Introduction
Ovarian cancer gene 1 (OVCA1) is a tumor
suppressor gene located on chromosome 17p13.3 (1). Loss of heterozygosity in the
OVCA1 gene occurs in 50–86% of cases of ovarian cancer
(1–5).
OVCA1 was originally identified in 1996 (1,6). Since its
sequence is highly similar to that of the yeast diphthamide
biosynthesis protein 2, the OVCA1 gene was previously
referred to as diphthamide synthesis protein 2-like (7). Previous studies have demonstrated that
OVCA1, also termed DPH1 (diphthamide biosynthesis 1),
is involved in the biosynthesis of diphthamide by interacting with
the eukaryotic translation elongation factor 3 (8–12). OVCA1
has an important role in the regulation of cell proliferation,
embryonic development and tumorigenesis (4,13–20). It inhibits the proliferation of
epithelial ovarian cancer cells and blocks the cell cycle at
G1 phase (4,19) by decreasing cyclin D1 and increasing
p16, a tumor suppressor protein (19). Previous studies have demonstrated that
OVCA1-mutant mice do not survive during embryonic
development or after birth, due to developmental delay and defects
in multiple organ systems, and that OVCA1 is involved in p53
deficiency-induced tumorigenesis (14,18).
Abnormality in OVCA1 occurs prior to defects in p53
and breast cancer 1 gene, and is therefore considered an early
event in ovarian tumorigenesis (6,14,18,21). The
close link between OVCA1 and p53 on human chromosome
17 suggests that they may have synchronized effects in cancer
development (14). However, the
biological function of OVCA1, and its role in tumor occurrence and
development have not yet been determined.
To reveal the biological function of OVCA1 and its
association with ovarian cancer, commercial antibodies were used to
try and detect OVCA1 in cells. However, the results demonstrated
that endogenous OVCA1 could not be observed by western blotting
with commercial antibodies, despite the large panel of anti-OVCA1
antibodies tested. The mRNA expression levels of OVCA1 were
detected by reverse transcription-quantitative polymerase chain
reaction (data not shown). Currently, it is well established that
the stability of tumor suppressors is positively associated with
their functions. In order to explain these phenomena and to further
understand the functions of OVCA1, the degradation pathway of OVCA1
and its half-life were investigated in this study.
Materials and methods
Cell lines, antibodies, and
plasmids
293, Hela and A2780 cell lines were purchased from
the Institute of Shanghai Biochemistry Cell Biology (Shanghai,
China). Mouse monoclonal anti-green fluorescence protein (GFP)
antibody (cat. no. TA06), mouse monoclonal anti-GAPDH antibody
(cat. no. TA08) and horseradish peroxidase labeled goat anti-mouse
immunoglobulin G antibody (catalog no. ZB-2305) were purchased from
ZSGB-BIO Company (Beijing, China). Mouse monoclonal anti-ubiquitin
antibody (cat. no. sc-8017) was purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). A GFP-tagged-OVCA1
plasmid was designed by inserting full-length OVCA1 into the
XhoI and EcoRI sites of a pEGFP-C1 vector (Clontech
Laboratories, Inc., Mountainview, CA, USA).
Cell culture and transfection
Hela and 293 cells were cultured in Dulbecco's
modified Eagle's medium (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) supplemented with 10% (v/v) newborn calf serum
(Gibco; Thermo Fisher Scientific, Inc.) 50 U/ml penicillin (Gibco;
Thermo Fisher Scientific, Inc.), and 50 µg/ml streptomycin (Gibco;
Thermo Fisher Scientific, Inc.). A2780 cells were cultured in
RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% (v/v) fetal bovine serum (Gibco; Thermo
Fisher Scientific, Inc.), 50 U/ml penicillin (Invitrogen, Thermo
Fisher Scientific, Inc.) and 50 µg/ml streptomycin (Invitrogen,
Shanghai, China). Cells were incubated at 37°C in a humidified
atmosphere containing 5% CO2. Cells were seeded at a
density of 3×105 cells/well into 12-well culture plates
and were transfected with Lipofectamine® 2000 reagent
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. Briefly, 1 µg plasmid DNA was diluted in
50 µl DMED and 4 µl Lipofectamine® 2000 reagent was
diluted in 50 µl DMEM. The diluted plasmid was mixed with the
diluted lipofectamine® reagent, and was incubated for 15
min at temperature. The mixture was added into a 70–90% confluent
cell monolayer. The cells were incubated at 37°C in a humidified
atmosphere containing 5% CO2. GFP and GFP-tagged-OVCA1
protein in the cells transfected with empty vector or
GFP-tagged-OVCA1 were observed directly with a fluorescence
microscope.
Cell viability assay
Cell viability was measured by MTT assay. Cells were
seeded into 96-well plates (Corning Incorporated, Corning, NY, USA)
at 2×104 cells/well. After 24 h, cells were treated with
various concentrations of carbobenzoxy-L-leucyl-L-leucyl-L-leucinal
(MG132; Merck KGaA, Darmstadt, Germany) for 24 h at 37°C in a
humidified atmosphere containing 5% CO2. Cell viability
was assessed using an MTT assay at 490 nm using a microplate
reader.
Flow cytometry
Cells were seeded into 6-well plates (Corning
Incorporated, Corning, NY, USA) at 1×106 cells/well.
After 24 h, cells were treated with various concentrations of MG132
for 24 h at 37°C in a humidified atmosphere containing 5%
CO2. Cells were then fixed with 70% ethanol at 4°C for
12 h, washed with PBS, and resuspended in 500 µl PBS containing 45
µl RNase A and 405 µl propidium iodide (PI) from the Cell Cycle
Detection kit (catalog no. KGA512; Nanjing KeyGen Biotech Co.,
Ltd., Nanjing, China), and incubated at room temperature for 30–60
min in the dark. Cell proliferation was determined by flow
cytometry (BD FACSCalibur; BD Biosciences, Franklin Lakes, NJ,
USA).
