Introduction
Head and neck cancer (HNC) is one of the most common
malignant neoplasms observed worldwide (1). Although early detection and therapeutic
strategies for HNC, including radiotherapy, chemotherapy,
immunotherapy and surgery, have substantially improved, the
outcomes for patients with HNC remain poor, with an overall 5-year
survival rate of only 50% (2,3). Therefore, it is important to find novel
treatment strategies for HNC. Uncontrolled invasion and metastasis
contribute to this poor prognosis, and recent study results have
suggested that epithelial-mesenchymal transition (EMT) serves an
essential role in cancer cell metastasis, invasion, radiotherapy
resistance, drug resistance, immune evasion and the cancer
stem-cell phenotype (4,5). Previous studies have demonstrated that a
number of critical biomarkers, including E-cadherin, N-cadherin and
vimentin, are involved in EMT (5,6). The
present study reported that that the expression levels of transient
receptor potential polycystic 2 (TRPP2, previously known as
polycystin-2, PKD2 or PC2), a nonselective cation channel encoded
by the PKD2 gene, are markedly increased in laryngeal
squamous cell carcinoma. It was also previously determined that
inhibition of TRPP2 protein expression via transfection with small
interfering RNA (siRNA) markedly decreased the expression levels of
vimentin and N-cadherin and increased E-cadherin expression levels
in Hep2 cells (a cell line originating from human laryngeal
squamous cell carcinoma) (5).
Targeted delivery using siRNA-based technology is a
promising strategy for the treatment of a variety of diseases
(7–10). However, certain characteristics of
siRNA, including its polyanionic charge, poor stability against
serum nuclease degradation, low permeability, immune response and
toxicity, make it difficult to use in clinical practice (10,11).
Exosomes, which are endogenous nano-sized vesicles that mediate
cell-to-cell communication, have been demonstrated to carry RNA and
freely enter cells (12–15). These characteristics provide an
opportunity for the use of exosomes to deliver therapeutic siRNA to
targeted cancer cells in cancer gene therapy.
In the present study, TRPP2 siRNA was delivered into
FaDu cells (a cell line originating from human pharyngeal squamous
cell carcinoma) using exosomes secreted from 293 cells. The
packaging capacity of exosomes for TRPP2 siRNA, stability of the
exosome/TRPP2 siRNA complex, and expression levels of EMT
biomarkers were determined, and cell migration and invasion were
assessed, in order to establish whether EMT is inhibited by
exosome-delivery of TRPP2 siRNA, and whether this strategy
warranted further development as a viable treatment option in
HNC.
Materials and methods
Cell culture
The FaDu cells were purchased from the American Type
Culture Collection (Manassas, VA, USA) and cultured in DMEM
supplemented with 10% fetal bovine serum (FBS; both Thermo Fisher
Scientific, Inc., Waltham, MA USA) depleted of exosomes, 100 U/ml
penicillin and 0.1 mg/ml streptomycin. The cells were incubated in
an atmosphere of 5% CO2 at 37°C.
Exosome purification
The exosomes were prepared from 293 cells purchased
from the American Type Culture Collection. Briefly, 5 ml DMEM with
10% exosome-depleted FBS was added to 60 mm diameter culture dish
containing 293 cells (2×106/ml). Following 48 h of
incubation, the cells were harvested and the culture medium was
centrifuged at 2,000 × g for 30 min at 4°C to eliminate cells and
cell debris. The remaining supernatant was mixed with polyethylene
glycol (PEG) (16,17). The exosomes were precipitated with an
equal volume of PEG buffer (160 g/l PEG and 1 M NaCl) overnight at
4°C and centrifuged at 10,000 × g for 1 h at 4°C. The supernatant
was removed, leaving the exosomes in the bottom of the tube. The
exosomes were resuspended in 10 µl PBS, and a bicinchoninic acid
protein assay kit (BestBio, Shanghai, China) was used to determine
the protein concentration. The exosomes were stored at −80°C until
use.
Transmission electron microscopy
The diameters of the exosome/siRNA
(5′-AACCUGUUCUGUGUGGUCAGGUUAU-3′; Biomics Biotechnologies Co.,
Ltd., Jiangsu, China) nanoparticles in water were analyzed at 25°C.
The exosome was fixed with 1 ml of 2.5% glutaraldehyde in 0.1 M
sodium cacodylate solution (pH 7.0) for 1 h at 4°C. Samples were
subsequently embedded in pure low viscosity embedding mixture using
Spurr Low Viscosity Embedding kit (cat. no. 01916-1; Polysciences
Inc. Warrington, PA, USA) using the embedding mold, according to
the manufacturer's protocols, and baked for 24 h at 65°C.
Transmission electron microscopy (HT7700; Hitachi, Ltd., Tokyo,
Japan) was used to obtain images, operating at an acceleration
voltage of 100 kV. The sample solution with TRPP2 siRNA
(exosome/siRNA, 4:5 µg/nM) was placed onto a 300-mesh copper grid
coated with carbon. Following deposition for 5 min, the surface
water was removed with filter paper and air-dried. A 4 wt% aqueous
uranyl acetate solution was used for positive staining at room
temperature for 20 min.
Agarose gel electrophoresis
In the present study, it was assessed whether the
exosome/TRPP2 siRNA complex enhanced the stability of TRPP2 siRNA
in the presence of RNA nucleases or serum obtained from mice.
Following incubation with nucleases for 0, 5, 15, 30, 60 and 120
min at 37°C, or incubation with serum for 120 min, heparin was
added to release the TRPP2 siRNA [4:5 ratio of exosomes to TRPP2
siRNA (µg/nM)] in the exosome/TRPP2 siRNA complex groups. Agarose
(0.9 g) was added to 100 ml Tris-acetate-EDTA buffer (40 mM Tris,
20 mM NaAc and 1 mM EDTA at pH 8.0) and dissolved by heating to
100°C. An ethidium bromide aqueous solution at a final
concentration of 0.5 µg/ml was added to the dissolved gel. As the
solution cooled to <50°C, the gel solidified. Once completely
solidified, the gel was placed in a tank filled with
electrophoresis solution. Electrophoresis was conducted at 30 V for
20 min, and the gel was placed under UV light to observe the siRNA
bands. Exosomes (20 or 40 µl) containing proteins (4 µg/µl) were
mixed with 200 nM TRPP2 siRNA, with or without serum obtained from
mice (cat. no. HQ30078; Hongquan Biotechnology Co., Guangzhou,
China).
Western blotting
Target proteins in FaDu cells were examined by
western blotting, which was performed as previously described
(18). The proteins were extracted
from lysates of FaDu cells with a detergent extraction buffer that
consisted of 150 mM NaCl, 50 mM Tris-HCl (pH 7.4), 1 mM disodium
salt of EDTA, 0.1% SDS, 1% Triton X-100, and 1% sodium
deoxycholate, sodium orthovanadate, sodium fluoride and leupeptin.
A total of 30 µg protein per lane was separated via SDS-PAGE on a
10% gel and transferred to a polyvinylidene difluoride membrane.
