Introduction
Cancer immunotherapy has gained interest due to the
clinical efficacy of immune checkpoint inhibitors, including
antibodies targeting programmed death-1 (PD-1), programmed death
ligand-1 (PD-L1) and cytotoxic T-lymphocyte-associated protein 4
(CTLA-4), in a number of cancer types, including melanoma,
colorectal cancer (CRC), renal cell carcinoma and non-small cell
lung carcinoma (1–4). Identification of factors that could
predict the curative effect of anti-PD-1/L1 therapy is a major
challenge. Mismatch repair protein (MMR) status has been reported
to be a primary indicator for anti-PD-1/L1 therapy in patients with
CRC, as only MMR-deficient (dMMR) patients are promising candidates
(2). However, to the best of our
knowledge, it is unknown why only dMMR patients are sensitive to
anti-PD-1/L1 therapy. In addition, only certain dMMR patients
benefit from the treatment (2), which
suggests that other predictive factors should also be explored.
The tumor microenvironment is a complicated system
that has the dual function of promoting and inhibiting tumor
growth, which therefore influences drug response. The tumor
microenvironment is composed of non-tumor cells, cytokines,
chemokines and the extracellular matrix. Immune cells, particularly
acquired immune cells, including T and B lymphocytes, are important
non-tumor cells (5). The current
study primarily focused on tumor infiltrating lymphocytes (TILs),
crucial acquired immune cells, and PD-L1 expressed by tumor cells,
which negatively affects the function of TILs. In human melanoma,
the classification of tumor microenvironments based on TIL presence
and PD-L1 expression has been proposed to predict and guide
immunotherapeutic approaches (6).
Among the four types of tumor microenvironments (TIL+
PD-L1+, TIL+ PD-L1−,
TIL− PD-L1+ and TIL−
PD-L1−), TIL+ PD-L1+ tumors
exhibit the best response to checkpoint blockade and are the most
inclined to benefit from single anti-PD-1/L1 agent therapy, as
pre-existing intratumor T cells in these tumors are turned off by
PD-L1 and reactivated by the agent (7). TIL− PD-L1+ and
TIL− PD-L1− tumors appear insensitive to
single checkpoint blockade therapy due to a lack of pre-existing
lymphocyte infiltrates, instead combination therapy is required to
attract T cells into tumors (8–10).
TIL+ PD-L1− tumors contain
TILs, but no PD-L1 expression; therefore, without evident adaptive
resistance, TIL+ PD-L1− tumors may not be
suitable for anti-PD-1/L1 therapy (11).
To the best of our knowledge, the typical TIL/PD-L1
status of patients with CRC has not previously been investigated.
The current study enrolled 243 patients with CRC who were defined
as pMMR or dMMR. The associations between MMR status, TIL
expression and PD-L1 expression were investigated. In addition, the
associations between TIL/PD-L1 status and clinicopathological
features, particularly MMR status, were investigated to evaluate
whether TIL/PD-L1 status could provide an explanation for the
improved response of dMMR patients to anti-PD-1/L1 therapy and
determine whether this classification method is suitable for
efficacy prediction.
Patients and methods
Patients and specimens
Pathological specimens of CRC tissue were collected
from 243 patients with CRC (121 pMMR patients and 122 dMMR
patients) of stage I to IV who underwent primary surgery between
March 2009 and December 2016 at Sun Yat-Sen University Cancer
Center (Guangzhou, China). The obtained specimens were fixed in 10%
formalin for 24 h at room temperature and embedded in paraffin for
further use. Written informed consent was obtained from every
patient. The study was performed according to the principles of the
Declaration of Helsinki and was approved by the Research Ethics
Committee of Sun Yat-Sen University (Gaungzhou, China).
Estimation of TILs
T-lymphocyte density within the cancer cell nests
and at the invasive margin, which denoted the interface between the
invading edge area of the tumor and the host stroma, was identified
and estimated using a hematoxylin and eosin (H&E) staining
method. Briefly, tumor samples were paraffin-embedded and sliced
into 4-µm sections. Slides were deparaffinized in xylene for 10 min
three times and rehydrated in a descending alcohol series (100, 95,
85 and 75%) for 5 min each at 25°C. Subsequently, the samples were
stained with H&E (cat no. 468802128; POCH S.A., Gliwice,
Poland) at room temperature for 20 min. The sections were then
observed with a light microscope (magnification, ×200). The
intensity of the lymphocyte infiltrate was scored as follows: Score
0, none; score 1, weak, rare lymphocytes; score 2, moderate, focal
infiltration; and score 3, severe, diffuse infiltration (12). Populations with scores of 0 and 1 were
defined as TIL−, and those with scores of 2 and 3 were
defined as TIL+.
Immunohistochemistry and scoring
The immunohistochemistry experiment was conducted as
previously described (13). To detect
MMR proteins, mouse monoclonal antibodies against MutL homolog 1
(MLH1) (cat. no. ES05; 1:500), MutS homolog 2 (MSH2) (cat. no.