Western blotting
Cells were lysed in lysis buffer (Beyotime Institute
of Biotechnology, Haimen, China) and centrifuged at 12,000 × g for
5 min. The supernatant was collected as whole cell lysates, and
protein concentration was assessed using the Bradford assay
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). The whole cell
lysate (40 µg) was separated by 10% SDS-PAGE and transferred to a
polyvinylidene difluoride membrane (Merck KGaA). After 2 h blocking
at room temperature with TBST [20 mmol/l Tris-HCl (pH 7.4), 137
mmol/l NaCl and 0.1% Tween-20] containing 5% (w/v) skimmed milk
powder, the membranes were incubated with primary antibody (diluted
to 1:1,000 with TBST containing 5% skimmed milk powder) overnight
at 4°C, and were later incubated with horseradish
peroxidase-conjugated secondary antibody (diluted to 1:5,000 with
TBST containing 5% skimmed milk powder) for 2 h at room
temperature. An ECL™ Prime Western Blotting system
(catalog no. RPN2232; Merck KGaA) was used to detect protein
bands.
Co-immunoprecipitation
Total protein lysate (200 µl) was incubated with 2
µl anti-GFP monoclonal antibody and 40 µl protein A+G agarose
(Beyotime, Institute of Biotechnology) at 4°C for 3 h. After 5 min
centrifugation at 2,500 × g, the supernatant was discarded and the
precipitate was washed five times with PBS and resuspended in 40 µl
1X SDS-PAGE loading buffer (Beyotime Institute of Biotechnology).
After boiling for 5 min, the supernatant was subjected to
immunoblotting.
Half-life measurement
After 24 h of transfection with OVCA1, cells
were cultured in medium containing MG132 (20 µmol/l for Hela and
293 cells, and 5 µmol/l for A2780 cells). After 24 h, cells were
cultured in fresh medium containing cycloheximide (CHX;
Sigma-Aldrich; Merck KGaA) at a final concentration of 50 µg/ml,
with or without MG132, for 0, 30, 60, 120, 180, 240 and 300 min,
and were subsequently lysed for western blotting. The bands of
OVCA1 and GAPDH were scanned, and the intensities of the bands were
semi-quantified by ImageJ 1.46r software (National Institutes of
Health, Bethesda, Maryland, USA). The relative concentration of
OVCA1 at time 0 was defined as 100%. The percentage of OVCA1 at
each indicated time point was normalized by comparing the relative
concentration of OVCA1 with that at time 0. The protein half-life
was calculated by linear regression analysis.
Statistical analysis
Data were from at least three independent
experiments were expressed as the mean ± standard deviation. Data
were analyzed by one-way analysis of variance followed by Fisher's
least significant difference or Tukey's test to compare the means
or by bivariate Pearson correlation analysis with SPSS 17.0. (SPSS,
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference. GraphPad Prism version 6
(GraphPad Software, Inc., La Jolla, IL, USA) was used to create the
illustrations.
Results
Detection of OVCA1 protein in
cells