The membranes were blocked with Tris-buffered saline solution
containing 10% nonfat milk and Tween-20 (0.1%) for 1 h at room
temperature to block nonspecific binding sites. For immunoblotting,
the membrane with the transferred proteins was incubated with
specific primary antibodies overnight at 4°C (BIOSS, Beijing,
China; anti-CD63, cat. no. bs-1523R) (Santa Cruz Biotechnology,
Dallas, Texas, USA; anti-TRPP2 cat. no. sc-25749; anti-vimentin
cat. no. sc-373717; anti-E-cadherin cat. no. sc-8426; and
anti-N-cadherin cat. no. sc-393933), diluted 1:200. Subsequently,
the membrane was washed with PBS in triplicate and incubated with
the respective horseradish peroxidase-conjugated secondary antibody
(dilution, 1:5,000; cat. no. E-AB-1003; Elabscience Biotechnology
Co., Ltd, Wuhan City, China) at room temperature for 1 h. The
resulting immunosignals were detected using an enhanced
chemiluminescence detection system (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). The optical densities of the protein bands were
analyzed using ImageJ 1.50 software (National Institutes of Health,
Bethesda, MD, USA). All protein bands were normalized to GAPDH
located in the same lane and the results are expressed as the
relative optical density.
Fluorescence assay
Purified exosomes were labeled with PKH26 red, a
fluorescent dye, using a linker kit according to the manufacturer's
instructions (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
(16). Briefly, 1 µl PKH26 was added
to 250 µl diluent C for 5 min at room temperature. An equal volume
of exosome-depleted serum was added to terminate the labeling
reaction, and PEG precipitation was used to purify the exosomes.
The PKH26-labeled exosomes were resuspended in 250 µl PBS. The
PKH26-labeled exosomes (5, 10 and 20 µl) were added to FaDu cell
culture medium in a 12-well plate with 1.8×106/ml
density and incubated at 37°C for 12 h. For the control group, 250
µl dilution buffer was used in place of the exosomes. Following
incubation, FaDu cells were washed with PBS. Uptake of the labeled
exosomes by FaDu cells was determined using fluorescence microscopy
(magnification, ×100).
To determine whether the exosomes were able to
deliver TRPP2 siRNA into FaDu cells, 40 µl exosomes (4 µg/µl) was
added to 50 ml Opti-MEM (Thermo Fisher Scientific, Inc.) for 5 min
at room temperature. In addition, 10 µl fluorescein amidite
(FAM)-labeled siRNA (1 µg/µl) was added to 50 ml Opti-MEM for 5 min
at room temperature. Following 5 min incubation, the two solutions
were mixed together and incubated for 20 min at room temperature,
prior to adding to FaDu cells for transfection. For the control
group, 40 µl dilution buffer replaced the exosomes. After 24 h from
transfection, the fluorescence signal of the FAM-labeled TRPP2
siRNA in the FaDu cells was observed using fluorescence
microscopy.
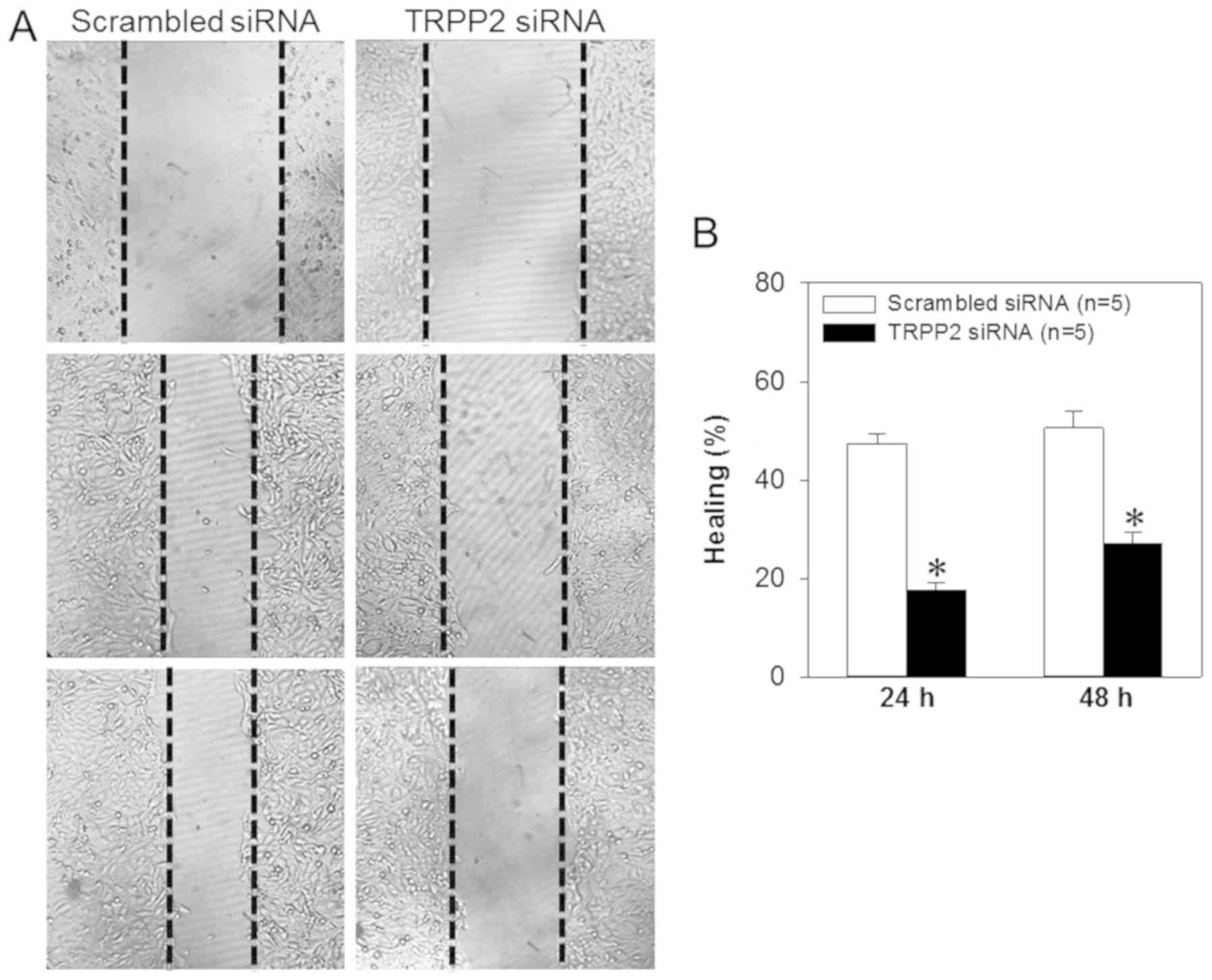
Wound healing assay
The FaDu cells were cultured in a 6-well plate until
100% confluent, washed with PBS and cultured overnight in low-serum
(0.1%) DMEM. The sterile tips of 200 µl pipettes were used to
create scratches or ‘wounds’ in the cell layer. The FaDu cells were
rinsed with PBS to remove floating cells and were cultured in DMEM
supplemented with 0.1% FBS. The lengths of the scratches in each
well were recorded by capturing images using a fluorescence
microscope (magnification, ×40; Nikon Corporation, Tokyo, Japan) at
the same configuration 0, 24 and 48 h following the creation of the
scratch. The relative distances that cells migrated through the
scratched area were measured, and a percentage of ‘healing’ was
calculated. The experiment was repeated four times.
