Introduction
Beclin 1 participates in the regulation of
autophagosome formation and is associated with multiple processes,
including tumor suppression, protection against certain cardiac and
neurological degenerative diseases, and lifespan extension
(1). Beclin 1 possesses B-cell
lymphoma 2 homology 3 (BH3) coiled-coil evolutionarily conserved
domains from the N- to the C-terminus (2). Functionally, Beclin 1 is involved in the
activation of autophagy and inhibition of proliferation by
modulating the formation of Beclin 1-vacuolar protein sorting
(Vps)34-Vps15 core complexes (1–4). When
Beclin 1 binds to anti-apoptotic proteins [e.g., B-cell lymphoma 2
(Bcl-2), B-cell lymphoma extra-large and Bcl-2-like protein 2],
cellular apoptosis may be inhibited and the basal autophagy level
may be maintained (3,4). The chemical modification of Beclin 1,
including phosphorylation or ubiquitination by phosphoinositide
3-kinase (PI3K) III or ubiquitin ligases respectively, disrupts the
interaction via the BH3 domain (5).
Although Beclin 1 induces autophagy-mediated cell death via
caspase-9 (6), caspase-3 and
caspase-8 may cleave Beclin 1 and suppress autophagy in the
mitochondrial pathway of apoptosis (7,8).
Beclin 1 is critical for cancer stem-like cell (CSC)
maintenance and tumor development in nude mice, whereas its
expression limits the development of tumors not enriched with
breast CSCs/progenitor cells (9).
Biallelic loss of Beclin 1 causes the embryonic mortality of mice,
whereas monoallelic loss of Beclin 1 results in an increased tumor
risk of lymphoma, and liver and lung cancer (10,11).
Previously, it has been identified that Beclin 1 expression is
associated with favorable prognosis in stage IIIB colon cancer as
an independent factor (12). Park
et al (13) identified that
Beclin 1 overexpression was independently associated with poorer
overall survival of the patients with colon cancer who received
5-fluorouracil-based adjuvant therapy. Koneri et al
(14) only demonstrated that Beclin 1
overexpression suppressed the cell proliferation and induced
G1 arrest of colon cancer cells, with cyclin E and
phosphorylated retinoblastoma levels decreased. In the present
study, the effects of Beclin 1 overexpression on cell
proliferation, apoptosis, autophagy, invasion, migration and
lamellipodia formation of colon cancer cells was analyzed with
consideration of the expression of phenotype-associated molecules.
Finally, the in vivo effects of Beclin 1 overexpression on
tumor growth were determined in nude mice.
Materials and methods
Cell culture
Colon cancer HCT-15 and HCT-116 cell lines were
obtained from by Professor Miyagi Yohei (Clinical Research
Institute, Kanagawa Cancer Center, Yokohama, Japan). The cell lines
were cultured as monolayers in RPMI-1640 medium (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10%
fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.),
100 U/ml penicillin and 100 µg/ml streptomycin at 37°C in a
humidified atmosphere containing 5% CO2. All cells were
harvested by centrifugation (1,500 × g for 10 min at 4°C) and
rinsed with PBS.
Plasmid construction and
transfection
Plasmid pT-Beclin 1 was constructed by amplification
of Beclin 1 using a DNA amplifier (Thermo Fisher Scientific, Inc.)
and a Takara Polymerase kit (Takara Bio, Inc., Otsu, Japan),
according to the manufacturer's protocols, with the primers
5′-CTGAGGGATGGAAGGGTCTAAG-3′ (sense) and
5′-CCCATTTGTTATAAAATTGTGAGG-3′ (antisense). PCR amplification of
cDNA was performed in 25 µl mixtures containing 0.125 µl Pfu
(Agilent Technologies, Inc., Santa Clara, CA, USA) with 2.0 mM
MgCl2, 2.5 µl 10X PCR buffer (Takara Bio, Inc.), 2 µl
dNTP mixture, 1 µM of each primer set, and 100 ng template cDNA.
PCR conditions were denaturation at 95°C for 10 min, followed by 30
cycles of denaturation at 95°C for 30 sec, annealing at 56°C for 30
sec and extension at 72°C for 50 sec. As a termination step, the
extension time of the last cycle was increased to 7 min. The
amplicons were purified, digested and inserted into His-tagged
pcDNA3.1 (Clontech Laboratories, Inc., Mountainview, CA, USA)
between EcoRI and XhoI restriction sites. The HCT-15
and HCT-116 cells were transfected with pcDNA3.1-Beclin 1 or
pcDNA3.1 vector following seeding on 6 cm-diameter dishes (NEST,
Wuxi, China), and selected by G418 solution with two cell clones
obtained.
Proliferation assay
The Cell Counting Kit-8 (CCK-8; Dojindo Molecular
Technologies, Inc., Kumamoto, Japan) was used to determine the
number of viable cells. Briefly, 2.5×103 cells/well were
seeded on 96-well plates and allowed to adhere. At various time
points (0, 12, 24, 48, 72 and 96 h), 10 µl CCK-8 solution was added
to each well, the plates were incubated at 37°C for 3 h and the
absorbance was determined at 450 nm.
Cell cycle analysis
Cells were digested with 0.25% trypsin, washed twice
with PBS and fixed in 10 ml ice-cold ethanol for >2 h.
Subsequently, cells were washed with PBS and incubated with 100
µg/ml RNase A at 37°C for 1 h. To stain the DNA, propidium iodide
(PI) was added to 50 µg/ml prior to incubation at 4°C in the dark
for 30 min. Finally, flow cytometry was performed to determine the
strength of the PI signal using DxFLEX (Beckman Coulter, Inc.,
Brea, CA, USA) and CytExpert software (Beckman Coulter, Inc.).
Apoptosis assay
Flow cytometry was performed with PI and fluorescein
isothiocyanate (FITC)-labeled Annexin V (Nanjing KeyGen Biotech
Co., Ltd., Nanjing, China) to detect phosphatidylserine
externalization as an endpoint indicator of early apoptosis. A
total of 1×106 cells was collected and washed with PBS
twice. FITC-labeled Annexin V (5 µl) and PI (5 µl) were added to
490 µl 1×105 cell suspension. Following incubation, the
cells were analyzed by flow cytometry using DxFLEX and CytExpert
software.
Wound healing assay
Cells were seeded at a density of 1.0×106
cells/well in 6-well culture plates. Once confluence was reached,
the cell monolayer was scraped with a pipette tip to create a
scratch, washed with PBS three times and cultured in the FBS-free
RPMI-1640 medium. Images of cells were captured at 24 and 48 h, and
the scratch distance was determined using ImageJ bundled with Java
1.8.0_172 (National Institutes of Health, Bethesda, MD, USA).
