Introduction
Ovarian cancer has the highest mortality rate in
gynecological malignancies at present (1). Due to the lack of typical clinical
symptoms and occult onset at early stage, most patients with
ovarian cancer have reached advanced stage by the time the
diagnosis is confirmed, and even some have already metastasized
(2). Clinically, ovarian cancer is
mainly treated with cytoreductive surgery and adjuvant
chemotherapy. However, for patients at advanced stage and with
metastasis, even surgical treatment is difficult to stabilize the
condition, and it easily relapse and metastasize (3,4). It shows
that improving the early diagnosis rate of ovarian cancer, as early
as possible to find the invasion and metastasis of cancer cells and
to take measures have important significance. Although the
mechanism of the disease is not yet fully understood, the research
regarding this respect has been the focus of medical workers around
the world (5).
Considerable research shows that the abnormal
expression of cytokines plays a key role in the occurrence and
development of ovarian cancer, which may be due to the expression
imbalance of varieties of cytokines (6–8). Cytokines
promote or inhibit the tumor cell differentiation through different
secretory ways, thereby affect the angiogenesis and nutrient supply
of the tumor, and regulate the immune response of tumor cells to
affect their clinical treatment (9).
Ovarian tumors are associated with many cytokines such as
interleukin-8 (IL-8) and IL-10, and their abnormal expression
affects the overall process of occurrence, development and
metastasis of ovarian cancer. IL-8 is a cytokine which can
accelerate cancer cells metastasis. IL-10 is a cytokine that can
reduce and change the immune activity of the body (10). It has been reported that the
expressions of IL-8 and IL-10 is enhanced in ovarian cancer
patients, and the abnormal expression of these two cytokines is
accompanied throughout the course of the disease (11).
The purpose of this investigation was to analyze the
relationship between the expressions of IL-8 and IL-10 and patients
with ovarian cancer undergoing chemotherapy, and to provide methods
and references for early diagnosis, disease evaluation and
treatment of ovarian cancer, which has certain clinical
significance.
Materials and methods
General information
The clinical data of 156 patients with
pathologically confirmed ovarian tumors who were treated at the
Yidu Central Hospital of Weifang (Weifang, China) from January 2012
to December 2014 was retrospectively analyzed. The average age was
50.84±13.82 years, including 92 cases in malignant group and 64
cases in benign group, with 58 healthy subjects as control group,
and the average age was 51.05±12.74 years. No significant
differences were found between the two groups (P>0.05). None of
the subjects were treated with endocrine therapy, biological
therapy, chemical therapy and radiation therapy before operation,
including 20 cases at stage I and II, and 72 cases at stage III and
IV. Patients with ovarian epithelial tumor all received
platinum-based chemotherapy after admission and were followed up
for 36 months. None of the subjects had taken immunomodulators or
hormone drugs in the past six months.
The study was approved by the Ethics Committee of
Yidu Central Hospital of Weifang. Signed informed consents were
obtained from the patients or the guardians. General information of
the patients is shown in Table I.
 | Table I.General information. |
Table I.
General information.
|
| n (%) |
|---|
|
|
|
|---|
| Factors | Malignant group
(n=92) | Benign group
(n=64) | Control group
(n=58) |
|---|
| Age (years) |
| ≥50 | 48 (52.17) | 34 (53.13) | 30 (51.72) |
|
<50 | 44 (47.83) | 30 (46.87) | 28 (48.28) |
| Tissue types |
| Serous
tumor | 27 (29.35) |
|
|
| Mucinous
tumor | 46 (50.00) |
|
|
|
Endometrioid tumors | 19 (20.65) |
|
|
| Tumor stages |
| I+II | 20 (21.74) |
|
|
|
III+IV | 72 (78.26) |
|
|
| Lymphatic
metastasis |
| Yes | 52 (56.52) |
|
|
| No | 40 (43.48) |
|
|
| Distant
metastasis |
| Yes | 43 (46.74) |
|
|
| No | 49 (53.26) |
|
|
| Tumor size |
| <4
cm | 35 (38.04) |
|
|
| ≥4
cm | 57 (61.96) |
|
|
| Differentiation
degree |
| Middle
and low | 42 (45.65) |
|
|
| High | 50 (54.35) |
|
|
Reagents and instruments
ELISA kit was purchased from Shanghai Xinran
Biotechnology Co., Ltd. (Shanghai, China), and Antus PHOMO
automatic microplate reader was purchased from Bio-Rad
Laboratories, Inc., (Hercules, CA, USA).
