Introduction
Retinoblastoma (RB) is the most common ocular
malignancy in infants and young children (1). Incidence of RB ranks second among all
malignancies that occur in infants and young children. At present,
the global incidence is about 1×6,000, and the number of new cases
is about 5,000 per year (2).
Statistics show that about 90% RB occurred before the age of 6, and
more than 60% RB was monocular (3).
In addition, the incidence was constant across race and region
(4). RB seriously affects children's
vision and even threatens their life. The key of the survival of RB
patients is early diagnosis and timely effective treatment
(5). According to literature, most
patients with RB in China were diagnosed at advanced stages and the
best time for non-surgical treatment is usually missed (6). However, the most common and effective
treatment in clinical practice is surgical removal of diseased
eyeballs (7).
Materials and methods
Since the internal organs of infants and young
children are at the stage of growth and development, the functions
of various systems are still immature. For example, they have a
weak immune system and low sense of autonomy as well as ability to
process outside information (8).
According to literature, safety risks of general anesthesia in
children undergoing surgery are much greater than in adults
(9). Anesthetic agents have a
pronounced impact on respiratory function in children. When
children are sedated, their small airways are prone to collapse.
Small airway closure leads to low lung ventilation, and children
are prone to intraoperative complications such as cough, airway
obstruction and even asphyxia (10).
It was reported that dexmedetomidine (Dex) has strong calming and
anti-anxiety effects while lacking respiratory depressant effects.
It causes few adverse reactions, including a light impact on
circulatory system. Dex is an excellent sedative and hypnotic drug
and has been widely used in clinical anesthesia (11). To the best of our knowledge, there is
no previous report on the effect of Dex on the respiratory system
in pediatric patients undergoing RB surgical resection. This study
aimed to investigate whether Dex can stabilize the respiratory
function in perioperative children and can avoid complications such
as arrhythmia and hypoxia caused by the surgical procedure.
Subjects
A total of 87 pediatric patients who underwent RB
resection were recruited into this study. General anesthesia was
first induced for all patients, of which 45 were randomly assigned
to the experimental group and received Dex through an intravenous
infusion pump to maintain general anesthesia. In the experimental
group, there were 24 males and 21 females, aged 1–8 years, with a
mean age of 4.37±0.78 years. The remaining 42 patients were
assigned to the control group and received saline through an
intravenous infusion pump. In the control group, there were 22
males and 20 females, aged 1–8 years, with a mean age of 4.41±0.82
years. The general clinical records are shown in Table I.
 | Table I.General clinical record (mean ±
standard deviation) [n (%)]. |
Table I.
General clinical record (mean ±
standard deviation) [n (%)].
| Variables | Experimental group
n=45 | Control group
n=42 | t or
χ2 | P-value |
|---|
| Age (year) | 4.37±0.78 | 4.41±0.82 | 0.233 | 0.816 |
| Body weight
(kg) | 8.59±5.37 | 8.72±4.83 | 0.118 | 0.906 |
| Sex |
|
Male | 24 (53.33) | 22 (52.38) | 0.008 | 0.929 |
|
Female | 21 (46.67) | 20 (47.62) |
|
|
| ASA
classification |
| Class
I | 28 (62.22) | 26 (61.90) | 0.001 | 0.976 |
| Class
II | 17 (37.78) | 16 (38.10) |
|
|
| Clinical stage |
| Group
D | 21 (46.67) | 18 (42.86) | 0.470 | 0.926 |
| Group
E | 12 (26.67) | 14 (33.33) |
|
|
|
Extraocular RB | 7
(15.56) | 6
(14.29) |
|
|
|
Metastatic RB | 5
(11.11) | 4 (9.52) |
|
|
| Clinical
manifestation |
|
Strabismus | 5
(11.11) | 5
(11.90) | 0.077 | 0.995 |
| White
pupillary reflex | 30 (66.67) | 28 (66.67) |
|
|
| Vision
problem | 5
(11.11) | 5
(11.90) |
|
|
| Eye
congestion | 5
(11.11) | 4 (9.52) |
|
|
| Affected eye |
| Left
eye | 17 (37.78) | 15 (35.71) | 0.054 | 0.973 |
| Right
eye | 16 (35.55) | 15 (35.71) |
|
|
|
Bilateral | 12 (26.67) | 12 (28.57) |
|
|
| Three generations
of immediate family members with RB |
|
Yes | 13 (28.89) | 11 (26.19) | 0.079 | 0.778 |
| No | 32 (71.11) | 31 (73.81) |
|
|
Patients who met the following criteria were
eligible for this study: i) patients who were assigned Class I or
Class II according to American Society of Anesthesiologists (ASA)
physical status classification (12);
ii) patients who were diagnosed with RB by fundus examination and
imaging tests such as B-ultrasound; and iii) patients who were
assigned D-E phase, extraocular phase, and metastasis phase
according to International Intraocular Retinoblastoma
Classification (IIRC) (12), or
patients who had extraocular RB or metastatic RB and experienced
severe eye pain and whose lesioned eyeballs had no chance for
recovery of vision. This study was approved by the Medical Ethics
Committee of Yidu Central Hospital of Weifang (Weifang, China).
Patients' families were informed of the specific contents of the
study and signed a completed informed consent form.
Patients who met the following criteria were
excluded from the study: i) patients who suffered from primary
disease affecting their respiratory function, such as bronchial
asthma and myasthenia gravis; ii) patients who had cardiopulmonary
disorders or liver/kidney dysfunction, associated with serious
infection; and iii) patients who had obvious surgical
contraindications such as coagulation disorders and allergy to
anesthetic agents.
Methods
Pharmaceutical drugs and
instruments
Pharmaceutical drugs used in this study were
purchased from various sources: atropine sulfate injection [SFDA
Approval no. H41021817; Kaifeng Pharmaceutical (Group) Co., Ltd.,
Kaifeng, China], phenobarbital tablets (SFDA Approval no.
H61021669; Xi'an Lijun Pharmaceutical Co., Ltd., Xi'an, China),
lidocaine hydrochloride injection (SFDA Approval no. H45020823;
Guilin Pharmaceutical Co., Ltd., Guilin, China), sufentanil citrate
injection (SFDA Approval no. H20054172; Yichang Humanwell
Pharmaceutical Co., Ltd., Yichang, China), propofol injection (SFDA
Approval no. H20123138; Jiangsu Nhwa Pharmaceutical Co., Ltd.,
Jiangsu, China) and Dex (SFDA Approval no. H20110086; Jiangsu Nhwa
Pharmaceutical Co., Ltd.), midazolam (SFDA Approval no.
