig. 6B). Kaplan-Meier
and Cox analysis revealed that ASPM mRNA expression was
significantly associated with PFS in PCa and RCC, based on TCGA
datasets (P<0.01; Fig. 6C and
D).
Discussion
In the present study, it was demonstrated that
ASPM was significantly overexpressed in bladder cancer and
was associated with invasive pathological characteristics,
including high grade and advanced TNM stage in bladder cancer,
based on the GEO and TCGA datasets. In the pooled analysis,
ASPM was significantly associated with poor OS and PFS in a
dose-dependent manner. In order to verify this result,
stratification and multivariate analyses were conducted to reduce
potential confounding effects. It was indicated that the clinical
and prognostic significance of ASPM was particularly
pronounced in the low-grade and papillary bladder cancer subtypes.
The prognostic significance of ASPM was also examined in six
bladder cancer gene datasets. Individual analysis results revealed
that the prognostic performance of ASPM for each dataset
varied. The percentage of low-grade and histological subtypes of
bladder cancer in each dataset was different, which explains the
variation in prognostic performance in each dataset. In addition,
it was also observed that ASPM could predict PFS in NMIBC
and OS in MIBC. In the present study, participant data were
collected from several countries with different socioeconomic
backgrounds, suggesting that the prognostic significance of
ASPM is universal. Overall, our current findings suggest
that ASPM may serve as a prognostic biomarker for bladder
cancer of the low-grade and papillary histological subtypes.
ASPM may also serve as a potential therapeutic biomarker for
bladder cancer treatment.
Our analysis further validated that ASPM was
associated with progression and poor outcomes of other two urologic
cancers, including prostate cancer (PCa) and renal cell carcinoma
(RCC) (Fig. 6). An association
between ASPM mRNA expression and biochemical recurrence in
PCa has been previously reported (20). Kaplan-Meier analysis of ASPM
mRNA in liver, endometrial, pancreatic and lung cancer was obtained
from the Human Protein Atlas (www.proteinatlas.org/ENSG00000066279-ASPM/pathology).
Furthermore, prognostic significance of ASPM was also
reported in hepatocellular carcinoma (10), ovarian cancer (18) and pancreatic cancer (19). These findings suggest that ASPM
may serve as a prognostic biomarker for a number of other cancer
types, in addition to bladder cancer.
The mechanism by which ASPM promotes
low-grade bladder cancer aggressiveness is not yet known. In the
present study, the top 11 proteins in the ASPM network were
examined (Fig. 5). The functions of
these genes are implicated in cell mitosis, DNA synthesis, DNA
three-dimensional folding and cell proliferation. The GSEA results
indicated that ASPM could enrich mitosis, differentiation
and metastasis gene signatures in bladder cancer (Fig. 4). Previous studies have reported that
ASPM maintains the migratory and pro-metastatic potential of
cancer stem cells (CSCs) and multiple glandular cancers, including
pancreatic, breast and prostate cancer (19). ASPM may augment canonical and
non-canonical Wnt signaling by positively regulating disheveled or
other upstream Wnt signaling components to activate Wnt signaling
and cancer aggressiveness in subpopulations of CSCs. Further
studies are required in order to elucidate the mechanism underlying
the role of ASPM in cancer cell division, undifferentiation
and invasion.
A limitation of the present study is a lack of
immunohistochemistry (IHC) data to support the conclusions. The
prognostic significance of ASPM protein expression for bladder
cancer was not evaluated due to non-specific staining on IHC
analysis (data not shown). The Human Protein Atlas validated the
ASPM antibody and also concluded that ASPM IHC results were
inconsistent with mRNA expression data (www.proteinatlas.org/ENSG00000066279-ASPM/antibody).
To date, data on ASPM protein expression and outcomes in cancer
have not been reported due to the poor performance of ASPM
antibodies on IHC staining. In the present study, RT-qPCR was used
to replace IHC in order to evaluate differences in expression
between normal and bladder cancer samples. Another limitation of
this study is that therapeutic significance of ASPM could
not be evaluated due to insufficient chemotherapy and radiotherapy
information. The prognostic and predictive performance of
ASPM will be investigated further in our future studies.
In summary, the present study investigated the
clinical significance and prognostic value of ASPM mRNA
expression in bladder cancer based on six gene expression datasets.
It was concluded that ASPM expression is significantly
associated with bladder cancer aggressiveness in the low-grade and
papillary histological subtypes. Therefore, ASPM appears to
be a promising prognostic biomarker and potential therapeutic
target in bladder cancer.