Detection of serum IL-8 and IL-10
Peripheral venous blood (3 ml) was taken from study
subjects following fasting in the morning as the first serum
sample. The second serum sample was taken from the patients treated
with chemotherapy on the second morning after the end of second
stage, and was centrifuged at 2,600 × g for 8 min at 22°C. Serum
IL-8, IL-10 and OD values at the wavelength of 450 nm were measured
by ELISA method. The operation was carried out strictly according
to the instructions.
Statistical analysis
The statistical software SPSS 19.0 (IBM Corp.,
Armonk, NY, USA) was used to carry out the analysis. The t-test was
applied in data measurement, and the comparison was made before and
after treatment with the paired t-test. The comparison among groups
was performed with One-way ANOVA analysis, and least significant
difference test, and Kaplan-Meier survival curves and the log-rank
test was used to draw survival curve in survival analysis.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Comparison of serum levels of IL-8 and
IL-10 among the three groups of patients
The expression level of serum IL-8 was 79.68±9.53
ng/l in benign group and 220.54±12.49 ng/l in malignant group, and
both were higher than the 54.31±10.26 ng/l in healthy control
group. The expression level of IL-10 was 7.05±2.37 ng/l in benign
group and 17.35±4.02 ng/l in malignant group, and both were higher
than the 3.81±0.52 ng/l in healthy control group. Serum expression
levels of IL-8 and IL-10 in benign and malignant groups both were
higher than those in healthy controls. The levels of IL-8 and IL-10
in malignant group were statistically significant higher than those
in benign group (P<0.001) (Table
II).
 | Table II.Comparison of IL-8 and IL-10 levels in
serum of patients among the three groups (ng/l). |
Table II.
Comparison of IL-8 and IL-10 levels in
serum of patients among the three groups (ng/l).
| Items | Control group | Benign group | Malignant group | F-value | P-value |
|---|
| Case number | 58 | 64 | 92 |
|
|
| IL-8 | 54.31±10.26 |
79.68±9.53a |
220.54±12.49b | 50.970 | <0.001 |
| IL-10 | 3.81±0.52 |
7.05±2.37a |
17.35±4.02b | 55.570 | <0.001 |
Comparison of serum IL-8 and IL-10
levels of patients with malignant ovarian tumors at clinical
stages
The IL-8 level of ovarian cancer I+II was
181.37±13.54 ng/l, and the level of IL-10 was 13.52±2.16 ng/l. The
IL-8 level of ovarian cancer III+IV was 228.41±6.79 ng/l, and the
level of IL-10 was 20.16±4.84 ng/l. The serum expression levels of
IL-8 and IL-10 at ovarian cancer III+IV stage were higher than
those at I+II stage (P<0.001) (Table
III).
 | Table III.Comparison of IL-8 and IL-10 levels in
serum of malignant ovarian tumor patients at different clinical
stages (ng/l). |
Table III.
Comparison of IL-8 and IL-10 levels in
serum of malignant ovarian tumor patients at different clinical
stages (ng/l).
| Clinical stages | Case number | IL-8 | IL-10 |
|---|
| I+II | 20 | 181.37±13.54 | 13.52±2.16 |
| III+IV | 72 | 228.41±6.79 | 20.16±4.84 |
| t value |
| 21.620 | 8.308 |
| P-value |
| <0.001 | <0.001 |
Changes of serum levels of IL-8 and
IL-10 of malignant ovarian tumor patients before and after
chemotherapy
Serum levels of IL-8 and IL-10 in malignant ovarian
tumor patients were respectively 220.54±12.49 and 17.35±4.02 ng/l
before chemotherapy, and were respectively 159.82±8.77 and
11.48±1.74 ng/l after chemotherapy. Serum levels of IL-8 and IL-10
of malignant ovarian tumor patients before chemotherapy both were
statistically significant higher than those after chemotherapy
(P<0.001) (Table IV).
 | Table IV.Changes of IL-8 and IL-10 levels in
serum of malignant ovarian tumor patients before and after
chemotherapy (ng/l). |
Table IV.
