Introduction
Systematic chemotherapy has been the mainstay of
treatment for advanced urothelial carcinoma (UC).
Cisplatin-containing combination therapy is considered as standard
first-line chemotherapy. However, many patients with advanced UC
have renal dysfunction and poor performance status (1). Due to the nephrotoxicity and
gastrointestinal toxicity associated with cisplatin, many patients
are often unfit for drug treatment, with the proportion in various
cohorts ranging from 30–60% (2,3).
Carboplatin is frequently substituted for cisplatin, although its
therapeutic efficacy is reportedly inferior to that of cisplatin
(4). To date, no standard therapy for
cisplatin-unfit patients has been established.
Nedaplatin is a platinum derivative that was
developed in Japan. It is more soluble than cisplatin, and has less
side effects in terms of renal function and gastrointestinal
symptoms (5). Nedaplatin has
antitumor activity in various cancer types, such as head and neck
cancer, lung cancer, cervical cancer, and ovarian cancer. In
advanced UC, the response rate with nedaplatin alone is 28.6%. In
terms of renal toxicity, serum creatinine elevation is observed in
15.1% of the patients (6). However,
the anticancer effect and the impact on renal function of
nedaplatin is poorly understood when it is included as part of a
combination regimen in UC.
To determine the extent of renal toxicity associated
with nedaplatin-containing combination chemotherapy, we designed
the current study to evaluate changes in renal function of
nedaplatin combination regimens in patients with advanced UC.
Materials and methods
Patients
A total of 35 UC patients who had received
nedaplatin-containing chemotherapy at the Shiga University of
Medical Science Hospital from 2001 to 2014 were studied
retrospectively. As comparative controls, we also examined 35
patients with the same disease who underwent cisplatin-containing
chemotherapy during the same period. The eligibility criteria were
as follows 1): UC with local invasion or metastasis, 2) cisplatin
ineligible due to impaired renal function, 3) cisplatin-related
adverse events during previous chemotherapy, and 4) an Eastern
Cooperative Oncology Group (ECOG) performance status of 2 or less.
In our institution, a cutoff value of measured creatinine clearance
<60 ml/min was used to exclude patients from receiving
cisplatin-containing chemotherapy.
The present study was approved by the ethics review
board of Shiga University of Medical Science (Shiga, Japan).
Nedaplatin-containing regimens and
treatment schedule