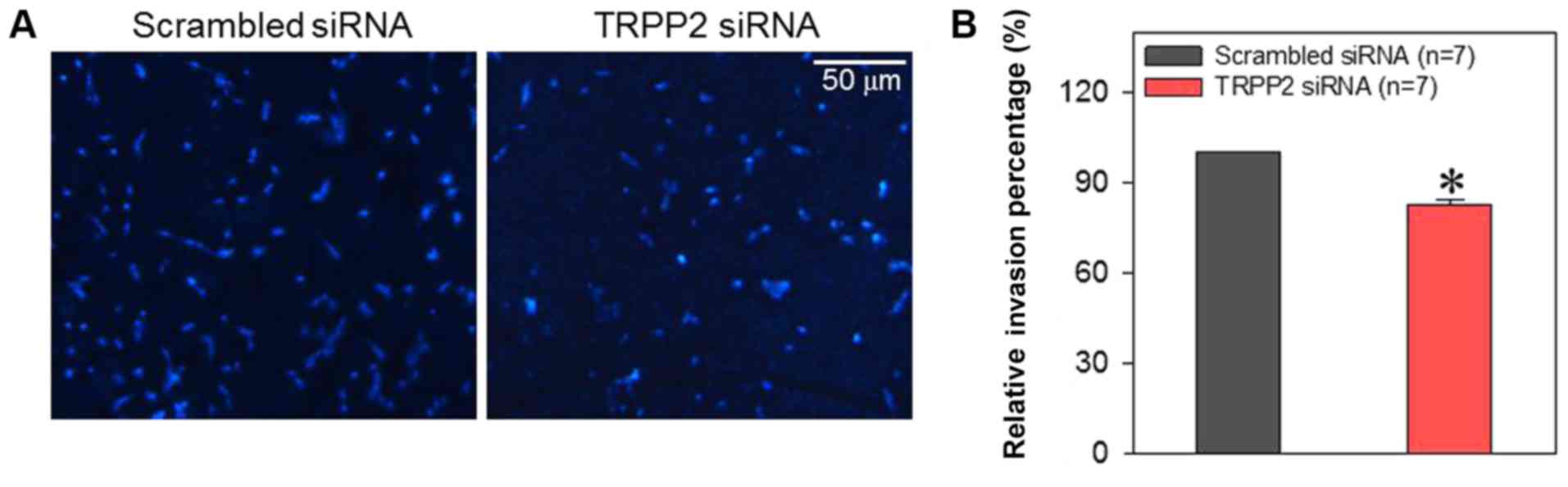
Cell migration and invasion assay
The 8-µm pore polycarbonate membranes (cat no. 3422;
Corning Inc., Corning, NY, USA) of 24-well Transwell inserts were
coated with Matrigel® (cat no. BD354277; BD Biosciences,
San Jose, CA, USA). In each well, 30 µl of Matrigel® was
added to the upper part of the insert and dried at 37°C to form a
thin gel layer for 20 min. Serum-free medium (DMEM; 0.2 ml) was
added to the upper chamber containing FaDu cells and medium (0.6
ml) supplemented with 20% FBS was added to the lower chamber. FaDu
cells were seeded at a density of 1×105 in the upper
part of the chamber. Following 48 h incubation in a 5%
CO2 incubator at 37°C, the Transwell inserts were
removed from the plates and the cells that had not migrated from
the top of the membrane were wiped away using a cotton swab. Cells
passing through the membrane pores were fixed with 4%
paraformaldehyde was infiltrated for 5 min at room temperature and
stained with DAPI for 3 min at room temperature. A fluorescence
microscope (magnification, ×40; Nikon Corporation, Tokyo, Japan)
was used to observe and count the number of cells that had
successfully migrated through the membrane in four randomly
selected areas.
Statistical analysis
SigmaPlot 13.0 software (Systat Software Inc., San
Jose, California, USA) was used to analyze the collected data. All
results are presented as the mean ± standard error of the mean.
Two-tailed Student's t-tests were used to compare the results of
different groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Characterizing the exosome/TRPP2 siRNA
complex
In order to view the morphology of exosome/TRPP2
siRNA, transmission electron microscopy was used to reveal
spherical nanoparticles with diameters of 50–100 nm (Fig. 1A). Membranes of exosomes are enriched
with numerous types of proteins, including cluster of
differentiation (CD)63, CD9, CD37, CD53 and CD81 (19–22); CD63
and CD9 are considered to be specific markers for exosomes
(20). In the present study, CD63 was
probed in order to determine whether exosomes has been successfully
extracted from 293 cells (Fig. 1B)
and a gel retardation assay was used in order to determine the
TRPP2 siRNA packaging capacity of the exosomes. As presented in
Fig. 1C, exosomes encapsulated TRPP2
siRNA in a concentration-dependent manner. As the exosome to TRPP2
siRNA weight/molar (µg/nM) ratio increased, enhanced retardation of