Cell migration and invasion assay
For the invasion assay, Matrigel-coated chambers (BD
Biosciences, Franklin Lakes, NJ, USA) were rehydrated in RPMI-1640
medium for cell seeding. The lower compartment contained 10% FBS as
a chemoattractant. Following culture for 24 h at 37°C, cells on the
membrane were removed with a cotton bud and the membranes were
washed with PBS. Cells migrating through the membrane were fixed in
100% methanol, stained with Giemsa dye at 37°C for 30 min and
quantified under light microscopy at a magnification of ×200. For
the migration assay, the procedures were the same except for
excluding the control-membrane insert Transwell chamber (BD
Biosciences).
Alkaline phosphatase (ALP)
activity
ALP activity was employed as a marker of colon cell
differentiation. The cells were harvested, broken up and subjected
to ALP reagent (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at
37°C for 2 h. The protein content of the samples was determined
using Bio-Rad protein assay kit (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA), according to the manufacturer's protocol.
Transmission electron microscopy
(TEM)
Specimens were immersed in 2% cacodylate-buffered
glutaraldehyde. Cells were then rinsed in cacodylate buffer
supplemented with 15% sucrose, post-fixed with 1%
phosphate-buffered OsO4 (pH 7.4), dehydrated with
alcohol, clarified in propylene oxide and embedded in Epon using
flat molds. Using an ultramicrotome, 1 nm-thick sections were
obtained, stained with uranyl acetate, followed by a saturated
solution of bismuth subnitrate and finally examined under a Hitachi
electron microscope (×1,000,000). In each group, sodium
butyrate-treated cells (10 mM for 24 h) were used as positive
controls.
Immunofluorescence
Cells were seeded on glass coverslips until they had
adhered, were fixed with 4% formaldehyde in PBS for 10 min and
permeabilized with 0.2% Triton X-100 in PBS for 10 min at room
temperature. Following washing with PBS, cells were blocked with 1%
bovine serum albumin for 30 min and subsequently incubated
overnight at 4°C with goat anti-Beclin 1 (Ab51031; Sigma-Aldrich;
Merck KGaA; 1:500) or anti-light chain 3B (LC-3B; wl01506; Cell
Signaling Technology, Inc., Danvers, MA, USA; 1:500). Following
washing with PBS, the slides were incubated with FITC-conjugated
anti-goat immunoglobulin G (IgG; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA; sc-2356; 1:1,000) antibody at room temperature for
1 h. To examine lamellipodia formation, the slides were directly
incubated overnight at 4°C with Alexa Fluor® 568
phalloidin (Invitrogen; Thermo Fisher Scientific, Inc.) following
washing with PBS 3 times. The cell nuclei were stained with 1 µg/ml
DAPI (Sigma-Aldrich; Merck KGaA) for 30 min at 37°C. Finally,
coverslips were mounted with SlowFade® Gold Antifade
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and observed
using a laser-scanning confocal microscope (Olympus Corporation,
Tokyo, Japan) at ×200 magnification.
Xenograft models
BALB/c nude 8-week male mice (25±1.3 g; n=20) were
maintained under specific pathogen-free conditions with food and
water available ad libitum. Mice were housed in plastic
cages with paper chips for bedding (3 mice/case) in a
temperature-controlled room (22–26°C) with a 12 h light/dark
illumination cycle. Housing and all procedures involving animals
were in compliance with the guidelines of the Committee for Animal
Experiments of China Medical University (Shenyang, China), who
approved the study. In total, 20 mice were arranged into the
control and Beclin 1-overexpressing groups, respectively.
Subcutaneous xenografts were established by bilateral injection of
1×106 cells/mouse. For each tumor, measurements were
made using calipers, and the tumor volume calculated as follows:
Length × width2 × 0.52. At day 8 post-injection, mice
were sacrificed, and part of the tumor was removed, fixed in 10%
formalin at room temperature for 2 days, embedded in paraffin at
65°C for 1 h and cut into 4-µm-thick sections. The remaining tumors
were frozen in liquid nitrogen and stored at −80°C.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from colon cancer cell lines
using a RNeasy mini kit (Qiagen, Inc., Valencia, CA, USA) and
reverse-transcribed into cDNA at 42°C for 1 h using avian
myeloblastosis virus reverse transcriptase (Takara Biotechnology
Co., Ltd., Tokyo, Japan). Oligonucleotide primers for PCR are
presented in Table I. qPCR
amplification of cDNA was performed using a SYBR® Premix
Ex Taq™ II kit (Takara Biotechnology Co., Ltd.), respectively.
GAPDH was used as the reference gene. The qPCR conditions were
denaturation at 95°C for 10 min, followed by 45 cycles of
denaturation at 95°C for 30 sec, annealing at 60°C for 30 sec and
extension at 55°C for 34 sec. The gene expression level was
expressed as 2−∆∆Cq, where ∆Cq=Cq (gene)-Cq (GAPDH)
(15). The expression level in
control cells was considered as 1.
 | Table I.Primer sequences for polymerase chain
reaction. |
Table I.
Primer sequences for polymerase chain
reaction.