Cisplatin was replaced with nedaplatin in select
combination regimens. Two regimens were performed. One was
methotrexate/epirubicin/nedaplatin (MEN) therapy, which is
essentially a modified version of methotrexate/epirubicin/cisplatin
(MEC) therapy (7). The other was
gemcitabine/nedaplatin (GN) therapy, which was based on a
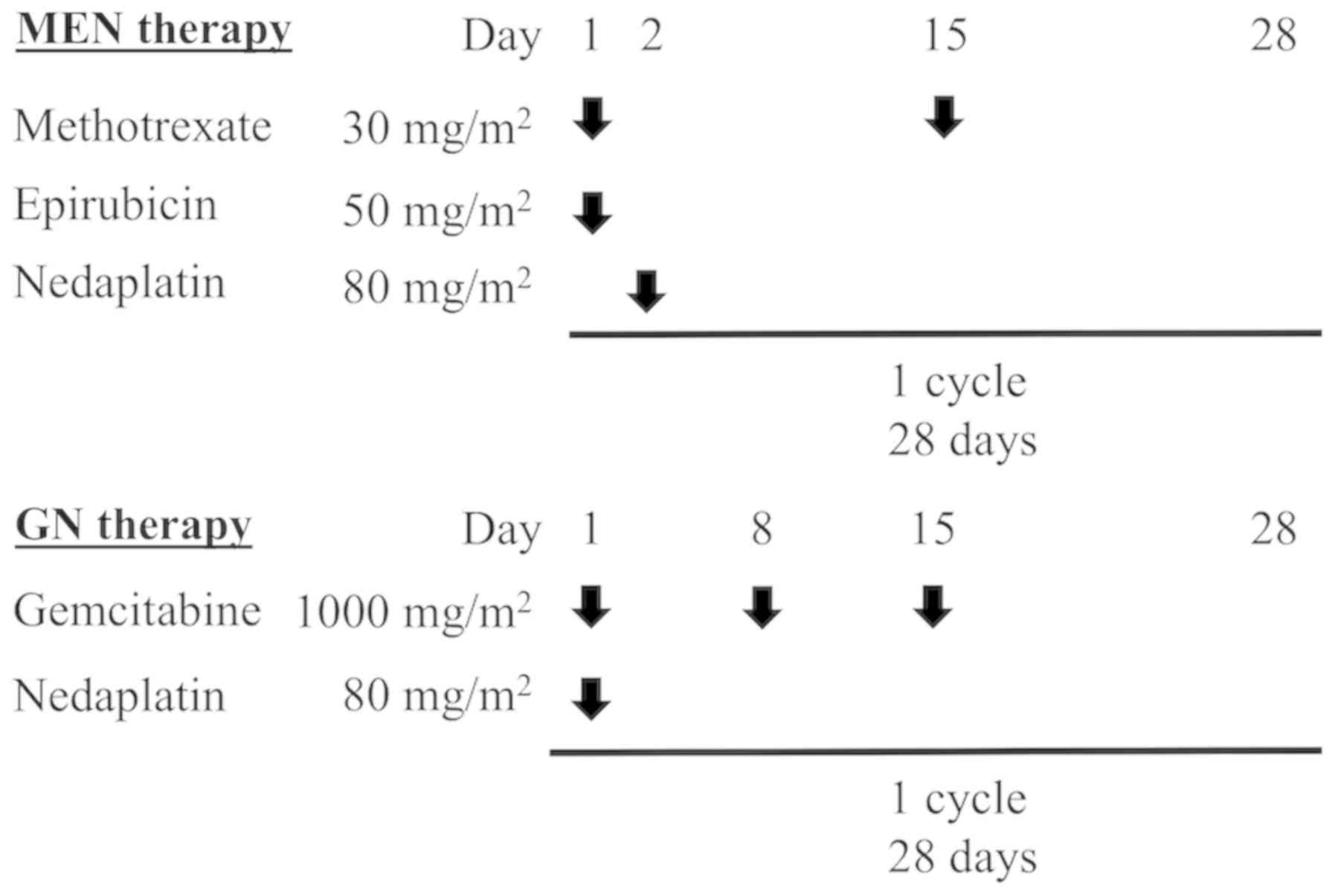
modification of gemcitabine/cisplatin (GC) therapy (8). Patients who were given MEN therapy
received methotrexate (30 mg/m2) on days 1 and 15,
epirubicin (50 mg/m2) on day 1 and nedaplatin (80
mg/m2) on day 2 intravenously (Fig. 1). Patients given GN therapy received
gemcitabine (1,000 mg/m2) on days 1, 8 and 15 and
nedaplatin (80 mg/m2) on day 1 intravenously. Both
regimens were repeated every 4 weeks. Physicians reduced the dose
of nedaplatin based on the occurrence of severe hematologic
toxicity and/or compromised renal function.
Evaluation of renal function and
therapeutic effect
We compared estimated glomerular filtration rate
(eGFR; ml/min/1.73 m2) before and after each cycle of
chemotherapy. The eGFR after administration was assessed just
before the next cycle, and was calculated using the following
formula reported by the Japanese Society of Nephrology in 2008.
eGFR=194×Cr−1.094xAge−0.287 if
male, or 194×Cr−1.094xAge0.739
if female (9).
Therapeutic effects were evaluated using the
response rate according to the Response Evaluation Criteria in
Solid Tumors (10). Toxicity was
monitored according to Common Terminology Criteria for Adverse
Events (CTCAE) v.4.0.
Statistical analysis
Differences between patients' characteristics were
estimated using a chi-square test and an independent sample t test.
Paired t tests were used to compare changes of eGFR between pre-
and post-administration in each cycle of chemotherapy. All data
were analyzed using SPSS software (v.22; IBM Corp., Armonk, NY,
USA) and P<0.05 was considered to indicate a statistically
significant difference.
Results
Patients' characteristics
Patients' characteristics are shown in Table I. The primary tumors in both groups
were four cases of renal pelvic carcinoma (11%), 13 cases of ureter
carcinoma (37%) and 18 cases of bladder carcinoma (51%). The median
ages of patients who underwent nedaplatin combination regimen
(nedaplatin group) and cisplatin combination regimen (cisplatin
group) were 64 and 68 years, respectively; this difference was not
statistically significant. On the other hand, the performance
status was significantly poorer in the nedaplatin group than in the
cisplatin group.
 | Table I.Patients' characteristics. |
Table I.
