Introduction
Leukemia is one of the malignant clonal disease
types involved with hematopoietic stem cells. In 2015 the incidence
rate of leukemia was reported to be 5.68 cases/100,000 individuals
in China (1). Leukemia is the leading
cause of cancer-associated mortalities globally in children and
adults <35 years old, and various studies have been conducted in
order to understand its mechanisms (1–4). Leukemia
is characterized by the upregulation of cell proliferation and its
failure to undergo differentiation into hematopoietic cells
(5–7).
The treatment strategy for leukemia consists of transplantation of
bone marrow, and chemo- and radiotherapy (8–10). Despite
these available treatment strategies, leukemia continues to be the
leading cause of mortality globally; therefore, clinicians and
researchers require novel drug candidates in order to treat
leukemia efficiently. Natural compounds isolated from diverse
sources act as therapeutic candidates for the treatment and
prevention of various disorders including cancer and arthritis
(11–14). Natural products have been determined
to act as neuroprotective, antioxidant (15), anti-inflammatory (16) and anti-apoptotic agents (17), as well as reducing autophagy (18). Sanguinarine is located in the plant
Sanguinaria canadensis. Sanguinarine is a member of the
alkaloid family and has been determined to act as a potential agent
against inflammation, tumor growth and hypertension (19,20).
The present study aimed to investigate the effect of
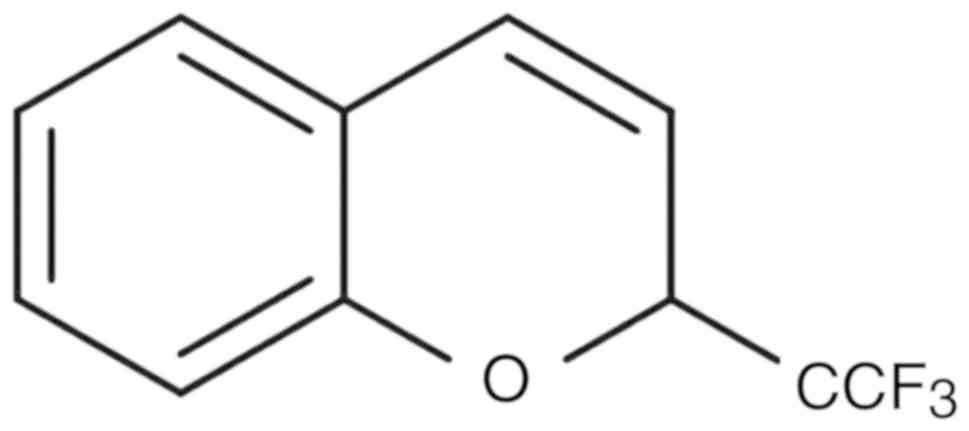
benzoxime (Fig. 1) on RBL-1 leukemia
cell proliferation and on leukemia Sprague-Dawley rat models. The
results demonstrated that benzoxime treatment reduced RBL-1
leukemia cell proliferation in vitro and prevented damage to
the spleen and liver, and changes in the biochemical profile of
blood in vivo.
Materials and methods
Cell culture
The leukemia RBL-1 cell line was supplied by the
Chinese Academy of Sciences (Shanghai, China). Cell culture was
performed in 75-cm2 tissue culture flasks, which
contained RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). L-glutamine (2 mM) and 10% fetal bovine serum
were added to the medium (Gibco; Thermo Fisher Scientific, Inc.).
The medium also contained 1% penicillin-streptomycin (100 U/ml
penicillin and 100 µg/ml streptomycin). The cells were incubated at
37°C in a humidified atmosphere of 5% CO2.
Analysis of cell viability
The effect of benzoxime on leukemia RBL-1 cell
viability was analyzed with an MTT assay. RBL-1 cells were seeded
onto 96-well cell culture plates at a density of 2×104
cells/well and cultured for 24 h. Benzoxime dissolved in dimethyl
sulfoxide (DMSO) at 2–14 µM doses was added to the RPMI-١٦٤٠ medium
and incubation was conducted for ٢٤ h. The conditions for
incubation used were a temperature of 37°C in an atmosphere
containing 5% CO2. Following incubation, the cells were
washed twice with PBS and subsequently exposed to 0.5 mg/ml MTT.
Incubation of the cells was continued for 4 h at 37°C and then the
culture medium was removed. DMSO was added to the plates for
solubilization of the formazan crystals. Measurement of the
absorbance values for each plate was performed in triplicate
independently at 485 nm. The microplate autoreader (BioTek
Instruments Inc., Winooski, VT, USA) was used for recording
absorbance.
Handling of animals
The male Sprague-Dawley rats (8-week old; weight,
~200 g; n=30) were purchased from the Guangzhou University's
Laboratory Animal Center for Traditional Chinese Medicine [license
no. scxk-129(Yue)2014-0129; Guangzhou, China]. The animals were
accommodated under 12-h light and dark cycles in an animal house
under conditions of controlled humidity and a temperature of 20°C.