To the best of our knowledge, only six studies have
demonstrated the detection of OVCA1 by western blot analysis
(4,8,13,14,18,22). Liang
et al (18) identified OVCA1
protein in mouse cells by western blotting using a commercial
anti-OVCA1 antibody (cat. no. ab40733; Abcam, Cambridge, UK). This
antibody and two other anti-OVCA1 antibodies: ab54777 (Abcam), and
bs-5714R (BIOSS, Beijing, China) were used in the preliminary
experiments. However, no endogenous OVCA1 was observed using these
three commercial antibodies by western blotting in any of the cell
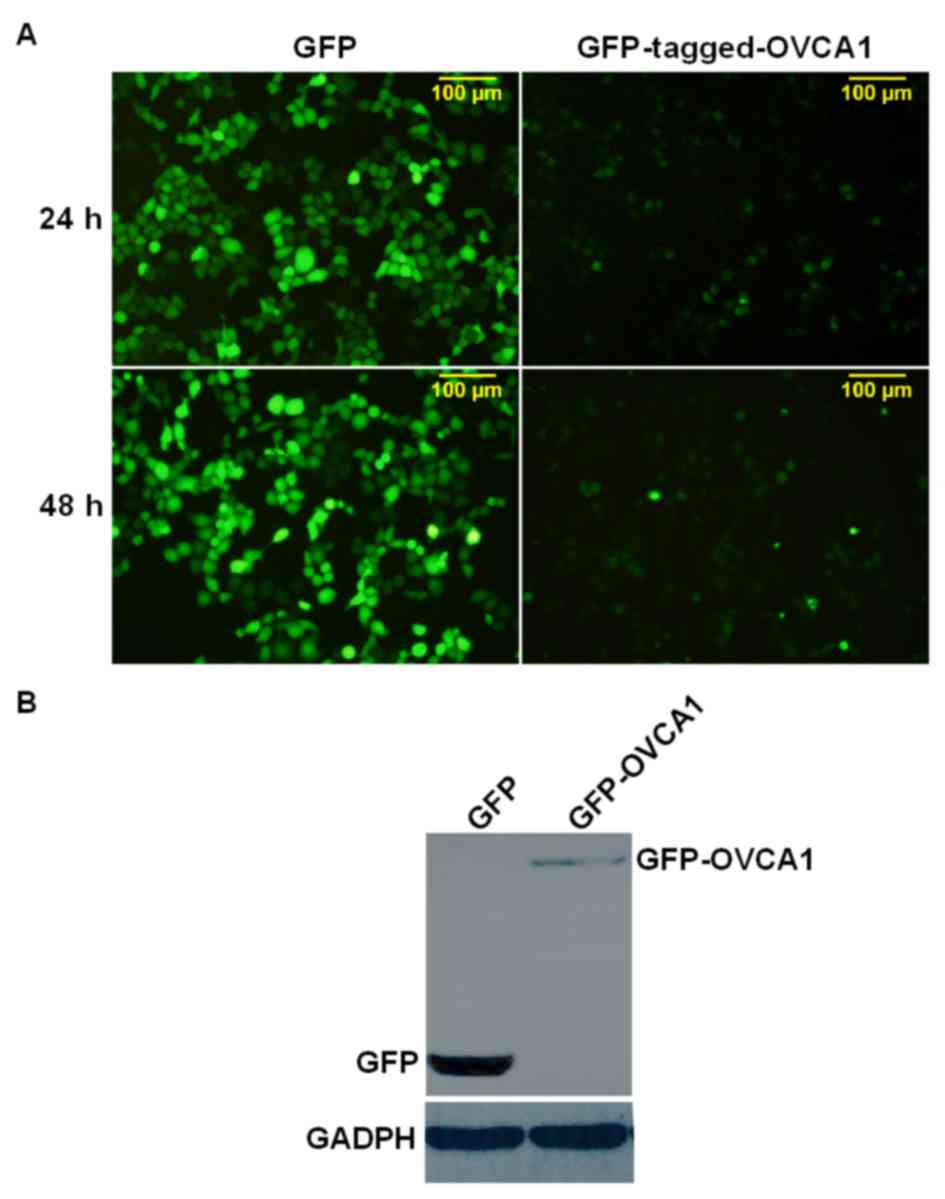
lines used (Hela, 293 and A2780 cell lines). Subsequently,
exogenous GFP-tagged-OVCA1, which was over expressed in Hela cells,
was used to assess these three commercial anti-OVCA1 antibodies
[cat. nos. ab40733 and ab54777 (Abcam) and BS-5714R (BIOSS)]. After
24 h transfection, only weak signals in the cells transfected with
GFP-tagged-OVCA1 were detected by fluorescence microscopy, and the
cell transfection score was lower than in the control cells, which
were transfected with a GFP vector only (Fig. 1A). After 48 h transfection, the
fluorescence intensity of the cells transfected with
GFP-tagged-OVCA1 was slightly increased compared with at 24 h
(Fig. 1A). Subsequently, the
GFP-tagged-OVCA1 protein from these transfected cells was detected
by western blotting; however no band was observed after membrane
incubation with the aforemention commercial anti-OVCA1 antibodies
(data not shown). The membranes were stripped and re-probed with
anti-GFP antibody, and a weak band of GFP-tagged-OVCA1 was observed
at ~75 kD (Fig. 1B). Due to the low
level of OVCA1 protein and the high death rate of transfected
cells, which was also described by Bruening et al (4), GFP-tagged-OVCA1 was used in this study
to help visually estimate the transfection efficiency and the
protein expression levels. Small tags, such as hemagglutinin and
Myc, cause less disturbance than GFP, but when no or a very weak
OVCA1 band is observed, it is difficult to differentiate whether it
is a result of high death rate or low transfection efficiency,
which may influence the reliability of the results. Therefore in
this study, exogenous GFP-OVCA1 was preferentially assessed, and
only the results detected with anti-GFP antibody were
presented.
OVCA1 degradation is inhibited by
MG132
To investigate the pathway of OVCA1 degradation,
MG132, a common 26S proteasome inhibitor, was used to inhibit the
proteasome degradation pathway, which is the main pathway for
cellular protein turnover (23).
Since MG132 inhibits cell proliferation and induces apoptosis
(24–27), the highest concentrations of MG132
that did not influence cell growth were assessed with MTT assay and
determined to be 20 µmol/l in Hela and 293 cells, and 5 µmol/l in
A2780 cells (Fig. 2Aa). The effects
of MG132 on cell proliferation was dose-dependent (Fig. 2Aa). These results were verified by
flow cytometry (Fig. 2Ab).