ZWK-135-13791; Shanghai ZZBio Co., Ltd., Shanghai, China), and
cisatracurium besylate [SFDA Approval no. H20060926; DongYing
(Jiangsu) Pharmaceutical Co., Ltd., Jiangsu, China]. The following
instruments were purchased from respective sources: the ECG monitor
(Article no. 6800-10; Shanghai Zhiheng Medical Devices Co., Ltd.,
Shanghai, China) the tracheal intubation catheter (Article no.
TK8976; Henan Zeyuan Medical Equipment Sales Co., Ltd., Beijing,
China, http://www.tdshoupin.com/) and the
anesthesia machine (Article no. 06; Beijing First Product Condar
Technology Co., Ltd., Beijing, China).
Anesthetic procedures
All pediatric patients underwent 8–12 h fasting and
water-deprivation before surgery. Atropine sulfate (0.01 mg/kg) and
phenobarbital tablets (2–3 mg/kg) were given via routine injection
and oral administration, respectively, 30 min before entering the
operating room to achieve preoperative stabilization. After the
child entered the operating room, the surgical site was routinely
disinfected before surgery. After performing local infiltration
anesthesia by injection of 1% lidocaine, an indwelling catheter was
placed on the right hand through radial artery puncture, followed
by establishment of a venous access. Patients were then connected
to an ECG monitor. A general anesthesia protocol was developed
after assessment of the patient's overall physical state. The
patient was gently pacified before anesthesia.
General anesthesia was induced for all subjects by
intravenous injection of fentanyl (2.0–3.5 µg/kg), propofol
(1.0–1.5 mg/kg), midazolam (0.05 mg/kg), and atracurium (0.5–0.8
mg/kg). During the same time, patients in the experimental group
were given Dex (1 µg/kg) through an intravenous infusion pump at a
rate of 0.2–0.8 µg/(kg•h) within 15 min, and patients in the
control group received 0.9% saline (0.25 ml/kg) as a reference,
through an intravenous infusion pump at a rate of 0.125 ml/(kg•h)
within 15 min. After sufficient anesthetic depth was reached,
tracheal intubation was performed to assist breathing using a
catheter for age +18. After that, patient were connected to an
anesthesia machine for breathing control. If the child still
maintained spontaneous breathing function, expansion and
contraction of the respiratory balloon, it could be seen with the
breath. If spontaneous breathing was not observed, the anesthesia
machine was then adjusted to provide a tidal volume of 14–20 ml/kg,
a respiratory rate (R) of 20–30 breaths/min, an
inspiration-to-expiration ratio (I:E) of 1:2, and an end-tidal
CO2 partial pressure of 35–40 mmHg. During the
operation, patients were checked regularly for a normal breath
sound and a respiratory undulation of the chest.
Observed indicators
i) Indexes of respiratory function and hemodynamics
at five time-points, i.e., before anesthesia induction (T0), 5 min
after injection of anesthetic agents (T1), before intubation (T2),
15 min after intubation (T3), and 30 min after extubation (T4),
were recorded for all subjects. The indexes of respiratory function
included R and blood oxygen saturation (SpO2) value. The
intraoperative hemodynamic indexes included heart rate (HR) and
mean arterial pressure (MAP) value. ii) Incidence of perioperative
cardiac and respiratory adverse events in both groups was recorded:
cardiac arrest, apnea, bradycardia (HR was more than 30% lower than
healthy children of the age group), tachycardia (HR was more than
30% higher than healthy children of the age group), vomiting,
hypoxia (SpO2 <90% at the end of 60 sec), and
laryngism (13,14). iii) Postoperative anesthesia recovery
of the experimental and control groups was recorded in detail:
recovery time of anesthesia (the time between discontinuation of
intravenous anesthesia in the two groups to the blink of children)
and the time of extubation (the time between discontinuation of
intravenous anesthesia and organ catheter extraction). Agitation
score (PAED) (15) was used to
evaluate the postoperative agitation.
Statistical analysis
Statistical analysis was performed using SPSS 19.0
statistics software system (IBM SPSS, Shanghai, China). Measurement
data were expressed as mean ± standard deviation. The t-test was
used for comparison between the two groups. Repeated measures ANOVA
and Least Significant Difference post hoc test were used for
comparison between multiple groups. Enumeration data were expressed
in percentage (%). The χ2 test was used for the analysis
of count data. P<0.05 was considered to indicate a statistically
significant difference.
Results
General clinical record
As shown in Table I,
there were no significant differences in sex, age, and ASA
classification between the experimental and control groups
(P>0.05).