The rats had free access to the fresh drinking water and standard
laboratory diet ad libitum. The working protocols involving
animals were approved by the Committee for Care and Use of Animal
of Guangzhou University of Traditional Chinese Medicine (approval
no. 2014A123).
Leukemia rat model development
The 30 Sprague-Dawley rats were randomly assigned
into three groups of 10 animals each. To induce malignancy,
1×106 RBL-1 cells in 200 µl sterile RPMI-1640 medium
were inoculated subcutaneously into the postauricular region of the
animals (18). The treatment group
was inoculated with 1×106 RBL-1 cells subcutaneously and
then treated with benzoxime (50 mg/kg/day) for 1 week through an
intravenous tail injection. The positive control group was
administered with an intravenous injection of normal saline alone
(100 µl). The animals in negative control group were given
١x١٠6 RBL-1 cells subcutaneously followed by
administration of 100 µl normal saline alone. During the study, the
rat body weight was recorded every week. The animals were
sacrificed on day ٣٥ of the study using established CO2
euthanasia method where the flow rate of CO2 displaced
>30% of the chamber volume/minute, in order to extract the liver
and spleen, and collect the blood samples. The liver and spleen of
each animal was weighed as previously reported (19,20). Tumor
diameter was measured using calipers, and the tumor volume was
calculated. The tumors were measured in 2 dimensions and tumor
volume was calculated according to the formula V=(D ×
d2)/2, in which D and d are the major and minor
perpendicular tumor diameters, respectively.
Immunofluorescence staining
The blood samples (~600 µl) from the rats were
collected and then treated with lysing buffer (Pharm Lyse; BD
Biosciences, San Jose, CA, USA). Following lysis of the blood
cells, the samples were subjected to centrifugation for ١٠ min at
4°C at 1,500 rpm to isolate the leukocytes. The leukocytes were
cultured on glass coverslips and fixed in 4% paraformaldehyde for
15 min at room temperature. Slips were washed in PBS three times
for 30 min at room temperature and incubated with 0.1% Triton X-100
for 30 min at room temperature. Following washing, the slips were
blocked in goat serum (10%; Thermo Fisher Scientific, Inc.) for 20
min at room temperature. The cells were then incubated with
anti-CD3 (cat. no. SAB4700040; dilution 1:200), anti-CD19 (cat. no.
SAB5500047; dilution 1:200) and anti-CD11b (cat. no. SAB4700386;
dilution 1:200; all from Sigma-Aldrich; Merck KGaA, Darsmtdt,
Germany) antibodies at 4°C overnight. Subsequently, the cells were
washed for 15 min twice with PBS at room temperature and incubated
with polyclonal peroxidase-conjugated goat anti-rabbit antibody
