Introduction
The giant cell tumor of the tendon sheath (GCTTS) is
a type of slow-growing benign soft tissue tumor that typically
arises from the synovium of the tendon sheath. The disease was
characterized by the proliferation of synovial-like mononuclear
cells mingled with dispersed multinucleate giant cells,
siderophages, and inflammatory cells (1,2). In terms
of its growth pattern according to the World Health Organization
classification, GCCTS can be divided into a localized type that
mainly occurs in the digits and a diffuse type associated with a
more aggressive growth and high recurrence rate that predominantly
occurs in large joints (1).
A solitary enchondroma is a benign bone tumor
comprising mature hyaline cartilage that centrally develops within
the tubular bone. It is typically asymptomatic and accidentally
found because of a deformity, fracture, or a more frequent imaging
[e.g., radiographs and magnetic resonance imaging (MRI)] (3).
GCTTS and enchondroma are categorized as one of the
most common benign soft tissue and bone tumors of the hand,
respectively, with the finger being the most common site among all
locations (4–7). However, the coexistence of both these
tumors in the finger, one in the phalangeal region, is exceedingly
rare and may mimic a malignant tumor, which makes the diagnosis
more challenging. Herein, we report an unusual case of the
simultaneous existence of GCTTS and enchondroma, which was
initially considered on the imaging results as a single primary or
secondary malignant bone tumor.
