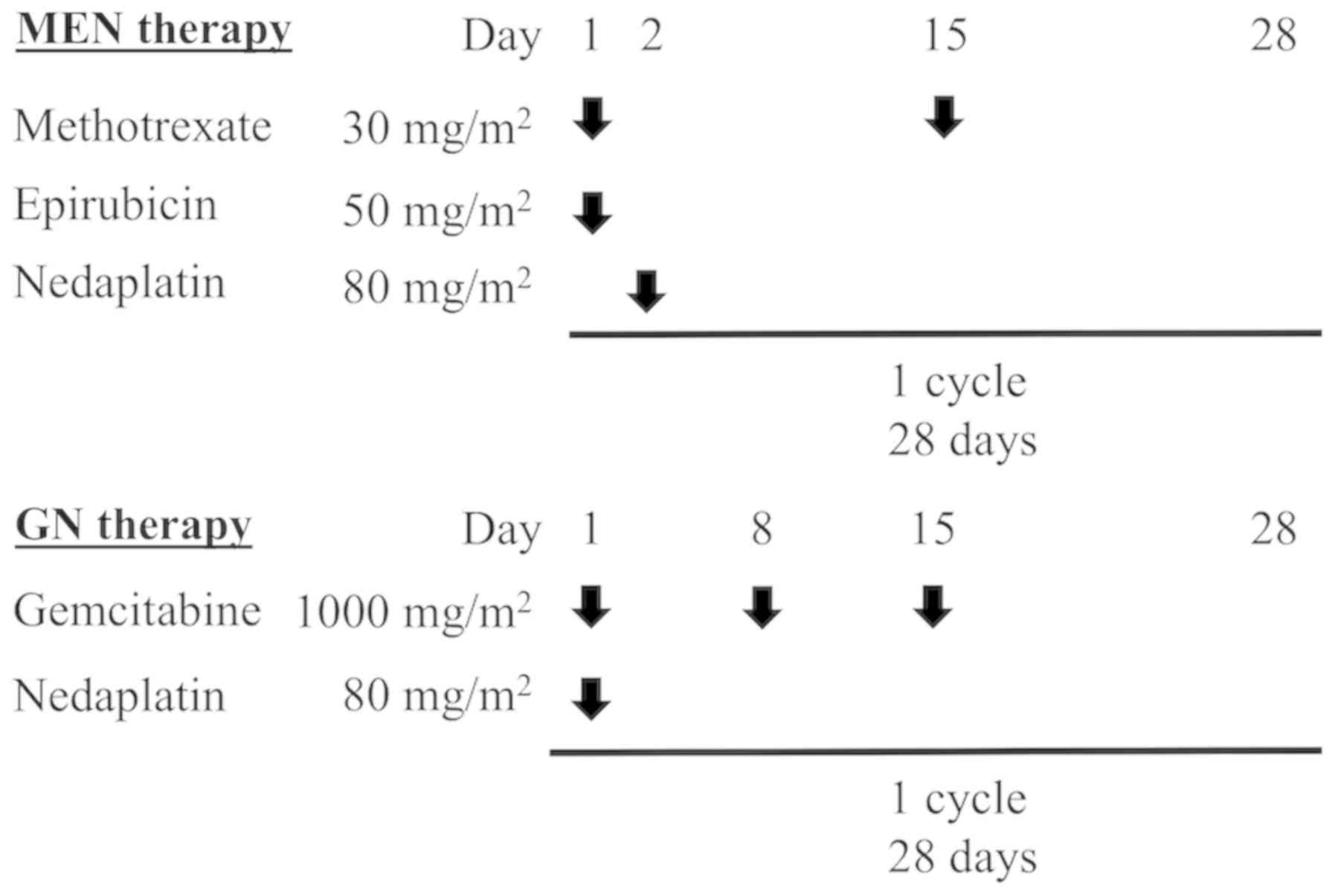
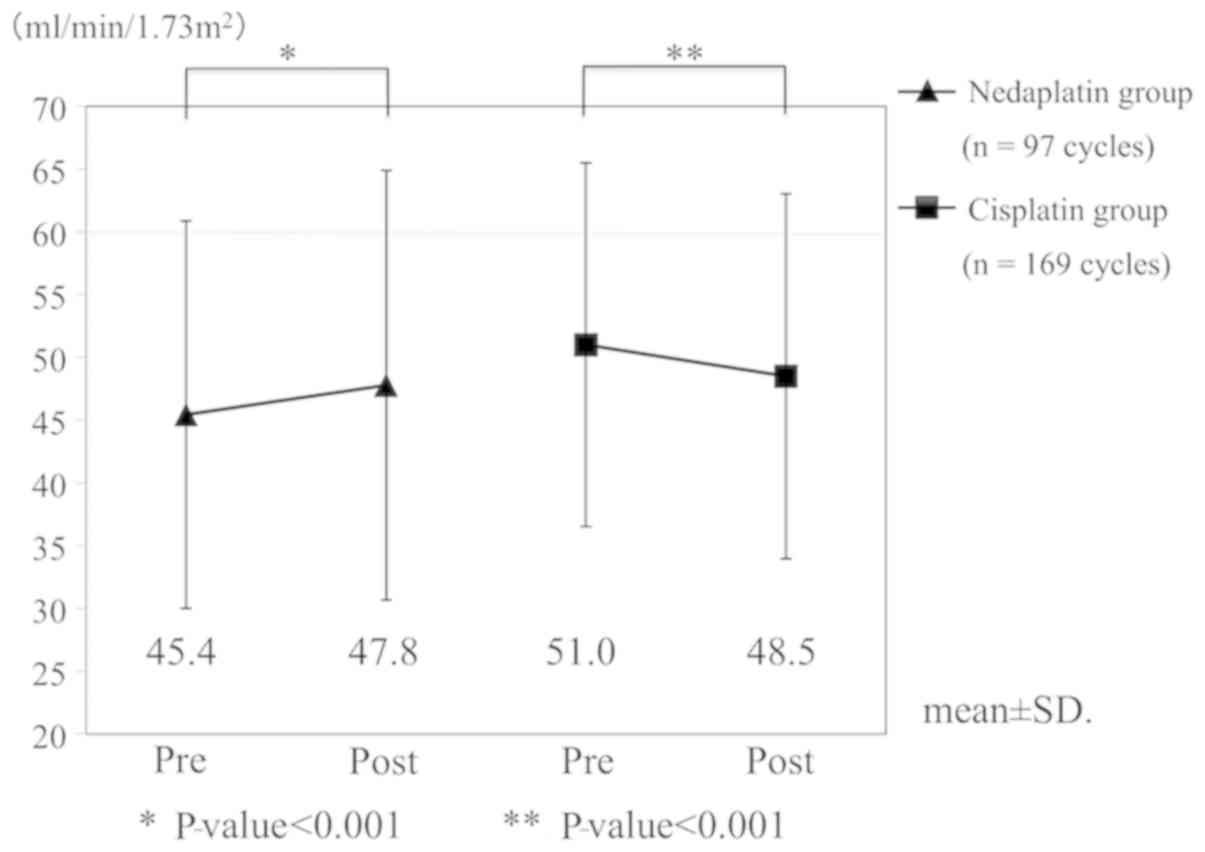
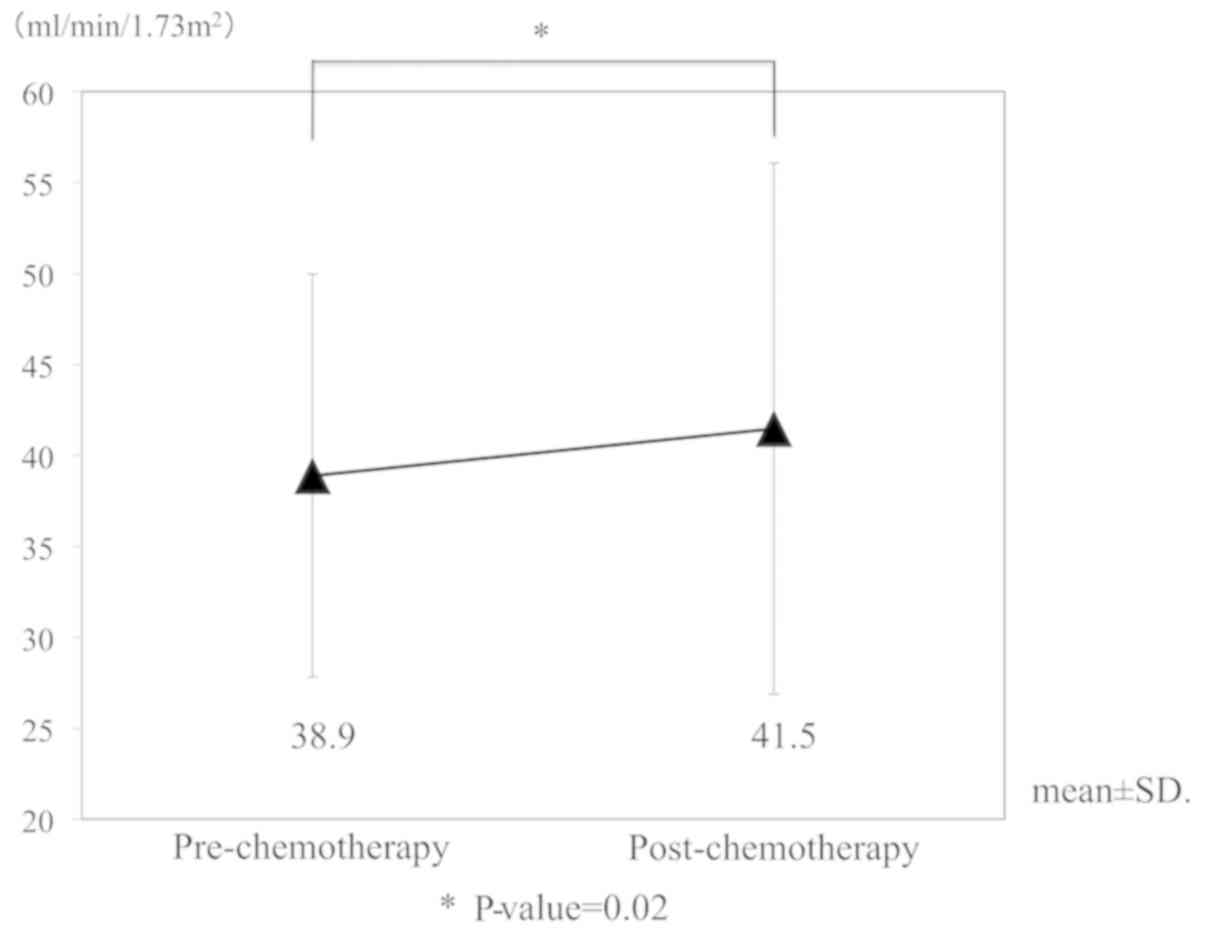
|
1
|
De Santis M, Bellmunt J, Mead G, Kerst JM,
Leahy M, Maroto P, Gil T, Marreaud S, Daugaard G, Skoneczna I, et
al: Randomized phase II/III trial assessing gemcitabine/carboplatin
and methotrexate/carboplatin/vinblastine in patients with advanced
urothelial cancer who are unfit for cisplatin-based chemotherapy:
EORTC study 30986. J Clin Oncol. 30:191–199. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dash A, Galsky MD, Vickers AJ, Serio AM,
Koppie TM, Dalbagni G and Bochner BH: Impact of renal impairment on
eligibility for adjuvant cisplatin-based chemotherapy in patients
with urothelial carcinoma of the bladder. Cancer. 107:506–513.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kaag MG, O'Malley RL, O'Malley P, Godoy G,
Chen M, Smaldone MC, Hrebinko RL, Raman JD, Bochner B, Dalbagni G,
et al: Changes in renal function following nephroureterectomy may
affect the use of perioperative chemotherapy. Eur Urol. 58:581–587.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Galsky MD, Chen GJ, Oh WK, Bellmunt J,
Roth BJ, Petrioli R, Dogliotti L, Dreicer R and Sonpavde G:
Comparative effectiveness of cisplatin-based and carboplatin-based
chemotherapy for treatment of advanced urothelial carcinoma. Ann
Oncol. 23:406–410. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shimada M, Itamochi H and Kigawa J:
Nedaplatin: A cisplatin derivative in cancer chemotherapy. Cancer
Manag Res. 5:67–76. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Akaza H, Togashi M, Nishio Y, Miki T,
Kotake T, Matsumura Y, Yoshida O and Aso Y: Phase II study of
cis-diammine(glycolato)platinum, 254-S, in patients with advanced
germ-cell testicular cancer, prostatic cancer, and
transitional-cell carcinoma of the urinary tract. 254-S urological
cancer study group. Cancer Chemother Pharmacol. 31:187–192. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kuroda M, Kotake T, Akaza H, Hinotsu S and
Kakizoe T: Efficacy of dose-intensified MEC (methotrexate,
epirubicin and cisplatin) chemotherapy for advanced urothelial
carcinoma: A prospective randomized trial comparing MEC and M-VAC
(methotrexate, vinblastine, doxorubicin and cisplatin). Japanese
urothelial cancer research group. Jpn J Clin Oncol. 28:497–501.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
von der Maase H, Hansen SW, Roberts JT,
Dogliotti L, Oliver T, Moore MJ, Bodrogi I, Albers P, Knuth A,
Lippert CM, et al: Gemcitabine and cisplatin versus methotrexate,
vinblastine, doxorubicin, and cisplatin in advanced or metastatic
bladder cancer: Results of a large, randomized, multinational,
multicenter, phase III study. J Clin Oncol. 18:3068–3077. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Matsuo S, Imai E, Horio M, Yasuda Y,
Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H and Hishida A;
Collaborators developing the Japanese equation for estimated GFR:
Revised equations for estimated GFR from serum creatinine in Japan.
Am J Kidney Dis. 53:982–992. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Alfred Witjes J, Lebret T, Compérat EM,
Cowan NC, De Santis M, Bruins HM, Hernández V, Espinós EL, Dunn J,
Rouanne M, et al: Updated 2016 EAU guidelines on muscle-invasive
and metastatic bladder cancer. Eur Urol. 71:462–475. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shinohara N, Harabayashi T, Suzuki S,
Nagao K, Seki H, Murakumo M, Mitsuhashi K, Demura T, Nagamori S,
Matsuyama H, et al: Salvage chemotherapy with paclitaxel,
ifosfamide, and nedaplatin in patients with urothelial cancer who