the TRPP2 siRNA band was observed, with the retardation of the
TRPP2 siRNA band apparent at a 4:5 ratio of exosomes to TRPP2 siRNA
(µg/nM) (Fig. 1C). These results
indicated that exosomes were capable of encapsulating TRPP2
siRNA.
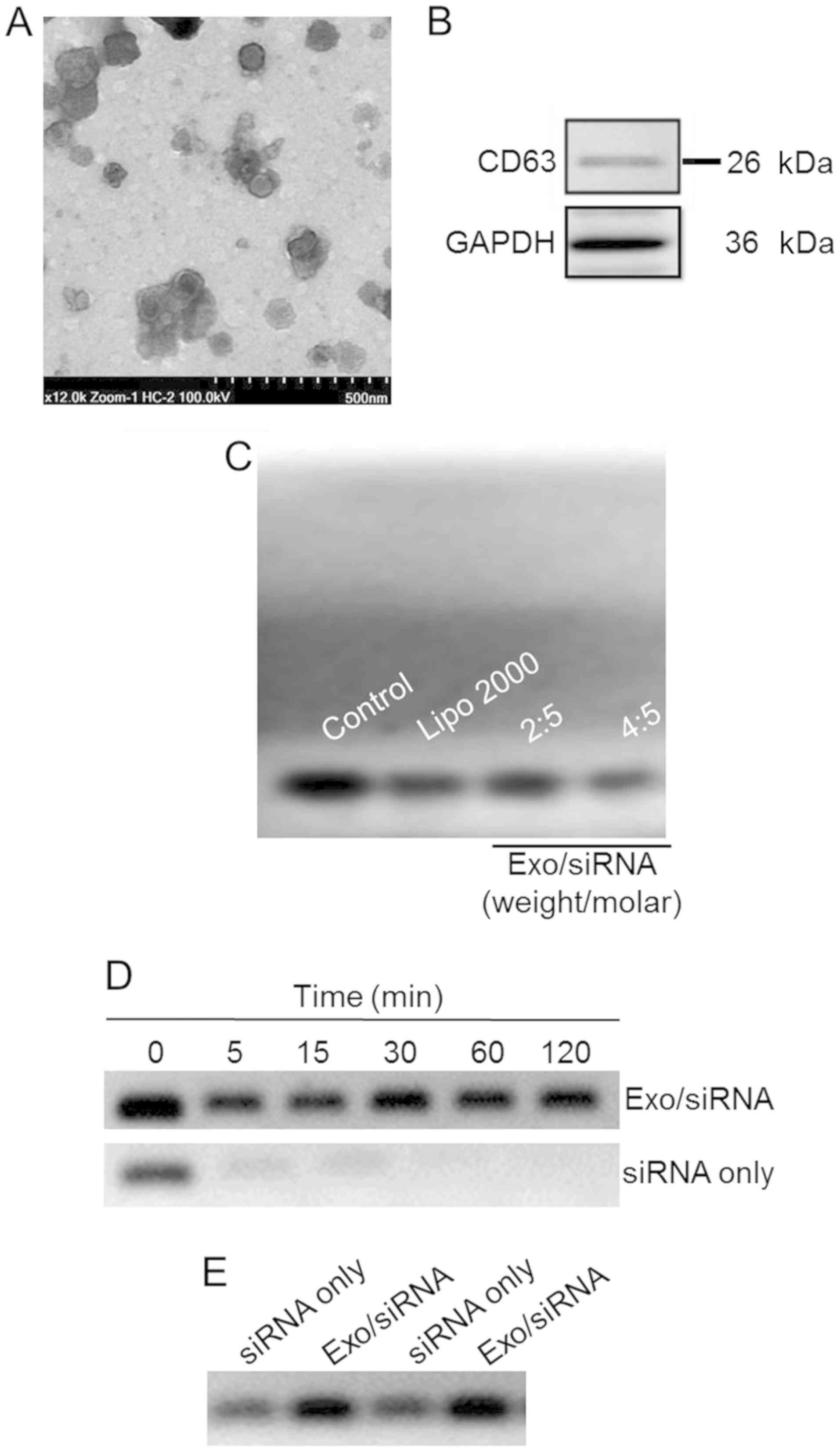 | Figure 1.Characterization of the exosome/TRPP2
siRNA complexes. (A) Representative transmission electron
microscopy image displaying exosome/TRPP2 siRNA particles
counterstained with 4% uranyl acetate. Scale bar, 500 nm. (B)
Representative image presenting the protein expression of CD63, an
exosomal marker. (C) Exosomes encapsulated TRPP2 siRNA (Exo/siRNA)
in a concentration-dependent manner. The TRPP2 siRNA packaging
capacity of exosomes was assessed. An agarose gel retardation assay
was performed at different weight/molar ratios of exosomes to TRPP2
siRNA (µg/nM; Exo/siRNA). (D) Stability of TRPP2 siRNA only and
TRPP2 siRNA encapsulated within exosomes against enzymatic
degradation following incubation with RNA nucleases for 0, 5, 15,
30, 60 and 120 min or (E) in serum for 120 min in a 4:5 ratio of
exosomes to TRPP2 siRNA (µg/nM) or naked siRNA (siRNA only). In the
Exo/siRNA group, heparin was added to the exosome/TRPP2 complexes
to release TRPP2 siRNA prior to conducting the gel retardation
assay. Exo, exosome; Lipo, Lipofectamine®; CD, cluster
of differentiation; siRNA, small interfering RNA; TRPP2, transient
receptor potential polycystic 2; Exo, exosome. |
Protection of the exosome/TRPP2 siRNA complexes
against nucleases and other enzymes in the bloodstream is critical
for their use in siRNA-based therapies. Agarose gel electrophoresis
was conducted to determine the stability of the exosome/TRPP2 siRNA
complex. The agarose gel electrophoresis results indicated that
naked (non-complexed; siRNA only) TRPP2 siRNA degraded within 5
min, whereas the TRPP2 siRNA encapsulated within exosomes remained
intact, as no decrease in the density of the TRPP2 siRNA band was
observed at any time examined (Fig. 1D
and E). Hence, exosomes effectively protected TRPP2 siRNA
against nuclease degradation and substantially enhanced TRPP2 siRNA
stability.
Exosomes deliver TRPP2 siRNA into FaDu
cells
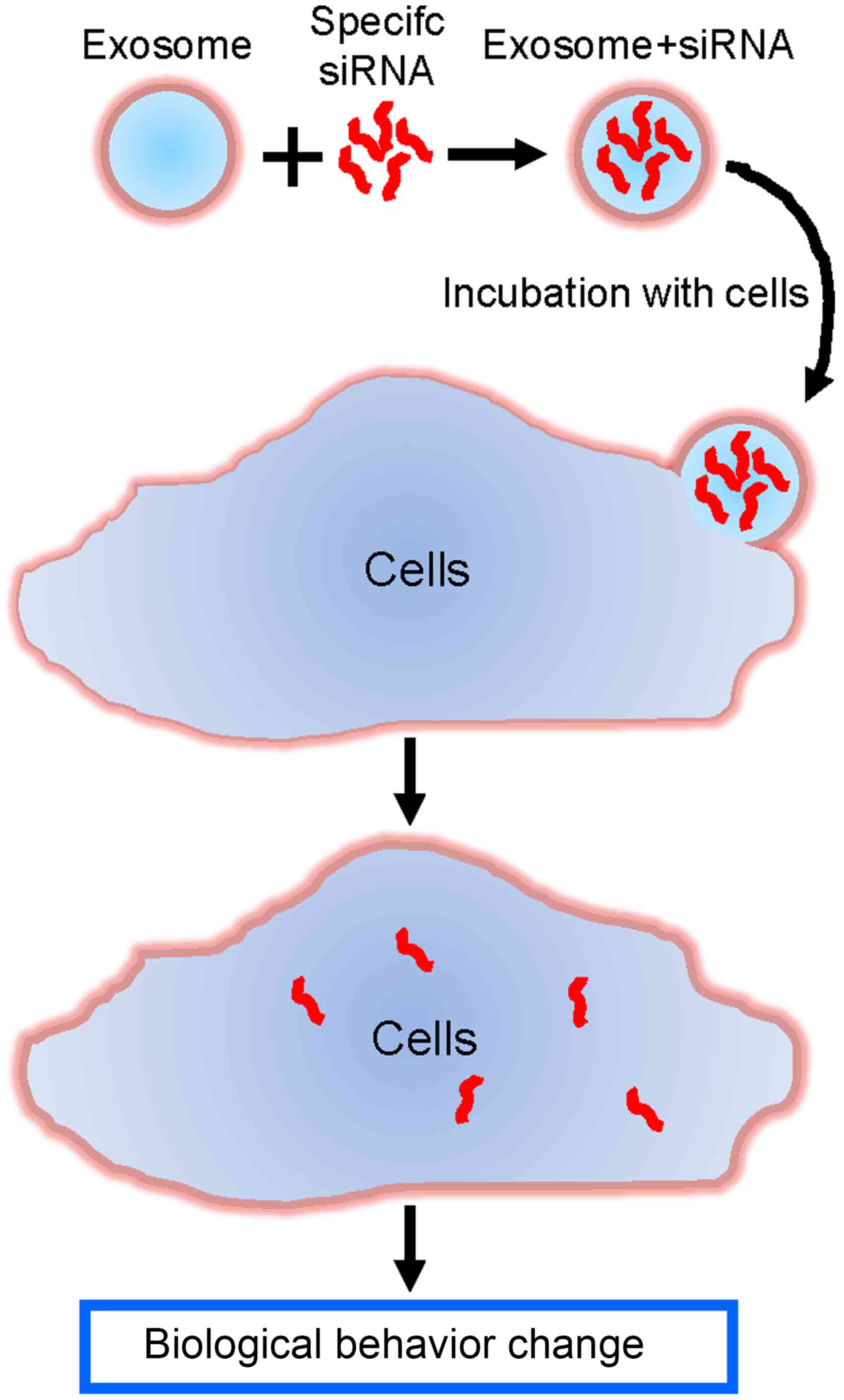
Exosomes/TRPP2 siRNA complexes were used as a tool
to deliver TRPP2 siRNA into FaDu cells in order to regulate
cellular biological behavior, and eventually develop a therapeutic
approach to treat HNC (Fig. 2).