Case report
A 79-year-old female, right hand dominant, presented
to our hospital with a 3-month history of a painless palpable
growing mass in the left little finger. Clinically, the mass was on
the volar aspect of the middle phalanx with the discoloration of
the overlying skin, measuring 12×9 mm, with a firm consistency and
was not tender. She had a past medical history of breast cancer,
which had been treated with a multidisciplinary approach (surgical
resection, chemotherapy, and radiation therapy) approximately 8
years prior. The patient was regularly followed-up by clinical
examination, additional imaging (mammography, ultrasound, computed
tomography, and positron emission tomography), and laboratory and
biomarker tests [e.g., carcinoembryonic antigen (CEA) and cancer
antigen (CA) 15–3] and showed no signs of recurrence, and no
metastases were detected. The patient denied any history of
preceding trauma, discharging sinuses, or constitutional symptoms.
General examination did not reveal any abnormality.
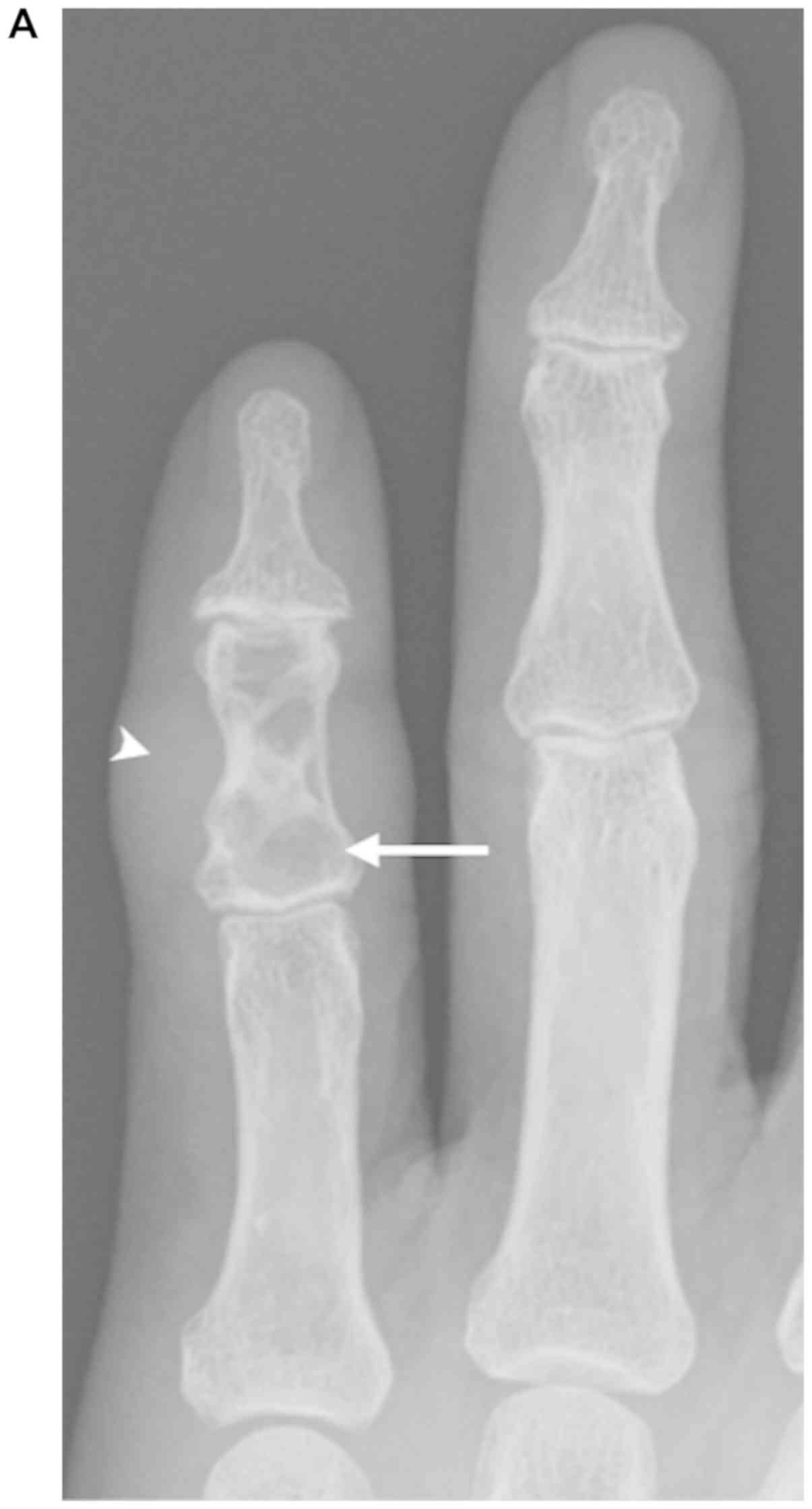
Radiographs of the middle phalanx in the little
finger revealed an ill-defined radiolucent lesion containing a
partially sclerotic rim and internal septations with a thinned
distal half of the anterior cortex. A soft tissue mass was
anteriorly and laterally identified. No calcification of the tumor
matrix, joint involvement, or periosteal reaction was identified
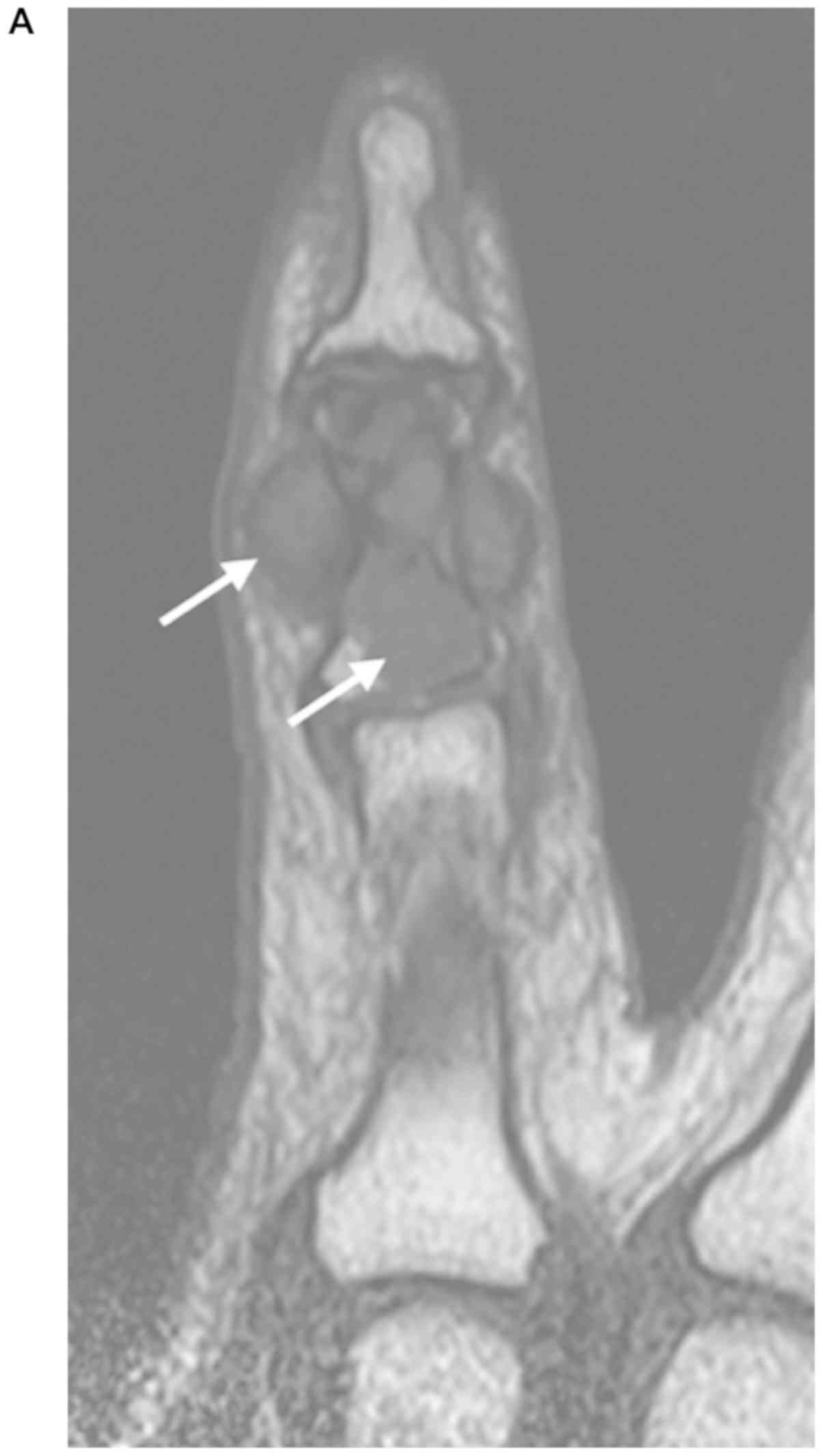
(Fig. 1). MRI was subsequently