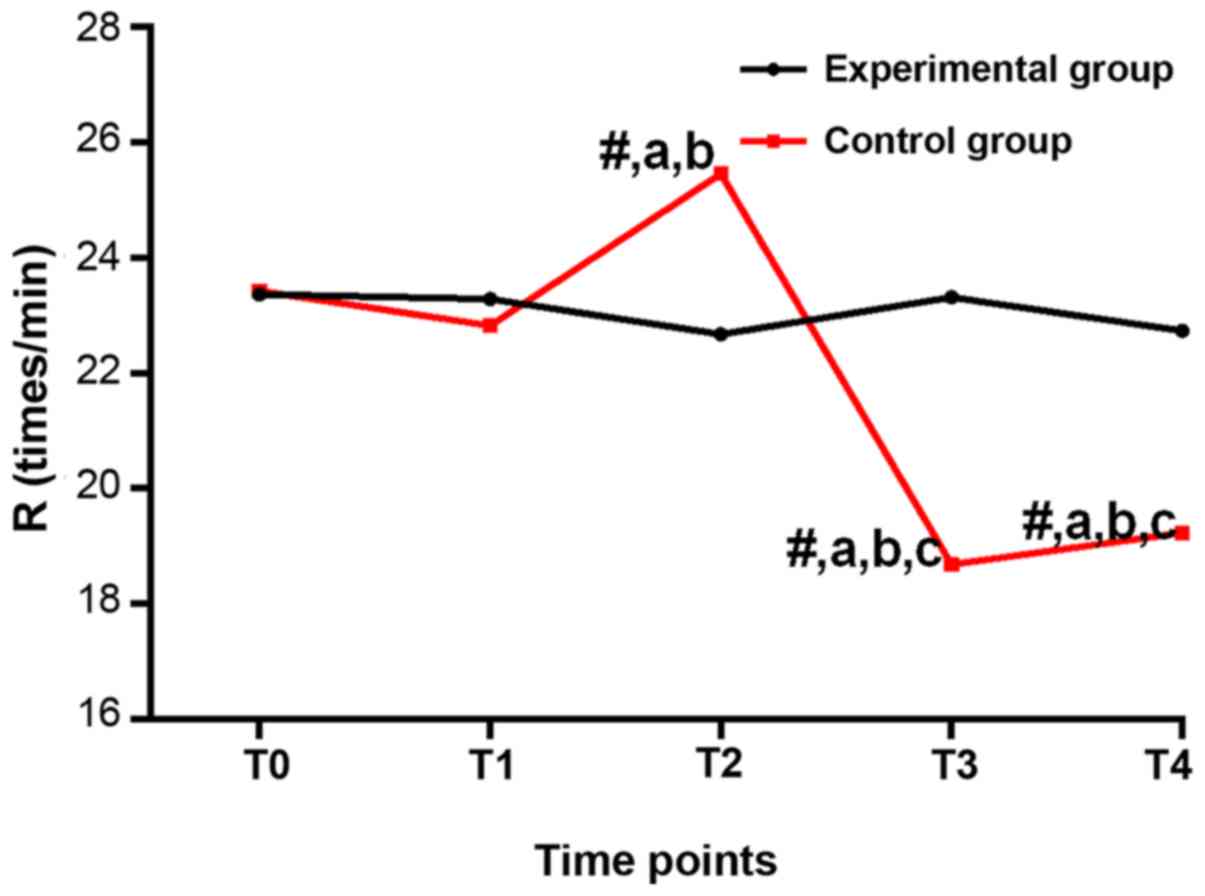
Respiratory rate (breaths/min) at
different time-points
Regarding the comparison of R, there was no
difference among T0-T4 in the experimental group (P>0.05); there
were differences among T0-T4 in the control group (P<0.05).
Compared with T0, the control group had a lower R at T2-T4
(P<0.05). Compared with T1, the control group had a lower R at
T2-T4 (P<0.05). Compared with T2, the control group had a lower
R at T3 and T4 (P<0.05). Compared with the experimental group at
the same time-point, R was higher at T2, and lower at T3 and T4 in
the control group (P<0.05), and no significant difference was
found at T0 and T1 (P>0.05; Table
II and Fig. 1).
 | Table II.Respiratory rate (breaths/min) at
different time-points. |
Table II.
Respiratory rate (breaths/min) at
different time-points.
| Time-points | Experimental
group | Control group | t | P-value |
|---|
| T0 | 23.36±3.86 | 23.42±3.78 | 0.073 |
0.942 |
| T1 | 23.28±3.97 | 21.83±2.86 | 0.535 |
0.5938 |
| T2 | 22.67±4.24 |
25.46±3.35a,b | 3.389 |
0.001 |
| T3 | 23.32±4.15 |
18.68±2.13a–c | 6.490 | <0.001 |
| T4 | 22.74±4.17 |
19.24±2.54a–c | 4.687 | <0.001 |
|
Fgroup |
8.298 | − | − | − |
|
Pgroup | <0.001 | − | − | − |
|
Fintercross | 21.311 | − | − | − |
|
Pintercross | <0.001 | − | − | − |
Blood oxygen saturation
(SpO2) (%) at different time-points
SpO2 of the experimental group was
slightly lower than that of T0 at T1-T4 (P>0.05). Compared with
T1, SpO2 of the experimental group decreased
significantly at T3 (P<0.05). Compared with T2, SpO2
of the experimental group decreased significantly at T3
(P<0.05). Compared with T3, SpO2 of the experimental
group decreased significantly at T4 (P<0.05). In the control
group, levels of SpO2 were significantly lower at T1-T4
than that at T0 (P<0.05). Compared with the experimental group
at the same time-point, SpO2 of the control group at
T1-T4 decreased significantly (P<0.05). Compared with T2,
SpO2 of the control group decreased significantly at T3
(P<0.05). Compared with T3, SpO2 of the control group
decreased significantly at T4 (P<0.05). Compared with the
experimental group at the same time-point, SpO2 of the
control group decreased significantly at T1-T4 (P<0.05), and
there was no significant difference between the two groups at T0
(P>0.05; Table III and Fig. 2).