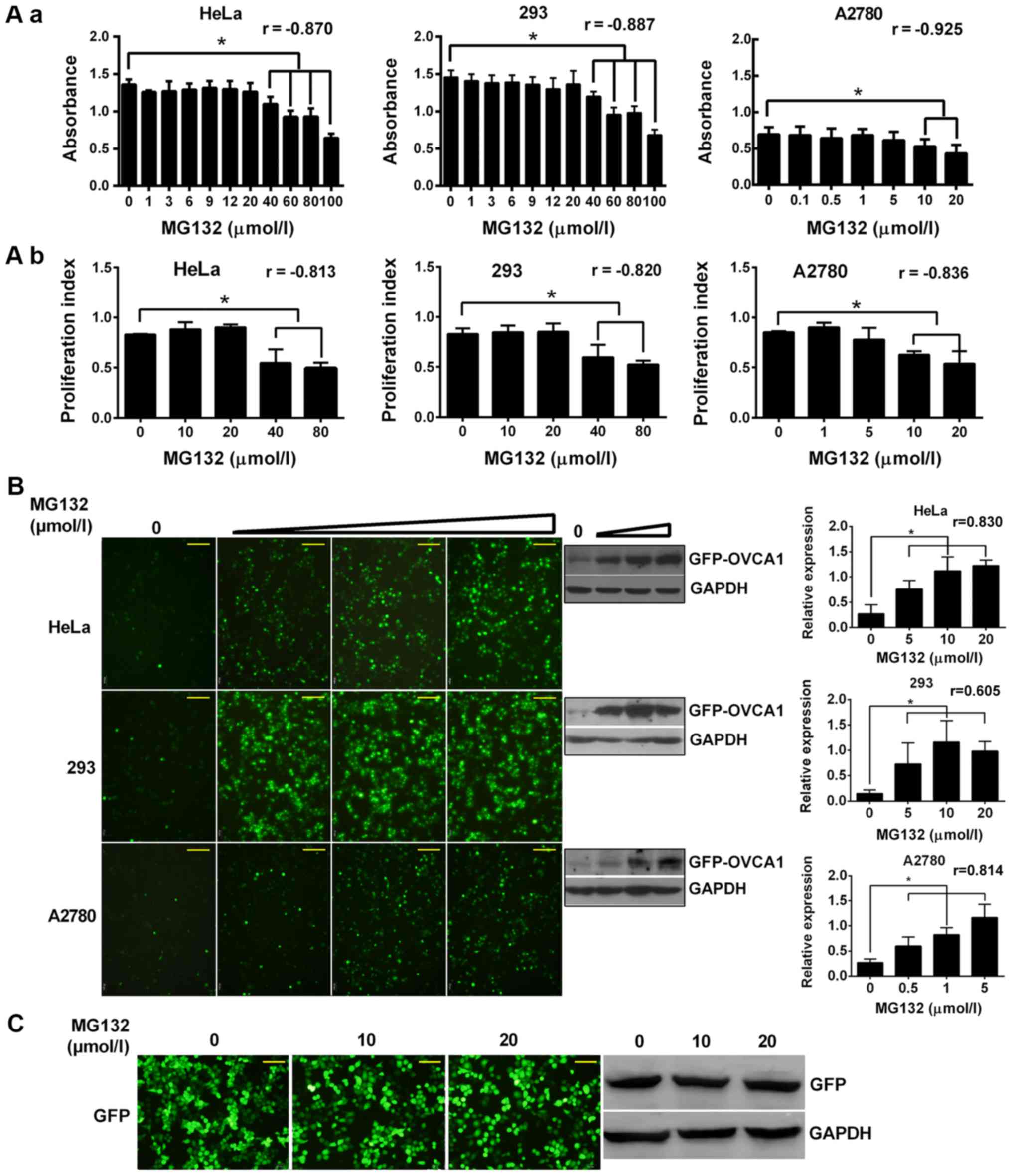 | Figure 2.(A) OVCA1 degradation was inhibited
by MG132. Cells were seeded and cultured for 24 h and were treated
with various final concentrations of MG132, as indicated, for 24 h.
The effects of MG132 on cell proliferation were then detected by
(Aa) MTT assay or by (Ab) flow cytometry after propidium iodide
staining. Pearson correlation coefficients (r) were indicated in
the figure. (B) OVCA1 degradation was blocked by MG132 in a
dose-dependent manner. Cells were transfected with
GFP-tagged-OVCA1, as indicated. After 24 h, transfected
cells were treated with various concentrations of MG132 (0, 5, 10
and 20 µmol/l for Hela and 293 cells; 0, 0.5, 1 and 5 µmol/l for
A2780 cells) for another 24 h. The fluorescence intensities were
observed by fluorescence microscopy and OVCA1 levels were detected
by western blotting using an anti-GFP antibody. Relative
GFP-tagged-OVCA1 expression was normalized to the internal control
GAPDH. (C) GFP protein level was not regulated by MG132. Hela cells
were transfected with empty pEGFP-C1 vector. After 24 h, cells were
treated with various concentrations of MG132, as indicated, for
another 24 h. The fluorescence intensities of GFP were observed by
fluorescence microscopy, and GFP protein levels were detected by
western blotting. Scale bar, 100 µm. *P<0.05. GFP, green
fluorescence protein; MG132,
carbobenzoxy-L-leucyl-L-leucyl-L-leucinal; OVCA1, ovarian
cancer gene 1. |
Hela, 293, and A2780 cells were transfected with
GFP-tagged-OVCA1. In the absence of MG132 treatment, the
fluorescence intensities of GFP-OVCA1 levels were low (Fig. 2B). Following treatment with increasing
doses of MG132, the fluorescence intensities of GFP-OVCA1 levels
were detected and the levels of GFP-tagged-OVCA1 protein in the
cells were significantly augmented in a dose-dependent manner
(Fig. 2B). The fluorescence
intensities and protein levels of GFP in the control cells, which
were transfected with empty vector, were not modified following
MG132 treatment (Fig. 2C), which was
already demonstrated by Gong et al (28). These findings demonstrated that OVCA1
degradation may be inhibited by MG132 in various cell lines.