performed to further evaluate the mass on the little finger. The
study demonstrated a 14×6×6-mm lesion within the fifth middle
phalangeal bone. The lesion extraosseously extended into the
adjacent soft tissue. A T1-weighted MRI revealed a lesion with a
homogenous low-signal intensity on the entire lesions with an
H-shaped lesion partially enveloping the tendon sheath (Fig. 2A and B). A T2-weighted image showed an
area of homogenous high-signal intensity on the proximal half
intraosseous region and low-signal intensity on the distal half
intraosseous, as well as the extraosseous extension (Fig. 2C). The lesions exhibited contrast
enhancement on the T1-weighted image after gadolinium (Gd) contrast
administration (Fig. 2D).
In the absence of antecedent injury and infection,
an ill-defined border and extraosseous extension was found on the
imaging evaluation, suggestive of a malignant tumor. Preoperative
differential diagnoses of the primary chondrosarcoma of the bone
invading the surrounding soft tissues or secondary breast cancer in
the bone was considered. Two distinct types of tumor lesions were
found during the open biopsy: 1) A soft tissue extraosseous lesion
with medullary invasion in the distal half of the phalangeal bone
and 2) a cartilaginous intraosseous lesion at the proximal half of
the bone without an extraosseous extension. Excisional biopsy of
the extraosseous lesion and curettage with an artificial bone graft
of the intraosseous lesion were subsequently performed after an
intraoperative pathology consultation.