| Gene | Accession
number | Primer
sequences | Region
amplified | Product size,
bp |
|---|
| Beclin 1 | NM_003766.3 | F:
5′-GATGGAAGGGTCTAAGACGTCCAA-3′ | 162–321 | 160 |
|
|
| R:
5′-TTTCGCCTGGGCTGTGGTAAG-3′ |
|
|
| c-Myc | X00676 | F:
5′-AGCGACTCTGAGGAGGAACA-3′ | 1,318-1,425 | 108 |
|
|
| R:
5′-TCCAGCAGAAGGTGATCCA-3′ |
|
|
| Cyclin D1 | NG_000002 | F:
5′-TGCCACAGATGTGAAGTTCATT-3′ | 776–937 | 162 |
|
|
| R:
5′-CAGTCCGGGTCACACTTGAT-3′ |
|
|
| Bax | DQ926869 | F:
5′-GATTGCCGCCGTGGAC-3′ | 306–393 | 88 |
|
|
| R:
5′-GCCCCAGTTGAAGTTGC-3′ |
|
|
| Survivin | DQ508252 | F:
5′-TTCTCAAGGACCACCGCATC-3′ | 159–320 | 162 |
|
|
| R:
5′-AGCCTTCCAGCTCCTTGAAG-3′ |
|
|
| β-catenin | X87838 | F:
5′-GCTTGGAATGAGACTGCTGA-3′ | 2,221-2,334 | 114 |
|
|
| R:
5′-CTGGCCATATCCACCAGAGT-3′ |
|
|
| IRE1 | AF059198 | F:
5′-ACTGGCTTCTGATAGGAC-3′ | 1,186-1,272 | 87 |
|
|
| R:
5′-GATGTTTGGGTAGATTGTT-3′ |
|
|
| MDR-1 | NM_000927 | F:
5′-ACACCTGGGCATCGT-3′ | 3,826-3,983 | 158 |
|
|
| R:
5′-TATTAGGCAGTGACTCGA-3′ |
|
|
| GRP78 | FJ436356 | F:
5′-GTTCTTGCCGTTCAAGGTGG-3′ | 600–780 | 181 |
|
|
| R:
5′-TGGTACAGTAACAACTGCATG-3′ |
|
|
| p21 | NM_000389.3 | F:
5′-ACTGTCTTGTACCCTTGTGCC-3′ | 464–571 | 108 |
|
|
| R:
5′-AAATCTGTCATGCTGGTCTGC-3′ |
|
|
| GAPDH | NM_ 002046.3 | F:
5′-CAATGACCCCTTCATTGACC-3′ | 201–335 | 135 |
|
|
| R:
5′-GGAAGATGGTGATGGGATT-3′ |
|
|
Western blot (WB) analysis
Protein was extracted from colon cancer cells and
tissue, and determined using a Bio-Rad assay kit. Denatured protein
was separated by SDS/PAGE (10% acrylamide) and transferred onto a
Hybond membrane (GE Healthcare, Chicago, IL, USA), which was
blocked overnight in 5% skimmed milk in Tris-buffered saline with
0.1% Tween-20 (TBST) at room temperature for 1 h. For
immunoblotting, the membrane was incubated with the primary
antibody (Table II) at room
temperature for 1 h. The membrane was rinsed with TBST and
incubated with anti-rabbit (abca2517726), anti-goat (abca2517747)
or anti-mouse (orb21692) IgG antibody conjugated to horseradish
peroxidase (1:1,000, Dako; Agilent Technologies, Inc.) at room
temperature for 1 h. Protein bands were visualized using X film and
Enhanced Chemiluminescence-Plus detection reagents (Santa Cruz
Biotechnology, Inc.). The membranes were washed with WB Stripping
Solution (Nacalai Tesque, Inc., Kyoto, Japan) and treated as
aforementioned.
 | Table II.Antibodies used in western blot
analysis. |
Table II.
Antibodies used in western blot
analysis.
| Target | Source | Cat. no. | Dilution | Supplier |
|---|
| His tag | Rabbit | sc-804 | 1:300 | Santa Cruz
Biotechnology, Inc., Dallas, TX, USA |
| JNK (FL) | Rabbit | sc-571 | 1:500 | Santa Cruz
Biotechnology, Inc. |
| Bax (B-9) | Mouse | sc-7480 | 1:300 | Santa Cruz
Biotechnology, Inc. |
| PI3K p110
(D-4) | Mouse | sc-8010 | 1:300 | Santa Cruz
Biotechnology, Inc. |
| TAK1 (D94D7) | Rabbit | 5206 | 1:1,000 | Cell Signaling
Technology, Inc., Danvers, MA, USA |
| Cdc25B (H-85) | Rabbit | sc-5619 | 1:500 | Santa Cruz
Biotechnology, Inc. |
| Cyclin B1
(GNS1) | Mouse | sc-245 | 1:500 | Santa Cruz
Biotechnology, Inc. |
| CDK4 (C-22) | Rabbit | sc-260 | 1:500 | Santa Cruz
Biotechnology, Inc. |
| c-Myc (9E10) | Mouse | sc-40 | 1:300 | Santa Cruz
Biotechnology, Inc. |
| Bcl-2 (C-21) | Rabbit | sc-783 | 1:500 | Santa Cruz
Biotechnology, Inc. |
| Cyclin E
(HE12) | Mouse | sc-247 | 1:500 | Santa Cruz
Biotechnology, Inc. |
| LC-3B | Rabbit | 2775 | 1:1,000 | Cell Signaling
Technology, Inc. |
| Beclin 1 | Rabbit | SAB2103299 | 1:1,000 | Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany |
| β-actin (C-4) | Mouse | sc-47778 | 1:2,000 | Santa Cruz
Biotechnology, Inc. |
Immunohistochemistry
Consecutive 4-µm-thick sections were deparaffinized
in xylene twice for 10 min, rehydrate in gradient ethanol (100, 90,
80, 70 and 60%) once for 2 min at room temperature and subjected to
antigen retrieval by irradiation in target retrieval solution
(Dako; Agilent Technologies, Inc.) in a microwave oven. The
sections were quenched with 3% H2O2 to block
endogenous peroxidase at room temperature for 15 min. Bovine serum
albumin (5%) was then applied to prevent non-specific binding. The
sections were incubated with rabbit anti-beclin 1 (1:100;
SAB2103299; Sigma-Aldrich; Merck KGaA) or anti-Ki67 (1:300;
Ab15580; Abcam, Cambridge, MA, USA) antibodies, prior to treatment
with the horseradish peroxidase-conjugated anti-rabbit secondary
antibody as aforementioned (1:100). All incubations were performed
in a microwave oven to allow intermittent irradiation. Following
each treatment, the slides were washed with TBST three times.
Binding sites were visualized with 3,3′-diaminobenzidine. Following
counterstaining with Mayer's hematoxylin at room temperature for 2
min, the sections were dehydrated and mounted with coverslips.
Omission of the primary antibody was used as a negative
control.
Terminal deoxynucleotidyl transferase
(TdT) dUTP nick-end labeling (TUNEL)
Cell apoptosis was assessed using TUNEL, a method
that is based on the specific binding of O-TdT to the 3-OH ends of
DNA, ensuring the synthesis of a polydeoxynucleotide polymer. For
this purpose, an ApopTag Plus Peroxidase In Situ Apoptosis
Detection kit (EMD Millipore, Billerica, MA, USA) was employed
according to the manufacturer's protocol. Omission of the
working-strength TdT enzyme was used as the negative control.
Statistical analysis
Results are representative of three independent
experiments and are expressed as the mean ± standard deviation.
Statistical evaluation was performed using Mann-Whitney U test to
differentiate between the means of different groups. P<0.05 was
considered to indicate a statistically significant difference. SPSS
software (version 10.0; SPSS, Inc., Chicago, IL, USA) was employed
to analyze all data.