Changes of IL-8 and IL-10 levels in
serum of malignant ovarian tumor patients before and after
chemotherapy (ng/l).
| Groups | Case number | IL-8 | IL-10 |
|---|
| Before
chemotherapy | 92 | 220.54±12.49 | 17.35±4.02 |
| After
chemotherapy | 92 | 159.82±8.77 | 11.48±1.74 |
| t value |
| 38.16 | 12.85 |
| P-value |
| <0.001 | <0.001 |
Changes of serum levels of IL-8 and
IL-10 of malignant ovarian tumor patients before and after
chemotherapy
Serum levels of IL-8 and IL-10 were, respectively
184.52±11.47 and 18.24±3.05 ng/l in malignant ovarian tumor
patients with recurrence and metastasis after chemotherapy, and
serum levels of IL-8 and IL-10 were, respectively 151.08±9.38 and
9.46±1.08 ng/l in malignant ovarian tumor patients in stable
condition after chemotherapy. The serum levels of IL-8 and IL-10 in
malignant ovarian tumor patients in stable condition after
chemotherapy both were statistically significant lower than those
in malignant ovarian tumor patients with recurrence and metastasis
after chemotherapy (P<0.001) (Table
V).
 | Table V.Comparison of IL-8 and IL-10 levels in
serum of malignant ovarian tumor patients with stable state or
recurrent and metastasis after chemotherapy (ng/l). |
Table V.
Comparison of IL-8 and IL-10 levels in
serum of malignant ovarian tumor patients with stable state or
recurrent and metastasis after chemotherapy (ng/l).
| Groups | Case number | IL-8 | IL-10 |
|---|
| Recurrence and
metastasis after chemotherapy | 59 | 184.52±11.47 | 18.24±3.05 |
| Stable condition
after chemotherapy | 33 | 151.08±9.38 | 9.46±1.08 |
| t value |
| 11.16 | 14.81 |
| P-value |
| <0.001 | <0.001 |
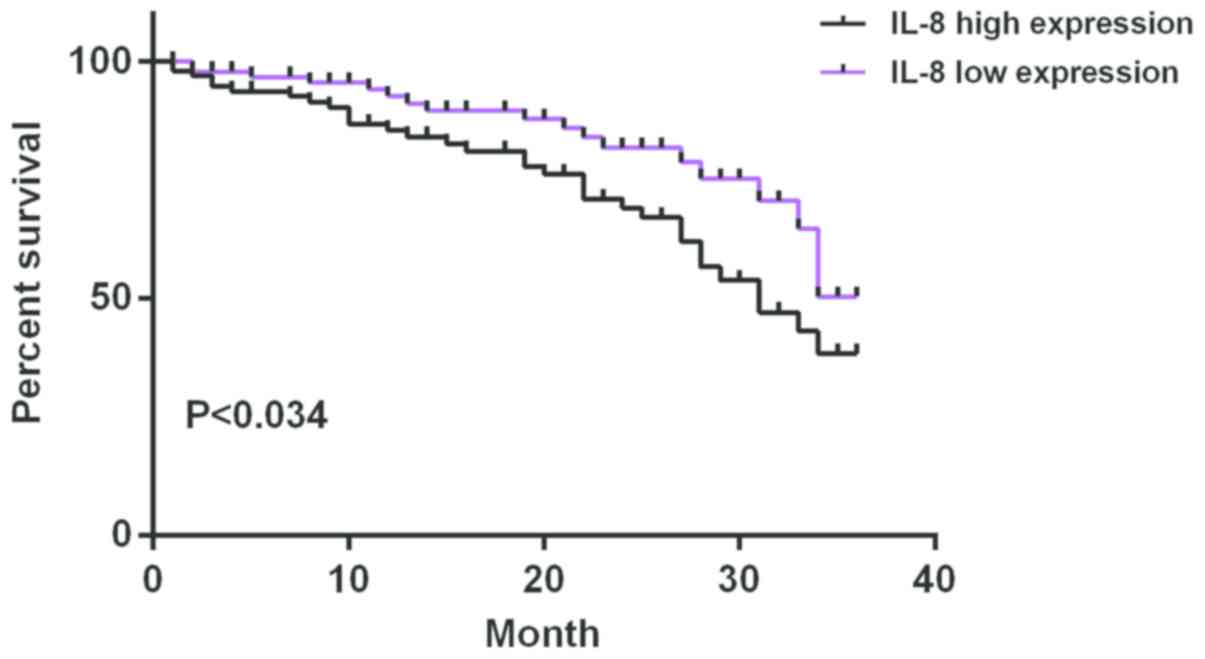
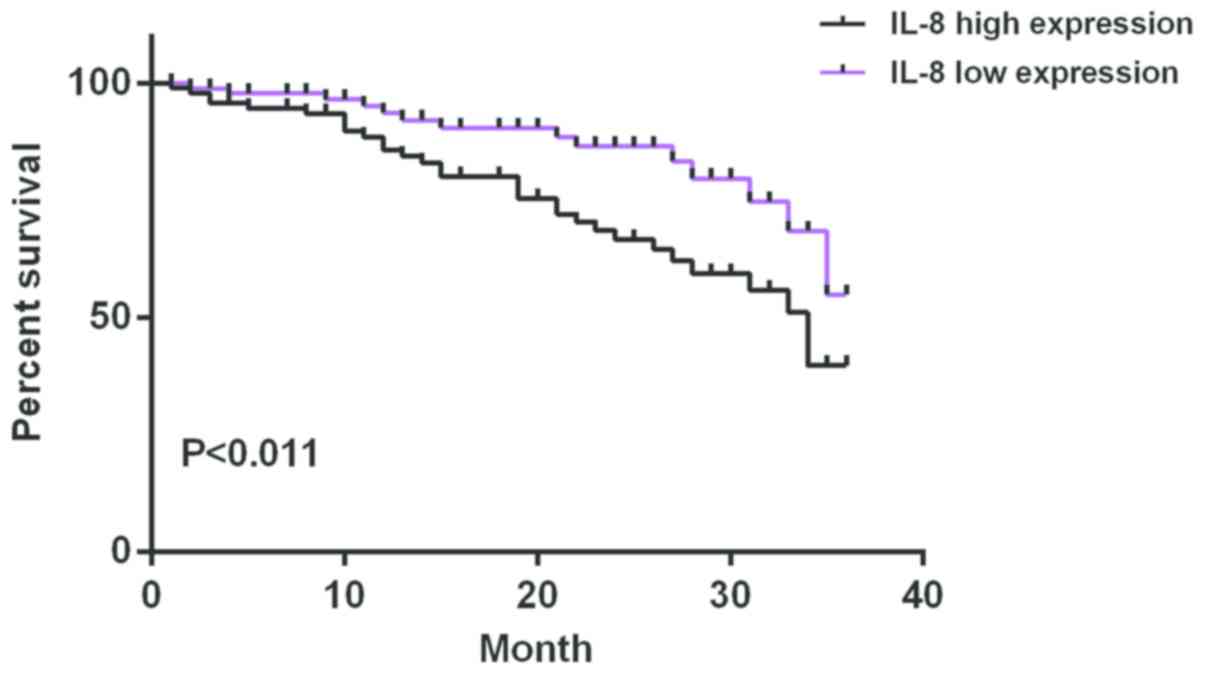
The median of serum expression level of IL-8 of