FE11; 1:500), MutS homolog 6 (MSH6; cat. no. EP49; 1:500) and PMS1
homolog 2 (PMS2; cat. no. EP51; 1:500) (all from Dako; Agilent
Technologies, Inc., Santa Clara, CA, USA) were used. Rabbit
monoclonal antibodies against PD-L1 (cat. no. 13684; 1:200; Cell
Signaling Technology, Inc., Danvers, MA, USA) and PD-1 (cat. no.
ab137132; 1:500; Abcam, Cambridge, UK) were also used to evaluate
the expression of PD-L1 and PD-1. Samples embedded in paraffin were
cut into 4-µm sections, deparaffinized in xylene, rehydrated
through graded ethanol and dipped in 0.3% hydrogen peroxide for
>15 min to inactivate the endogenous peroxidase. The slides were
then processed for antigen retrieval with high pressure cooking at
120°C for ~10 min in citrate antigen retrieval solution, followed
by incubation with primary antibody at 4°C overnight. Following
three washes with PBS, the slides were subsequently co-incubated
with goat anti-rabbit or anti-mouse biotin-linked secondary
antibodies for 30 min at 37°C (cat. no. SP-9000; 1:2,000; OriGene
Technologies, Inc., Rockville MD, USA), counterstained with
hematoxylin for 15 min at room temperature for color reaction and
ultimately fixed in mounting media. The sections were observed with
a light microscope (magnification, ×200). Staining without primary
antibody served as a negative control. Each slide was examined by
three pathologists.
Tumors expressing all four proteins, MLH1, MSH2,
PMS2 and MSH6, were defined as pMMR and all other tumors were
considered to be dMMR. The expression of PD-L1 in tumor cells was
divided into two groups: <5% positive cells was considered as
negative and ≥5% was considered as positive (3,14). PD-1
expression in TILs was scored as follows: Score 0, none (0% of
lymphocytes); score 1, isolated (<5% of lymphocytes); score 2,
moderate (5–50% of lymphocytes; and 3, severe (>50% of
lymphocytes. Scores of 0 and 1 were considered as low expression
and score of 2 and3 were considered as high expression (7,15).
Statistical analysis of the data
All statistical analyses were performed using SPSS
22.0 software (IBM Corp., Armonk, NY, USA). Pearson's χ2
test was used to assess the association between categorical
variables. Multivariable multinomial logistic regression analysis
was used for multivariate analysis to predict the odds ratio (OR)
of individual factors for TIL+ PD-L1+ status.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Patient characteristics
The clinicopathological characteristics of all 243
patients are presented in Table I.
Patients were selected according to pMMR (121 patients) and dMMR
(122 patients) status, including 189 patients with colon cancer
(left, 90 patients; right, 99 patients) and 54 patients with rectum
cancer. Table II presents the
frequency of aberrant MMR protein expression in dMMR patients. The
median age at the time of diagnosis was 60 years (range, 22–84
years), and 58.4% of patients were male and 41.6% were female.
According to the American Joint Committee on Cancer (AJCC) 7th
edition TNM staging system, pathologically confirmed stage I–IV
disease was identified in 48 (19.8%), 135 (55.6%), 46 (18.9%) and
14 (5.8%) patients respectively. Moderately differentiated
adenocarcinoma accounted for 58.4% of cases and well-differentiated