Results
Effects of Beclin 1 expression on the
phenotypes of colon cancer cell lines and associated molecules
Beclin 1-expressing plasmid was successfully
transfected into HCT-15 and HCT-116 cells, as confirmed using
RT-qPCR (Fig. 1A), immunofluorescence
(Fig. 1B) and western blot analysis
using anti-Beclin 1 or anti-His tag antibody (Fig. 1C). The transfectants exhibited
significantly decreased viability as indicated by CCK-8 staining
(P<0.05; Fig. 1D), significantly
increased differentiation as observed by ALP activity (P<0.05;
Fig. 1E) and markedly increased
autophagy as visualized using TEM (Fig.
1F) and LC-3B immunoreactivity (Fig.
1G). The PI staining revealed that ectopic Beclin 1
overexpression led to significant G2 arrest in HCT-15
cells and G1 arrest in HCT-116 cells (P<0.05;
Fig. 1H). Apoptosis observed by
Annexin V-FITC staining was increased following Beclin 1
overexpression (P<0.05; Fig. 1I)
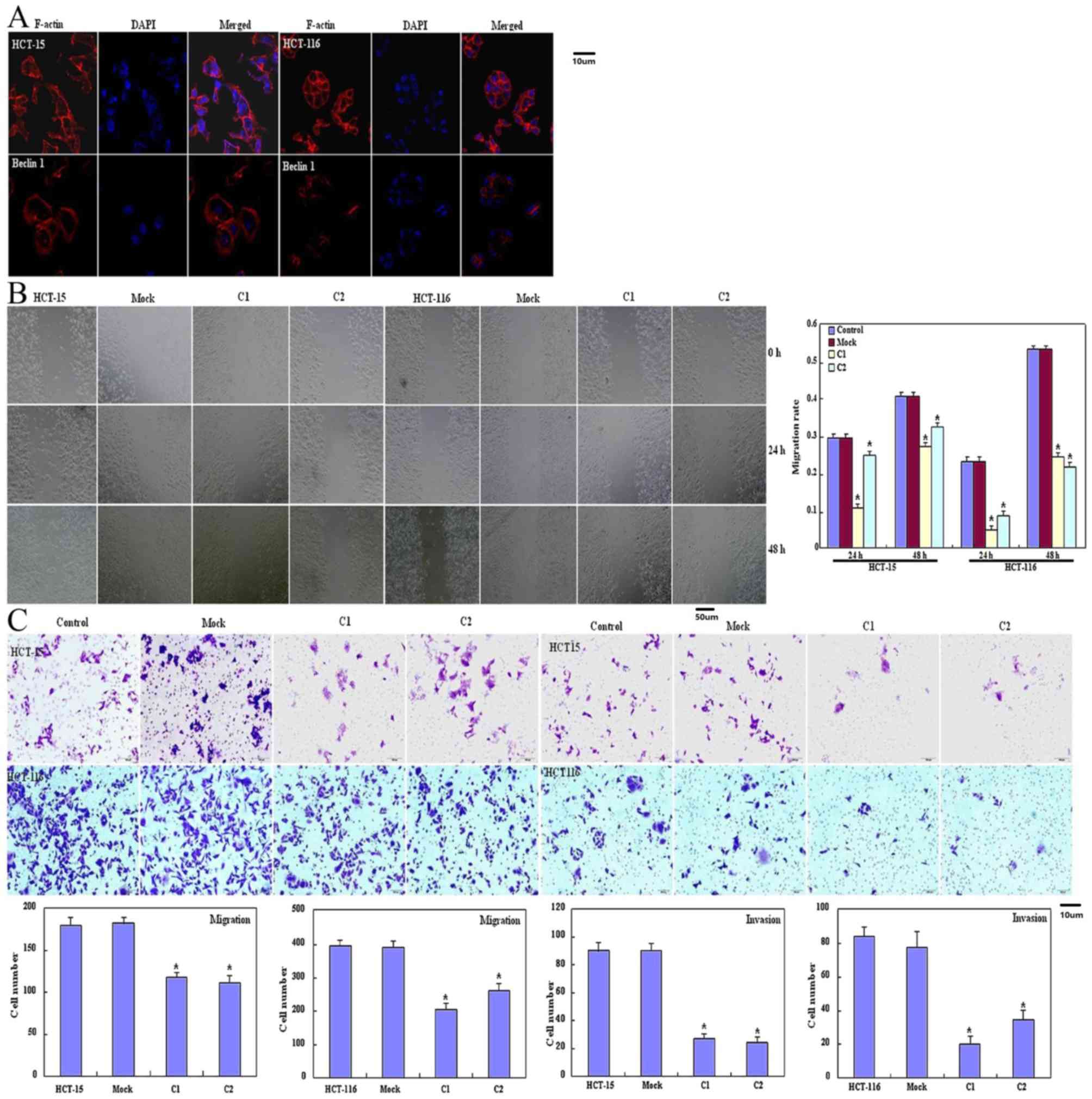
and lamellipodia formation was weaker as revealed using filamentous
actin staining (Fig. 2A) compared
with the control and mock cells. Significantly decreased migration
and invasion were observed using a wound healing assay (P<0.05;
Fig. 2B) or Transwell chamber assay
(P<0.05; Fig. 2C) in HCT-15 and
HCT-116 transfectants compared with the control and mock cells.