ovarian cancer patients after chemotherapy was 167.43 ng/l and 46
were in the low expression and the high expression group,
respectively. The median of serum expression level of IL-10 of
ovarian cancer patients after chemotherapy was 13.54 ng/l and 46
were in the low expression group and the high expression group,
respectively. The average survival time of IL-8 low expression
group was 33.12±1.08 months and that of IL-8 high expression group
was 28.82±2.84 months. The average survival time of IL-10 low
expression group was 32.16±1.88 months and that of IL-10 high
expression group was 29.86±2.02 months. Survival analysis showed
that the survival time of patients with high expression of IL-8 and
IL-10 was significantly shorter than that of patients with low
expression of IL-8 and IL-10 (P<0.05) (Figs. 1 and 2).
Discussion
The pathogenesis of ovarian cancer is not yet clear
and there is a lack of appropriate and accurate early diagnosis.
Therefore, the 5-year survival rate of ovarian cancer does not
exceed 30% (12). According to
statistics, >120,000 people worldwide die of this disease every
year (13). The process of cancer
cell growth and diffusion is extremely complex, and the possible
influencing factors are various, but mainly tumor angiogenesis
(14). IL-8 can accelerate tumor
angiogenesis and promote the progress of ovarian cancer. It has
been widely accepted that IL-8 plays an important role in tumor
angiogenesis (15). When a person is
healthy and in good condition, the IL-10 content in the body is
extremely low, but if someone is sick or stimulated by external
environmental factors, such as pregnancy, tumor and infectious
disease the IL-10 content will increase rapidly, and it only
affects the human body. The reason is that there is no homologous
sequence in other organism cells (16).