Patients' characteristics.
| Characteristic | Cisplatin group | Nedaplatin group | P-value |
|---|
| Age (years) |
|
| 0.052 |
|
Median | 68 | 64 |
|
|
Range | 55–82 | 54–83 |
|
| Sex (n) |
|
| 0.78 |
| Male | 26 | 27 |
|
|
Female | 9 | 8 |
|
| ECOG Performance
status (n) |
|
| 0.02a |
| 0 | 15 | 8 |
|
| 1 | 20 | 20 |
|
| 2 | 0 | 7 |
|
| Primary site (n) |
|
| 1 |
| Upper
urinary tract | 17 | 17 |
|
|
Bladder | 18 | 18 |
|
| Purpose of
chemotherapy (n) |
|
| 0.15 |
|
Neoadjuvant | 10 | 5 |
|
|
Adjuvant | 10 | 16 |
|
|
Metastasis | 25 | 18 |
|
| Prior chemotherapy, n
(cycles) |
|
|
|
| None |
| 17 |
|
| MEC |
| 13 (21) |
|
| GC |
| 9 (36) |
|
| Regimen (cycles) |
|
|
|
| MEC | 24 | 66 |
|
|
GC/GN | 145 | 31 |
|
All patients of the cisplatin group and 97% (34 of
35) of the nedaplatin group received these drugs as first-line
therapy. In the nedaplatin group, approximately half of patients
had received a prior cisplatin combination regimen (57%) and were
switched to nedaplatin due to cisplatin-unfitness. Only one case
was treated as the second-line therapy for relapse after a prior
cisplatin combination regimen.
Patients treated with the nedaplatin combination
regimen underwent 66 cycles of MEN therapy and 31 cycles of GN
therapy. The most common reason for using nedaplatin was chronic
renal failure (18 cases, 51%); the second was impaired renal
function caused by the cisplatin combination regimen (13 cases,
37%). Twenty-four patients had an eGFR of 30–60 ml/min/1.73
m2, and four patients had an eGFR of less than 30
ml/min/1.73 m2 before induction of nedaplatin
chemotherapy.
Changes in renal function before and
after chemotherapy
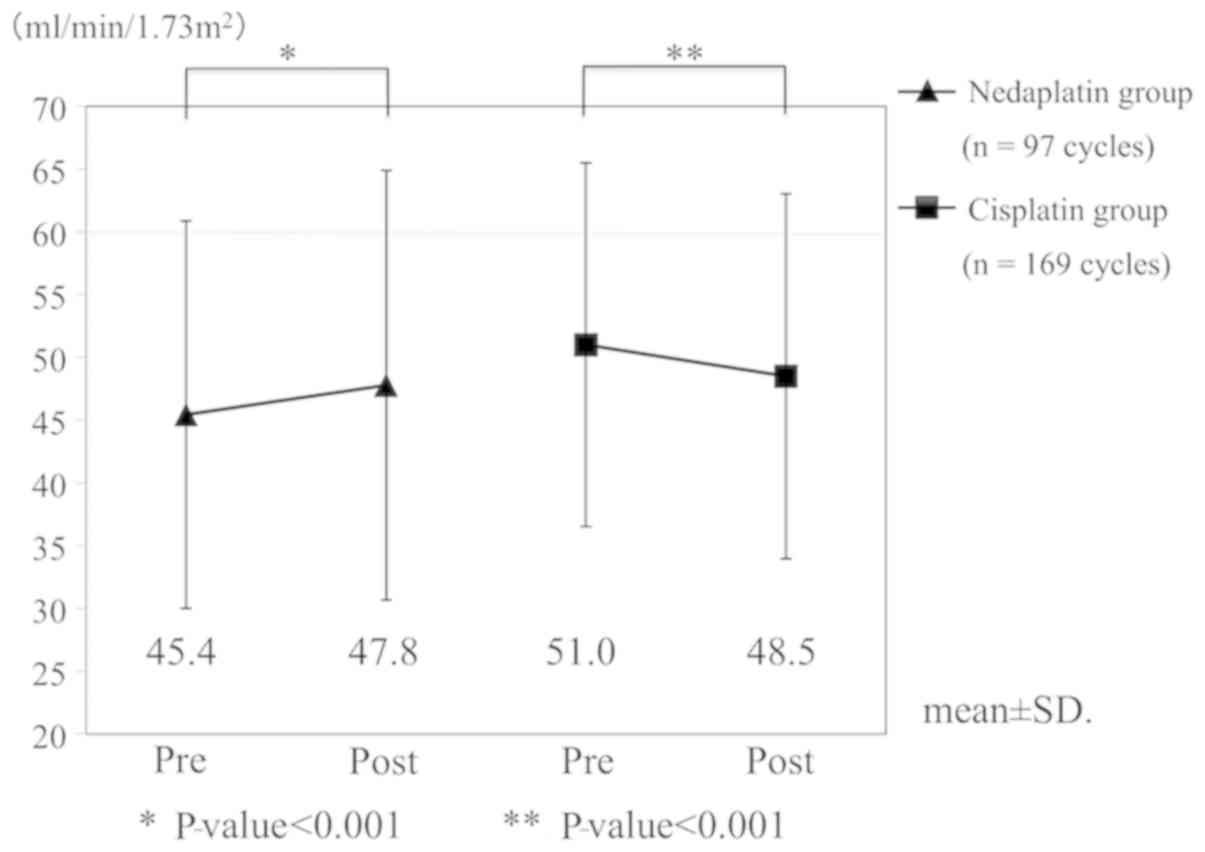
We compared changes in eGFR at each cycle of
chemotherapy (Fig. 2). In the
nedaplatin group (n=97 cycles), the mean eGFRs before and