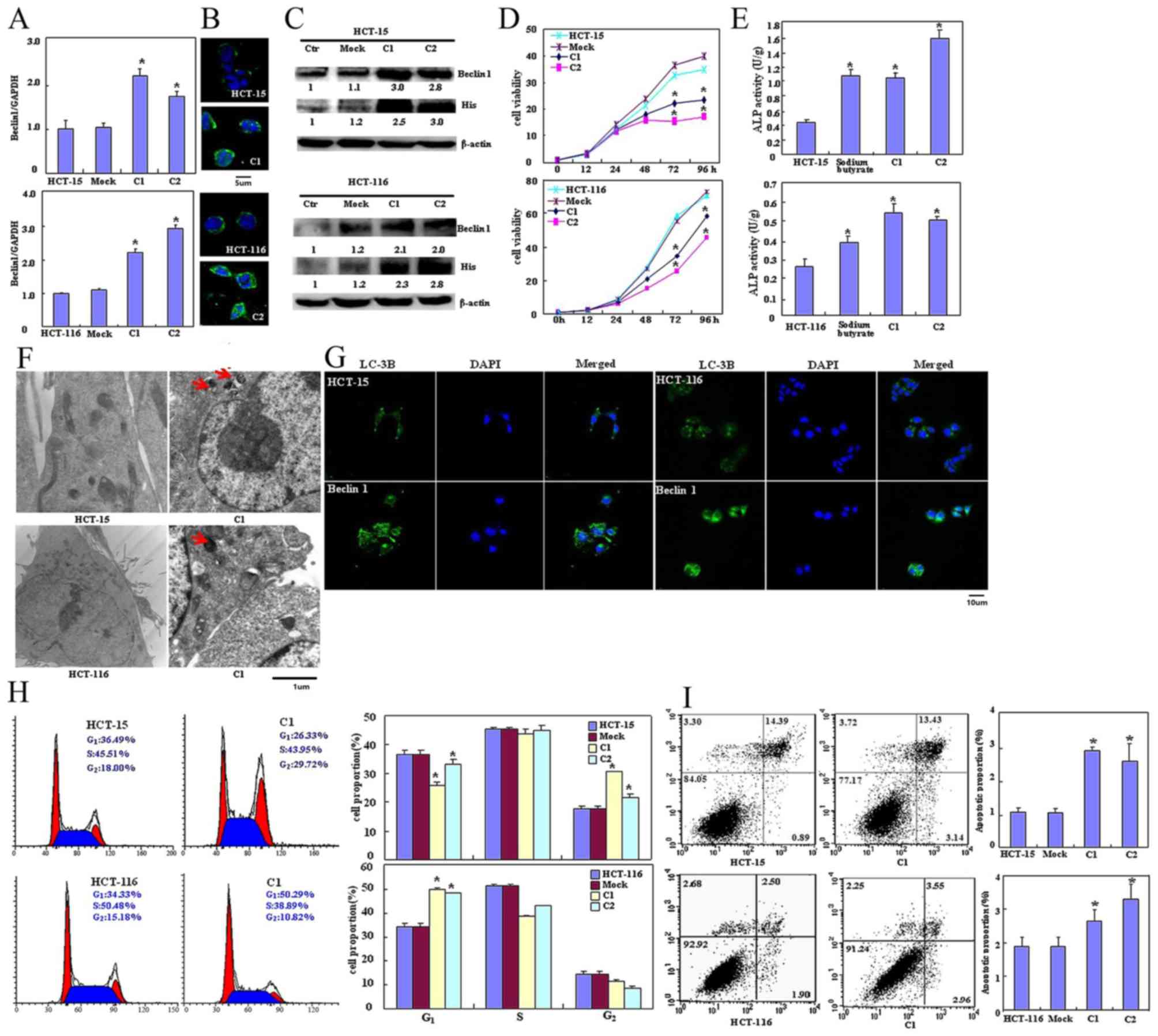 | Figure 1.Effects of Beclin 1 expression on
proliferation, apoptosis, autophagy and differentiation of HCT-15
and HCT-116 cells. Beclin 1 expression in HCT-15 and HCT-116 cells
was determined following transfection with pcDNA3.1-Beclin 1 using
(A) reverse transcription-quantitative polymerase chain reaction,
(B) immunofluorescence and (C) western blotting. (D) The
transfectants exhibited a decrease in viability in comparison with
the control and mock. Beclin 1 overexpression may (E) improve the
differentiation of HCT-15 and HCT-116 cells as identified by ALP
activity and promote autophagy as determined by (F) electron
microscopy (red arrow indicates the autophagosome) and (G) marked
LC-3B staining. (H) Forced Beclin 1 expression induced the G2
arrest of HCT-15 cells, but the G1 arrest of HCT-116 cells. (I)
Apoptosis was increased by Beclin 1 overexpression, as identified
using an Annexin V assay. Results are representative of three
independent experiments and are expressed as the mean ± standard
deviation. *P<0.05 vs. mock and control groups. ALP, alkaline
phosphatase; LC-3B, light chain 3B; Ctr, control; C1, clone 1; C2,
clone 2. |
As presented in Fig.
3A, HCT-15 Beclin 1 transfectants exhibited decreased
expression of survivin and MDR-1 (P<0.05), but increased
expression of c-Myc, cyclin D1, Bcl-2-associated X protein (Bax),
β-catenin, insulin-response element 1 (IRE1) and GRP78 compared
with the control and mock cells by qPCR (P<0.05). As presented
in Fig. 3B, Beclin 1 transfectants of
HCT-116 cells exhibited decreased expression of MDR-1 (P<0.05),
but increased expression of c-Myc, p21, cyclin D1, β-catenin, IRE1
and GRP78 compared with the control and mock cells by qPCR
(P<0.05). At the protein level, Beclin 1 overexpression
decreased the expression of cyclin D1 and E (P<0.05), but
increased the expression of c-Jun N-terminal kinase (JNK), Bax,
PI3K, transforming growth factor β-activated kinase 1 (TAK1),
cyclin-dependent kinase 4 (CDK4), Bcl-2, cyclin B1 and LC-3B in
HCT-15 transfectants (P<0.05, Fig.
3C). Beclin 1 overexpression in HCT-116 transfectants increased
the expression of CDK4 and LC-3B (P<0.05), but decreased the
expression of JNK, Bax, PI3K, TAK1, c-Myc, Bcl-2, cyclin B1 and
cyclin E (P<0.05; Fig. 3D).