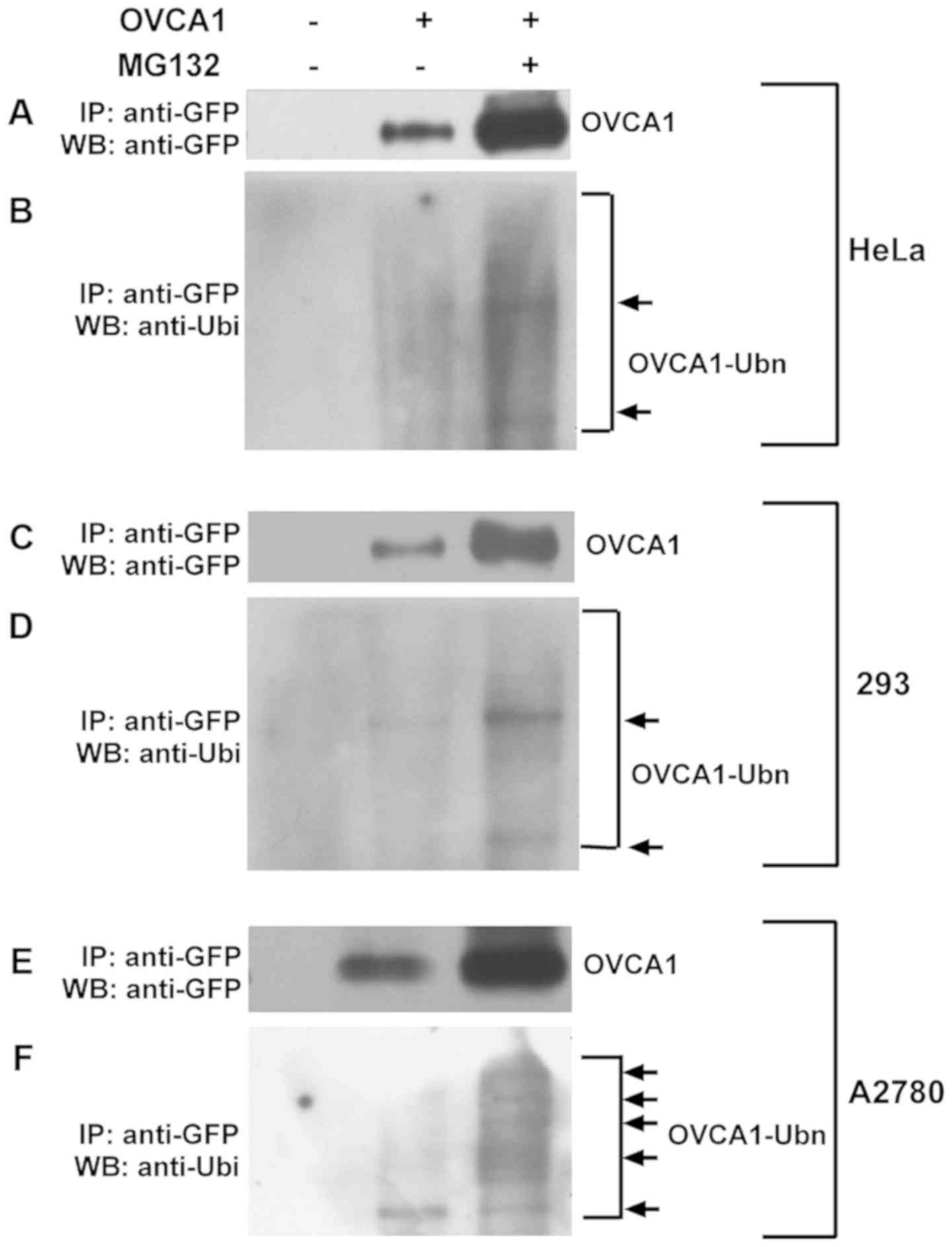
OVCA1 is degraded by the
ubiquitin-proteasome pathway (UPP)
To confirm that OVCA1 degradation is mediated by the
UPP, the interaction between OVCA1 and ubiquitin was determined by
co-immunoprecipitation. Hela, 293 and A2780 cells were transfected
with GFP-tagged-OVCA1. To overcome the problem of low
GFP-tagged-OVCA1 protein detection, cells treated with MG132 were
also used (Fig. 3). GFP-tagged-OVCA1
protein in total cell lysates was pulled down with anti-GFP
antibody, and the proteins were analyzed with anti-GFP antibody
(Fig. 3A, C and E) to check the
pull-down effect. The interaction of GFP-tagged-OVCA1 with
ubiquitin was checked with anti-ubiquitin antibody (Fig. 3B, D and F). The protein pull down was
also attempted with anti-ubiquitin antibody and by analyzing the
interaction with anti-GFP antibody; unfortunately, no band was
detected (data not shown). This may be due to a low level of OVCA1
proteins in the cell lysates and therefore, a low percentage of
ubiquitinated OVCA1 protein among the pulled-down ubiquitinated
proteins. The results indicated that OVCA1 binds to ubiquitin and
forms poly-ubiquitinated OVCA1.
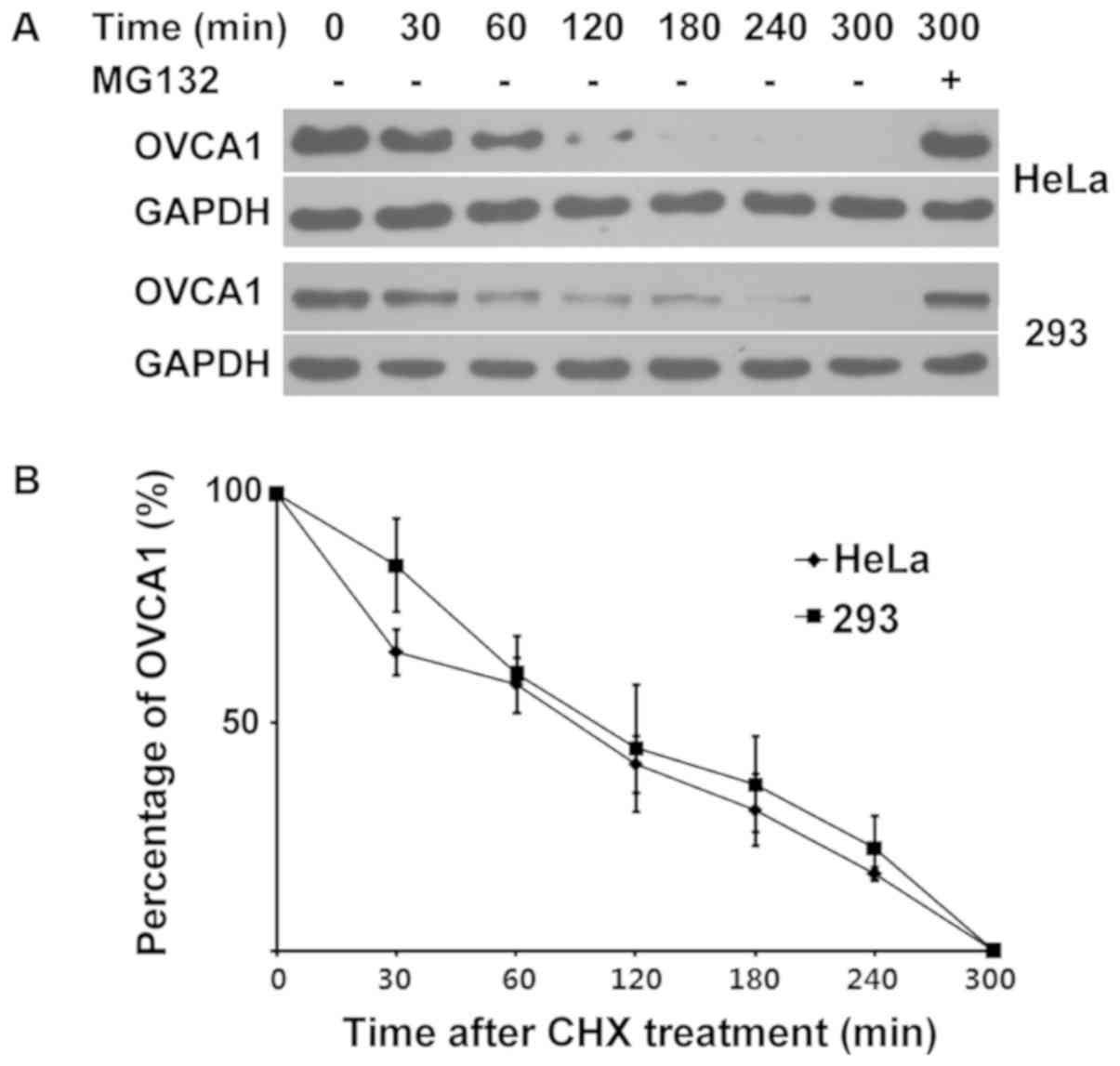
OVCA1 may be a short half-life
protein
To reveal the half-life of OVCA1 degradation in
cells, CHX, which inhibits protein synthesis in eukaryotic
organisms by disturbing the translocation step (29), was used to block the synthesis of
proteins in cells. Hela, 293 and A2780 cells were transfected with
GFP-tagged-OVCA1. Prior to cell incubation with CHX, cells
were treated with MG132, in order to block OVCA1 protein
degradation and therefore allow the protein detection necessary to
carry out the subsequent experiments. After 24 h of treatment with
MG132, cells were treated with 50 µg/ml CHX alone for 0, 30, 60,
120, 180, 240 and 300 min. Cells were treated with CHX and MG132
together for 300 min as a control. The levels of GFP-tagged-OCVA1
at these time points were detected. Because A2780 cells died during
CHX treatment, this experiment was only performed in Hela and 293
cells. The results demonstrated that the protein expression levels
of OVCA1 in cells decreased with time and had completely
disappeared after 300 min of CHX treatment. However, when the cells
were treated with CHX and MG132 together for 300 min, the protein
expression levels of GFP-tagged-OVCA1 remained high (Fig. 4A), thus suggesting that the loss of
GFP-tagged-OVCA1 with time may be caused by ubiquitin-mediated
protein degradation. The linear regression analysis demonstrated
that the GFP-tagged-OVCA1 half-life was of 105 and 118 min in Hela
and 293 cells, respectively (Fig.
4B). These results demonstrated that the OVCA1 protein may have