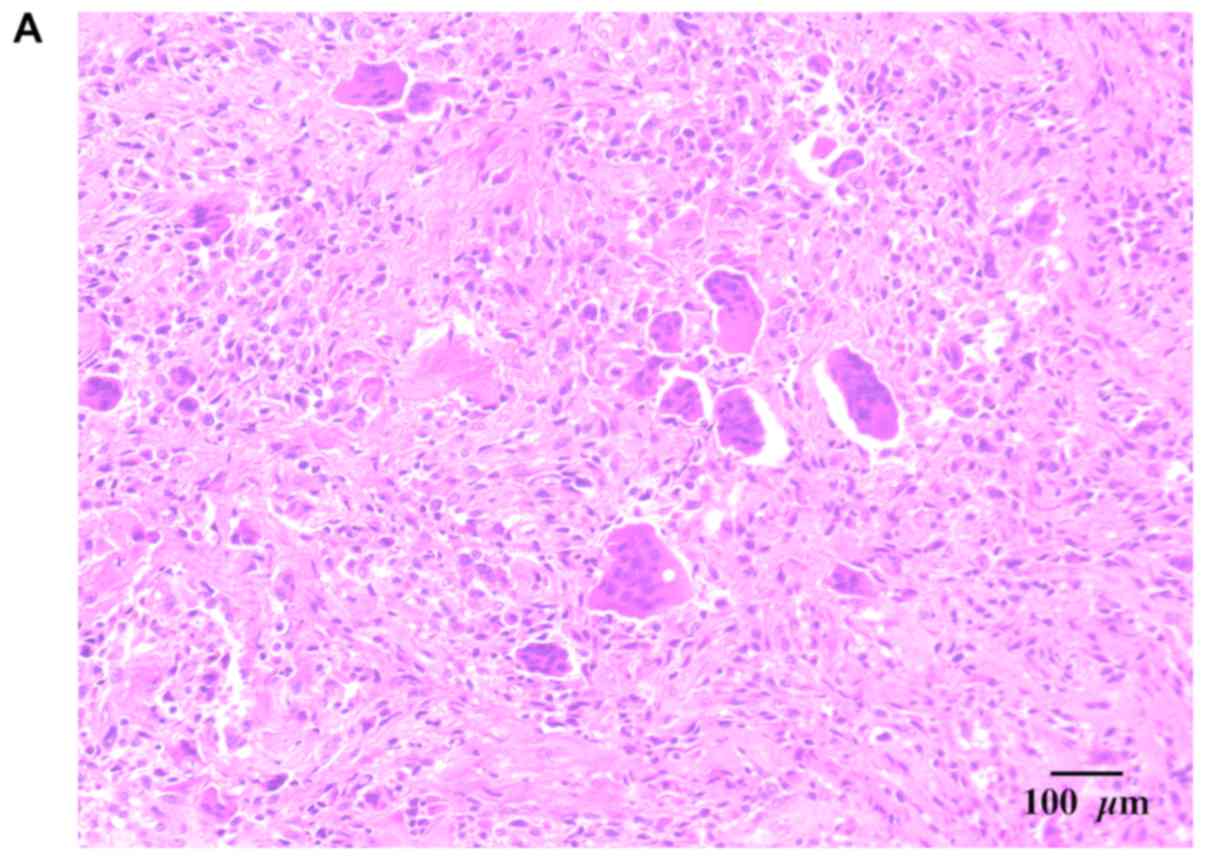
Two distinct characteristics of the gross specimen
were examined. One gross specimen, the soft tissue extraosseous
mass, was a circumscribed yellowish brown piece of tissue measuring
12×8×6 mm. Microscopically, this tissue lesion comprised an uneven
and sparse distribution of osteoclast-like multinucleated giant and
mononuclear cells. The mononuclear cells varied from oval
histiocytoid or epithelioid cells to plump spindle cells. Both had
an eosinophilic cytoplasm and central nuclei that closely resembled
the nuclei within adjacent osteoclast-like cells. The histiocytoid
cells frequently exhibited indented or folded nuclei. The spindle
mononuclear cells were arranged in storiform patterns and were
associated with collagen production. The cartilaginous intraosseous
lesion was a white cartilaginous tumor measuring approximately 5
mm3 in the aggregate. Microscopically, the tumor
comprised mature cartilage lobules surrounded by mature bone with
chondrocytes and displayed no obvious atypia (Fig. 3). In both specimens, no malignant
features were observed (e.g., high number of mitoses and
pleomorphic nuclei). The soft tissue extraosseous mass was
considered a benign soft tissue tumor, GCTTS, with intramedullary
invasion into the distal half of the phalangeal bone, whereas the
proximal cartilaginous intraosseous lesion was considered a primary
benign bone tumor (enchondroma). A final histopathological
diagnosis of concurrent giant cell tumor of tendon sheath and an
enchondroma of the middle phalanx was established. During the most
recent follow-up visit at 21 months postoperatively, no evidence of
recurrence was observed on MRI. We obtained written informed
consent from the patient for publication of this case report.