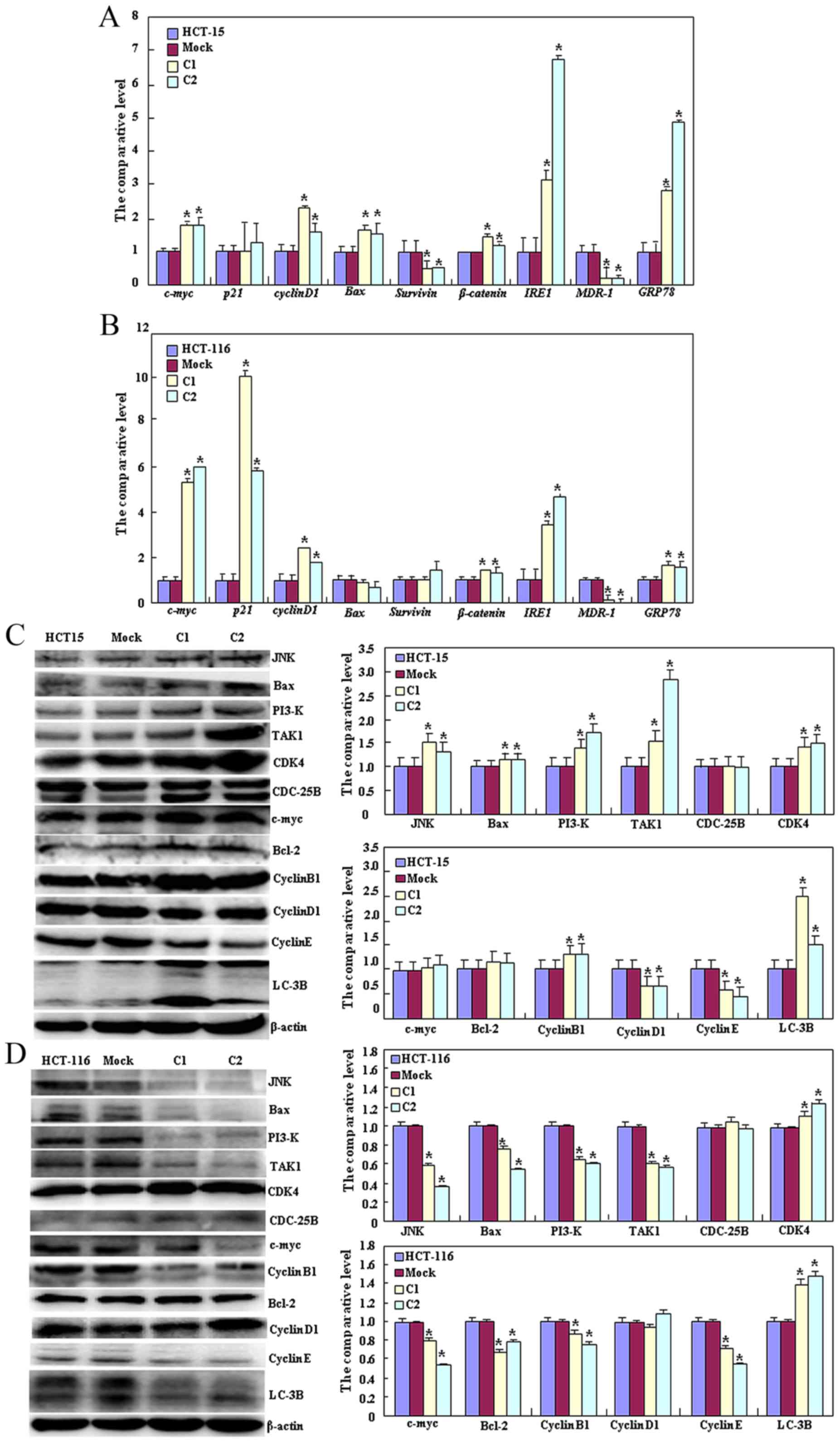 | Figure 3.Effects of Beclin 1 expression on
phenotype-associated molecules of HCT-15 and HCT-116 cells. Reverse
transcription-quantitative polymerase chain reaction analysis of
(A) HCT-15 and (B) HCT-116 cells, and western blot analysis of (C)
HCT-15 and (D) HCT-116 cells were employed to determine the
expression of phenotype-associated molecules in the control, vector
mock and Beclin 1 transfectants. Results are representative of
three independent experiments and are expressed as the mean ±
standard deviation. *P<0.05 vs. the mock and control groups.
JNK, c-Jun N-terminal kinase; Bax, Bcl-2-associated X protein;
TAK1, transforming growth factor β-activated kinase 1; CDK4,
cyclin-dependent kinase 4; Bcl-2, B-cell lymphoma 2; LC-3B, light
chain 3B; C1, clone 1; C2, clone 2. |
Beclin 1 suppresses the viability of
colon cancer cells
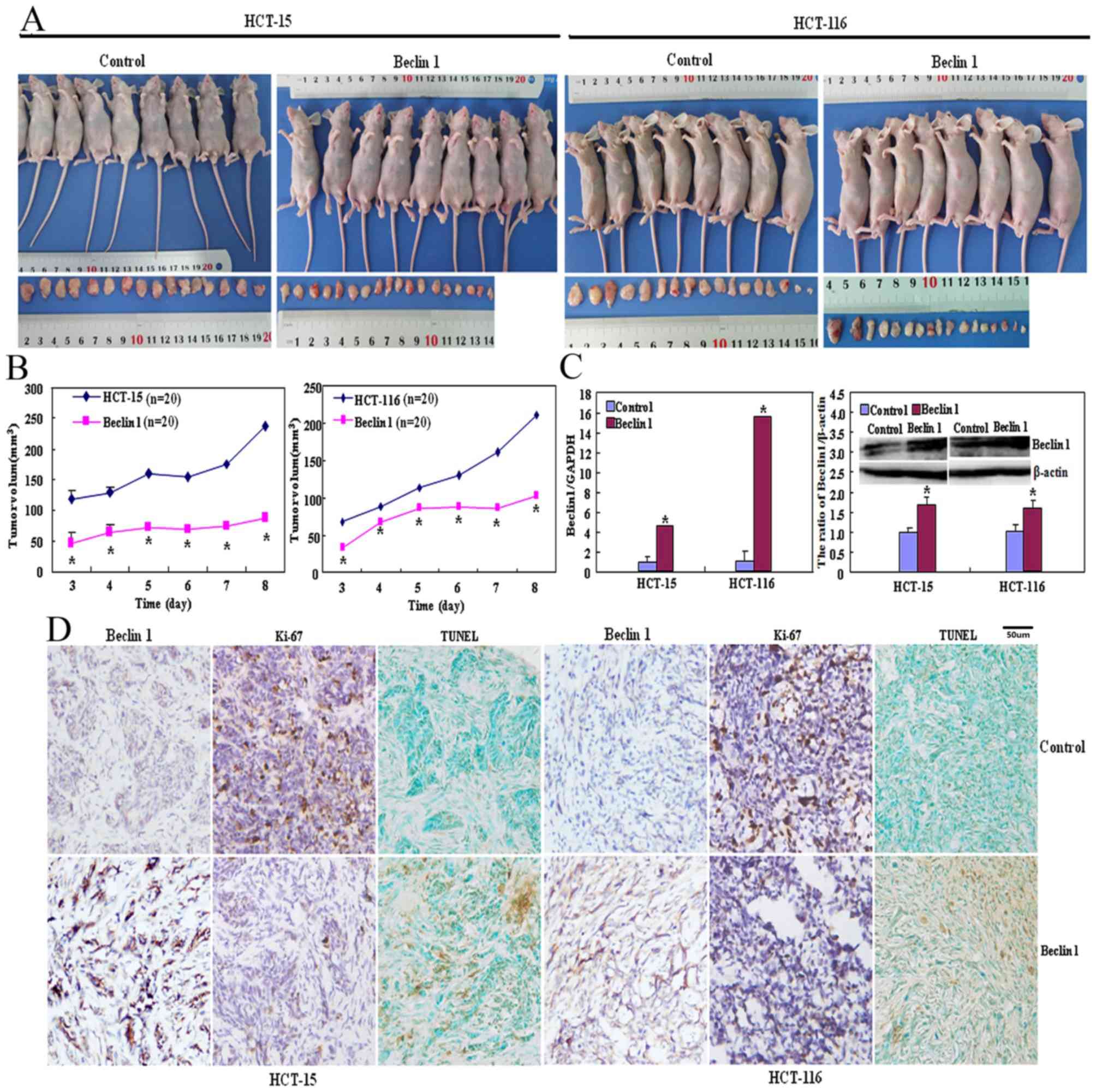
As presented in Fig. 4A
and B, the tumor volumes of HCT-15 and HCT-116 cells xenografts
were increased compared with those of their Beclin 1 transfectants
(P<0.05) although the same number of cancer cells was injected
into mice in the two groups. There was significantly increased
Beclin 1 mRNA and protein expression in transfectant xenograft
tumors of Beclin 1 compared with that of the respective control
cells (Fig. 4C; P<0.05).