after chemotherapy were 45.4 ml/min/1.73 m2 and 47.8
ml/min/1.73 m2, respectively. The post-chemotherapy eGFR
was significantly increased (P<0.001). The eGFR in the
nedaplatin group was significantly elevated, even when cases with
eGFR <60 ml/min/1.73 m2 were analyzed (n=87
cycles). On the other hand, in the cisplatin group (n=169
cycles), the mean eGFRs before and after chemotherapy were 51.0
ml/min/1.73 m2 and 48.5 ml/min/1.73 m2,
respectively. This decrease was statistically significant
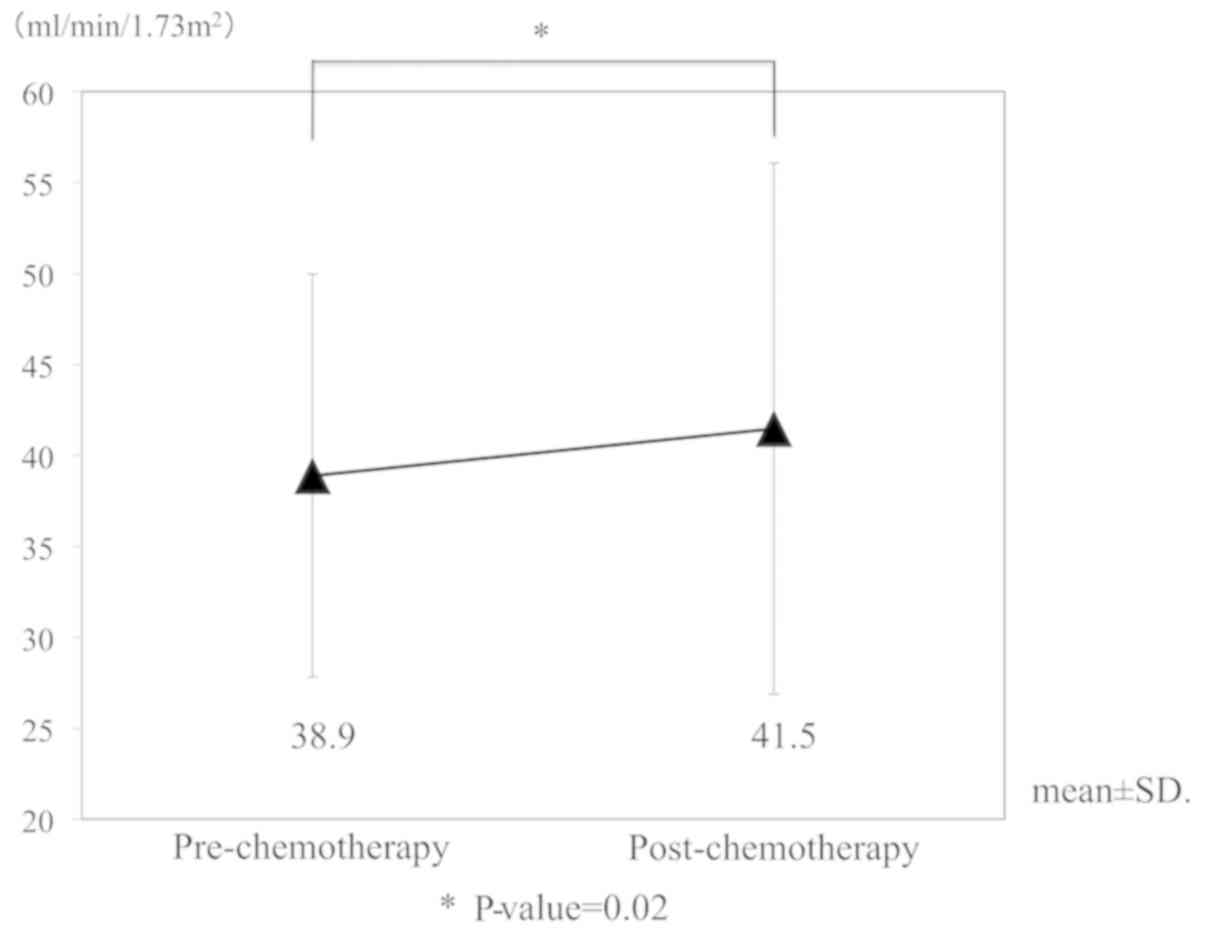
(P<0.001). As a sub-analysis in the nedaplatin group, eGFR
alterations in patients whose renal function was impaired by prior
cisplatin administration were investigated (n=33 cycles in
13 patients). No decrease in renal function following nedaplatin
administration was observed in this sub-population (Fig. 3). In a subgroup of patients where
nedaplatin dose reduction was not required, the mean eGFR values
before and after chemotherapy were 59.2 ml/min/1.73 m2
and 63.2 ml/min/1.73 m2, respectively (n=16
cycles in 11 patients). Thus, renal function was not compromised in
patients receiving the standard full dose of nedaplatin.
Treatment evaluation
In the nedaplatin group, the median number of times
the nedaplatin combination regimens administered was two cycles
(1–8 cycles, Table II). The median
dose of nedaplatin per cycle was 60.0 mg/m2, and the
median relative dose intensity (RDI) was 63.0%. In the cisplatin
group, RDI and number of chemotherapy cycles were significantly
larger than in the nedaplatin group (P<0.001). Of the 23
patients who had measurable lesions in the nedaplatin group, one
complete response (CR) (4.3%) and six partial responses (PRs)
(26.1%) were observed. The overall response rate (CR+PR) was 30.4%