Discussion
GCTTS is one of the most common tumors involving the
hand and accounts for 74.2% of all benign soft tissue tumors in
this region (4). GCTTS has also
presented as localized nodular tenosynovitis, pigmented
villonodular synovitis (8), and
fibrous xanthoma (9). In a study of
207 GCTTS cases, the finger was the most common site (75.8%)
(10), with a predominant involvement
of the distal joint (5,9). For localized GCTTS, radiographic
features typically display a soft tissue mass with or without bone
changes, including bone pressure erosion, osseous invasion, cystic
change, degenerative changes, periosteal reaction, and
calcification (2,5,11–13). GCTTS with phalangeal bone involvement
in the hand region has also been reported (12,14,15). In
the form of an intraosseous lytic lesion on radiography, GCTTS may
mimic a primary bone tumor, as observed in our present case
(11).
Enchondroma is the most common benign bone tumor of
the hand, accounting for 35–65% of cases (6). In the digit distribution meta-analysis
of 327 cases conducted by Gaulke et al, the little finger
was the most common site (7). This
tumor can usually be diagnosed with radiographs, which show a
well-defined central osteolytic lesion with or without
calcification. In the present case, the patient presented with a
mass in the little finger with a radiographic feature of central
lucency with mild endosteal scalloping but lacking the typical
calcification at the base of the middle phalanx, which
intraoperatively corresponded to the enchondroma lesion site.
GCTTS with an intramedullary bone invasion, which
may mimic primary bone tumor, is considered rare (11). The concurrent presence of the
intraosseous extension of GCTTS and enchondroma, a primary bone
tumor, in the same phalangeal bone is extremely rare. Our
literature search revealed that GCTTS has not been previously
described in association with enchondroma. The coincidence of these
two entities can mimic malignancy due to its intramedullary
accompanying lesion with soft tissue mass involvement, making the
diagnosis more challenging. In the present case, the lesions were
primarily centered in the phalangeal bone, which involved the
entire intraosseous region, with mixed signal intensity and diffuse
contrast enhancement associated with an extensive soft tissue mass.
Considering the patient's age, history of previous cancer, and
these imaging findings, a malignancy including primary bone tumor
(chondrosarcoma) and bone metastases from breast cancer was
initially considered.
Chondrosarcoma is the most important condition to be
differentiated. This tumor, located at the phalangeal bone, is
locally aggressive and exhibits minimal metastatic potential
(16). Moreover, the distribution of
the tumor site in the hand is similar between chondrosarcoma and
enchondroma (17). The type of
aggressive chondrosarcoma (high grade) generally displays an
intraosseous ill-defined lytic area with a mouth-eaten or
permeative pattern, periosteal reaction, and large soft tissue
invasion through cortical destruction (18). However, in this case, an ill-defined
intraosseous lesion was found on imaging findings due to
synchronous double tumors, which was not characteristic of a
high-grade pattern. Typical features of high-grade chondrosarcoma,
including a lobulated high T2 signal and a ring-and-arc enhancement
pattern, were not identified. The evidence that there were two
distinct lesions, with the isolated cartilaginous intraosseous
lesion being unrelated to the surrounding soft tissue mass (GCTTS),
and no breach of the cortex on this lesion site was
intraoperatively identified, which suggested that no soft tissue
invasion arose from the intraosseous cartilaginous lesion.