Immunohistochemically, Beclin 1 overexpression was observed in
Beclin 1 transfectants in comparison with HCT-15 and HCT-116 cells
(Fig. 4D). HCT-15 and HCT-116 cells
exhibited increased proliferation by the Ki-67 marker compared with
their transfectants (Fig. 4D).
Increased apoptosis in Beclin 1-overexpressing transfectants was
observed compared with the control group using TUNEL (Fig. 4D).
Discussion
In previous studies, Beclin 1 downregulation was
identified to be involved in the carcinogenesis and subsequent
progression, including invasion and metastasis (16–25).
Therefore, we hypothesized that Beclin 1 could be employed as a
molecule target for the treatment of cancer, such as in colon
cancer. In the present study, the cell function assay revealed that
Beclin 1 overexpression may lead to decreased proliferation, cell
cycle arrest, increased autophagy and apoptosis in HCT-15 and
HCT-116 cells, similar to a previous report (14). In the xenograft model, Beclin 1
overexpression suppressed tumor growth of colon cancer cells by
inhibiting proliferation and inducing apoptosis in line with the
in vivo data. Pirtoli et al (21) also reported that overexpression of
Beclin 1 protein was positively associated with apoptosis, and
negatively associated with cell proliferation in high-grade glioma.
Additionally, in vitro Beclin 1 overexpression decreased
cell migration, invasion and lamellipodia formation of colon cancer
cells, indicating that Beclin 1 suppresses invasion and metastasis
of colon cancer by inhibiting cellular migration, invasion and
mobility. Beclin 1 overexpression also increased differentiation of
HCT-15 and HCT-116 cells as revealed by increased ALP activity.
Taken together, these results indicate that ectopic Beclin 1
expression may reverse the aggressive phenotypes and have potential
for gene therapy for colon cancer. The results of the present study
also provide a good explanation for downregulated Beclin 1
expression during carcinogenesis (18–20,22,24)
and support the inverse correlation of Beclin 1 with aggressiveness
and worse prognosis of cancers (16–18,23–25).
It is noteworthy that ectopic Beclin 1 expression
resulted in G2 arrest of HCT-15 transfectants and
G1 arrest of HCT-116 transfectants, which may be due to
differential p21 mRNA expression in the different cell types, as
cell cycle G1 arrest results from increased expression
of p21cip1/waf1 (26).
Cyclin E and D1 activate CDKs, and serve an essential function in
the transition between G1 and S phase (27,28).
Therefore, CDK4 overexpression may account for the G2
arrest in HCT-15 transfectants despite the downregulation of cyclin
D1 and E expression. In the two cell lines, Beclin 1 overexpression
resulted in high levels of MDR-1, IRE1 and GRP78 mRNA, suggesting a
function of Beclin 1 in endoplasmic reticulum (ER) stress and
drug-resistant suppression as MDR-1, GRP78 and IRE1 have been
identified to be involved in protein folding of ER and decreased
drug accumulation (29,30). The effect of Beclin 1 on the
transcription of c-Myc, cyclin D1 and β-catenin requires further
investigation. Bcl-2 interacts with Bax on the mitochondrial
membrane to suppress apoptosis as Bax is hypothesized to open the
mitochondrial voltage-dependent anion channel for apoptosis
(31). Consequently, Bax
overexpression in HCT-15 cells and Bcl-2 hypoexpression in HCT-116
cells may explain the induction of apoptosis by Beclin 1 in the two
cell lines. It has been reported that TAK1 phosphorylates
mitogen-activated protein kinase kinase 4 and 3/6, which activate
JNK (32). The upregulated expression
of TAK1 and JNK in HCT-116 transfectants indicated that Beclin 1
may strengthen this signal pathway.
In summary, Beclin 1 overexpression suppresses
proliferation, migration and invasion, yet induces apoptosis,
autophagy and differentiation of colon cancer cells. Therefore,
Beclin 1 is a potential candidate for the future target gene
therapy for colon cancer.
Acknowledgements
Not applicable.
Funding
The present study was supported by A Project
Supported by Scientific Research Fund of Liaoning Provincial
Education Department (grant nos. LJQ2014093 and L2014333), Liaoning
BaiQianWan Talents Program, Outstanding Scientific Fund of
Shengjing Hospital, Award for Liaoning Distinguished Professors,
Shenyang Science and Technology Grand (grant no. 18-013-0-59) and
the National Natural Scientific Foundation of China (grant nos.
81472544 and 81672700).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MYZ, LYW, SZ, XCG, HL and YQX conducted the
experiments and analyzed the data. HCZ and ZHZ designed the study
and wrote the manuscript.
Ethics approval and consent to
participate
The animal experiments were approved by the
Committee for Animal Experiments of China Medical University
(Shenyang, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Liang XH, Jackson S, Seaman M, Brown K,
Kempkes B, Hibshoosh H and Levine B: Induction of autophagy and
inhibition of tumorigenesis by Beclin 1. Nature. 402:672–676. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liang XH, Yu J, Brown K and Levine B:
Beclin 1 contains a leucine-rich nuclear export signal that is
required for its autophagy and tumor suppressor function. Cancer
Res. 61:3443–3449. 2001.PubMed/NCBI
|
|
3
|
Kang R, Livesey KM, Zeh HJ, Loze MT and
Tang D: HMGB1: A novel Beclin 1-binding protein active in
autophagy. Autophagy. 6:1209–1211. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kang R, Zeh HJ, Lotze MT and Tang D: The
Beclin 1 network regulates autophagy and apoptosis. Cell Death
Differ. 18:571–580. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Abrahamsen H, Stenmark H and Platta HW:
Ubiquitination and phosphorylation of Beclin 1 and its binding
partners: Tuning class III phosphatidylinositol 3-kinase activity
and tumor suppression. FEBS Lett. 586:1584–1591. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang ZH, Xu L, Duan ZL, Zeng LQ, Yan NH
and Peng ZL: Beclin 1-mediated macroautophagy involves regulation
of caspase-9 expression in cervical cancer HeLa cells. Gynecol
Oncol. 107:107–113. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li H, Wang P, Sun Q, Ding WX, Yin XM,
Sobol RW, Stolz DB, Yu J and Zhang L: Following cytochrome c
release, autophagy is inhibited during chemotherapy-induced
apoptosis by caspase 8-mediated cleavage of Beclin 1. Cancer Res.