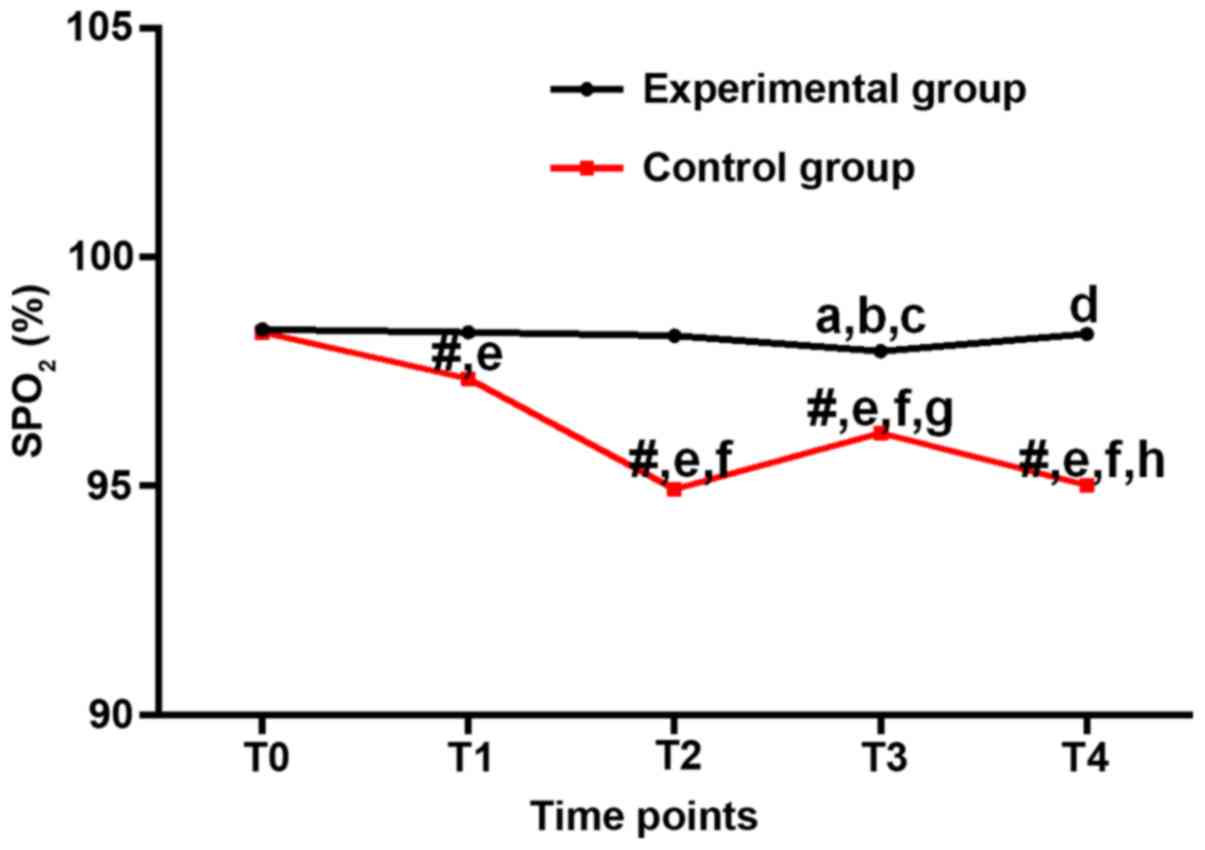 | Figure 2.Blood oxygen saturation (%) at
different time-points. In the experimental group, the differences
in SpO2 value were not statistically significant across
the time-points (P>0.05). In between-group comparison, the
SpO2 values were all higher in the experimental group,
and the difference at T2 was statistically significant (P<0.05).
#P<0.05, compared with the same time-point in the
experimental group; aP<0.05, compared with T0 in the
experimental group; bP<0.05, compared with T1 in the
experimental group; cP<0.05, compared with T2 in the
experimental group; dP<0.05, compared with T3 in the
experimental group; eP<0.05, compared with T0 in the
control group; fP<0.05, compared with T1 in the
control group; gP<0.05, compared with T2 in the
control group; hP<0.05, compared with T3 in the
control group. |
 | Table III.Blood oxygen saturation (%) at
different time-points. |
Table III.
Blood oxygen saturation (%) at
different time-points.
| Time-points | Experimental
group | Control group | t | P-value |
|---|
| T0 | 98.42±0.35 | 98.36±0.41 |
0.736 |
0.464 |
| T1 | 98.36±0.29 |
97.34±0.26e | 17.230 | <0.001 |
| T2 | 98.28±0.37 |
94.93±0.14e,f | 55.090 | <0.001 |
| T3 |
97.94±0.83a–c |
96.15±0.21e–g | 13.570 | <0.001 |
| T4 |
98.32±0.44d |
95.02±0.14e,f,h | 46.450 | <0.001 |
|
Fgroup | 82.000 | − | − | − |
|
Pgroup | <0.001 | − | − | − |
|
Fintercross | 82.000 | − | − | − |
|
Pintercross | <0.001 | − | − | − |
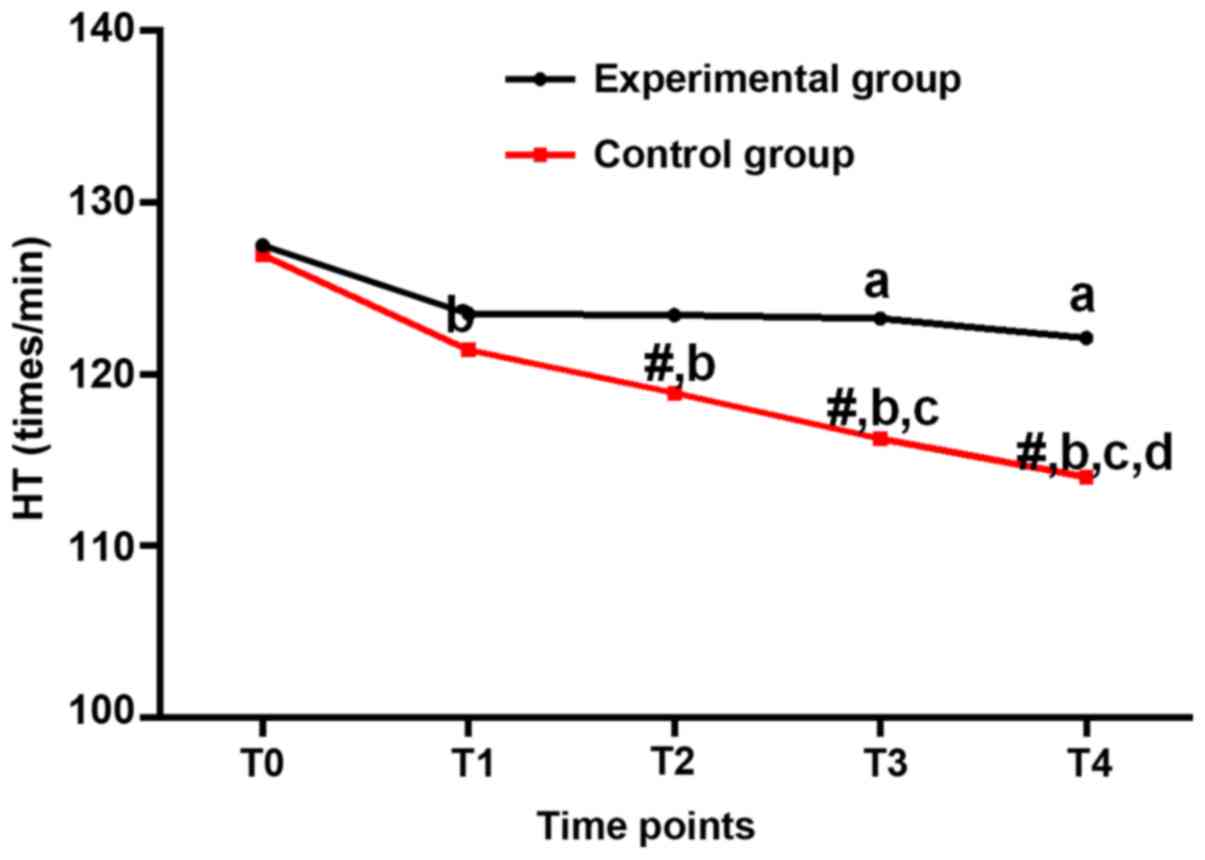
HR (beats per minute) at different
time-points
HR of the experimental group were lower at T3 and T4
than that at T0 (P<0.05). HR of the experimental and control
groups were lower at T1-T4 than that at T0 (P<0.05). HR of the
experimental group was higher than that of the control group at
T1-T4 (P<0.05). Compared with T1, HR of the control group
decreased significantly at T3 and T4 (P<0.05). Compared with T2,
HR of the control group decreased significantly at T4 (P<0.05).