Additionally, histological examination of intraosseous and
extraosseous lesions revealed no cytological atypia featuring
high-grade chondrosarcoma. Since the type of low-grade
chondrosarcoma (grade I) exhibits only minimal histological atypia,
it is difficult to histologically distinguish it from enchondroma
(18,19). Radiographically, cortical thickening
or disruption may be evident if soft tissue is involved (20). In our case, because no cortical
thickening or destruction was observed on imaging and
intraoperative findings in the cartilaginous lesion site, a
diagnosis of low-grade chondrosarcoma could not be made.
Metastases to the hand from a primary tumor
elsewhere are very rare, with an incidence of 0.007–0.3% (21). In a literature review of 163 cases
conducted by Kerin et al, lung carcinoma was the most common
primary malignancy to metastasize (42%), followed by the kidney
(13%) and breast (11%) (22).
According to their radiological features, bone metastases are
classified as either osteoblastic or osteolytic (bone-destruction).
The latter can be radiographically observed only if at least 50% of
the bone material has been destroyed (23). In the present case, from a clinical
point of view, based on the patient's advanced age and
particularly, the history of preceding breast cancer, the
development of a bone metastasis in the ill-defined osteolytic
finger lesion may be considered, despite its rarity. Bone
metastases can appear in any pattern on radiographic findings using
X-rays (24). Osseous metastases
typically display T1 low-signal intensity, T2 high-signal
intensity, and gadolinium enhancement, as were found in our case
(25). However, regular follow-up
care that showed no evidence of recurrence and distant metastases
warranted a confirmatory biopsy, which was consistent with dual
benign primary tumors.
In conclusion, the simultaneous presentation of
GCTTS with intramedullary invasion and an enchondroma on the
phalangeal bone has not been previously reported and can initially
present as a single intrinsic osseous lesion mimicking malignancy
on imaging findings. As a result, their coexistence must be
considered in the differential diagnosis of a poorly margined
intramedullary lytic lesion associated with a soft tissue mass in
the fingers, and a meticulous preoperative MRI investigation is
required.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Author's contributions
MPJ, TK, TF, TS and NA were responsible for the
study concepts. Data acquisition, analysis, and interpretation was
undertaken by MPJ, TK, TF, TS and NA. Drafting of the manuscript
was the responsibility of MPJ, TK, TF, TS and NA. MPJ, TK, TF, TS
and NA gave final approval of the manuscript to be published, and
MPJ, TK, TF, TS and NA are in agreement to be accountable for all
aspects of the work.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for publication of this case report.
Competing interests
The authors declare they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
GCTTS
|
giant cell tumor of the tendon
sheath
|
|
MRI
|
magnetic resonance imaging
|
|
CEA
|
carcinoembryonic antigen
|
|
CA
|
cancer antigen
|
|
Gd
|
gadolinium
|
References
|
1
|
Fletcher CDM, Bridge JA, Hogendoorn PCW
and Mertens F: WHO Classification of Tumours of Soft Tissue and
Bone. International Agency for Research on Cancer. fourth. Lyon:
2013
|
|
2
|
Murphey MD, Rhee JH, Lewis RB,
Fanburg-Smith JC, Flemming DJ and Walker EA: Pigmented villonodular
synovitis: Radiologic-pathologic correlation. Radiographics.
28:1493–1518. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stomp W, Reijnierse M, Kloppenburg M, de
Mutsert R, Bovée JV, den Heijer M and Bloem JL; NEO study group, :
Prevalence of cartilaginous tumours as an incidental finding on MRI
of the knee. Eur Radiol. 25:3480–3487. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Myhre-Jensen O: A consecutive 7-year
series of 1331 benign soft tissue tumours. Clinicopathologic data.