a short half-life.
Discussion
The deletion or mutation of tumor suppressor genes
has an important role in the development of cancer. OVCA1 is
a tumor suppressor gene, which may be deleted or mutated in ovarian
and breast cancer (3,4,6). In
addition, the overexpression of OVCA1 inhibits cell
proliferation (4,14,19,20). In
the present study, the cellular OVCA1 protein levels were
demonstrated to be very low. Controlling the stability of cellular
proteins is a fundamental way of regulating cell proliferation,
survival and development, particularly tumor suppressors. The
assessment of a protein half-life is one of the first steps to
check whether the function of a protein is regulated by proteolysis
under specific physiological conditions. The half-life of OVCA1
measured in this study was 105 and 118 min in Hela and 293 cells,
respectively, thus suggesting that OVCA1 may be a short half-life
protein. In the present study, the endogenous protein levels of
OVCA1 in cells were too low to be detected by western blotting,
transfection efficiency was also low and the death rate of
OVCA1-transfected cells was high (data not shown).
Consequently, the GFP-fused OVCA1 protein was used to observe
transfection efficiency and to avoid alterations in OVCA1 protein
levels caused by low transfection efficiency or cell death.
Although the stability of GFP is not affected by MG132 (28), and it is often used as a tag protein
for studies on ubiquitin-mediated protein degradation (30,31), being
able to measure either endogenous OVCA1 or a suitable smaller tag
fusion protein would be more convincing.
Cellular proteins are degraded through various
pathways, including the lysosomal pathway, the UPP and the caspase
pathway (32,33). The UPP is a specific protein
degradation pathway, which is essentially responsible for the
degradation of most intracellular proteins (32). Ubiquitin first attaches to a target
protein, involving three critical enzymes, the ubiquitin activating
enzyme, the ubiquitin-conjugating enzyme and the ubiquitin ligase,
and forms protein complexes. The protein complexes are then
recognized by the 26S proteasome, a large multi-enzyme complex
responsible for protein degradation (34–36). MG132
is an inhibitor commonly used to block the proteolytic activity of
the 26S proteasome complex (35). In
the present study, cellular OVCA1 protein levels were significantly
increased following MG132 treatment, thus indicating that OVCA1 may
be degraded by the UPP. The subsequent co-immunoprecipitation
experiment confirmed that OVCA1 can interact with ubiquitin in
cells. These results suggested that the UPP may be one of the
pathways allowing the OVCA1 protein degradation.