adenocarcinoma accounted for 0.8% of all cases. A total of 20
(8.2%) and 28 (11.5%) patients exhibited vascular and nerve
invasion, respectively.
 | Table I.Association analysis between MMR
status and clinicopathological features using a χ2
test. |
Table I.
Association analysis between MMR
status and clinicopathological features using a χ2
test.
| Characteristic | pMMR, n (%) | dMMR, n (%) | C | P-value |
|---|
| Age at diagnosis,
years |
|
| 0.190 | 0.003 |
|
<60 | 54 (44.6) | 78 (63.9) |
|
|
|
≥60 | 67 (55.4) | 44 (36.1) |
|
|
| Sex |
|
|
| 0.22 |
|
Male | 66 (54.5) | 76 (62.3) |
|
|
|
Female | 55 (45.5) | 46 (37.7) |
|
|
| Primary tumor
site |
|
| 0.18 | 0.017 |
| Left
colon | 55 (45.5) | 35 (28.7) |
|
|
| Right
colon | 40 (33.1) | 59 (48.4) |
|
|
|
Rectum | 26 (21.5) | 28 (23.0) |
|
|
| Stagea |
|
|
| 0.514 |
| I | 22 (18.2) | 26 (21.3) |
|
|
| II | 73 (60.3) | 62 (50.8) |
|
|
|
III | 20 (16.5) | 26 (21.3) |
|
|
| IV | 6 (5.0) | 8 (6.6) |
|
|
| Tumor stage |
|
| 0.204 | 0.033 |
| T1 | 6 (5.0) | 5 (3.1) |
|
|
| T2 | 21 (17.4) | 24 (14.7) |
|
|
| T3 | 82 (67.8) | 63 (51.6) |
|
|
|
T4a | 9 (7.4) | 22 (18.0) |
|
|
|
T4b | 3 (2.5) | 8 (6.6) |
|
|
| Node stage |
|
|
| 0.458 |
|
Negative | 97 (80.2) | 93 (76.2) |
|
|
|
Positive | 24 (19.8) | 29 (23.8) |
|
|
| Tumor histological
grade |
|
| 0.186 | 0.033 |
|
Well-differentiated | 1 (0.8) | 1 (0.8) |
|
|
|
Moderately differentiated | 80 (66.1) | 62 (50.8) |
|
|
| Poorly
differentiated | 19 (15.7) | 18 (14.8) |
|
|
|
Mucinous | 21 (17.4) | 41 (33.6) |
|
|
| Vascular
invasion |
|
| 0.149 | 0.019 |
| No | 106 (87.6) | 117 (95.9) |
|
|
|
Yes | 15 (12.4) | 5 (4.1) |
|
|
| Nerve invasion |
|
| 0.179 | 0.005 |
| No | 100 (82.6) | 115 (94.3) |
|
|
|
Yes | 21 (17.4) | 7 (5.7) |
|
|
| NLRb |
|
|
| 0.699 |
| Low
(≤5) | 109 (91.6) | 110 (90.2) |
|
|
| High
(>5) | 10 (8.4) | 12 (9.8) |
|
|
| CRP, mg/l |
|
| 0.201 | 0.001 |
| Low
(≤5) | 85 (72.0) | 63 (52.1) |
|
|
| High
(>5) | 33 (28.0) | 58 (47.9) |
|
|
| PD-1
expression |
|
|
| 0.805 |
|
Low | 43 (44.8) | 40 (43.0) |
|
|
|
High | 53 (55.2) | 53 (57.0) |
|
|
| TIL expression |
|
| 0.193 | 0.002 |
|
TIL− | 60 (49.6) | 37 (30.3) |
|
|
|
TIL+ | 61 (50.4) | 85 (69.7) |
|
|
| PD-L1
expression |
|
| 0.144 | 0.023 |
|
PD-L1− | 89 (73.6) | 73 (59.8) |
|
|
|
PD-L1+ | 32 (26.4) | 49 (40.2) |
|
|
 | Table II.Aberrant protein expression in
MMR-deficient patients. |
Table II.
Aberrant protein expression in
MMR-deficient patients.
| Aberrant protein
expression | Cases, n (%) |
|---|
| MMR (1) | 44 (36.1) |
|
MLH1 | 9
(7.4) |
|
MSH2 | 4
(3.3) |
|
MSH6 | 25 (20.5) |
|
PMS2 | 6
(4.9) |
| MMR (2) | 68 (55.7) |
| MMR (3) | 7
(5.7) |
| MMR (4) | 3
(2.5) |
Association between MMR status, TILs,
PD-L1 expression and other clinicopathological features
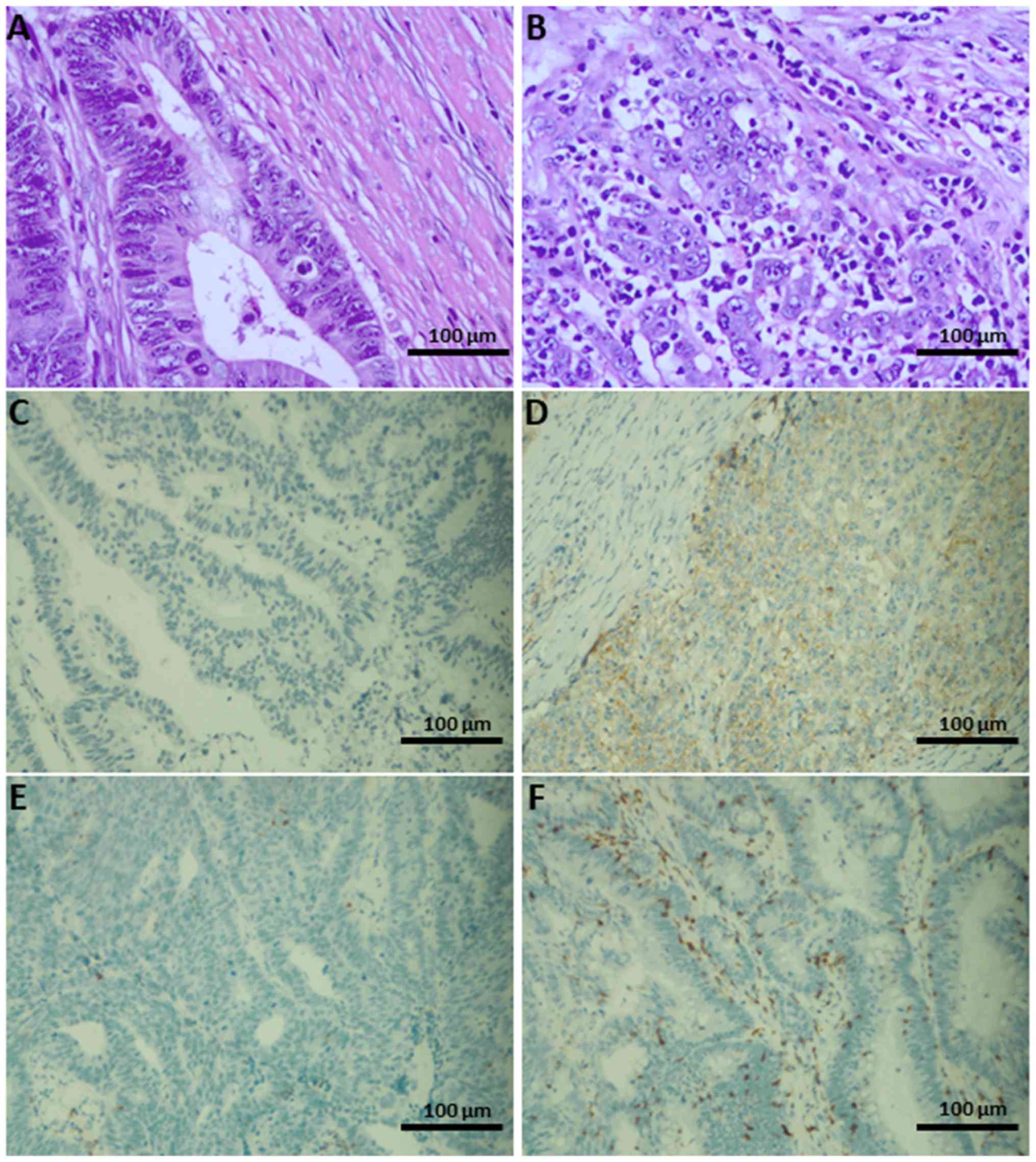
Representative images obtained to determine TIL
presence and PD-L1 expression are presented in Fig. 1. The presence of TILs was classified
as positive or negative according to H&E staining. The degree
of PD-L1 expression was based on IHC (negative, <5% positive
tumor cells; positive, ≥5% positive tumor cells). The associations
of MMR status with TIL presence and PD-L1 expression are presented