Acknowledgements
The authors would like to thank NIH Gene Expression
Omnibus, ArrayExpress (www.ebi.ac.uk/arrayexpress) and The Cancer Genome
Atlas pilot platform (established by NCI and NHGRI; cancergenome.nih.gov), which made the genomic and
clinical bladder cancer data available.
Funding
This project was supported by the Key Project of the
Science and Technology Program of Zhejiang Province (grant no.
2014C03028), Donyang Hospital Research Supporting Program and the
Sino-America Cancer Foundation.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZX collected data and interpreted the results of
data analysis. QZ performed statistical and bioinformatics analysis
for bladder cancer datasets. FL provided scientific suggestions on
study design and English editing of the manuscript. BJ designed the
study. XL analyzed the data, and was a major contributor to writing
the manuscript. All authors have read and approved the final
version of this manuscript for publication.
Ethics approval and consent to
participate
Ten paired bladder cancer tissue samples were
collected from the Affiliated Dongyan People's Hospital, Wenzhou
Medical University (Dongyang, China), according to a protocol
approved by the Institutional Review Board. Informed consent was
obtained from each participant. All other high-throughput gene
expression data and clinical information of bladder cancer were
obtained from public genomic databases. All identifying information
had been removed from downloaded datasets. Our research meets the
publication guidelines provided by TCGA (cancergenome.nih.gov/publications/publicationguidelines).
Patient consent for publication
All identifying information had been removed.
Informed consent was obtained from each participant.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ASPM
|
abnormal spindle-like microcephaly-
associated protein
|
|
MIBC
|
muscle-invasive bladder cancer
|
|
NMIBC
|
non-muscle-invasive bladder cancer
|
|
GEO
|
Gene Expression Omnibus
|
|
TCGA
|
The Cancer Genome Atlas
|
|
GSEA
|
gene set enrichment analysis
|
|
OS
|
overall survival
|
|
PFS
|
progression-free survival
|
|
HR
|
hazard ratio
|
|
95% CI
|
95% confidence interval
|
|
RCC
|
renal cell carcinoma
|
|
PCa
|
prostate cancer
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Babjuk M, Burger M, Zigeuner R, Shariat
SF, van Rhijn BW, Compérat E, Sylvester RJ, Kaasinen E, Böhle A,
Palou Redorta J, et al: EAU guidelines on non-muscle-invasive
urothelial carcinoma of the bladder: Update 2013. Eur Urol.
64:639–653. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Velázquez N: Bladder cancer academy 2018
selected summaries. Rev Urol. 20:31–37. 2018.PubMed/NCBI
|
|
4
|
Darling D, Luxmanan C, O'Sullivan P, Lough
T and Suttie J: Clinical utility of cxbladder for the diagnosis of
urothelial carcinoma. Adv Ther. 34:1087–1096. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kouprina N, Pavlicek A, Collins NK, Nakano
M, Noskov VN, Ohzeki J, Mochida GH, Risinger JI, Goldsmith P,
Gunsior M, et al: The microcephaly ASPM gene is expressed in
proliferating tissues and encodes for a mitotic spindle protein.
Hum Mol Genet. 14:2155–2165. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kaindl AM, Passemard S, Kumar P, Kraemer
N, Issa L, Zwirner A, Gerard B, Verloes A, Mani S and Gressens P:
Many roads lead to primary autosomal recessive microcephaly. Prog
Neurobiol. 90:363–383. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Higgins J, Midgley C, Bergh AM, Bell SM,
Askham JM, Roberts E, Binns RK, Sharif SM, Bennett C, Glover DM, et
al: Human ASPM participates in spindle organisation, spindle
orientation and cytokinesis. BMC Cell Biol. 11:852010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Desir J, Cassart M, David P, Van Bogaert P
and Abramowicz M: Primary microcephaly with ASPM mutation shows
simplified cortical gyration with antero-posterior gradient pre-
and post-natally. Am J Med Genet A. 146A:1439–1443. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kouprina N, Pavlicek A, Mochida GH,
Solomon G, Gersch W, Yoon YH, Collura R, Ruvolo M, Barrett JC,
Woods CG, et al: Accelerated evolution of the ASPM gene controlling
brain size begins prior to human brain expansion. PLoS Biol.
2:E1262004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lin SY, Pan HW, Liu SH, Jeng YM, Hu FC,
Peng SY, Lai PL and Hsu HC: ASPM is a novel marker for vascular
invasion, early recurrence, and poor prognosis of hepatocellular
carcinoma. Clin Cancer Res. 14:4814–4820. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hagemann C, Anacker J, Gerngras S, Kühnel
S, Said HM, Patel R, Kämmerer U, Vordermark D, Roosen K and Vince
GH: Expression analysis of the autosomal recessive primary
microcephaly genes MCPH1 (microcephalin) and MCPH5 (ASPM, abnormal
spindle-like, microcephaly associated) in human malignant gliomas.