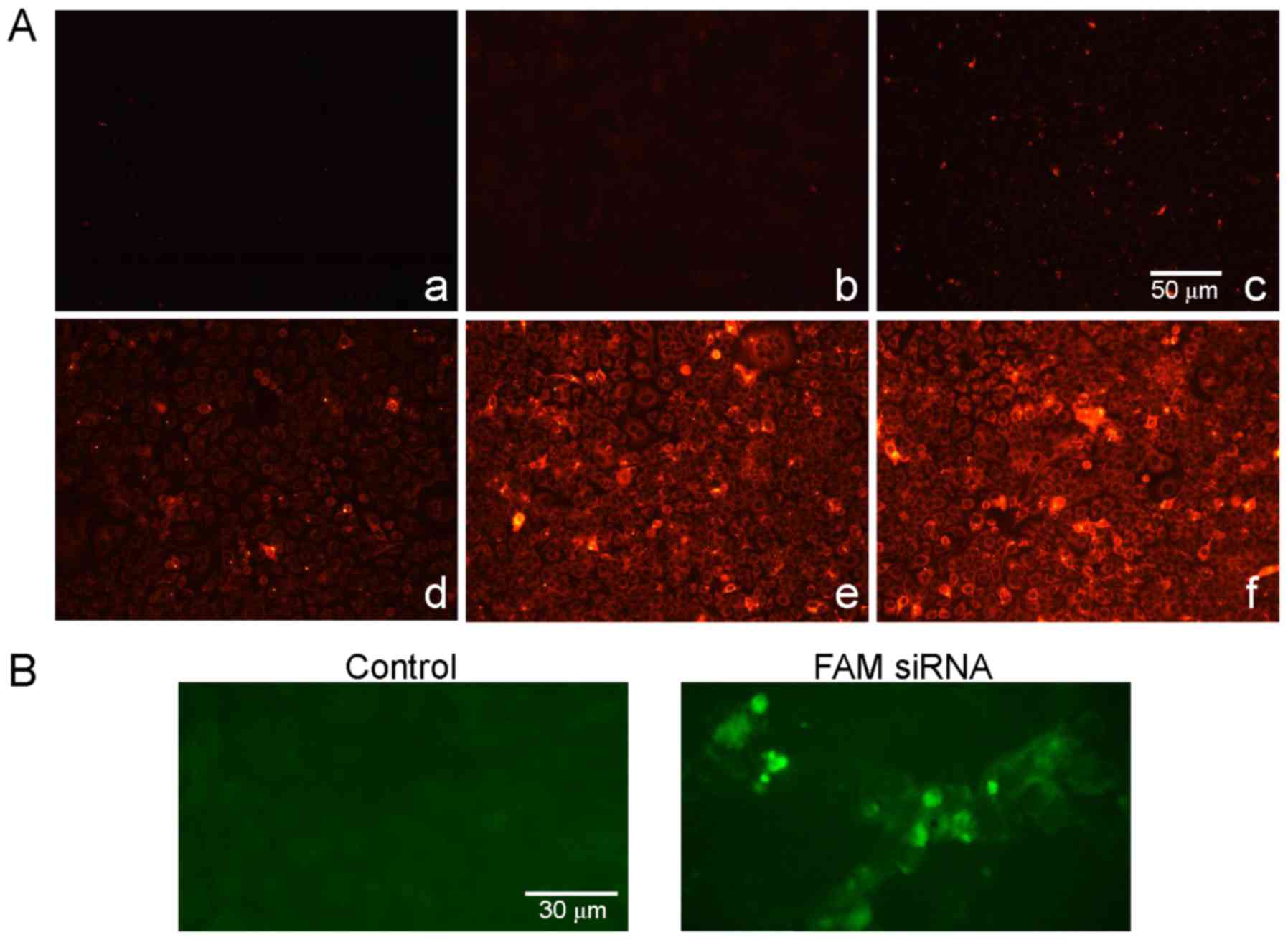
A fluorescence assay was used in order to determine
whether FaDu cells were able to effectively uptake exosomes and
whether exosomes were able to deliver TRPP2 siRNA into the FaDu
cells. As presented in the fluorescence microscopy images in
Fig. 3A, red fluorescence signals
from the PKH26-labeled exosomes were observed in FaDu cells
following transfection (Fig. 3Ad-f),
whereas no red fluorescence signals were observed in the control
group (Fig. 3Aa-c). Green
fluorescence signals from FAM-labeled exosome/TRPP2 siRNA
complexes, were observed in FaDu cells and not from controls
(Fig. 3B). Together, these results
demonstrated that FaDu cells uptake exosomes and that exosomes may
have the ability to encapsulate and deliver TRPP2 siRNA into FaDu
cells.
Exosome/TRPP2 siRNA complexes suppress
TRPP2 expression in FaDu cells
TRPP2, a nonselective cation channel encoded by the
PKD2 gene, serves an important role in a number of cellular
processes (23). A previous study
demonstrated that TRPP2 expression levels are increased
significantly in human laryngeal squamous cell carcinoma and that
TRPP2 enhances metastasis in these cells by regulating EMT
(5). The results presented above
indicated that exosomes may be capable of delivering TRPP2 siRNA
into FaDu cells in vitro; however, it was unknown as to
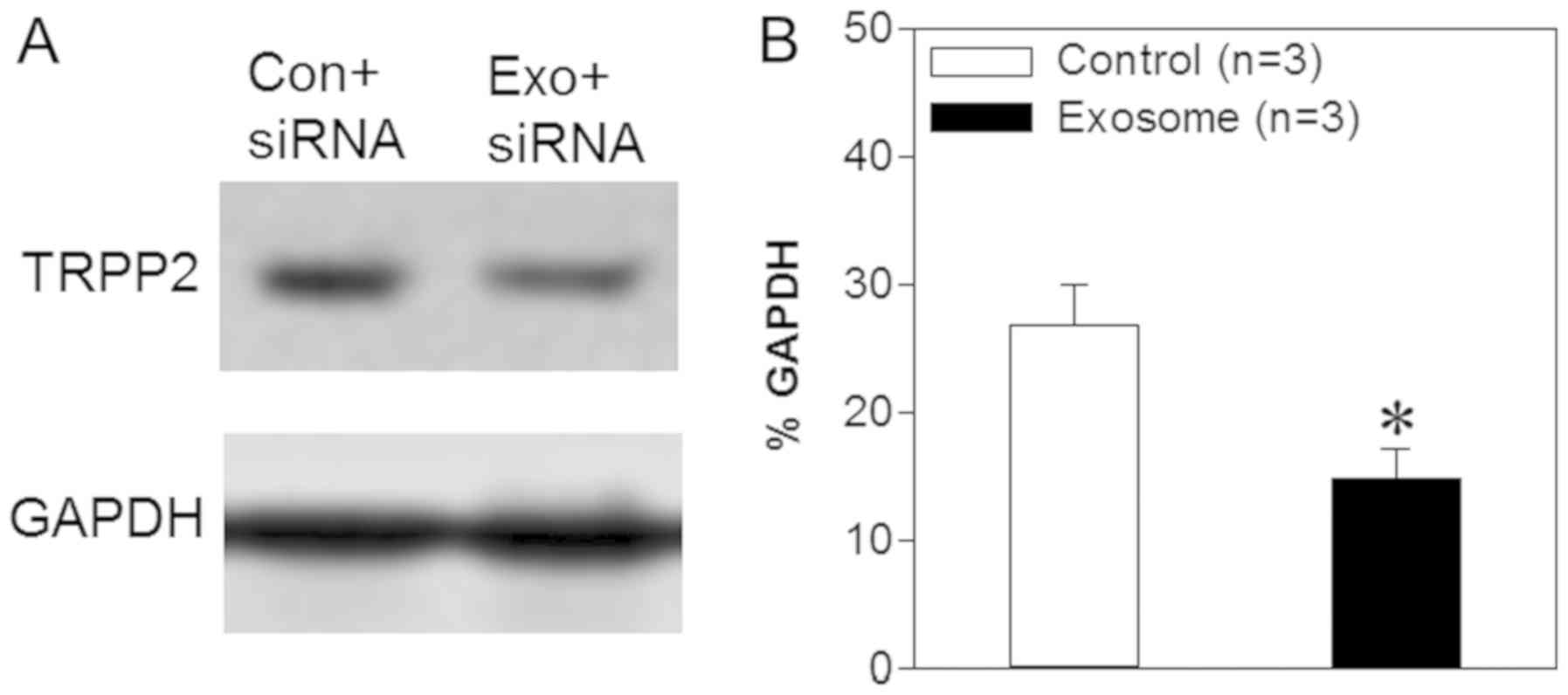
whether TRPP2 siRNA achieved TRPP2-specific gene silencing. Thus,
western blot analyses were used in order to assess the expression
levels of TRPP2 in FaDu cells following transfection. The results
demonstrated that TRPP2 expression levels were significantly
decreased following the transfection of TRPP2 siRNA into FaDu cells
compared with the control (FaDu cells transfected with scrambled
siRNA) (Fig. 4). Thus, these findings
indicated that exosomes may successfully deliver TRPP2 siRNA into
FaDu cells and that TRPP2 siRNA may significantly suppress TRPP2
expression in FaDu cells.