in Table I. The dMMR group exhibited
a significantly higher frequency of TIL+ (85/122 vs.
61/121) and PD-L1+ expression (49/122 vs. 32/121)
compared with the pMMR group (P<0.05). Other clinicopathological
characteristics, including age, primary tumor site, tumor stage,
histological grade, C-reactive protein (CRP) level, vascular
invasion and nerve invasion were also significantly associated with
MMR status (P<0.05).
Association of TILs with PD-L1
expressed by CRC cells
The association between TIL frequency and PD-L1
expression by CRC cells was investigated in the pMMR and dMMR
groups. As presented in Table III,
PD-L1+ expression was identified in 42.4% of
TIL+ cases in the dMMR group, while PD-L1+
expression was only identified in 18.0% of TIL+ cases in
the pMMR group. A significant association was revealed between the
presence of TILs and PD-L1 expression in the pMMR group
(P<0.05), but not in the dMMR group (P>0.05).
 | Table III.Association analysis between TILs and
PD-L1 expression in colorectal cancer cells. |
Table III.
Association analysis between TILs and
PD-L1 expression in colorectal cancer cells.
|
| TIL+, n
(%) | TIL−, n
(%) |
|
|
|---|
|
|
|
|
|
|
|---|
| MMR status |
PD-L1+ |
PD-L1− |
PD-L1+ |
PD-L1− | C | P-value |
|---|
| pMMR | 11 (18.0) | 50 (82.0) | 21 (35.0) | 39 (65.0) | 0.189 | 0.034 |
| dMMR | 36 (42.4) | 49 (57.6) | 13 (35.1) | 24 (64.9) |
| 0.455 |
| All | 47 (32.2) | 99 (67.8) | 34 (35.1) | 63 (64.9) |
| 0.643 |
Association between TIL/PD-L1 status
and clinicopathological features
A total of 4 distinct groups were determined
according to the presence of TILs and PD-L1: The presence of TILs
and PD-L1 (TIL+ PD-L1+), the presence of TILs
without PD-L1 (TIL+ PD-L1−), PD-L1 expression
without TILs (TIL− PD-L1+) and the absence of
TILs and PD-L1 expression (TIL− PD-L1−). The
current study investigated whether TIL/PD-L1 status was associated
with other clinicopathological features, particularly MMR status.
As presented in Table IV, TIL/PD-L1
status was significantly associated with AJCC stage, tumor stage,
node stage, tumor histological grade, nerve invasion, neutrophil
lymphocyte ratio, CRP level, PD-1 expression and MMR status
(P<0.05). These significantly associated features were then
further analyzed by multivariable multinomial logistic regression
analysis (Table V). The results
demonstrated that PD-1 expression and MMR status were significantly
associated with TIL/PD-L1 status. Taking TIL+
PD-L1+ as a reference, low PD-1 expression compared with
high expression was associated with a higher likelihood of
presenting as TIL− PD-L1− [OR, 10.473; 95%
confidence interval (CI), 3.005–36.503; P<0.001] and
TIL+ PD-L1− (OR, 3.443; 95% CI, 1.145–10.352;
P=0.028). pMMR status compared with dMMR status was associated with
an increased likelihood of being TIL− PD-L1−
(OR, 11.536; 95% CI, 3.223–41.290; P<0.001), TIL−
PD-L1+ (OR, 14.523; 95% CI, 3.358–62.803; P<0.001)
and TIL+ PD-L1− (OR 4.718; 95% CI,
1.607–13.848; P=0.005). Compared with the pMMR group, the dMMR
group was more likely to present with a TIL+
PD-L1+ status (76.6 vs. 23.4%; Table IV).
 | Table IV.Association analysis of TIL/PD-L1
status with clinicopathological features using a χ2
test. |
Table IV.