(cat. no. ZDR-5306; dilution 1:200, ZSGB-BIO) at room temperature
for 1 h. The cells were observed under a fluorescence microscope
(BX53; Olympus) at ×250 magnification. Flow cytometry was used for
the analysis of surface markers using the previously reported
procedures (21–23).
Determination of biochemical
profiles
The level of various components, including serum
glutamate pyruvate transaminase (sGPT), serum glutamate oxaloacetic
transaminase (sGOT) and blood urea nitrogen (BUN), in the rat blood
serum samples was determined using the previously described
procedures (24,25).
Statistical analysis
The presented data are the mean ± standard error of
the mean of three experiments performed independently. The data
were analyzed using one-way analysis of variance followed by
Student-Newman-Keuls test for multiple comparisons. All statistical
analyses were performed using SPSS 17.0 software package (SPSS,
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Benzoxime has an inhibitory effect on
RBL-1 cell viability
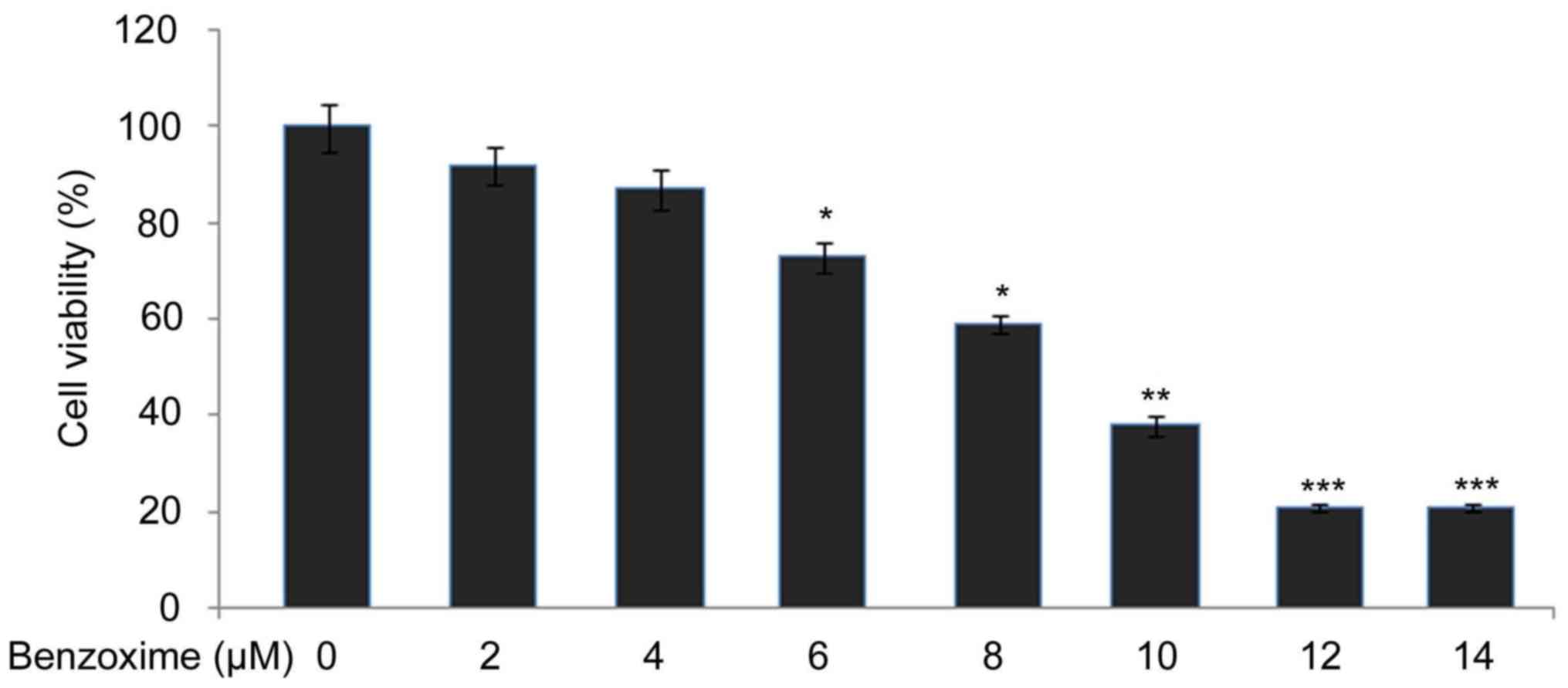
RBL-1 cells were exposed for 24 h to a range of
benzoxime doses from 2–14 µM and the effect on viability of the
cells was examined using an MTT assay. It was observed that an
increase in the dosage of benzoxime from 2 to 12 µM reduced RBL-١