in the nedaplatin group, and 66.7% in the cisplatin group. The
overall response rate of all cases and metastatic cases was higher
in the cisplatin group than in the nedaplatin group (P<0.001).
In the nedaplatin group, the overall response rates in patients
with and without prior cisplatin chemotherapy were 23.1% (3 of 13
cases) and 40.0% (4 of 10 cases), respectively.
 | Table II.Amount of treatment and best overall
response. |
Table II.
Amount of treatment and best overall
response.
| Variable | Cisplatin group | Nedaplatin group | P-value |
|---|
| Relative dose
intensity (%) |
|
|
|
|
Median | 96.3 | 63 |
|
|
Range | 50–100 | 34.5–143.0 | 0.0004b |
| Number of cycle
(cycles) |
|
|
|
|
Median | 4 | 2 |
|
|
Range | 1–13 | 1–8 | 0.0008b |
| Overall response
rate, % (n) |
|
|
|
| All
cases | 66.7 (20) | 30.4 (7) | 0.0090a |
|
Metastatic cases | 65 (13) | 22.2 (4) | 0.0080a |
|
Neoadjuvant chemotherapy
cases | 70 (7) | 60 (3) | 0.7000 |
Adverse events
In both groups, myelosuppression was the most common
side effect (Table III). Leucopenia
of Grade 3 or more was observed in 68.6 and 65.7% in the cisplatin
and nedaplatin groups, respectively. The incidence of febrile
neutropenia also occurred at frequencies of 20.0 and 22.9% in the
cisplatin and nedaplatin groups, respectively. The incidences of
Grade 2 and 3 acute kidney injuries were 8.6% (3 patients) and 2.9%
(1 patient) in the cisplatin group, whereas in the nedaplatin group
there was no acute kidney injury of Grade 2 or higher. The
frequency of gastrointestinal side effects such as anorexia was
lower in the nedaplatin group than in the cisplatin group, although
this difference was not statistically significant.
 | Table III.Adverse Events (Common Terminology
Criteria for Adverse Events version 4.0, Grade 3 and above). |
Table III.