HR was higher in the experimental group than in the control group
at T2-T4 (P<0.05), but no significant differences were found at
T0 and T1 (P>0.05; Table IV and
Fig. 3).
 | Table IV.Heart rates (beats/min) at different
time-points. |
Table IV.
Heart rates (beats/min) at different
time-points.
| Time-points | Experimental
group | Control group | t | P-value |
|---|
| T0 | 127.52±8.74 | 126.94±8.56 | 0.312 |
0.756 |
| T1 | 123.52±7.36 |
121.43±6.35b | 1.414 |
0.161 |
| T2 | 123.45±7.42 |
118.93±6.14b | 3.083 |
0.003 |
| T3 |
122.25±7.38a |
116.25±5.21b,c | 5.078 | <0.001 |
| T4 |
122.12±7.25a |
114.02±5.15b–d | 5.969 | <0.001 |
|
Fgroup | 27.461 | − | − | − |
|
Pgroup | <0.001 | − | − | − |
|
Fintercross |
1.528 | − | − | − |
|
Pintercross |
0.199 | − | − | − |
MAP (mmHg) at different
time-points
As shown in Table V,
MAP of the experimental and control groups were lower at T1-T4 than
that at T0 (P<0.05). Compared with T1, MAP of the experimental
group decreased at T3 and T4 (P<0.05). Compared with T1, MAP of
the control group decreased at T2-T4 (P<0.05). Compared with T2,
MAP of the control group decreased at T3 and T4 (P<0.05). MAP of
the control group was higher than that of the experimental group at
T2 but lower than that of the experimental group at T1-T4, no
significant differences were found at T0 (Fig. 4).
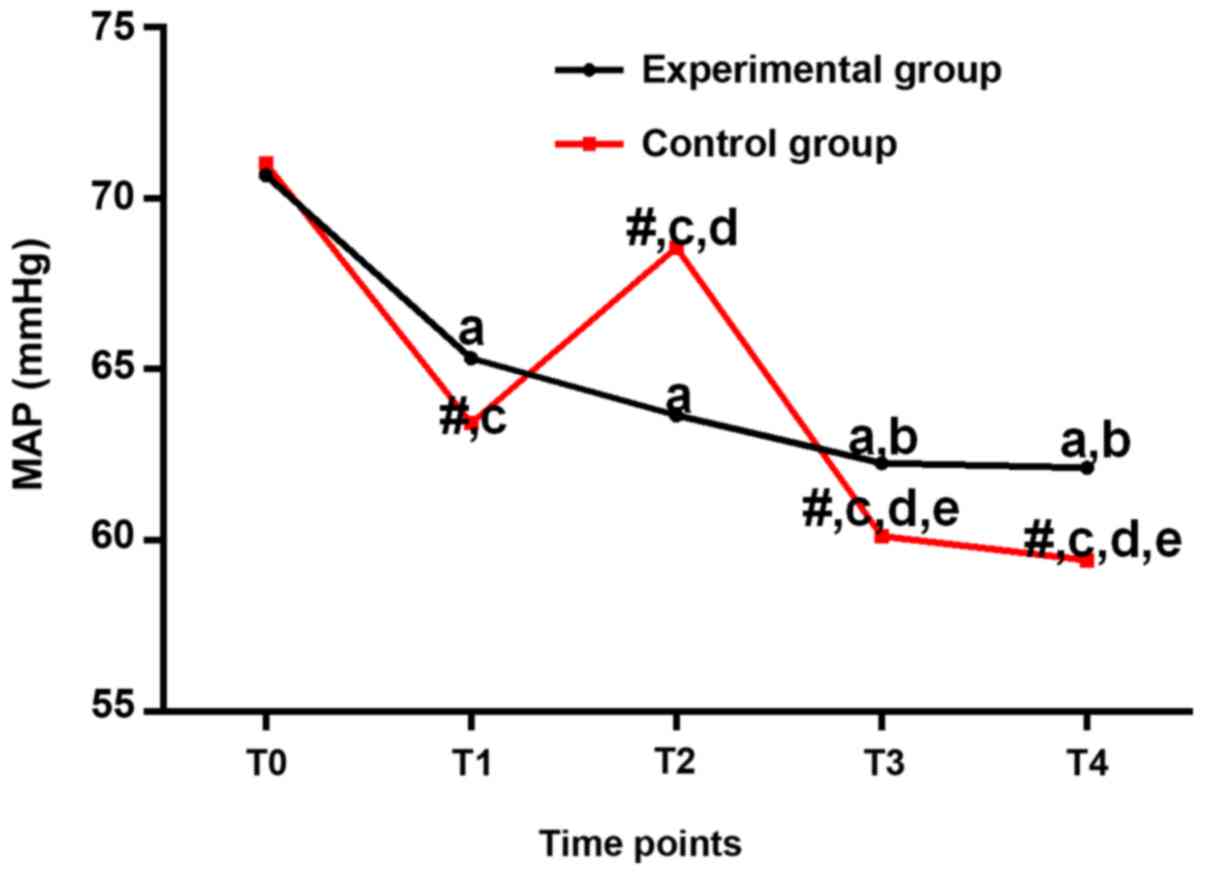 | Figure 4.Mean arterial pressures (mmHg) at
different time-points. In the experimental group, the MAP values
from T1 to T4 were all lower than that at T0 (P<0.05). In the
in-between-group comparison, the MAP value was lower at T2 but
higher at T1, T3 and T4 in the experimental group, and the
differences were statistically significant (P<0.05).