cell viability from ٩٢ to ٢١٪. Further increase in benzoxime
concentration did not significantly decrease the viability
inhibition, compared with 12 µM benzoxime. The viability of the
RBL-1 cells following treatment with 14 µM benzoxime was determined
to be 24% after 24 h (Fig. 2).
Development of leukemia in
Sprague-Dawley rats is inhibited following treatment with
benzoxime
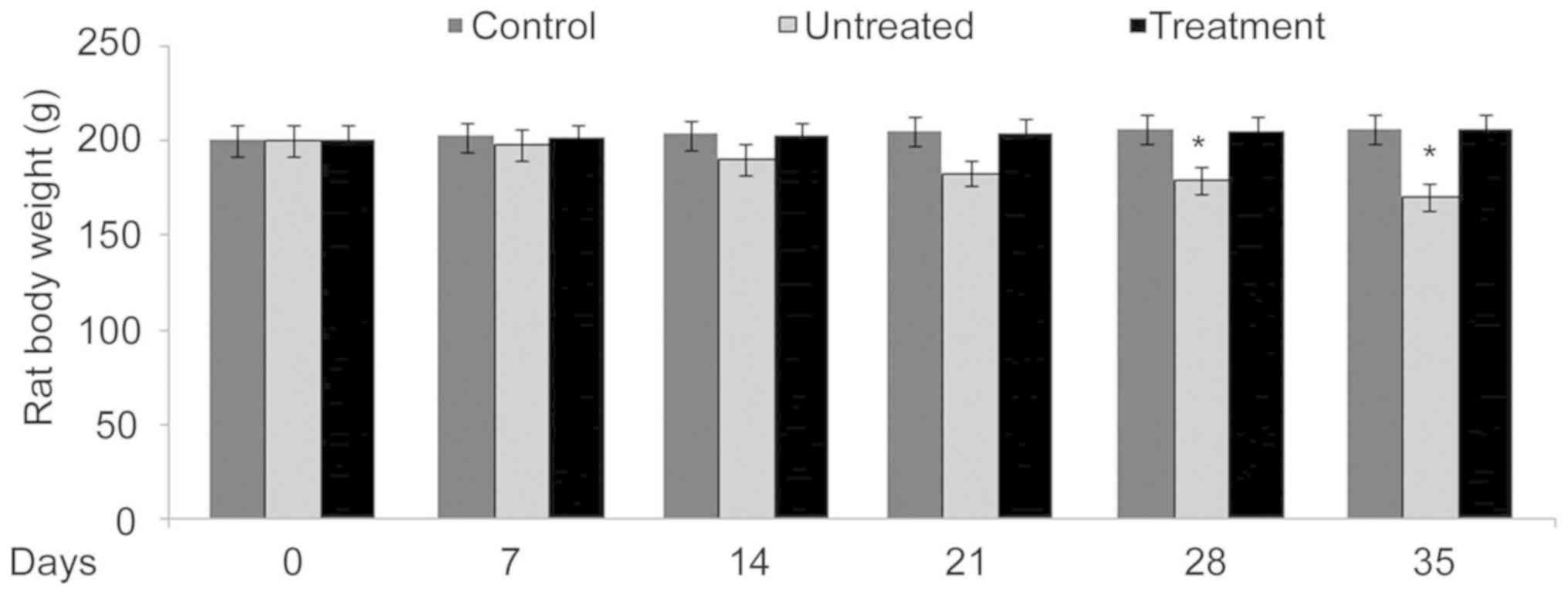
In benzoxime-treated rats, body weight was
determined to be similar to that of the rats in the negative
control group. Compared with the negative control group rats, the
positive control group presented with significantly (P<0.05)
reduced body weight (Fig. 3). The
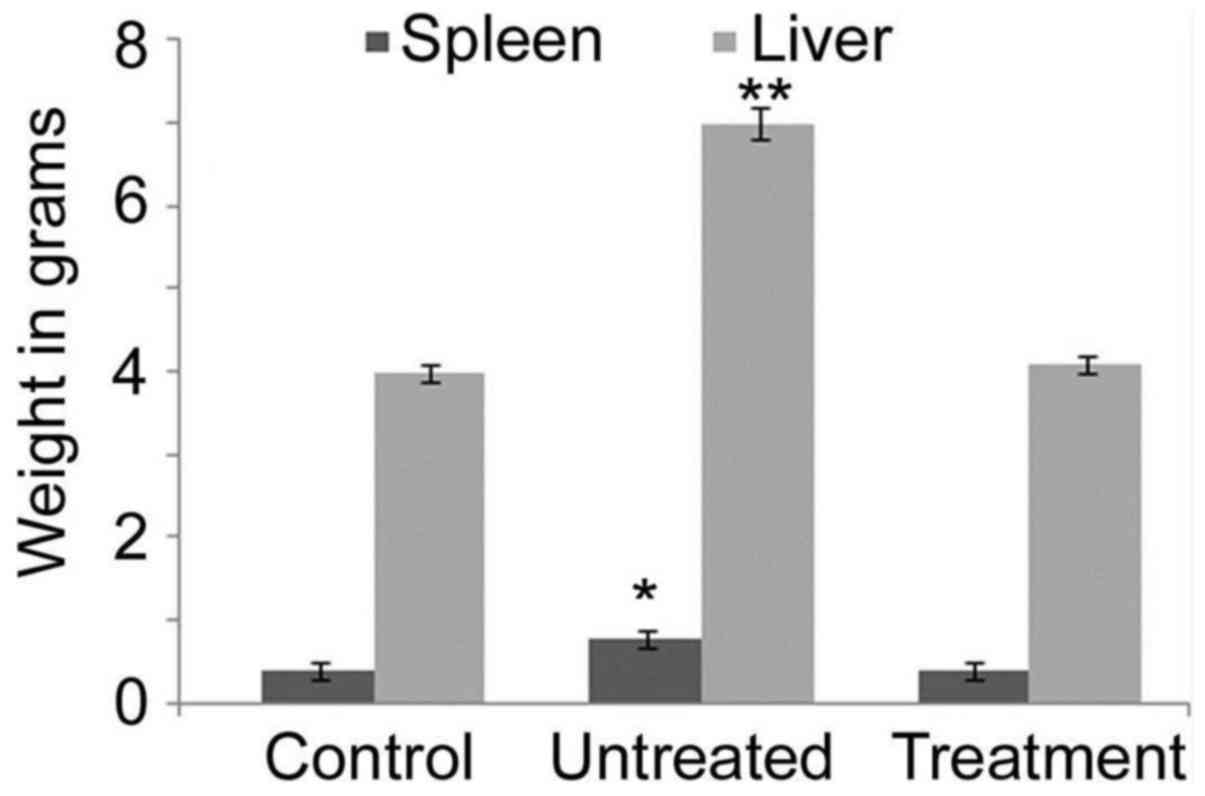
weights of the spleen and liver were determined to be significantly
increased in the positive control rats compared with those in the
negative control and benzoxime-treated groups, after 35 days
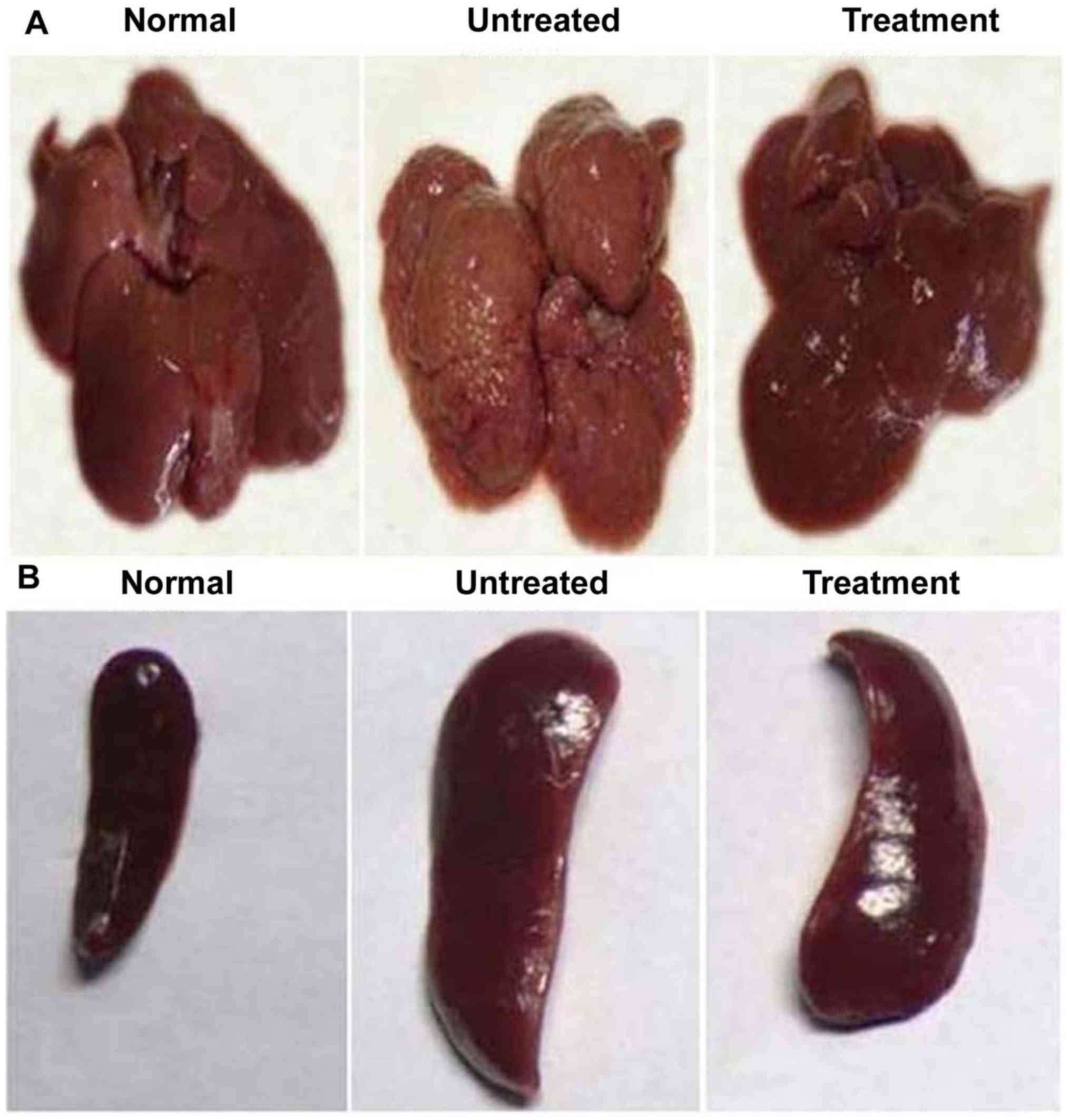
(Fig. 4). The liver and spleen were
also determined to be enlarged in the positive control rats
compared with those in the negative control and benzoxime-treated
groups, after 35 days (Fig. 5). The
average tumor size in the liver of the positive control group was
540 mm3, while no tumor growth was observed in the rats
of the negative control and treatment groups. In the spleen of the