Adverse Events (Common Terminology
Criteria for Adverse Events version 4.0, Grade 3 and above).
|
| Cisplatin
group | Nedaplatin
group |
|
|---|
|
|
|
|
|
|---|
| Adverse event | No. | % | No. | % | P-value |
|---|
|
Myelosuppression |
|
|
|
|
|
|
Anemia | 17 | 48.6 | 18 | 51.4 | 0.810 |
|
Leukopenia | 24 | 68.6 | 23 | 65.7 | 0.800 |
|
Thrombocytopenia | 25 | 71.4 | 17 | 48.6 | 0.051 |
| Febrile
neutropenia | 7 | 20.0 | 8 | 22.9 | 0.770 |
| Nausea | 0 | 0 | 1 | 2.9 | 0.310 |
| Anorexia | 5 | 14.3 | 1 | 2.9 | 0.088 |
| Oral mucositis | 0 | 0 | 1 | 2.9 | 0.310 |
| Colonic
obstruction | 1 | 2.9 | 0 | 0 | 0.310 |
| Acute kidney
injury | 1 | 2.9 | 0 | 0 | 0.310 |
Discussion
Cisplatin combination chemotherapy is a standard
treatment for advanced UC. However, many patients with advanced UC
are unfit for cisplatin therapy, and standard treatments for such
patients have not been established. The proportion of
cisplatin-unfit patients due to renal impairment is 28% in patients
with advanced bladder cancer. In upper urinary tract cancer, 51 and
81% of patients had renal dysfunction before and after
nephroureterectomy (2,3). For cisplatin-unfit patients, EAU
guidelines recommend carboplatin combination regimens, and
particular those that incorporate gemcitabine (11). However, the overall response rate of
gemcitabine and carboplatin combination therapy for cisplatin-unfit
metastatic UC is reported as 42%, and antitumor efficacy is limited
(1).
Nedaplatin was developed in Japan to provide a
treatment with a similar efficacy to cisplatin, but with less renal
and gastrointestinal toxicities (5).
Nedaplatin is approximately 10-fold more water-soluble than
cisplatin, and this in part explains its reduced nephrotoxicity.
Nedaplatin combination therapy elicited less nephrotoxicity than
did cisplatin combination therapy. In the present study, no
decrease in eGFR was observed after administration of the
nedaplatin combination regimen; rather, eGFR was significantly
increased. Similar renal function changes were found not only in
the cases with eGFR <60 ml/min/1.73 m2, but also in
the case with renal failure due to previous cisplatin chemotherapy.
On the other hand, the cisplatin combined regimen reduced eGFR,
indicating that nedaplatin is less nephrotoxic compared to
cisplatin, and thus safe to use in patients with renal impairment.
Regarding the increase of eGFR in the nedaplatin group, it is
unlikely that nedaplatin itself had a positive effect on renal
function. There were three cases in which hydronephrosis was
improved due to the therapeutic effect of nedaplatin, and cases who
had improved renal function over time after acute renal failure
following cisplatin administration. We infer that these cases
affected the significant increase in eGFR after nedaplatin
combination regimen.
In the present study, the overall response rate was
lower than that observed in other reports of nedaplatin (Table IV) (6,12–14). This may be due to a difference in the
drugs that were used in combination with nedaplatin. We currently
use gemcitabine in combination with nedaplatin, because an in
vivo study suggested a synergistic inhibition of lung cancer
cell growth using this combination (15). However, it is unknown whether synergy
between nedaplatin and gemcitabine would also be observed in UC. In
a meta-analysis of non-small cell lung cancer, docetaxel or
paclitaxel plus nedaplatin produced a longer overall and
progression-free survival than gemcitabine plus nedaplatin
(16). In other studies, nedaplatin
was used in combination with paclitaxel to treat UC (12,13), which
is different from the combinations used in our regimens. We note
that patients with lymph node metastasis alone are generally more
responsive to anticancer drugs than patients with visceral
metastases. Our study had fewer cases with lymph node metastasis
alone, which may have masked any association between this disease
stage and treatment efficacy. Thirdly, 57% of patients in our
nedaplatin group had previously received treatment with a cisplatin
combination regimen. Nedaplatin has the same amine carrier ligand
as cisplatin, binds to DNA, and inhibits DNA replication and
transcription in a similar manner to cisplatin. Therefore,
cross-resistance to nedaplatin can be induced by prior cisplatin
treatment (5), and in this study the
overall response rate of patients after a cisplatin containing
regimen was as low as 23.1%. Finally, a low median dose of 60.0
mg/m2 may lead to a decrease in response rate, although
the nedaplatin setting dose is 80.0 mg/m2.
 | Table IV.Clinical trials of nedaplatin regimen
in patients with urothelial carcinoma. |
Table IV.