Association analysis of TIL/PD-L1
status with clinicopathological features using a χ2
test.
| Characteristic | TIL+
PD-L1+ b | TIL+
PD-L1− b | TIL−
PD-L1+ b | TIL−
PD-L1− b | C | P-value |
|---|
| Age at diagnosis,
years |
|
|
|
|
| 0.751 |
|
<60 | 28 (59.6) | 51 (51.5) | 20 (58.8) | 33 (52.4) |
|
|
|
≥60 | 19 (40.4) | 48 (48.5) | 14 (41.2) | 30 (47.6) |
|
|
| Sex |
|
|
|
|
| 0.271 |
|
Male | 23 (48.9) | 63 (63.6) | 22 (64.7) | 34 (54.0) |
|
|
|
Female | 24 (51.1) | 36 (36.4) | 12 (35.3) | 29 (46.0) |
|
|
| Primary tumor
site |
|
|
|
|
| 0.297 |
| Left
colon | 11 (23.4) | 37 (37.4) | 14 (41.2) | 28 (44.4) |
|
|
| Right
colon | 26 (55.3) | 37 (37.4) | 13 (38.2) | 23 (36.5) |
|
|
|
Rectum | 10 (21.3) | 25 (25.3) | 7
(20.6) | 12 (19.0) |
|
|
| Stagea |
|
|
|
| 0.389 | <0.001 |
| I | 13 (27.7) | 33 (33.3) | 1
(2.9) | 1
(1.6) |
|
|
| II | 29 (61.7) | 47 (47.5) | 20 (58.8) | 39 (61.9) |
|
|
|
III | 4
(8.5) | 17 (17.2) | 8
(23.5) | 17 (27.0) |
|
|
| IV | 1
(2.1) | 2
(2.0) | 5
(14.7) | 6
(9.5) |
|
|
| Tumor stage |
|
|
|
| 0.396 | <0.001 |
| T1 | 2
(4.3) | 9
(9.1) | 0
(0.0) | 0
(0.0) |
|
|
| T2 | 12 (25.6) | 30 (30.3) | 1
(2.9) | 2
(3.2) |
|
|
| T3 | 27 (57.4) | 51 (51.5) | 24 (70.6) | 43 (68.3) |
|
|
|
T4a | 5
(10.6) | 8
(8.1) | 6
(17.6) | 12 (19.0) |
|
|
|
T4b | 1
(2.1) | 1
(1.0) | 3
(8.8) | 6
(9.5) |
|
|
| Node stage |
|
|
|
| 0.227 | 0.004 |
|
Negative | 43 (91.5) | 82 (82.8) | 23 (67.6) | 42 (66.7) |
|
|
|
Positive | 4
(8.5) | 17 (17.2) | 11 (32.4) | 21 (33.3) |
|
|
| Tumor histological
grade |
|
|
|
| 0.279 | 0.015 |
|
Well-differentiated | 0
(0.0) | 2
(2.0) | 0
(0.0) | 0
(0.0) |
|
|
|
Moderately differentiated | 30 (63.8) | 65 (65.7) | 14 (41.2) | 33 (52.4) |
|
|
| Poorly
differentiated | 5
(10.6) | 18 (18.2) | 8
(23.5) | 6
(9.5) |
|
|
|
Mucinous | 12 (25.5) | 14 (14.1) | 12 (35.3) | 24 (38.1) |
|
|
| Vascular
invasion |
|
|
|
|
| 0.166 |
| No | 46 (97.9) | 92 (92.9) | 29 (85.3) | 56 (88.9) |
|
|
|
Yes | 1
(2.1) | 7
(7.1) | 5
(14.7) | 7
(11.1) |
|
|
| Nerve invasion |
|
|
|
| 0.256 | 0.001 |
| No | 45 (95.7) | 94 (94.9) | 28 (82.4) | 48 (76.2) |
|
|
|
Yes | 2
(4.3) | 5
(5.1) | 6
(17.6) | 15 (23.8) |
|
|
| NLR |
|
|
|
| 0.235 | 0.003 |
| Low
(≤5) | 41 (87.2) | 98 (99.0) | 27 (81.8) | 53 (85.5) |
|
|
| High
(>5) | 6
(12.8) | 1
(1.0) | 6 (18.2%) | 9
(14.5) |
|
|
| CRP, mg/l |
|
|
|
| 0.311 | <0.001 |
| Low
(≤5) | 23 (50.0) | 80 (80.8) | 15 (48.4) | 30 (47.6) |
|
|
| High
(>5) | 23 (50.0) | 19 (19.2) | 16 (51.6) | 33 (52.4) |
|
|
| PD-1
expression |
|
|
|
| 0.315 | <0.001 |
|
Low | 7
(18.9) | 33 (43.4) | 9
(36.0) | 34 (66.7) |
|
|
|
High | 30 (81.1) | 43 (56.6) | 16 (64.0) | 17 (33.3) |
|
|
| MMR status |
|
|
|
| 0.268 | <0.001 |
|
pMMR | 11 (23.4) | 50 (50.5) | 21 (61.8) | 39 (61.9) |
|
|
|
dMMR | 36 (76.6) | 49 (49.5) | 13 (38.2) | 24 (38.1) |
|
|
 | Table V.Multivariate multinomial logistic
regression analysis of risk factors for TIL+
PD-L1+ status. |
Table V.