This study shows that serum expression levels of
IL-8 and IL-10 in benign ovarian disease group and malignant
ovarian tumor group both were statistically significantly higher
than those in healthy control group (P<0.001). The serum
expression levels of IL-8 and IL-10 at ovarian cancer III+IV stage
were statistically significantly higher than those at ovarian
cancer I+II stage (P<0.001). The results of Charbonneau et
al (11) are consistent with
ours. The expression of IL-8 and IL-10 in ovarian cancer was
positively correlated with clinical stages, and the occurrence and
development of ovarian cancer was closely related to IL-8 which is
a special marker (17). The high
expression of IL-10 in tumor microenvironment inactivate the cell
lesion, however, tumor cells cannot be effectively eliminated.
Therefore, the measurement of IL-8 and IL-10 levels has an
important reference value in judging the malignant degree and
staging of ovarian cancer (17–19). Serum
levels of IL-8 and IL-10 in malignant ovarian tumor patients before
chemotherapy both were statistically significant higher than those
after chemotherapy (P<0.001). Serum levels of IL-8 and IL-10 of
malignant ovarian tumor patients in stable condition after
chemotherapy were statistically significantly lower than those in
patients with recurrence and metastasis after chemotherapy
(P<0.001). Our results are similar to those of Matte et
al (20). Studies show that
polyphenols can enhance the sensitivity of ovarian cancer cell
CAOV-3 to cisplatin. The cells produce high levels of IL-8, IL-10,
and have poor sensitivity to cisplatin. Polyphenols can inhibit
CAOV-3 cells to produce IL-8 and IL-10. Therefore, the patients
with metastasis and recurrence after chemotherapy may be due to the
high levels of IL-8 and IL-10 in vivo reducing the
sensitivity of cancer cells to chemotherapy, thus leading to
recurrence and metastasis. The average survival time of IL-8 in low
expression group was 33.12±1.08 months, and that of IL-8 in high
expression group was 28.82±2.84 months. The average survival time
of IL-10 in low expression group was 32.16±1.88 months and that of
IL-10 in high expression group was 29.86±2.02 months. Survival
analysis showed that the survival time of patients with high
expression of IL-8 and IL-10 was significantly shorter than that of
patients with low expression of IL-8 and IL-10 (log-rank,
P<0.05). The results of follow-up were consistent with the
results of Table V. Serum expression
of IL-8 and IL-10 in patients with poor chemotherapy effect and
those in deceased patients were higher, which indicated that the
expressions of IL-8 and IL-10 played a certain role in predicting
and evaluating the survival prognosis of ovarian cancer patients
after chemotherapy.
However, the study has some limitations. Although
the follow-up results of ovarian cancer patients after chemotherapy
are consistent with the results of our study, which shows that the
high expression of IL-8 and IL-10 can shorten the survival time of
patients. Nevertheless, there may be still other factors which need
to be further studied.
In conclusion, serum high expression of IL-8 and
IL-10 is involved in the occurrence and development of ovarian
cancer, and also have an effect on sensitivity to chemotherapy. The
changes of IL-8 and IL-10 levels indirectly reflect the biological
behavior and prognosis of ovarian cancer, which has certain
clinical significance.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
LZ drafted the manuscript. LZ and WL were mainly
devoted to collecting and interpreting the general data. XinboW and
XiaoliW performed ELISA. LZ and HS were responsible for statistical
analysis. All authors read and approved the final study.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Yidu Central Hospital of Weifang (Weifang, China). Signed informed
consents were obtained from the patients or the guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhao BB, Yang ZJ, Wang Q, Pan ZM, Zhang W
and Li L: Clinical validation of multiple biomarkers suspension
array technology for ovarian cancer. Zhonghua Fu Chan Ke Za Zhi.