Exosome/TRPP2 siRNA complexes reduce
EMT in FaDu cells
Previous studies have demonstrated that TRPP2
enhances metastasis and invasion by regulating EMT in human
laryngeal squamous cell carcinoma (5). During EMT, E-cadherin expression levels,
regarded as a prognostic biomarker for patients with numerous types
of cancer, are significantly decreased, and cells produce more
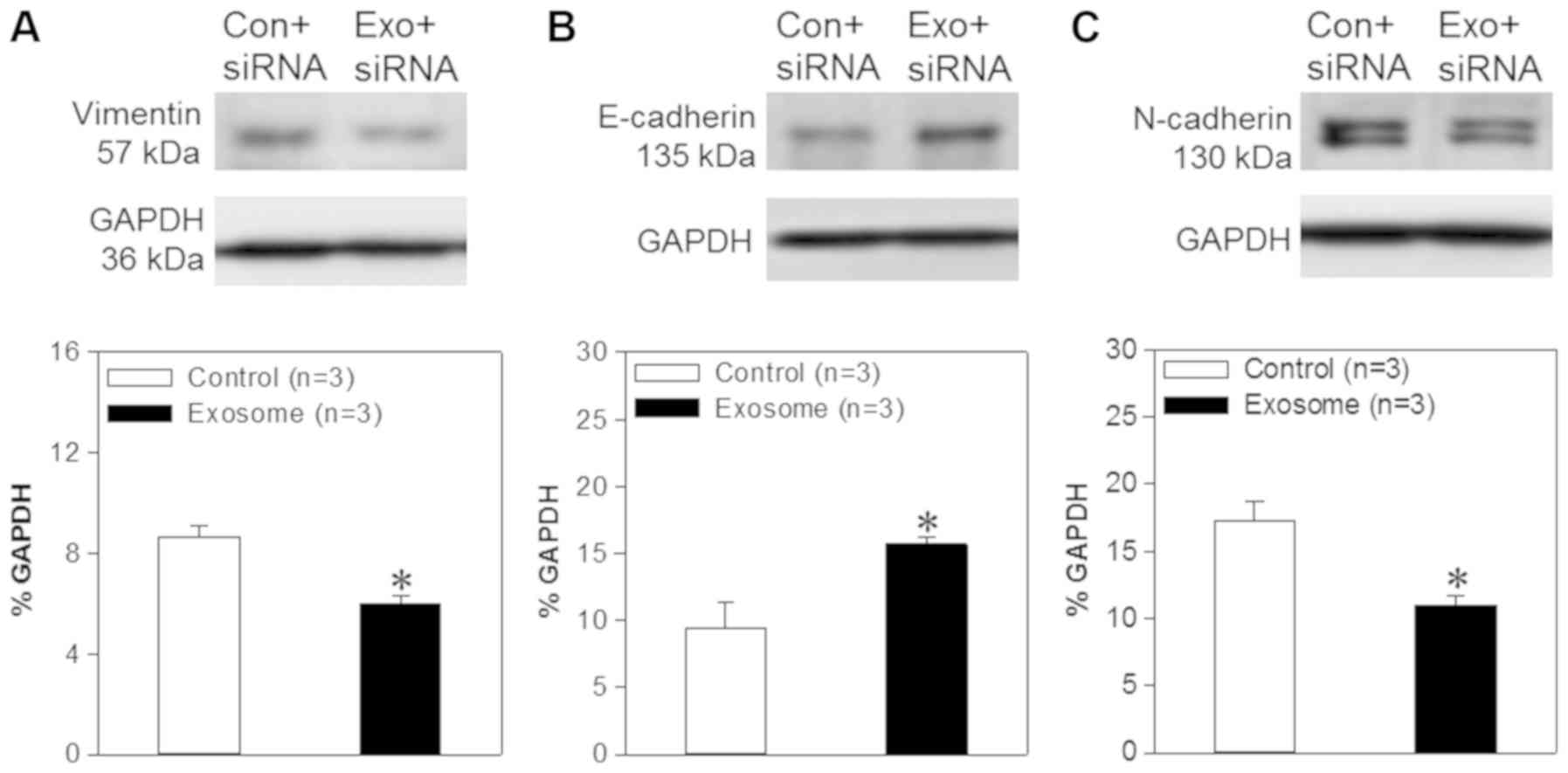
vimentin. In the present study, western blotting analyses were used
in order to assess vimentin, E-cadherin and N-cadherin expression
levels. The results demonstrated that the expression levels of
N-cadherin and vimentin were decreased significantly in FaDu cells
transfected with exosome/TRPP2 siRNA, compared with FaDu cells
transfected with scrambled siRNA. Furthermore, E-cadherin levels
were significantly increased in FaDu cells transfected with
exosome/TRPP2 siRNA, compared with FaDu cells transfected with
scrambled siRNA (Fig. 5), suggesting
that exosome/TRPP2 siRNA complexes may reduce metastasis and
invasion by inhibiting EMT. Taken together, these findings
indicated that exosomes may be ideal vectors to deliver TRPP2 siRNA
into FaDu cells, and that the exosome/TRPP2 siRNA complex is a
potential siRNA-based therapy for HNC.
Exosome/TRPP2 siRNA complexes inhibit
migration and invasion of FaDu cells
Cell migration and invasion are critically involved
in the metastasis of laryngeal cancer. Therefore, FaDu cells were
treated with exosome/TRPP2 siRNA complexes in order to determine
whether this treatment slowed cell migration and invasion in
vitro. When FaDu cells were transfected with exosome/TRPP2
siRNA complexes, wound healing data indicated that the migration
speed of the FaDu cells was significantly decreased (Fig. 6). To investigate the effect of
exosome/TRPP2 siRNA complexes on cell invasion, exosome/TRPP2 siRNA
complex-transfected FaDu cells were cultured in Matrigel-coated
Transwell inserts to simulate invasion through an extracellular
matrix. The results demonstrated that compared with FaDu cells
transfected with scrambled control siRNA, fewer of the cells
transfected with exosome/TRPP2 siRNA complexes moved through the
matrix (Fig. 7). These results
indicated that exosome/TRPP2 siRNA complexes may inhibit FaDu cell
migration and invasion in vitro.