Multivariate multinomial logistic
regression analysis of risk factors for TIL+
PD-L1+ status.
|
| TIL−
PD-L1− | TIL−
PD-L1+ | TIL+
PD-L1- |
|---|
|
|
|
|
|
|---|
| Factor | OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value |
|---|
| Stage (reference,
stage IV) |
| All >0.05 |
| All >0.05 | All P>0.05 | All >0.05 |
| Tumor stage
(reference, T4b) |
| All >0.05 |
| All >0.05 | All P>0.05 | All >0.05 |
| Negative lymph node
metastasis (reference, positive) | 1.889
(0.030–120.105) | 0.764 | 1.072
(0.014–83.342) | 0.975 | 21.414
(0.093–4946.861) | 0.764 |
| Tumor histological
grade (reference, mucinous) |
| All >0.05 |
| All >0.05 |
| All >0.05 |
| No nerve invasion
(reference, nerve invasion) | 6.533
(0.935–45.646) | 0.058 | 1.058
(0.100–11.239) | 0.963 | 0.417
(0.036–4.826) | 0.484 |
| Low NLR level
(reference, high NLR level) | 0.677
(0.100–4.569) | 0.689 | 0.588
(0.082–4.214) | 0.598 | N/A |
|
| Low CRP level
(reference, high CRP level) | 0.735
(0.209–2.585) | 0.631 | 1.409
(0.343–5.786) | 0.634 | 2.697
(0.871–8.351) | 0.085 |
| Low PD-1 expression
(reference, high expression) | 10.473
(3.005–36.503) | <0.001 | 2.017
(0.490–8.295) | 0.331 | 3.443
(1.145–10.352) | 0.028 |
| pMMR (reference,
dMMR) | 11.536
(3.223–41.290) | <0.001 | 14.523
(3.358–62.803) | <0.001 | 4.718
(1.607–13.848) | 0.005 |
Discussion
Classification of tumor microenvironments according
to the presence of TILs and PD-L1 expression has been established
in certain cancer types, particularly human melanoma, and applied
to predict the effect of anti-PD-1/L1 and select the optimum
immunotherapy strategies (6,11). However, to the best of our knowledge,
the occurrence of the four types of immune microenvironments in CRC
and their association with MMR status remains to be investigated.
The current study performed this investigation and revealed a
difference in TIL/PD-L1 status between pMMR and dMMR groups, with
dMMR patients more likely to exhibit a TIL+
PD-L1+ tumor microenvironment.
Compared with the pMMR group, the dMMR group more
frequently exhibited a TIL+ PD-L1+ phenotype.
Previous studies have also demonstrated that tumors from dMMR
patients contain a greater TIL density and higher PD-L1 expression
compared with tumors from pMMR patients (16–19). The
extent of DNA mutation directly or indirectly correlates with the
strength of immunogenicity in tumors (20); therefore, the higher density of TILs
in dMMR tumors may be due to the accumulation of frameshift
mutations and the production of neo-antigens, which activates the
immune system of the host (21,22).
TILs upregulate PD-L1 expression through the release
of interferon-γ, which mediates an adaptive immune-resistance
mechanism by inhibiting local effector T cell activity (6,23–25). This suggests that PD-L1 expression is
an adaptive approach for tumor escape from cytokine-mediated T-cell
killing. In accordance with previous studies, the current study
revealed that PD-L1+ expression was significantly higher
in the TIL+ dMMR group compared with that in the
TIL+ pMMR group, which indicated that TILs may induce
the expression of PD-L1 in the dMMR group. In addition, a negative
association was identified between TILs and PD-L1 expression in the
pMMR group, implying a complexity in the immune tolerance mechanism
in this group that cannot be explained by the current study.
Previous studies have divided the tumor immune
microenvironments into 4 groups namely
TIL+PD-L1+, TIL+
PD-L1−, TIL− PD-L1+ and
TIL− PD-L1−, according to PD-L1 expression
and the presence or absence of TILs. The association of TILs with
PD-L1 expression is considered to be more valuable for predicting
the response to anti-PD-1/L1 therapy compared with TILs or PD-L1
expression alone (26). In
TIL+ PD-L1+ tumors, a sufficient number of T
cells exist inside the tumor that can induce adaptive expression of
PD-L1, which in turn suppresses the function of T cells and may
support an effective response to PD-1/L1 blockade therapy (6,7).
TIL+ PD-L1− tumors account for ~20% of
melanoma cases and contain TILs, but no PD-L1 expression, which
indicates a lack of adaptive resistance and suggests that PD-1/L1
blockade is ineffective in this tumor type (11). No definite immunotherapy approaches
have been applied in the clinic for patients with these tumors;
therefore, novel immunosuppressive strategies should be developed
in the future (11). In
TIL− PD-L1+ tumors, PD-L1 is expressed by
tumor cells through oncogenic signaling instead of in response to
TILs. It is unlikely that blocking PD-1 or PD-L1 alone is effective
in this group due to a lack of T cell involvement (11). Radiotherapy or chemotherapy that
induces cell death and the release of neo-antigens to induce a
T-cell-mediated antitumor response has been used in combination
with anti-PD-1 agents (8,27,28).