positive control group, the tumor size was determined to be 435
mm3, but no tumor was present in the rats of the
negative control and treatment groups (Fig. 5).
Blood cell surface markers in rats
with leukemia are affected by benzoxime
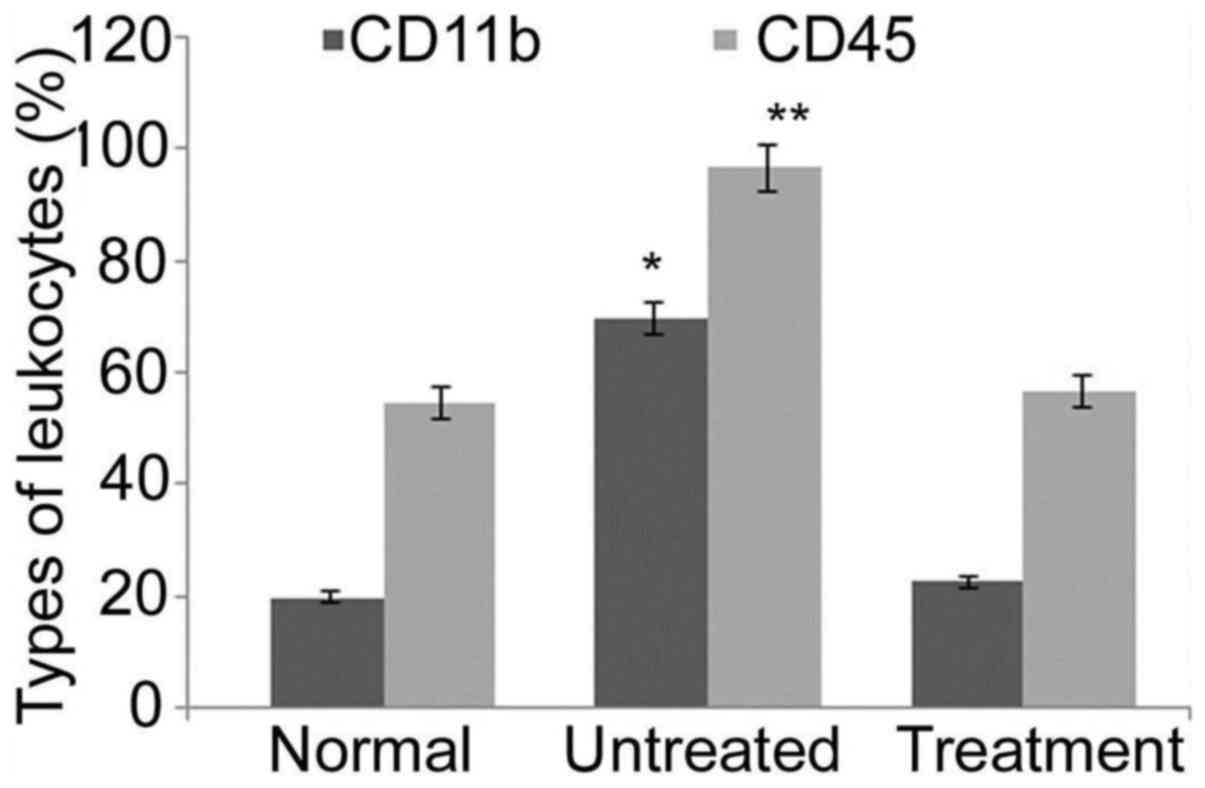
Analysis of leukocytes from positive control rats
after 35 days demonstrated a significant increase (P<0.05) in
CD11b and CD45 levels compared with those in negative control rats.
The level of leukocyte surface markers CD11b and CD45 was
determined to be similar in the rats of the benzoxime treatment and
negative control groups (Fig. 6).
Benzoxime treatment prevents
alteration in hematological, renal and hepatic parameters in rats
with leukemia
Determination of general body weight, and weight of
spleen and liver demonstrated no significant changes between rats
of the benzoxime treatment and negative control groups.
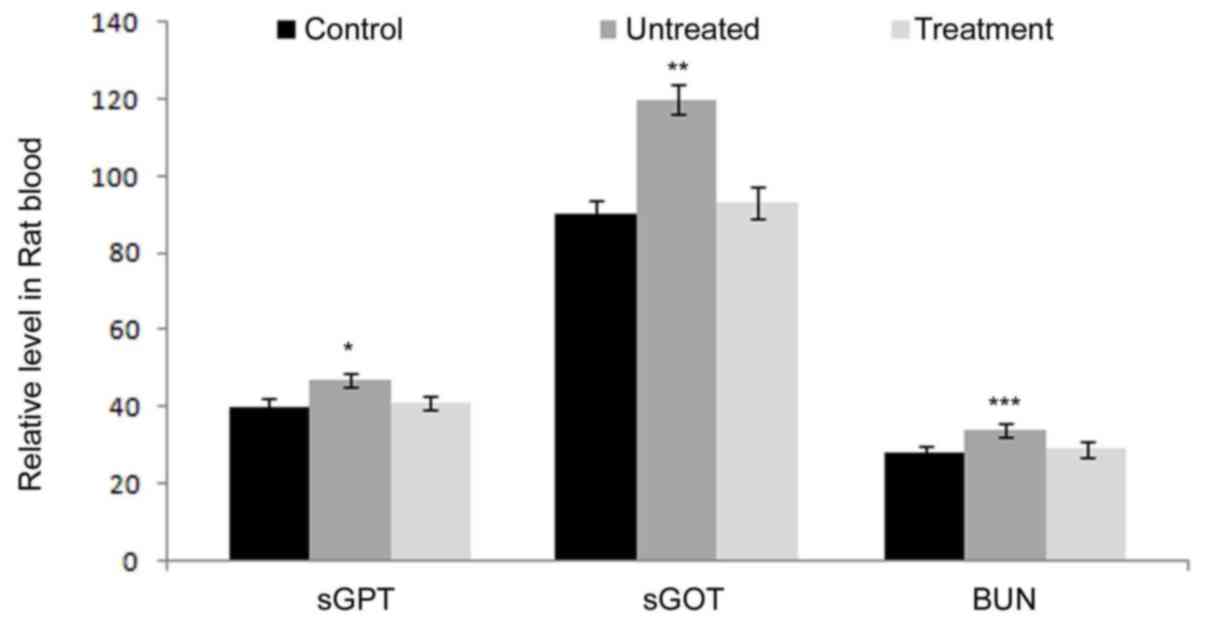
Additionally, analysis of the level of sGPT, sGOT and BUN indicated
that all the three components had no significant changes between
the rats of the benzoxime treatment and negative control groups. At
35 days, the levels of these three components in rats treated with
benzoxime were close to those in the control animals (Fig. 7). These data demonstrated that the
liver and kidneys are not influenced by benzoxime in rats with
leukemia.