Discussion
The present study investigated whether exosomes have
the potential to be used for the delivery of siRNA into FaDu cells,
and the role of the exosome/TRPP2 siRNA complex in the metastatic
processes of these cells. The principal findings were as follows:
i) Exosomes from 293 cells effectively encapsulated TRPP2 siRNA in
a concentration-dependent manner, and the exosome/TRPP2 siRNA
complex was stable in the presence of nucleases or serum obtained
from mice; ii) FaDu cells were able to uptake exosome/TRPP2 siRNA
complexes; iii) knockdown of the TRPP2 gene via exosome/TRPP2 siRNA
transfection reduced TRPP2 expression in FaDu cells; iv)
exosome/TRPP2 siRNA complex transfection significantly increased
E-cadherin while significantly decreasing N-cadherin and vimentin
protein expression levels; and v) exosome/TRPP2 siRNA complexes
inhibited the ability of FaDu cells to migrate and invade. Taken
together, these findings indicated that exosomes are able to
deliver TRPP2 siRNA into FaDu cells. Furthermore, exosome/TRPP2
siRNA complexes may inhibit metastasis in HNC by regulating EMT.
Therefore, these results provide evidence that further examination
of the exosome/TRPP2 siRNA complex is required for the treatment of
HNC.
HNC is a leading cause of cancer-associated illness
and mortality worldwide, and >600,000 cases are diagnosed every
year (24). Cetuximab, platinum and
fluorouracil, the most common first-line drugs for the treatment of
metastatic and recurrent HNC, are associated with a median overall
survival of only 10 months (25,26).
Although recent developments in immunotherapy, including checkpoint
inhibitors, have been a breakthrough in the treatment of HNC,
checkpoint inhibitors only benefit a minority of patients with
relapsed and metastatic HNC (27,28).
Therefore, effective therapies are still lacking in the treatment
of HNC. Previous studies have demonstrated that TRPP2 knockdown
significantly decreases ATP-induced Ca2+ release and
vimentin and N-cadherin expression levels, yet enhances E-cadherin
expression levels in Hep2 cells, suggesting that migration and
invasion may be reduced through the inhibition of EMT (29). Those novel findings provided a
potential therapeutic target in HNC. However, delivery of siRNA
into cancer cells in vivo remained a major barrier due to
the limiting characteristics of siRNA, including its polyanionic
charge, low cell membrane permeability and low stability in the
presence of serum nucleases (30,31). A
number of siRNA delivery vehicles, including nonreplicating
viruses, oncolytic virus platforms, adenovirus and proteins, have
been widely investigated in order to address these challenges
(32–35). Although a number of creative vectors
have given promising results, their safety, biocompatibility and
low transduction efficiency are notable concerns for the delivery
of siRNA (30,31). Exosomes, produced endogenously from
endosomes by numerous types of cells, are able to fuse with cancer
cell membranes naturally, with promising safety, biocompatibility
and transduction efficiency (30,36). The
results of the present study strongly indicated that exosomes
encapsulated TRPP2 siRNA in a concentration-dependent manner and
effectively protected TRPP2 siRNA from nuclease degradation.
Furthermore, it was demonstrated that FaDu cells took up the
exosome/TRPP2 siRNA complexes. These results support the idea of
exosomes being ideal carriers for the delivery of siRNA to specific
cells. Although this particular exosome delivery system may not
represent targeted delivery, the present study provides evidence to
suggest that exosome delivery is a potential novel tool for the
delivery of siRNA into the cells.
EMT, in which cell adhesion is reduced and cell
motility is enhanced, is a common phenomenon in cancer invasion and
metastasis, and is associated with decreased E-cadherin expression
levels and increased N-cadherin and vimentin expression levels. To
inhibit EMT, TRPP2 siRNA was delivered into FaDu cells via exosomes
isolated from 293 cells. The results demonstrated that TRPP2
expression levels were decreased significantly following TRPP2
siRNA transfection, and that decreased TRPP2 expression led to
significantly decreased N-cadherin and vimentin expression levels
and significantly increased E-cadherin expression levels. Together,
these findings demonstrated that exosome/TRPP2 siRNA complexes may
enter FaDu cells in order to reduce EMT, and may therefore inhibit
the invasion and metastasis of FaDu cells. This RNA-based therapy
provides a novel approach to the treatment of HNC, with improved
biocompatibility and safety in addition to higher efficiency
compared with radiotherapy, chemotherapy or immunotherapy.
In summary, it was demonstrated that exosomes
isolated from 293 cells encapsulated TRPP2 siRNA in a
concentration-dependent manner, and the exosome/TRPP2 siRNA complex
remained stable in the presence of nucleases and serum. The FaDu
cells effectively took up the exosome/TRPP2 siRNA complexes.
Treatment with exosome/TRPP2 siRNA complexes markedly suppressed
TRPP2 expression, EMT processes, and migration and invasion in FaDu
cells. On the basis of these results, it may be hypothesized that
the development of exosome/TRPP2 siRNA complexes as an RNA-based
gene therapy in the treatment of HNC is warranted.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from
National Natural Science Foundation of China (grant nos. 81371284,
81570403, 81600286 and U1732157); Anhui Provincial Natural Science
Foundation (grant nos. 1408085MH157 and 1708085MH187); and the
Science and Technology Research Project of Anhui Province (grant
no. 1501041147).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CW, YH and LC isolated and characterized the
exosomes and wrote the manuscript. KL, TF, AJ and RZ performed the
biological activity tests. XX, BS, JD and YL conceived and
initiated the study. YL finalized the manuscript and supervised the
entire study.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Liu W, Zhang B, Chen G, Wu W, Zhou L, Shi
Y, Zeng Q, Li Y, Sun Y, Deng X and Wang F: Targeting miR-21 with
sophocarpine inhibits tumor progression and reverses
epithelial-mesenchymal transition in head and neck cancer. Mol
Ther. 25:2129–2139. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Elzakra N, Cui L, Liu T, Li H, Huang J and
Hu S: Mass spectrometric analysis of SOX11-binding proteins in head
and neck cancer cells demonstrates the interaction of SOX11 and
HSP90α. J Proteome Res. 16:3961–3968. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xiang C, Lv Y, Wei Y, Wei J, Miao S, Mao
X, Gu X, Song K and Jia S: Effect of EphA7 silencing on
proliferation, invasion and apoptosis in human laryngeal cancer
cell lines Hep-2 and AMC-HN-8. Cell Physiol Biochem. 36:435–445.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Su Z, Li G, Liu C, Ren S, Deng T, Zhang S,
Tian Y, Liu Y and Qiu Y: Autophagy inhibition impairs the
epithelial-mesenchymal transition and enhances cisplatin
sensitivity in nasopharyngeal carcinoma. Oncol Lett. 13:4147–4154.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu K, Shen B, Jiang F, Xia L, Fan T, Qin
M, Yang L, Guo J, Li Y, Zhu M, et al: TRPP2 enhances metastasis by
regulating epithelial-mesenchymal transition in laryngeal squamous
cell carcinoma. Cell Physiol Biochem. 39:2203–2215. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Natarajan J, Chandrashekar C and
Radhakrishnan R: Critical biomarkers of epithelial-mesenchymal
transition in the head and neck cancers. J Cancer Res Ther.