Similar to that in TIL− PD-L1+ tumors, single
checkpoint blockade agents appear to be ineffective in
TIL− PD-L1− tumors due to the lack of T-cell
infiltrates. Combination therapy strategies that aim to attract T
cells into tumors and prevent inhibition of T cells should be
considered. CTLA-4, an inducer of numerous T-cell responses, in
combination with anti-PD-1, has been confirmed to effective in a
clinical trial, regardless of PD-L1 expression (9,10). Based
on the recognition that TIL+ PD-L1+ tumors
exhibit the best response to PD-1/L1 blockade therapy, the current
study assumed that dMMR patients contain a higher proportion of
TIL+ PD-L1+ tumors compared with pMMR
patients. The results confirmed this hypothesis and demonstrated
that dMMR status is an independent risk factor for TIL+
PD-L1+ status. This could provide immunological evidence
for an improved response to anti-PD-1/L1 therapy in dMMR patients
compared with that in pMMR patients.
Immune checkpoint blockade is predominantly applied
for patients with stage IV CRC (2,3),
therefore, the current study attempted to investigate dMMR patients
with an advanced stage of CRC. However, dMMR patients account for
only a small percentage of patients with stage IV disease and
obtaining pathological specimens from these patients was limited
due to the loss of surgical opportunities. Although some
immunological evidence could be provided, the current study would
be more valuable if a higher number of stage IV patients were
included. The current study aimed to collect samples from multiple
centers to solve this problem, however, this was difficult due to
objective factors. This is a notable limitation of the present
study, therefore, further investigations are required in the
future.
In conclusion, the current study indicated that dMMR
patients are more likely to express TILs and PD-L1, and present
with a TIL+ PD-L1+ status compared with pMMR
patients. Therefore, the tumor type identified by this
classification method can partially explain the improved response
of dMMR patients to PD-1/L1 blockade. However, the response of each
tumor type to PD-1/L1 blockade requires further investigation.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural
Science Foundation of Guangdong Province (grant no.
2017A030310337), the Natural Science Foundation of Guangdong
Province (grant no. 2015A030313010), the Science and Technology
Program of Guangzhou, China (grant no. 1563000305) and the National
Natural Science Foundation of China (grant nos. 81272641 and
81572409).
Availability of data and materials
All data generated and analyzed during this study
are included in this manuscript.
Authors' contributions
SL, PK, CJ, QQ, QX and LX designed and performed the
study. XW, LY, WH and JH collected and analyzed the data. SL, BZ
and XX analyzed the results and wrote the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The study was performed according to the principles
of the Declaration of Helsinki and was approved by the Research
Ethics Committee of Sun Yat-sen University (Guangzhou, China).
Written informed consent was obtained from each patient.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hodi FS, O'Day SJ, McDermott DF, Weber RW,
Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel
JC, et al: Improved survival with ipilimumab in patients with
metastatic melanoma. N Engl J Med. 363:711–723. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Le DT, Uram JN, Wang H, Bartlett BR,
Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, et
al: PD-1 blockade in tumors with mismatch-repair deficiency. N Engl
J Med. 372:2509–2520. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Topalian SL, Hodi FS, Brahmer JR,
Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD,
Sosman JA, Atkins MB, et al: Safety, activity, and immune
correlates of anti-PD-1 antibody in cancer. N Engl J Med.
366:2443–2454. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Herbst RS, Soria JC, Kowanetz M, Fine GD,
Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger
SN, et al: Predictive correlates of response to the anti-PD-L1
antibody MPDL3280A in cancer patients. Nature. 515:563–567. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lin WF, Lu JY, Cheng BB and Ling CQ:
Progress in research on the effects of traditional Chinese medicine
on the tumor microenvironment. J Integr Med. 15:282–287. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Taube JM, Anders RA, Young GD, Xu H,
Sharma R, McMiller TL, Chen S, Klein AP, Pardoll DM, Topalian SL
and Chen L: Colocalization of inflammatory response with B7-h1
expression in human melanocytic lesions supports an adaptive
resistance mechanism of immune escape. Sci Transl Med.