Modification of ubiquitination serves an important
role in regulating protein stability and activity, and is closely
associated with the regulation of biological processes and the
development of numerous diseases, such as cancer, neurodegenerative
diseases and autoimmune diseases (37). The ubiquitin-proteasome system has
therefore become an important target for drug screening, and
research on the ubiquitination process has become crucial in drug
development (38,39). In this study, the OVCA1 was
demonstrated to be a short half-life protein that was degraded by
the UPP. The molecules involved in the UPP-associated OVCA1
degradation are currently being investigated. Novel molecules
targeting the UPP, and hence, regulating the stability or
degradation of tumor suppressors have already shown great promise
in the treatment of some types of cancer (38,39).
Regulating OVCA1 protein degradation may therefore represent a
novel target in the treatment of ovarian cancer.
In conclusion, the present study demonstrated that
the OVCA1 protein was degraded by the UPP and may be a short
half-life protein. These findings provided a potential novel
direction for ovarian cancer therapy by regulating OVCA1 protein
via UPP. Since endogenous OVCA1 levels were too low to be detected,
GFP-tagged OVCA1 was determined in the study. The development of
novel techniques for the detection of endogenous OVCA1 is therefore
crucial. The signaling pathways of OVCA1 degradation through the
UPP will be further investigated in order to provide potential
novel targets for the treatment of ovarian cancer.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Science and
Technology governor of Liaoning Province of China (grant no.
2013023013).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YWL and FDK participated in the design of the study,
the writing and revising of the manuscript, the generation of the
figures, cloning of the gene and flow cytometry. YL performed MTT,
immunoprecipitation, cloning of the gene and western blotting
experiments. YHW partly performed the statistical analysis and flow
cytometry, and LS partly performed western blotting experiments.
CYZ contributed to the conception and design of the study, was
involved in drafting and revising the manuscript, and gave final
approval of the manuscript to be published. All authors read and
approved the final manuscript.
Ethical approval and consent to
participate
Not applicable.
Patient consent for application
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CHX
|
cycloheximide
|
|
ECL
|
enhanced chemi-luminescence
|
|
MG132
|
carbobenzoxy-L-leucyl-L-leucyl-L-leucinal
|
|
UPP
|
ubiquitin-proteasome pathway
|
|
PI
|
propidium iodide
|
References
|
1
|
Schultz DC, Vanderveer L, Berman DB,
Hamilton TC, Wong AJ and Godwin AK: Identification of two candidate
tumor suppressor genes on chromosome 17p13.3. Cancer Res.
56:1997–2002. 1996.PubMed/NCBI
|
|
2
|
Phillips N, Ziegler M, Saha B and Xynos F:
Allelic loss on chromosome 17 in human ovarian cancer. Int J
Cancer. 54:85–91. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Phillips NJ, Ziegler MR, Radford DM, Fair
KL, Steinbrueck T, Xynos FP and Donis-Keller H: Allelic deletion on
chromosome 17p13.3 in early ovarian cancer. Cancer Res. 56:606–611.
1996.PubMed/NCBI
|
|
4
|
Bruening W, Prowse AH, Schultz DC,
Holgado-Madruga M, Wong A and Godwin AK: Expression of OVCA1, a
candidate tumor suppressor, is reduced in tumors and inhibits
growth of ovarian cancer cells. Cancer Res. 59:4973–4983.
1999.PubMed/NCBI
|
|
5
|
Li AJ and Karlan BY: Genetic factors in
ovarian carcinoma. Curr Oncol Rep. 3:27–32. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Phillips NJ, Zeigler MR and Deaven LL: A
cDNA from the ovarian cancer critical region of deletion on
chromosome 17p13.3. Cancer Lett. 102:85–90. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schultz DC, Balasara BR, Testa JR and
Godwin AK: Cloning and localization of a human diphthamide
biosynthesis-like protein-2 gene, DPH2L2. Genomics. 52:186–191.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu S, Milne GT, Kuremsky JG, Fink GR and
Leppla SH: Identification of the proteins required for biosynthesis
of diphthamide, the target of bacterial ADP-ribosylating toxins on
translation elongation factor 2. Mol Cell Biol. 24:9487–9497. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mayer K, Schröder A, Schnitger J, Stahl S
and Brinkmann U: Influence of DPH1 and DPH5 protein variants on the
synthesis of diphthamide, the target of ADPRibosylating toxins.
Toxins (Basel). 9:E782017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Stahl S, da Silva Mateus Seidl AR, Ducret
A, Kux van Geijtenbeek S, Michel S, Racek T, Birzele F, Haas AK,
Rueger R, Gerg M, et al: Loss of diphthamide pre-activates NF-κB
and death receptor pathways and renders MCF7 cells hypersensitive
to tumor necrosis factor. Proc Natl Acad Sci USA. 112:10732–10737.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Webb TR, Cross SH, McKie L, Edgar R, Vizor
L, Harrison J, Peters J and Jackson IJ: Diphthamide modification of
eEF2 requires a J-domain protein and is essential for normal
development. J Cell Sci. 121:3140–3145. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nobukuni Y, Kohno K and Miyagawa K: Gene
trap mutagenesis-based forward genetic approach reveals that the
tumor suppressor OVCA1 is a component of the biosynthetic pathway
of diphthamide on elongation factor 2. J Biol Chem.