10:512–518. 2014.PubMed/NCBI
|
|
7
|
Fire A, Xu S, Montgomery MK, Kostas SA,
Driver SE and Mello CC: Potent and specific genetic interference by
double-stranded RNA in Caenorhabditis elegans. Nature. 391:806–811.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kurreck J: RNA interference: From basic
research to therapeutic applications. Angew Chem Int Ed Engl.
48:1378–1398. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
López-Fraga M, Martinez T and Jiménez A:
RNA interference technologies and therapeutics. From basic research
to products BioDrugs. 23:305–332. 2009.PubMed/NCBI
|
|
10
|
Ozcan G, Ozpolat B, Coleman RL, Sood AK
and Lopez-Berestein G: Preclinical and clinical development of
siRNA-based therapeutics. Adv Drug Deliv Rev. 87:108–119. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang H, Chen W, Xie H, Wei X, Yin S, Zhou
L, Xu X and Zheng S: Biocompatible, chimeric peptide-condensed
supramolecular nanoparticles for tumor cell-specific siRNA delivery
and gene silencing. Chem Commun (Camb). 50:7806–7809. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lai RC, Yeo RW, Tan KH and Lim SK:
Exosomes for drug delivery-a novel application for the mesenchymal
stem cell. Biotechnol Adv. 31:543–551. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vlassov AV, Magdaleno S, Setterquist R and
Conrad R: Exosomes: Current knowledge of their composition,
biological functions, and diagnostic and therapeutic potentials.
Biochim Biophys Acta. 1820:940–948. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tan A, Rajadas J and Seifalian AM:
Exosomes as nano-theranostic delivery platforms for gene therapy.
Adv Drug Deliv Rev. 65:357–367. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Valadi H, Ekström K, Bossios A, Sjöstrand
M, Lee JJ and Lötvall JO: Exosome-mediated transfer of mRNAs and
microRNAs is a novel mechanism of genetic exchange between cells.
Nat Cell Biol. 9:654–659. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang Y, Zhang L, Li Y, Chen L, Wang X, Guo
W, Zhang X, Qin G, He SH, Zimmerman A, et al:
Exosomes/microvesicles from induced pluripotent stem cells deliver
cardioprotective miRNAs and prevent cardiomyocyte apoptosis in the
ischemic myocardium. Int J Cardiol. 192:61–69. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rider MA, Hurwitz SN and Meckes DG Jr:
ExtraPEG: A polyethylene glycol-based method for enrichment of
extracellular vesicles. Sci Rep. 6:239782016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao R, Zhou M, Li J, Wang X, Su K, Hu J,
Ye Y, Zhu J, Zhang G, Wang K, et al: Increased TRPP2 expression in
vascular smooth muscle cells from high-salt intake hypertensive
rats: The crucial role in vascular dysfunction. Mol Nutr Food Res.
59:365–372. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hurwitz SN, Nkosi D, Conlon MM, York SB,
Liu X, Tremblay DC and Meckes DG Jr: CD63 regulates epstein-barr
virus LMP1 exosomal packaging, enhancement of vesicle production,
and noncanonical NF-κB signaling. J Virol. 91(pii): e02251–16.
2017.PubMed/NCBI
|
|
20
|
Khushman M, Bhardwaj A, Patel GK, Laurini
JA, Roveda K, Tan MC, Patton MC, Singh S, Taylor W and Singh AP:
Exosomal markers (CD63 and CD9) expression pattern using
immunohistochemistry in resected malignant and nonmalignant
pancreatic specimens. Pancreas. 46:782–788. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Escola JM, Kleijmeer MJ, Stoorvogel W,
Griffith JM, Yoshie O and Geuze HJ: Selective enrichment of
tetraspan proteins on the internal vesicles of multivesicular
endosomes and on exosomes secreted by human B-lymphocytes. J Biol
Chem. 273:20121–20127. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mathivanan S, Fahner CJ, Reid GE and
Simpson RJ: ExoCarta 2012: Database of exosomal proteins, RNA and
lipids. Nucleic Acids Res. 40:(Database Issue). D1241–D1244. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tsiokas L: Function and regulation of
TRPP2 at the plasma membrane. Am J Physiol Renal Physiol.
297:F1–F9. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vermorken JB, Mesia R, Rivera F, Remenar
E, Kawecki A, Rottey S, Erfan J, Zabolotnyy D, Kienzer HR, Cupissol
D, et al: Platinum-based chemotherapy plus cetuximab in head and
neck cancer. N Engl J Med. 359:1116–1127. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Argiris A, Harrington KJ, Tahara M,
Schulten J, Chomette P, Ferreira Castro A and Licitra L:
Evidence-based treatment options in recurrent and/or metastatic
squamous cell carcinoma of the head and neck. Front Oncol.
7:722017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ferris RL, Blumenschein G Jr, Fayette J,
Guigay J, Colevas AD, Licitra L, Harrington K, Kasper S, Vokes EE,
Even C, et al: Nivolumab for recurrent squamous-cell carcinoma of
the head and neck. N Engl J Med. 375:1856–1867. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chow LQ, Haddad R, Gupta S, Mahipal A,
Mehra R, Tahara M, Berger R, Eder JP, Burtness B, Lee SH, et al:
Antitumor activity of pembrolizumab in biomarker-unselected
patients with recurrent and/or metastatic head and neck squamous
cell carcinoma: Results from the phase Ib KEYNOTE-012 expansion
cohort. J Clin Oncol. 34:3838–3845. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wu J, Guo J, Yang Y, Jiang F, Chen S, Wu
K, Shen B, Liu Y and Du J: Tumor necrosis factor alpha accelerates
Hep-2 cells proliferation by suppressing TRPP2 expression. Sci
China Life Sci. 60:1251–1259. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee K, Jang B, Lee YR, Suh EY, Yoo JS, Lee
MJ, Lee JY and Lee H: The cutting-edge technologies of siRNA
delivery and their application in clinical trials. Arch Pharm Res.
41:867–874. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu F, Wang C, Gao Y, Li X, Tian F, Zhang
Y, Fu M, Li P, Wang Y and Wang F: Current transport systems and
clinical applications for small interfering RNA (siRNA) drugs. Mol
Diagn Ther. Jun 20–2018;(Epub ahead of print).
|
|
32
|
Xia H, Mao Q, Paulson HL and Davidson BL:
siRNA-mediated gene silencing in vitro and in vivo. Nat Biotechnol.
20:1006–1010. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang YA, Nemunaitis J, Samuel SK, Chen P,
Shen Y and Tong AW: Antitumor activity of an oncolytic
adenovirus-delivered oncogene small interfering RNA. Cancer Res.
66:9736–9743. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
DeGroot LJ and Zhang R: Viral mediated
gene therapy for the management of metastatic thyroid carcinoma.
Curr Drug Targets Immune Endocr Metabol Disord. 4:235–244. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yao YD, Sun TM, Huang SY, Dou S, Lin L,
Chen JN, Ruan JB, Mao CQ, Yu FY, Zeng MS, et al: Targeted delivery
of PLK1-siRNA by ScFv suppresses Her2+ breast cancer growth and
metastasis. Sci Transl Med. 4:130ra1482012. View Article : Google Scholar
|
|
36
|
Darband SG, Mirza-Aghazadeh-Attari M,
Kaviani M, Mihanfar A, Sadighparvar S, Yousefi B and Majidinia M:
Exosomes: Natural nanoparticles as bio shuttles for RNAi delivery.
J Control Release. 289:158–170. 2018. View Article : Google Scholar : PubMed/NCBI
|