had received prior cisplatin-based therapy. Cancer Chemother
Pharmacol. 58:402–407. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kitamura H, Taguchi K, Kunishima Y, Yanase
M, Takahashi A, Shigyo M, Tanaka T, Mutoh M, Fukuta F, Masumori N
and Tsukamoto T: Paclitaxel, ifosfamide, and nedaplatin as
second-line treatment for patients with metastatic urothelial
carcinoma: A phase II study of the SUOC group. Cancer Sci.
102:1171–1175. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Umemoto S, Miyoshi Y, Nakaigawa N,
Makiyama K, Ogawa T, Uemura H, Yao M and Kubota Y: A pilot study of
combination chemotherapy of gemcitabine and nedaplatin for
urological cancer. Jpn J Clin Urol. 61:903–908. 2007.
|
|
15
|
Matsumoto M, Takeda Y, Maki H, Hojo K,
Wada T, Nishitani Y, Maekawa R and Yoshioka T: Preclinical in vivo
antitumor efficacy of nedaplatin with gemcitabine against human
lung cancer. Jpn J Cancer Res. 92:51–58. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tian Y, Liu Q and Wu K, Chu Q, Chen Y and
Wu K: Meta-analysis comparing the efficacy of nedaplatin-based
regimens between squamous cell and non-squamous cell lung cancers.
Oncotarget. 8:62330–62338. 2017. View Article : Google Scholar : PubMed/NCBI
|