Oncol Rep. 20:301–308. 2008.PubMed/NCBI
|
|
12
|
Horvath S, Zhang B, Carlson M, Lu KV, Zhu
S, Felciano RM, Laurance MF, Zhao W, Qi S, Chen Z, et al: Analysis
of oncogenic signaling networks in glioblastoma identifies ASPM as
a molecular target. Proc Natl Acad Sci USA. 103:17402–17407. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bikeye SN, Colin C, Marie Y, Vampouille R,
Ravassard P, Rousseau A, Boisselier B, Idbaih A, Calvo CF, Leuraud
P, et al: ASPM-associated stem cell proliferation is involved in
malignant progression of gliomas and constitutes an attractive
therapeutic target. Cancer Cell Int. 10:12010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Peyre M, Commo F, Dantas-Barbosa C,
Andreiuolo F, Puget S, Lacroix L, Drusch F, Scott V, Varlet P,
Mauguen A, et al: Portrait of ependymoma recurrence in children:
Biomarkers of tumor progression identified by dual-color
microarray-based gene expression analysis. PLoS One. 5:e129322010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Drozdov I, Bornschein J, Wex T, Valeyev
NV, Tsoka S and Malfertheiner P: Functional and topological
properties in hepatocellular carcinoma transcriptome. PLoS One.
7:e355102012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jung HM, Choi SJ and Kim JK: Expression
profiles of SV40-immortalization-associated genes upregulated in
various human cancers. J Cell Biochem. 106:703–713. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kabbarah O, Nogueira C, Feng B, Nazarian
RM, Bosenberg M, Wu M, Scott KL, Kwong LN, Xiao Y, Cordon-Cardo C,
et al: Integrative genome comparison of primary and metastatic
melanomas. PLoS One. 5:e107702010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Brüning-Richardson A, Bond J, Alsiary R,
Richardson J, Cairns DA, McCormack L, Hutson R, Burns P, Wilkinson
N, Hall GD, et al: ASPM and microcephalin expression in epithelial
ovarian cancer correlates with tumour grade and survival. Br J
Cancer. 104:1602–1610. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang WY, Hsu CC, Wang TY, Li CR, Hou YC,
Chu JM, Lee CT, Liu MS, Su JJ, Jian KY, et al: A gene expression
signature of epithelial tubulogenesis and a role for ASPM in
pancreatic tumor progression. Gastroenterology. 145:1110–1120.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xie JJ, Zhuo YJ, Zheng Y, Mo RJ, Liu ZZ,
Li BW, Cai ZD, Zhu XJ, Liang YX, He HC and Zhong WD: High
expression of ASPM correlates with tumor progression and predicts
poor outcome in patients with prostate cancer. Int Urol Nephrol.
49:817–823. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim WJ, Kim EJ, Kim SK, Kim YJ, Ha YS,
Jeong P, Kim MJ, Yun SJ, Lee KM, Moon SK, et al: Predictive value
of progression-related gene classifier in primary non-muscle
invasive bladder cancer. Mol Cancer. 9:32010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Riester M, Taylor JM, Feifer A, Koppie T,
Rosenberg JE, Downey RJ, Bochner BH and Michor F: Combination of a
novel gene expression signature with a clinical nomogram improves
the prediction of survival in high-risk bladder cancer. Clin Cancer
Res. 18:1323–1333. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
El Behi M, Krumeich S, Lodillinsky C,
Kamoun A, Tibaldi L, Sugano G, De Reynies A, Chapeaublanc E,
Laplanche A, Lebret T, et al: An essential role for decorin in
bladder cancer invasiveness. EMBO Mol Med. 5:1835–1851. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hedegaard J, Lamy P, Nordentoft I, Algaba
F, Høyer S, Ulhøi BP, Vang S, Reinert T, Hermann GG, Mogensen K, et
al: Comprehensive transcriptional analysis of early-stage
urothelial carcinoma. Cancer Cell. 30:27–42. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Subramanian A, Tamayo P, Mootha VK,
Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub
TR, Lander ES and Mesirov JP: Gene set enrichment analysis: A
knowledge-based approach for interpreting genome-wide expression
profiles. Proc Natl Acad Sci USA. 102:15545–15550. 2005. View Article : Google Scholar : PubMed/NCBI
|