52:11–19. 2017.(In Chinese). PubMed/NCBI
|
|
2
|
Hennessy BT, Coleman RL and Markman M:
Ovarian cancer. Lancet. 374:1371–1382. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dubosq F, Ploussard G, Soliman H, Turpin
E, Latil A, Desgrandchamps F, de The H and Mongiat-Artus P:
Identification of a three-gene expression signature of early
recurrence in non-muscle-invasive urothelial cell carcinoma of the
bladder. Urol Oncol. 30:833–840. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cho KR and Shih IeM: Ovarian cancer. Annu
Rev Pathol. 4:287–313. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Holschneider CH and Berek JS: Ovarian
cancer: Epidemiology, biology, and prognostic factors. Semin Surg
Oncol. 19:3–10. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gorelik E, Landsittel DP, Marrangoni AM,
Modugno F, Velikokhatnaya L, Winans MT, Bigbee WL, Herberman RB and
Lokshin AE: Multiplexed immunobead-based cytokine profiling for
early detection of ovarian cancer. Cancer Epidemiol Biomarkers
Prev. 14:981–987. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mazzucchelli I, Avanzini MA, Ciardelli L,
Pagani S, Greco R, Belloni C, Castellazzi A, Marconi M, Rondini G
and Polatti F: Human amniotic fluid cells are able to produce IL-6
and IL-8. Am J Reprod Immunol. 51:198–203. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gorelik E, Landsittel DP, Marrangoni AM,
Modugno F, Velikokhatnaya L, Winans MT, Bigbee WL, Herberman RB and
Lokshin AE: Multiplexed immunobead-based cytokine profiling for
early detection of ovarian cancer. Cancer Epidemiol Biomarkers
Prev. 14:981–987. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Duan ZG and Yang WM: Analysis of cytokines
(IL-2, IL-8, IL-10) in the expressed prostatic secretions of
chronic prostatitis. Zhonghua Nan Ke Xue. 11:201–203. 2005.(In
Chinese). PubMed/NCBI
|
|
10
|
Redford PS, Murray PJ and O'Garra A: The
role of IL-10 in immune regulation during M. tuberculosis
infection. Mucosal Immunol. 4:261–270. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Charbonneau B, Goode EL, Kalli KR, Knutson
KL and Derycke MS: The immune system in the pathogenesis of ovarian
cancer. Crit Rev Immunol. 33:137–164. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Block MS, Maurer MJ, Goergen K, Kalli KR,
Erskine CL, Behrens MD, Oberg AL and Knutson KL: Plasma immune
analytes in patients with epithelial ovarian cancer. Cytokine.
73:108–113. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vergote I, Rustin GJ, Eisenhauer EA,
Kristensen GB, Pujade-Lauraine E, Parmar MK, Friedlander M,
Jakobsen A and Vermorken JB: Re: New guidelines to evaluate the
response to treatment in solid tumors [ovarian cancer]. Gynecologic
Cancer Intergroup. J Natl Cancer Inst. 92:1534–1535. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xiong W, Cao LL, Jiang LP, Xia H and Liang
ZQ: Clinical comparative analysis of comprehensive laparoscopic and
laparotomic staging of early-stage epithelial ovarian cancer.
Zhonghua Fu Chan Ke Za Zhi. 52:103–109. 2017.(In Chinese).
PubMed/NCBI
|
|
15
|
Lokshin AE, Winans M, Landsittel D,
Marrangoni AM, Velikokhatnaya L, Modugno F, Nolen BM and Gorelik E:
Circulating IL-8 and anti-IL-8 autoantibody in patients with
ovarian cancer. Gynecol Oncol. 102:244–251. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
O'Keefe GM, Nguyen VT and Benveniste EN:
Class II transactivator and class II MHC gene expression in
microglia: Modulation by the cytokines TGF-beta, IL-4, IL-13 and
IL-10. Eur J Immunol. 29:1275–1285. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lane D, Matte I, Rancourt C and Piché A:
Prognostic significance of IL-6 and IL-8 ascites levels in ovarian
cancer patients. BMC Cancer. 11:210. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang T, Ma Z, Wang R, Wang Y, Wang S,
Cheng Z, Xu H, Jin X, Li W and Wang X: Thrombin facilitates
invasion of ovarian cancer along peritoneum by inducing monocyte
differentiation toward tumor-associated macrophage-like cells.
Cancer Immunol Immunother. 59:1097–1108. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nishio H, Yaguchi T, Sugiyama J, Sumimoto
H, Umezawa K, Iwata T, Susumu N, Fujii T, Kawamura N, Kobayashi A,
et al: Immunosuppression through constitutively activated NF-κB
signalling in human ovarian cancer and its reversal by an NF-κB
inhibitor. Br J Cancer. 110:2965–2974. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Matte I, Lane D, Laplante C, Rancourt C
and Piché A: Profiling of cytokines in human epithelial ovarian
cancer ascites. Am J Cancer Res. 2:566–580. 2012.PubMed/NCBI
|