Discussion
The present study aimed to investigate the effect of
benzoxime on leukemia RBL-1 cell viability in vitro and in
RBL-1 cell rat leukemia allograft models in vivo.
Upregulation of proliferation and failure to undergo
differentiation into hematopoietic cells comprise the
characteristic features of leukemia (5–7);
therefore, suppression of leukemia cell proliferation is considered
to be of notable importance for its treatment. The present study
demonstrated that the synthetic compound benzoxime has the
potential to inhibit the proliferation of leukemia RBL-1 cells.
Benzoxime inhibited the proliferation of RBL-1 cells in a
dose-dependent manner without inducing any harmful effects in
vivo. These data indicated that benzoxime should be evaluated
for its potential as an anti-leukemia agent; thus, an in
vivo leukemia rat model was established by transplantation of
leukemia RBL-1 cells into Sprague-Dawley rats using the previously
reported procedures (22,23). Anti-leukemic studies for the
evaluation of various molecules are generally performed using
murine allograft models, due to the quick and easy developmental
procedures (26,27).
Numerous studies have evaluated the anti-leukemic
potential of a number of chemotherapeutic agents such as
2-benzyloxybenzaldehyde, chloroquinine and chrysin; however,
leukemia continues to be a challenge for clinicians and researchers
(22,27,28). The
present study demonstrated that benzoxime has an anti-leukemia
effect in leukemia RBL-1 cell rat models in vivo. Benzoxime
treatment of the leukemic rat model resulted in the prevention of
loss of body weight compared with the positive control group. The
body weight in the positive control rats was significantly reduced
compared with the benzoxime treatment and negative control rats.
The weight of the liver and spleen was significantly increased in
the positive control rats compared with that in the negative
control group. It was determined that the level of monocyte surface
marker CD11b and CD45 in the positive control rats was
significantly increased compared with that in the negative control
group; however, a significant increase in the level of CD11b was
prevented by the treatment of leukemia rats with benzoxime.
In conclusion, the present study demonstrated that
benzoxime reduces leukemia RBL-1 cell proliferation in vitro
without causing any harmful effects in vivo. It also
prevented damage to the spleen and liver, and changes in sGPT, sGOT
and BUN. Thus, the present study demonstrated that benzoxime acts
as a potential candidate for the treatment of leukemia. However,
further experiments need to be performed to confirm these
results.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YL designed the study and wrote the paper. HW, RZ
and GZ conducted the experiments. YY and ZL performed the
literature study and compiled the data. All the authors wrote and
approved the article for publication.
Ethics approval and consent to
participate
The working protocols involving animals were
approved by the Committee for Care and Use of Animal of Guangzhou
University of Traditional Chinese Medicine (approval no.
2014A123).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of
interest.
References
|
1
|
Liu YQ, Zhao FJ, Chen WQ, et al: An
analysis of incidence and mortality of leukemia in China, 2009.
China Cancer. 7:528–534. 2013.
|
|
2
|
ESPíRITO Santo AE, Chacim S, Ferreira I,
Leite L, Moreira C, Pereira D, Dantas Brito MD, Nunes M, Domingues
N, Oliveira I, et al: Effect of therapy-related acute myeloid
leukemia on the outcome of patients with acute myeloid leukemia.
Oncol Lett. 12:262–268. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li WX, Li YK and Lin HT: Correlation
between survivin polymorphism and acute leukemia of children. Expt
Ther Med. 15:2941–2945. 2018.
|
|
4
|
Jiang KL, Ma PP, Yang XQ, Zhong L, Wang H,
Zhu XY and Liu BZ: Neutrophil elastase and its therapeutic effect
on leukemia cells. Mol Med Rep. 12:4165–4172. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yildirim R, Gundogdu M, Ozbıcer A, Kiki I,
Erdem F and Dogan H: Acute promyelocytic leukemia, centre,
experience, Turkey. Transfus Apher Sci. 48:45–49. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guo J, Chang CK and Li X: Recent advances
of molecular mechanisms influencing prognosis of myelodysplastic
syndrome-review. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 20:1020–1024.
2012.(In Chinese). PubMed/NCBI
|
|
7
|
Kinoshita K and Funauchi M: Therapeutic
effect of retinoic acid in lupus nephritis. Nihon Rinsho Meneki
Gakkai Kaishi. 35:1–7. 2012.(In Japanese). View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Flatt T, Neville K, Lewing K and Dalal J:
Successful treatment of fanconi anemia and T-cell acute
lymphoblastic leukemia. Case Report Hematol. 2012:3963952012.
|
|
9
|
Estey EH: Acute myeloid leukemia: 2012
update on diagnosis, risk stratification, and management. Am J
Hematol. 87:89–99. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang TT and Chen BA: Leukemia
stem/progenitor cells and target therapy for leukemia-review.
Zhongguo Shi Yan Xue Ye Xue Za Zhi. 18:1654–1658. 2010.(In
Chinese). PubMed/NCBI
|
|
11
|
Zhai YK, Pan YL, Niu YB, Li CR, Wu XL, Fan
WT, Lu TL, Mei QB and Xian CJ: The importance of the prenyl group
in the activities of osthole in enhancing bone formation and
inhibiting bone resorption in vitro. Int J Endocrinol.