Clinical trials of nedaplatin regimen
in patients with urothelial carcinoma.
| Author, year | No. of
patients | No. of patients
with prior chemotherapy | Regimen | Dose of nedaplatin
(mg/m2) | Overall response
rate (%) | Lymph node
metastases alone/lymph node and other metastases (%) | (Refs.) |
|---|
| Akaza et al,
1992 | 35 | 13 | Nedaplatin | 100 | 28.6 | NA/34.3 | (6) |
| Shinohara et
al, 2006 | 32 | 32 | Nedaplatin,
Paclitaxel, Ifosfamide | 70 | 75.0 | 50/75 | (12) |
| Kitamura et
al, 2011 | 45 | 45 | Nedaplatin,
Paclitaxel, Ifosfamide | 70 | 40.0 | 42.2/68.9 | (13) |
| Umemoto et
al, 2007 | 12 | 10 | GN | 80 | 41.7 | NA/50 | (14) |
| Present study | 23 | 13 | MEN, GN | 80 | 30.4 | 27.8/66.7 | – |
There was no significant difference in the incidence
of adverse events induced by cisplatin and nedaplatin. Among the
adverse events, the frequency of bone marrow suppression was the
highest. Some patients developed febrile neutropenia and required
platelet transfusions, but these adverse events were manageable in
both groups.
There are some limitations in our study. First, it
is a retrospective analysis, and the RDI of the nedaplatin group
was low. Therefore, the apparent antitumor efficacy of nedaplatin
is comparatively weak. Second, our data were obtained from a small
number of patients at a single institution. Third,
carboplatin-containing chemotherapy is the most used for
cisplatin-unfit patients, but there has been no study comparing the
efficacy of carboplatin with that of nedaplatin. We therefore
propose that future prospective studies should directly compare the
efficacy and safety of nedaplatin and carboplatin.
In conclusion, nedaplatin-containing chemotherapy
for cisplatin-unfit patients with advanced UC can be performed
safely and is not associated with renal toxicity.
Nedaplatin-containing chemotherapy may be available for
cisplatin-unfit patients with cancer types other than UC. In order
to optimize the use of nedaplatin as an alternative to cisplatin,
the optimal drug combinations and the appropriate dose setting of
nedaplatin should be elucidated in order to improve its antitumor
effect.
Acknowledgements
Not applicable.
Funding
The present study was supported in part by a Grant
in Aid for Young Scientists (B) from JSPS KAKENHI (grant no.
17K16787).
Availability of data and material
The data generated during the current study are
available from the corresponding author on reasonable request.