280:10572–10577. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen CM and Behringer RR: Cloning,
structure, and expression of the mouse Ovca1 gene. Biochem Biophys
Res Commun. 286:1019–1026. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen CM and Behringer RR: Ovca1 regulates
cell proliferation, embryonic development, and tumorigenesis. Genes
Dev. 18:320–332. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jensen MR and Helin K: OVCA1: Emerging as
a bona fide tumor suppressor. Genes Dev. 18:245–248. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
L'Allemain G: Ovca1 gene, deleted in
ovarian cancer is a special tumor suppressor. Bull Cancer.
91:301–302. 2004.(In French). PubMed/NCBI
|
|
17
|
Chen CM and Behringer RR: OVCA1: Tumor
suppressor gene. Curr Opin Genet Dev. 15:49–54. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liang M, Ayanga B, Du S, Godwin AK,
Hartsock JK and Evans SC: Ovca1, a candidate gene of the genetic
modifier of Tp53, Mop2, affects mouse embryonic lethality. Genes
Chromosomes Cancer. 47:315–325. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kong F, Tong R, Jia L, Wei W, Miao X, Zhao
X, Sun W, Yang G and Zhao C: OVCA1 inhibits the proliferation of
epithelial ovarian cancer cells by decreasing cyclin D1 and
increasing p16. Mol Cell Biochem. 354:199–205. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yu YR, You LR, Yan YT and Chen CM: Role of
OVCA1/DPH1 in craniofacial abnormalities of Miller-Dieker syndrome.
Hum Mol Genet. 23:5579–5596. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wiper DW, Zanotti KM, Kennedy AW, Belinson
JL and Casey G: Analysis of allelic imbalance on chromosome 17p13
in stage I and stage II epithelial ovarian cancers. Gynecol Oncol.
71:77–82. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Prowse AH, Vanderveer L, Milling SW, Pan
ZZ, Dunbrack RL, Xu XX and Godwin AK: OVCA2 is downregulated and
degraded during retinoid-induced apoptosis. Int J Cancer.
99:185–192. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rock KL, Gramm C, Rothstein L, Clark K,
Stein R, Dick L, Hwang D and Goldberg AL: Inhibitors of the
proteasome block the degradation of most cell-proteins and the
generation of peptides presented on MHC class-I molecules. Cell.
78:761–771. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jullig M, Zhang WV, Ferreira A and Stott
NS: MG132 induced apoptosis is associated with p53-independent
induction of pro-apoptotic Noxa and transcriptional activity of
beta-catenin. Apoptosis. 11:627–641. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Han YH, Moon HJ, You BR and Park WH: The
effect of MG132, a proteasome inhibitor on HeLa cells in relation
to cell growth, reactive oxygen species and GSH. Oncol Rep.
22:215–221. 2009.PubMed/NCBI
|
|
26
|
Han YH and Park WH: MG132 as a proteasome
inhibitor induces cell growth inhibition and cell death in A549
lung cancer cells via influencing reactive oxygen species and GSH
level. Hum Exp Toxicol. 29:607–614. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Guo N and Peng Z: MG132, a proteasome
inhibitor, induces apoptosis in tumor cells. Asia Pac J Clin Oncol.
9:6–11. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gong Y, Wang D, Dar JA, Singh P, Graham L,
Liu W, Ai J, Xin Z, Guo Y and Wang Z: Nuclear export signal of
androgen receptor (NESAR) regulation of androgen receptor level in
human prostate cell lines via ubiquitination and
proteasome-dependent degradation. Endocrinology. 153:5716–5725.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Watanabe-Asano T, Kuma A and Mizushima N:
Cycloheximide inhibits starvation-induced autophagy through mTORC1
activation. Biochem Biophys Res Commun. 445:334–339. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fu C, Chen D, Chen R, Hu Q and Wang G: The
schizophrenia-related protein dysbindin-1A is degraded and
facilitates NF-Kappa B activity in the nucleus. PLoS One.
10:e01326392015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nakagawa K and Yokosawa H: Degradation of
transcription factor IRF-1 by the ubiquitin-proteasome pathway. The
C-terminal region governs the protein stability. Eur J Biochem.
267:1680–1686. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lecker SH, Goldberg AL and Mitch WE:
Protein degradation by the ubiquitin-proteasome pathway in normal
and disease states. J Am Soc Nephrol. 17:1807–1819. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Knecht E, Aguado C, Cárcel J, Esteban I,
Esteve JM, Ghislat G, Moruno JF, Vidal JM and Sáez R: Intracellular
protein degradation in mammalian cells: Recent developments. Cell
Mol Life Sci. 66:2427–2443. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Groll M, Ditzel L, Löwe J, Stock D,
Bochtler M, Bartunik HD and Huber R: Structure of 20S proteasome
from yeast at 2.4 A resolution. Nature. 386:463–471. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Doherty FJ, Dawson S and Mayer RJ: The
ubiquitin-proteasome pathway of intracellular proteolysis. Essays
Biochem. 38:51–63. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fuchs SY: The role of ubiquitin-proteasome
pathway in oncogenic signaling. Cancer Biol Ther. 1:337–341. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Schmidt M and Finley D: Regulation of
proteasome activity in health and disease. Biochim Biophys Acta.
1843:13–25. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu J, Shaik S, Dai X, Wu Q, Zhou X, Wang
Z and Wei W: Targeting the ubiquitin pathway for cancer treatment.
Biochim Biophys Acta. 1855:50–60. 2015.PubMed/NCBI
|
|
39
|
D'Arcy P and Linder S: Proteasome
deubiquitinases as novel targets for cancer therapy. Int J Biochem
Cell Biol. 44:1729–1738. 2012. View Article : Google Scholar : PubMed/NCBI
|