#P<0.05, compared with the same time-point in the
experimental group; aP<0.05, compared with T0 in the
experimental group; bP<0.05, compared with T1 in the
experimental group; cP<0.05, compared with T0 in the
control group; dP<0.05, compared with T1 in the
control group; eP<0.05, compared with T2 in the
control group. |
 | Table V.Mean arterial pressure value (mmHg)
at different time-points. |
Table V.
Mean arterial pressure value (mmHg)
at different time-points.
| Time-points | Experimental
group | Control group | t | P-value |
|---|
| T0 | 70.67±4.83 | 71.02±4.26 | 0.357 |
0.722 |
| T1 |
65.32±4.36a |
63.43±3.35c | 2.256 |
0.027 |
| T2 |
63.65±4.26a |
68.55±3.24c,d | 6.006 | <0.001 |
| T3 |
62.25±4.72a,b |
60.13±3.16c–e | 2.444 |
0.017 |
| T4 |
62.12±4.23a,b |
59.42±3.07c–e | 3.387 |
0.001 |
| F | 27.790 | 65.820 |
|
|
| P-value | <0.001 | <0.001 |
|
|
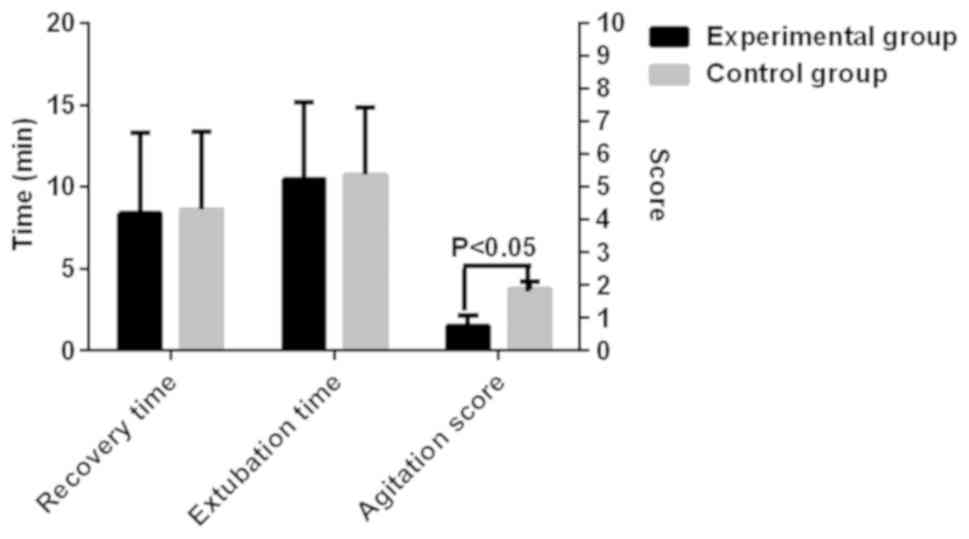
Post-anesthesia recovery in the two
groups of children
The recovery time of the experimental and control
groups were 8.37±4.93 and 8.63±4.74 min, respectively. There was no
significant difference between the two groups (P>0.05). The
extubation time of the two groups was 10.48±4.72 and 10.74±4.13
min, respectively. There was no difference in the extubation time
between the two groups (P>0.05). Agitation scores of the two
groups were 1.52±0.63 and 3.74±0.45, respectively. Agitation was
more severe in the control group than in the experimental group
(P<0.05; Fig. 5).
Discussion
Since there is no universal screening for common eye
diseases in newborns and preschool children in China, most RBs are
diagnosed as advanced intraocular RB group D or above at diagnosis
(16). Therefore, RB still poses a
significant health threat to infants and young children in China.
Currently, the clinical treatment of advanced RB is still the
enucleation of the affected eyeball to avoid further progression of
the disease (17). However, for
infants and young children whose organ systems are not fully
developed, conventional general anesthesia often has an inhibitory
effect on respiratory function (18).
For example, propofol is a widely used opioid analgesic in clinical
practice with an excellent analgesic effect (19). However, propofol can decrease vagal
tone, resulting in low extent of pulmonary expansion and collapse
due to participation of vagus nerve in pulmonary stretch reflexes.
Eventually the tidal volume decreases, and the respiratory function
is suppressed (20). Another example
is sufentanil, a strong opioid analgesic commonly used in clinical
practice. It has the advantage of fast onset and short half-life
(21). However, sufentanil has a
strong affinity and selectivity for the µ receptors, which are
distributed in the respiratory control center located in medulla
oblongata, resulting in alterations in the heart rate and tidal
volume. Eventually the respiratory function is affected (22).
Related studies showed that Dex is a highly
selective α adrenergic receptor agonist. It has excellent
anti-anxiety as well as strong analgesic and sedative effects
(23). According to literature, Dex
can reduce the output of sympathetic nerves by increasing the
output of parasympathetic nerves, thereby inhibiting the activity
of sympathetic nerves (24). The use
of Dex during the surgical procedure, can reduce the fluctuation of
hemodynamic parameters due to intraoperative events such as
intubation, extubation, awakening and stress response, and also
relieve the respiratory depression associated with the action of
other drugs (25). To the best of our
knowledge, there is no report in literature on the use of Dex in RB
surgical resection and its effect on improving the respiratory
function of pediatric patients with RB.
There were no significant differences in sex, age
and ASA classification between the two groups. Patients in the
experimental group received Dex. Their heart rates from T1 to T4
were all slightly lower than that at T0, but the differences were
not statistically significant. Patients in the control group
received saline, and their heart rate at T2 was significantly
higher than that at T0 (P<0.05), but the heart rates at T3 and
T4 were all significantly lower than that at T0 (P<0.05).