Comparison with sarcomas. Acta Orthop Scand. 52:287–293. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lancigu R, Rabarin F, Jeudy J, Saint Cast
Y, Cesari B, Fouque PA and Raimbeau G: Giant cell tumors of the
tendon sheaths in the hand: Review of 96 patients with an average
follow-up of 12 years. Orthop Traumatol Surg Res. 99 Suppl
4:S251–S254. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dahlin DC: Bone Tumors: General Aspects
and Data on 6,221 Cases. 3rd. Charles C. Thomas Pub; Springfield,
IL: pp. 325–326. 1978
|
|
7
|
Gaulke R: The distribution of solitary
enchondromata at the hand. J Hand Surg Br. 27:444–445. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jaffe HL, Lichtenstein L and Sutro CS:
Pigmented villonodular synovitis, bursitis, and tenosynovitis. Arch
Pathol. 31:731–765. 1941.
|
|
9
|
Jones FE, Soule EH and Coventry MB:
Fibrous xanthoma of synovium (giant-cell tumor of tendon sheath,
pigmented nodular synovitis). A study of one hundred and eighteen
cases. J Bone Joint Surg Am. 51:76–86. 1969. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ushijima M, Hashimoto H, Tsuneyoshi M and
Enjoji M: Giant cell tumor of the tendon sheath (nodular
tenosynovitis). A study of 207 cases to compare the large joint
group with the common digit group. Cancer. 57:875–884. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lantos JE, Hameed M, Healey JH and Hwang
S: Giant cell tumor of the tendon sheath mimicking a primary
intramedullary metatarsal tumor. Skeletal Radiol. 42:589–593. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
De Schepper AM, Hogendoorn PC and Bloem
JL: Giant cell tumors of the tendon sheath may present
radiologically as intrinsic osseous lesions. Eur Radiol.
17:499–502. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kitagawa Y, Ito H, Amano Y, Sawaizumi T
and Takeuchi T: MR imaging for preoperative diagnosis and
assessment of local tumor extent on localized giant cell tumor of
tendon sheath. Skeletal Radiol. 32:633–638. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Alves Mde P: Excision of giant cell tumor
of tendon sheath with bone involvement by means of double access
approach: Case report. Rev Bras Ortop. 46:101–106. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Singh R, Rohilla RK, Magu S, Siwach R,
Magu NK, Sangwan SS and Arora B: Giant cell tumor of the tendon
sheath with intraosseous phalangeal involvement. Curr Orthop Pract.
19:693–697. 2008. View Article : Google Scholar
|
|
16
|
Bovee JV, van der Heul RO, Taminiau AH and
Hogendoorn PC: Chondrosarcoma of the phalanx: A locally aggressive
lesion with minimal metastatic potential: A report of 35 cases and
a review of the literature. Cancer. 86:1724–1732. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dahlin DC and Salvador AH: Chondrosarcomas
of bones of the hands and feet-a study of 30 cases. Cancer.
34:755–760. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gelderblom H, Hogendoorn PC, Dijkstra SD,
van Rijswijk CS, Krol AD, Taminiau AH and Bovée JV: The clinical
approach towards chondrosarcoma. Oncologist. 13:320–329. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Geirnaerdt MJ, Hermans J, Bloem JL, Kroon
HM, Pope TL, Taminiau AH and Hogendoorn PC: Usefulness of
radiography in differentiating enchondroma from central grade 1
chondrosarcoma. AJR Am J Roentgenol. 169:1097–1104. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Crim J, Schmidt R, Layfield L, Hanrahan C
and Manaster BJ: Can imaging criteria distinguish enchondroma from
grade 1 chondrosarcoma? Eur J Radiol. 84:2222–2230. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
van Veenendaal LM, de Klerk G and van der
Velde D: A painful finger as first sign of a malignancy. Geriatr
Orthop Surg Rehabil. 5:18–20. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kerin R: The hand in metastatic disease. J
Hand Surg Am. 12:77–83. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Choi J and Raghavan M: Diagnostic imaging
and image-guided therapy of skeletal metastases. Cancer Control.
19:102–112. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ellmann S, Beck M, Kuwert T, Uder M and
Bäuerle T: Multimodal imaging of bone metastases: From preclinical
to clinical applications. J Orthop Translat. 3:166–177. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
O'Sullivan GJ, Carty FL and Cronin CG:
Imaging of bone metastasis: An update. World J Radiol. 7:202–211.
2015. View Article : Google Scholar : PubMed/NCBI
|