71:3625–3634. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhu Y, Zhao L, Liu L, Gao P, Tian W, Wang
X, Jin H, Xu H and Chen Q: Beclin 1 cleavage by caspase-3
inactivates autophagy and promotes apoptosis. Protein Cell.
1:468–477. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gong C, Bauvy C, Tonelli G, Yue W,
Deloménie C, Nicolas V, Zhu Y, Domergue V, Marin-Esteban V,
Tharinger H, et al: Beclin 1 and autophagy are required for the
tumorigenicity of breast cancer stem-like/progenitor cells.
Oncogene. 32:2261–2272. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Qu X, Yu J, Bhagat G, Furuya N, Hibshoosh
H, Troxel A, Rosen J, Eskelinen EL, Mizushima N, Ohsumi Y, et al:
Promotion of tumorigenesis by heterozygous disruption of the Beclin
1 autophagy gene. J Clin Invest. 112:1809–1820. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yue Z, Jin S, Yang C, Levine AJ and Heintz
N: Beclin 1, an autophagy gene essential for early embryonic
development, is a haploinsufficient tumor suppressor. Proc Natl
Acad Sci USA. 100:15077–15082. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li BX, Li CY, Peng RQ, Wu XJ, Wang HY, Wan
DS, Zhu XF and Zhang XS: The expression of beclin 1 is associated
with favorable prognosis in stage IIIB colon cancers. Autophagy.
5:303–306. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Park JM, Huang S, Wu TT, Foster NR and
Sinicrope FA: Prognostic impact of Beclin 1, p62/sequestosome 1 and
LC3 protein expression in colon carcinomas from patients receiving
5-fluorouracil as adjuvant chemotherapy. Cancer Biol Ther.
14:100–107. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Koneri K, Goi T, Hirono Y, Katayama K and
Yamaguchi A: Beclin 1 gene inhibits tumor growth in colon cancer
cell lines. Anticancer Res. 27:1453–1457. 2007.PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen Y, Lu Y, Lu C and Zhang L: Beclin-1
expression is a predictor of clinical outcome in patients with
esophageal squamous cell carcinoma and correlated to
hypoxia-inducible factor (HIF)-1alpha expression. Pathol Oncol Res.
15:487–493. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dong LW, Hou YJ, Tan YX, Tang L, Pan YF,
Wang M and Wang HY: Prognostic significance of Beclin 1 in
intrahepatic cholangiocellular carcinoma. Autophagy. 7:1222–1229.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Duan ZL, Peng ZL and Wang ZH: Expression
and involved signal transduction pathway of autophagy gene Beclin
1in epithelial ovarian cancer. Sichuan Da Xue Xue Bao Yi Xue Ban.
38:239–242. 2007.(In Chinese). PubMed/NCBI
|
|
19
|
Huang X, Bai HM, Chen L, Li B and Lu YC:
Reduced expression of LC3B-II and Beclin 1 in glioblastoma
multiforme indicates a down-regulated autophagic capacity that
relates to the progression of astrocytic tumors. J Clin Neurosci.
17:1515–1519. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jiang ZF, Shao LJ, Wang WM, Yan XB and Liu
RY: Decreased expression of Beclin-1 and LC3 in human lung cancer.
Mol Biol Rep. 39:259–267. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pirtoli L, Cevenini G, Tini P, Vannini M,
Oliveri G, Marsili S, Mourmouras V, Rubino G and Miracco C: The
prognostic role of Beclin 1 protein expression in high-grade
gliomas. Autophagy. 5:930–936. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shi YH, Ding ZB, Zhou J, Qiu SJ and Fan J:
Prognostic significance of Beclin 1-dependent apoptotic activity in
hepatocellular carcinoma. Autophagy. 5:380–382. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wan XB, Fan XJ, Chen MY, Xiang J, Huang
PY, Guo L, Wu XY, Xu J, Long ZJ, Zhao Y, et al: Elevated Beclin 1
expression is correlated with HIF-1alpha in predicting poor
prognosis of nasopharyngeal carcinoma. Autophagy. 6:395–404. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang ZH, Peng ZL, Duan ZL and Liu H:
Expression and clinical significance of autophagy gene Beclin 1 in
cervical squamous cell carcinoma. Sichuan Da Xue Xue Bao Yi Xue
Ban. 37:860–863. 2006.(In Chinese). PubMed/NCBI
|
|
25
|
Won KY, Kim GY, Lim SJ and Kim YW:
Decreased Beclin-1 expression is correlated with the growth of the
primary tumor in patients with squamous cell carcinoma and
adenocarcinoma of the lung. Hum Pathol. 43:62–68. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pajalunga D, Mazzola A, Franchitto A,
Puggioni E and Crescenzi M: The logic and regulation of cell cycle
exit and reentry. Cell Mol Life Sci. 65:8–15. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Müller GA and Engeland K: The central role
of CDE/CHR promoter elements in the regulation of cell
cycle-dependent gene transcription. FEBS J. 277:877–893. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang C, Lisanti MP and Liao DJ: Reviewing
once more the c-myc and Ras collaboration: Converging at the cyclin
D1-CDK4 complex and challenging basic concepts of cancer biology.
Cell Cycle. 10:57–67. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang LH, Song YB, Zheng WL, Jiang L and Ma
WL: The association between polymorphisms in the MDR1 gene and risk
of cancer: A systematic review and pooled analysis of 52
case-control studies. Cancer Cell Int. 13:462013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zheng HC, Takahashi H, Li XH, Hara T,
Masuda S, Guan YF and Takano Y: Overexpression of GRP78 and GRP94
are markers for aggressive behavior and poor prognosis in gastric
carcinomas. Hum Pathol. 39:1042–1049. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Adams JM and Cory S: The Bcl-2 apoptotic
switch in cancer development and therapy. Oncogene. 26:1324–1337.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ninomiya-Tsuji J, Kishimoto K, Hiyama A,
Inoue J, Cao Z and Matsumoto K: The kinase TAK1 can activate the
NIK-I kappaB as well as the MAP kinase cascade in the IL-1
signaling pathway. Nature. 398:252–256. 1999. View Article : Google Scholar : PubMed/NCBI
|