Authors' contributions
MaN, SuK and AK conceived and designed the present
study. MaN, TY, YO, ShK, AW, KT, KK, RM and TT obtained the
patients' data and performed experiments/evaluations. MaN, SuK, KJ
and MiN analyzed and interpreted the data. MaN and SuK drafted the
manuscript. SuK, KJ, MiN and AK revised the manuscript. SuK and AK
supervised the study. MaN acquired funding. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the ethics review
board of Shiga University of Medical Science (Shiga, Japan) and was
performed in accordance with the Declaration of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
De Santis M, Bellmunt J, Mead G, Kerst JM,
Leahy M, Maroto P, Gil T, Marreaud S, Daugaard G, Skoneczna I, et
al: Randomized phase II/III trial assessing gemcitabine/carboplatin
and methotrexate/carboplatin/vinblastine in patients with advanced
urothelial cancer who are unfit for cisplatin-based chemotherapy:
EORTC study 30986. J Clin Oncol. 30:191–199. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dash A, Galsky MD, Vickers AJ, Serio AM,
Koppie TM, Dalbagni G and Bochner BH: Impact of renal impairment on
eligibility for adjuvant cisplatin-based chemotherapy in patients
with urothelial carcinoma of the bladder. Cancer. 107:506–513.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kaag MG, O'Malley RL, O'Malley P, Godoy G,
Chen M, Smaldone MC, Hrebinko RL, Raman JD, Bochner B, Dalbagni G,
et al: Changes in renal function following nephroureterectomy may
affect the use of perioperative chemotherapy. Eur Urol. 58:581–587.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Galsky MD, Chen GJ, Oh WK, Bellmunt J,
Roth BJ, Petrioli R, Dogliotti L, Dreicer R and Sonpavde G:
Comparative effectiveness of cisplatin-based and carboplatin-based
chemotherapy for treatment of advanced urothelial carcinoma. Ann
Oncol. 23:406–410. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shimada M, Itamochi H and Kigawa J:
Nedaplatin: A cisplatin derivative in cancer chemotherapy. Cancer
Manag Res. 5:67–76. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Akaza H, Togashi M, Nishio Y, Miki T,
Kotake T, Matsumura Y, Yoshida O and Aso Y: Phase II study of
cis-diammine(glycolato)platinum, 254-S, in patients with advanced
germ-cell testicular cancer, prostatic cancer, and
transitional-cell carcinoma of the urinary tract. 254-S urological
cancer study group. Cancer Chemother Pharmacol. 31:187–192. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kuroda M, Kotake T, Akaza H, Hinotsu S and
Kakizoe T: Efficacy of dose-intensified MEC (methotrexate,
epirubicin and cisplatin) chemotherapy for advanced urothelial
carcinoma: A prospective randomized trial comparing MEC and M-VAC
(methotrexate, vinblastine, doxorubicin and cisplatin). Japanese
urothelial cancer research group. Jpn J Clin Oncol. 28:497–501.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
von der Maase H, Hansen SW, Roberts JT,
Dogliotti L, Oliver T, Moore MJ, Bodrogi I, Albers P, Knuth A,
Lippert CM, et al: Gemcitabine and cisplatin versus methotrexate,
vinblastine, doxorubicin, and cisplatin in advanced or metastatic
bladder cancer: Results of a large, randomized, multinational,
multicenter, phase III study. J Clin Oncol. 18:3068–3077. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Matsuo S, Imai E, Horio M, Yasuda Y,
Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H and Hishida A;
Collaborators developing the Japanese equation for estimated GFR:
Revised equations for estimated GFR from serum creatinine in Japan.
Am J Kidney Dis. 53:982–992. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Alfred Witjes J, Lebret T, Compérat EM,
Cowan NC, De Santis M, Bruins HM, Hernández V, Espinós EL, Dunn J,
Rouanne M, et al: Updated 2016 EAU guidelines on muscle-invasive
and metastatic bladder cancer. Eur Urol. 71:462–475. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shinohara N, Harabayashi T, Suzuki S,
Nagao K, Seki H, Murakumo M, Mitsuhashi K, Demura T, Nagamori S,
Matsuyama H, et al: Salvage chemotherapy with paclitaxel,
ifosfamide, and nedaplatin in patients with urothelial cancer who
had received prior cisplatin-based therapy. Cancer Chemother
Pharmacol. 58:402–407. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kitamura H, Taguchi K, Kunishima Y, Yanase
M, Takahashi A, Shigyo M, Tanaka T, Mutoh M, Fukuta F, Masumori N
and Tsukamoto T: Paclitaxel, ifosfamide, and nedaplatin as
second-line treatment for patients with metastatic urothelial
carcinoma: A phase II study of the SUOC group. Cancer Sci.
102:1171–1175. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Umemoto S, Miyoshi Y, Nakaigawa N,
Makiyama K, Ogawa T, Uemura H, Yao M and Kubota Y: A pilot study of
combination chemotherapy of gemcitabine and nedaplatin for
urological cancer. Jpn J Clin Urol. 61:903–908. 2007.
|
|
15
|
Matsumoto M, Takeda Y, Maki H, Hojo K,
Wada T, Nishitani Y, Maekawa R and Yoshioka T: Preclinical in vivo
antitumor efficacy of nedaplatin with gemcitabine against human
lung cancer. Jpn J Cancer Res. 92:51–58. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tian Y, Liu Q and Wu K, Chu Q, Chen Y and
Wu K: Meta-analysis comparing the efficacy of nedaplatin-based
regimens between squamous cell and non-squamous cell lung cancers.
Oncotarget. 8:62330–62338. 2017. View Article : Google Scholar : PubMed/NCBI
|