Overall changes in heart rate in the control group were larger than
those in the experimental group. In terms of SpO2,
values from T1 to T4 in the experimental group fluctuated slightly
compared with that at T0, but the differences were not
statistically significant (P>0.05). SpO2 values from
T1 to T4 in the control group were significantly lower than that at
T0 (P<0.05), which was consistent with a trend demonstrated by
large fluctuations of respiratory indexes in the control group. The
intense and repeated tracheal stimulation resulted from tracheal
intubation may cause fluctuations in the respiratory indexes. In
the experimental group, however, the difference in the respiratory
index between time-points T1 and T2 was not statistically
significant, which suggested that Dex had a significant effect on
relieving respiratory depression after the induction of general
anesthesia. According to literature, the sedative effect of Dex
does not impact spontaneous breathing (26). Dex exerts a mild analgesic effect and
induces natural non-eye-movement sleep by agonizing α-adrenergic
receptors in the brainstem locus coeruleus. Subjects in this
sedated state can still be awakened (27). We think that this excitatory mechanism
may not impact the vagal tone, and thus, it avoids the suppression
signal from the respiratory control center in the medulla
oblongata. In a word, Dex avoids perioperative respiratory
depression from the port.
In terms of hemodynamics, our results showed that HR
and MAP values from T1 to T4 were all lower than those at T0 in
both the experimental and control groups. HR values in the
experimental group were obviously higher than those at the same
time-point in the control group. Compared with the control group,
MAP value was lower at T2, but higher at T1-T4 in the experimental
group. MAP values in the experimental group fluctuated within a
smaller range compared with those in the control group, suggesting
that Dex can counter abnormal decreases of heart rate and blood
pressure caused by other drugs, thereby stabilizing hemodynamic
parameters. According to literature, slow pump infusion of Dex can
prevent activation of the q receptors located in the vascular
smooth muscle, thereby relieving vasoconstriction (28). In addition, Dex can also counter
abnormal increases of blood pressure and heart rate caused by
stimulation of other drugs of the sympathetic nerves due to its
inhibitory effect on sympathetic nerve activity (29). Our findings suggested that Dex can
inhibit the release of catecholamines by acting on α receptors,
thereby attenuating the physiological effects of catecholamines
such as increases in myocardial contractility, cardiac stroke
volume, and heart rate (30). In this
way, Dex stabilizes intraoperative hemodynamics.
Analysis of post-anesthesia resuscitation showed no
significant difference in both the recovery and extubation time
between the two groups, but agitation was more severe in the
control group than in the experimental group. Clinical studies have
shown that children are often prone to anxiety and confusion after
anesthesia. It has been reported that Dex can reduce the incidence
of mental confusion in children (31). Compared with other commonly used
sedative drugs such as Mita, Dex reduces the incidence of
postoperative agitation in children (32). In the pediatric strabismus anesthesia
for the same eye surgery, it has been reported that (33) intraoperative low-dose injection of Dex
can reduce the frequency of fentanyl rescue, and the maximum value
of intraoperative anxiety and discussion scores are smaller than
those in the normal saline group. These studies have shown that Dex
may significantly improve postoperative agitation in children.
In this study, the effect of Dex on respiratory
function in pediatric patients undergoing RB resection was
explored. In our future studies, interaction between multiple
anesthetic sedatives will be considered. Animal models will also be
established to explore the underlying mechanism. This study did not
include the prognosis of respiratory function in children with RB,
which should also be included in our future studies.
In summary, Dex can decrease surgical difficulties
and increase anesthetic safety by improving the respiratory
function and maintaining hemodynamic stability during anesthesia
induction in children undergoing RB resection, so as to reduce
surgical difficulty and increase anesthesia safety.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XR and CS recorded and analyzed indexes of
respiratory function and hemodynamics. FZ and JZ were responsible
for anesthetic procedures. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Yidu Central Hospital of Weifang (Weifang, China). Parents of the
child patients who participated in this research signed an informed
consent and the children had complete clinical data.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Schlößer HA, Drebber U, Kloth M, Thelen M,
Rothschild SI, Haase S, Garcia-Marquez M, Wennhold K, Berlth F,
Urbanski A, et al: Immune checkpoints programmed death 1 ligand 1
and cytotoxic T lymphocyte associated molecule 4 in gastric
adenocarcinoma. OncoImmunology. 5:e11007892015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kamihara J, Ma C, Fuentes Alabi SL,
Garrido C, Frazier AL, Rodriguez-Galindo C and Orjuela MA:
Socioeconomic status and global variations in the incidence of
neuroblastoma: call for support of population-based cancer
registries in low-middle-income countries. Pediatr Blood Cancer.
64:321–323. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Friedman DL, Krailo M, Villaluna D, Gombos
D, Langholz B, Jubran R, Shields C, Murphree L, O'Brien J, Kessel
S, et al: Systemic neoadjuvant chemotherapy for Group B intraocular
retinoblastoma (ARET0331): a report from the Children's Oncology
Group. Pediatr Blood Cancer. 64:e263942017. View Article : Google Scholar
|
|
4
|
Kelly KR, DeSimone KD, Gallie BL and
Steeves JK: Increased cortical surface area and gyrification
following long-term survival from early monocular enucleation.
Neuroimage Clin. 7:297–305. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dimaras H, Corson TW, Cobrinik D, White A,
Zhao J, Munier FL, Abramson DH, Shields CL, Chantada GL, Njuguna F,
et al: Retinoblastoma. Nat Rev Dis Primers. 1:150212015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chantada G and Schaiquevich P: Management
of retinoblastoma in children: current status. Paediatr Drugs.
17:185–198. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mathew AA, Sachdev N, Staffieri SE,
McKenzie JD and Elder JE: Superselective intra-arterial
chemotherapy for advanced retinoblastoma complicated by metastatic
disease. J AAPOS. 19:72–74. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gomez de Agüero M, Ganal-Vonarburg SC,
Fuhrer T, Rupp S, Uchimura Y, Li H, Steinert A, Heikenwalder M,
Hapfelmeier S, Sauer U, et al: The maternal microbiota drives early
postnatal innate immune development. Science. 351:1296–1302. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Taenzer A, Walker BJ, Bosenberg AT, Krane
EJ, Martin LD, Polaner DM, Wolf C and Suresh S: Interscalene
brachial plexus blocks under general anesthesia in children: is
this safe practice?: a report from the Pediatric Regional
Anesthesia Network (PRAN). Reg Anesth Pain Med. 39:502–505. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Davis PJ and Cladis FP: Smith's Anesthesia
for Infants and Children (e-book). 9th edition. Elsevier,
Philadelphia. 2017.
|
|
11
|
Wang X, Zhao B and Li X: Dexmedetomidine
attenuates isoflurane-induced cognitive impairment through
antioxidant, anti-inflammatory and anti-apoptosis in aging rat. Int
J Clin Exp Med. 8:17281–17288. 2015.PubMed/NCBI
|
|
12
|
Sankar A, Johnson SR, Beattie WS, Tait G
and Wijeysundera DN: Reliability of the American Society of
Anesthesiologists physical status scale in clinical practice. Br J
Anaesth. 113:424–432. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Akbulut UE, Saylan S, Sengu B, Akcali GE,
Erturk E and Cakir M: A comparison of sedation with
midazolam-ketamine versus propofol-fentanyl during endoscopy in
children: a randomized trial. Eur J Gastroenterol Hepatol.