4:127ra372012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Taube JM, Klein A, Brahmer JR, Xu H, Pan
X, Kim JH, Chen L, Pardoll DM, Topalian SL and Anders RA:
Association of PD-1, PD-1 ligands, and other features of the tumor
immune microenvironment with response to anti-PD-1 therapy. Clin
Cancer Res. 20:5064–5074. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kalbasi A, June CH, Haas N and Vapiwala N:
Radiation and immunotherapy: A synergistic combination. J Clin
Invest. 123:2756–2763. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huang RR, Jalil J, Economou JS,
Chmielowski B, Koya RC, Mok S, Sazegar H, Seja E, Villanueva A,
Gomez-Navarro J, et al: CTLA4 blockade induces frequent tumor
infiltration by activated lymphocytes regardless of clinical
responses in humans. Clin Cancer Res. 17:4101–4109. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wolchok JD, Kluger H, Callahan MK, Postow
MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K,
et al: Nivolumab plus ipilimumab in advanced melanoma. N Engl J
Med. 369:122–133. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Teng MW, Ngiow SF, Ribas A and Smyth MJ:
Classifying cancers based on t-cell infiltration and PD-L1. Cancer
Res. 75:2139–2145. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Klintrup K, Mäkinen JM, Kauppila S, Väre
PO, Melkko J, Tuominen H, Tuppurainen K, Mäkelä J, Karttunen TJ and
Mäkinen MJ: Inflammation and prognosis in colorectal cancer. Eur J
Cancer. 41:2645–2654. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shen SJ, Zhang YH, Gu XX, Jiang SJ and Xu
LJ: Yangfei Kongliu Formula, a compound Chinese herbal medicine,
combined with cisplatin, inhibits growth of lung cancer cells
through transforming growth factor-β1 signaling pathway. J Integr
Med. 15:242–251. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Droeser RA, Hirt C, Viehl CT, Frey DM,
Nebiker C, Huber X, Zlobec I, Eppenberger-Castori S, Tzankov A,
Rosso R, et al: Clinical impact of programmed cell death ligand 1
expression in colorectal cancer. Eur J Cancer. 49:2233–2242. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang Y, Sun Z, Mao X, Wu H, Luo F, Wu X,
Zhou L, Qin J, Zhao L and Bai C: Impact of mismatch-repair
deficiency on the colorectal cancer immune microenvironment.
Oncotarget. 8:85526–85536. 2017.PubMed/NCBI
|
|
16
|
Park JH, Powell AG, Roxburgh CS, Horgan
PG, McMillan DC and Edwards J: Mismatch repair status in patients
with primary operable colorectal cancer: Associations with the
local and systemic tumour environment. Br J Cancer. 114:562–570.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Smyrk TC, Watson P, Kaul K and Lynch HT:
Tumor-infiltrating lymphocytes are a marker for microsatellite
instability in colorectal carcinoma. Cancer. 91:2417–2422. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Inaguma S, Lasota J, Felisiak-Golabek A,
Kowalik A, Wang Z, Zieba S, Kalisz J, Ikeda H and Miettinen M:
Histopathological and genotypic characterization of metastatic
colorectal carcinoma with PD-L1 (CD274)-expression: Possible roles
of tumour micro environmental factors for CD274 expression. J
Pathol Clin Res. 3:268–278. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rosenbaum MW, Bledsoe JR, Morales-Oyarvide
V, Huynh TG and Mino-Kenudson M: PD-L1 expression in colorectal
cancer is associated with microsatellite instability, BRAF
mutation, medullary morphology and cytotoxic tumor-infiltrating
lymphocytes. Mod Pathol. 29:1104–1112. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen DS, Irving BA and Hodi FS: Molecular
pathways: Next-generation immunotherapy-inhibiting programmed
death-ligand 1 and programmed death-1. Clin Cancer Res.
18:6580–6587. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Woerner SM, Gebert J, Yuan YP, Sutter C,
Ridder R, Bork P and von Knebel Doeberitz M: Systematic
identification of genes with coding microsatellites mutated in DNA
mismatch repair-deficient cancer cells. Int J Cancer. 93:12–19.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Angelova M, Charoentong P, Hackl H,
Fischer ML, Snajder R, Krogsdam AM, Waldner MJ, Bindea G, Mlecnik
B, Galon J and Trajanoski Z: Characterization of the
immunophenotypes and antigenomes of colorectal cancers reveals
distinct tumor escape mechanisms and novel targets for
immunotherapy. Genome Biol. 16:642015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dong H, Zhu G, Tamada K and Chen L: B7-H1,
a third member of the B7 family, co-stimulates T-cell proliferation
and interleukin-10 secretion. Nat Med. 5:1365–1369. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Freeman GJ, Long AJ, Iwai Y, Bourque K,
Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne
MC, et al: Engagement of the PD-1 immunoinhibitory receptor by a
novel B7 family member leads to negative regulation of lymphocyte
activation. J Exp Med. 192:1027–1034. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tseng SY, Otsuji M, Gorski K, Huang X,
Slansky JE, Pai SI, Shalabi A, Shin T, Pardoll DM and Tsuchiya H:
B7-DC, a new dendritic cell molecule with potent costimulatory
properties for T cells. J Exp Med. 193:839–846. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sznol M and Chen L: Antagonist antibodies
to PD-1 and B7-H1 (PD-L1) in the treatment of advanced human
cancer. Clin Cancer Res. 19:1021–1034. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Aboudaram A, Modesto A, Chaltiel L,
Gomez-Roca C, Boulinguez S, Sibaud V, Delord JP, Chira C, Delannes
M, Moyal E and Meyer N: Concurrent radiotherapy for patients with
metastatic melanoma and receiving anti-programmed-death 1 therapy:
A safe and effective combination. Melanoma Res. 27:485–491. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Karaca B, Yayla G, Erdem M and Gürler T:
Electrochemotherapy with anti-PD-1 treatment induced durable
complete response in heavily pretreated metastatic melanoma
patient. Anticancer Drugs. Dec 21–2017.(Epub ahead of print). doi:
10.1097/CAD.0000000000000580. View Article : Google Scholar
|