2014:9219542014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yogesh HS, Chandrashekhar VM, Katti HR,
Ganapaty S, Raghavendra HL, Gowda GK and Goplakhrishna B:
Anti-osteoporotic activity of aqueous-methanol extract of Berberis
aristata in ovariectomized rats. J Ethnopharmacol. 134:334–338.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee WS, Lee EG, Sung MS and Yoo WH:
Kaempferol inhibits IL-1β-stimulated, RANKL-mediated
osteoclastogenesis via downregulation of MAPKs, c-Fos, and NFATc1.
Inflammation. 37:1221–1230. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tyagi AM, Srivastava K, Singh AK, Kumar A,
Changkija B, Pandey R, Lahiri S, Nagar GK, Yadav DK, Maurya R, et
al: Formononetin reverses established osteopenia in adult
ovariectomized rats. Menopause. 19:856–863. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ates O, Cayli S, Altinoz E, Gurses I,
Yucel N, Sener M, Kocak A and Yologlu S: Neuroprotection by
resveratrol against traumatic brain injury in rats. Mol Cell
Biochem. 294:137–144. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gatson JW, Liu MM, Abdelfattah K,
Wigginton JG, Smith S, Wolf S and Minei JP: Resveratrol decreases
inflammation in the brain of mice with mild traumatic brain injury.
J Trauma Acute Care Surg. 74:470–475. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lin CJ, Chen TH, Yang LY and Shih CM:
Resveratrol protects astrocytes against traumatic brain injury
through inhibiting apoptotic and autophagic cell death. Cell Death
Dis. 5:e11472014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lin C, Yang JS, Tsai SC, Lin CF and Lee
MR: In vivo evaluation of the synthesized novel
2-benzyloxybenzaldehyde analog CCY-1a-E2 for the treatment of
leukemia in the BALB/c mouse WEHI-3 allograft model. Oncol Lett.
5:777–782. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Alayev A, Sun Y, Snyder RB, Berger SM, Yu
JJ and Holz MK: Resveratrol prevents rapamycin-induced upregulation
of autophagy and selectively induces apoptosis in TSC2-deficient
cells. Cell Cycle. 13:371–382. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li H, Zhai Z, Liu G, Tang T, Lin Z, Zheng
M, Qin A and Dai K: Sanguinarine inhibits osteoclast formation and
bone resorption via suppressing RANKL-induced activation of NF-κB
and ERK signaling pathways. Biochem Biophys Res Commun.
430:951–956. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mackraj I, Govender T and Gathiram P:
Sanguinarine. Cardiovasc Ther. 26:75–83. 2008.PubMed/NCBI
|
|
22
|
Chung JG, Yang JS, Huang LJ, Lee FY, Teng
CM, Tsai SC, Lin KL, Wang SF and Kuo SC: Proteomic approach to
studying the cytotoxicity of YC-1 on U937 leukemia cells and
antileukemia activity in orthotopic model of leukemia mice.
Proteomics. 7:3305–3317. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lu CC, Yang JS, Chiang JH, Hour MJ, Lin
KL, Lin JJ, Huang WW, Tsuzuki M, Lee TH and Chung JG: Novel
quinazolinone MJ-29 triggers endoplasmic reticulum stress and
intrinsic apoptosis in murine leukemia WEHI-3 cells and inhibits
leukemic mice. PLoS One. 7:e368312012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chiang JH, Yang JS, Ma CY, Yang MD, Huang
HY, Hsia TC, Kuo HM, Wu PP, Lee TH and Chung JG: Danthron, an
anthraquinone derivative, induces DNA damage and caspase
cascades-mediated apoptosis in SNU-1 human gastric cancer cells
through mitochondrial permeability transition pores and
Bax-triggered pathways. Chem Res Toxicol. 24:20–29. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hsu SC, Ou CC, Li JW, Chuang TC, Kuo HP,
Liu JY, Chen CS, Lin SC, Su CH and Kao MC: Ganoderma tsugae
extracts inhibit colorectal cancer cell growth via G(2)/M cell
cycle arrest. J Ethnopharmacol. 120:394–401. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang JS, Wu CC, Kuo CL, Lan YH, Yeh CC, Yu
CC, Lien JC, Hsu YM, Kuo WW, Wood WG, et al: Solanum lyratum
extracts induce extrinsic and intrinsic pathways of apoptosis in
WEHI-3 murine leukemia cells and inhibit allograft tumor. Evid
Based Complement Alternat Med. 2012:2549602012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li J and Sartorelli AC: Synergistic
induction of the differentiation of WEHI-3B D+ myelomonocytic
leukemia cells by retinoic acid and granulocyte colony-stimulating
factor. Leuk Res. 16:571–576. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Barr RD and Harnish D: Induction of
differentiation of HL-60 and WEHI-3B D+ leukemia cells by lithium
chloride. Leuk Res. 17:1017–1018. 1993. View Article : Google Scholar : PubMed/NCBI
|