29:112–118. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bellolio MF, Puls HA, Anderson JL, Gilani
WI, Murad MH, Barrionuevo P, Erwin PJ, Wang Z and Hess EP:
Incidence of adverse events in paediatric procedural sedation in
the emergency department: a systematic review and meta-analysis.
BMJ Open. 6:e0113842016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bong CL, Lim E, Allen JC, Choo WL, Siow
YN, Teo PB and Tan JS: A comparison of single-dose dexmedetomidine
or propofol on the incidence of emergence delirium in children
undergoing general anaesthesia for magnetic resonance imaging.
Anaesthesia. 70:393–399. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
de Jong MC, de Graaf P, Brisse HJ,
Galluzzi P, Göricke SL, Moll AC, Munier FL, Popovic MB, Moulin AP,
Binaghi S, et al: European Retinoblastoma Imaging Collaboration
(ERIC) The potential of 3T high-resolution magnetic resonance
imaging for diagnosis, staging, and follow-up of retinoblastoma.
Surv Ophthalmol. 60:346–355. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim JY and Park Y: Treatment of
retinoblastoma: The role of external beam radiotherapy. Yonsei Med
J. 56:1478–1491. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lerman J, Coté CJ and Steward DJ: Manual
of pediatric anesthesia. Springer; New York, NY: 2016, View Article : Google Scholar
|
|
19
|
Baradari AG, Alipour A, Habibi MR,
Rashidaei S and Emami Zeydi A: A randomized clinical trial
comparing hemodynamic responses to ketamine-propofol combination
(ketofol) versus etomidate during anesthesia induction in patients
with left ventricular dysfunction undergoing coronary artery bypass
graft surgery. Arch Med Sci. 13:1102–1110. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Andolfatto G, Abu-Laban RB, Zed PJ,
Staniforth SM, Stackhouse S, Moadebi S and Willman E:
Ketamine-propofol combination (ketofol) versus propofol alone for
emergency department procedural sedation and analgesia: a
randomized double-blind trial. Ann Emerg Med. 59:504–512, e501-502.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xiao F, Xu WP, Zhang YF, Liu L, Liu X and
Wang LZ: The dose-response of intrathecal ropivacaine
co-administered with sufentanil for cesarean delivery under
combined spinal-epidural anesthesia in patients with scarred
uterus. Chin Med J (Engl). 128:2577–2582. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Youssef N, Orlov D, Alie T, Chong M, Cheng
J, Thabane L and Paul J: What epidural opioid results in the best
analgesia outcomes and fewest side effects after surgery?: a
meta-analysis of randomized controlled trials. Anesth Analg.
119:965–977. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang H, Yan X, Wang DG, Leng YF, Wan ZH,
Liu YQ and Zhang Y: Dexmedetomidine relieves formaldehyde-induced
pain in rats through both α2 adrenoceptor and imidazoline receptor.
Biomed Pharmacother. 90:914–920. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Julien C, Oréa V, Quintin L, Piriou V and
Barrès C: Renal sympathetic nerve activity and vascular reactivity
to phenylephrine after lipopolysaccharide administration in
conscious rats. Physiol Rep. 5:52017. View Article : Google Scholar
|
|
25
|
Song J, Ji Q, Sun Q, Gao T, Liu K and Li
L: The opioid-sparing effect of intraoperative dexmedetomidine
infusion after craniotomy. J Neurosurg Anesthesiol. 28:14–20. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sato N, Saiki C, Tamiya J, Imai T and
Sunada K: Imidazoline 1 receptor activation preserves respiratory
drive in spontaneously breathing newborn rats during
dexmedetomidine administration. Paediatr Anaesth. 27:506–515. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim DJ, Kim SH, So KY and Jung KT: Effects
of dexmedetomidine on smooth emergence from anaesthesia in elderly
patients undergoing orthopaedic surgery. BMC Anesthesiol.
15:1392015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li Q, Chen C, Chen X, Han M and Li J:
Dexmedetomidine attenuates renal fibrosis via α2-adrenergic
receptor-dependent inhibition of cellular senescence after renal
ischemia/reperfusion. Life Sci. 207:1–8. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mandal D, Das A, Chhaule S, Halder PS,
Paul J, RoyBasunia S, Chattopadhyay S and Mandal SK: The effect of
dexmedetomidine added to preemptive (2% lignocaine with adrenaline)
infiltration on intraoperative hemodynamics and postoperative pain
after ambulatory maxillofacial surgeries under general anesthesia.
Anesth Essays Res. 10:324–331. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mikami M, Zhang Y, Kim B, Worgall TS,
Groeben H and Emala CW: Dexmedetomidine's inhibitory effects on
acetylcholine release from cholinergic nerves in guinea pig
trachea: a mechanism that accounts for its clinical benefit during
airway irritation. BMC Anesthesiol. 17:522017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Whitman TM: Emergence delirium in
children: review and rationale for the use of dexmedetomidine for
prevention. J Pediatr Surg Nurs. 7:41–46. 2018. View Article : Google Scholar
|
|
32
|
Pasin L, Febres D, Testa V, Frati E,
Borghi G, Landoni G and Zangrillo A: Dexmedetomidine vs midazolam
as preanesthetic medication in children: a meta-analysis of
randomized controlled trials. Paediatr Anaesth. 25:468–476. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim J, Kim SY, Lee JH, Kang YR and Koo BN:
Low-dose dexmedetomidine reduces emergence agitation after
desflurane anaesthesia in children undergoing strabismus surgery.
Yonsei Med J. 55:508–516. 2014. View Article : Google Scholar : PubMed/NCBI
|