Introduction
Neurofibromatosis type 1 (NF1), also known as von
Recklinghausen disease, is an autosomal dominant disorder that
manifests as a number of symptoms, including pigmentary changes
(café-au-lait spots, skin fold freckling and Lisch nodules) and
neurofibromas (1). NF1 is known to
occur by heterozygous germ-line mutation of the NF1 gene,
which is located on the pericentromeric region of the long arm of
chromosome 17 and encodes the 327-kd protein known as neurofibromin
(2,3).
The function of neurofibromin remains unknown. Patients with NF1
have an increased risk for the development of malignancies,
particularly malignant peripheral nerve sheath tumors, optic and
other glioma types, including cerebellar astrocytomas, ependymomas
third-ventricle astrocytomas, cerebral astrocytomas, brain stem
gliomas and spinal cord tumors, and leukemia and breast cancer
(4). The characteristics of patients
with breast cancer with NF1 have been reported to include negative
estrogen receptor (ER), negative progesterone receptor (PgR),
amplified human epidermal growth factor receptor 2 (HER2), higher
tumor grade, and low 5-year survival rate compared with the control
group (5). As the NF1 gene and
the breast cancer susceptibility gene 1 (BRCA1) DNA
repair-associated gene are located on the long arm of chromosome
17, an association between the two genes has been reported
(6).
The present study reports the case of a female
patient who exhibited typical NF1 and was diagnosed with
metachronous bilateral breast cancer. In addition, a literature
review is presented of reported Japanese cases.
Case report
A 67-year-old female was referred to the National
Defense Medical College Hospital (Saitama, Japan) due to a palpable
lump in the right breast in December 2016. The past medical history
of the patient included NF1 at the age of 25 years and a Halsted
radical mastectomy for left-sided breast cancer (T2N0M0 stage IIA)
at the age of 33 years. Following this original surgery, the
patient did not receive adjuvant chemotherapy.
During the present physical examination, a 2-cm
tumor was palpated in the upper-outer quadrant of the right breast.
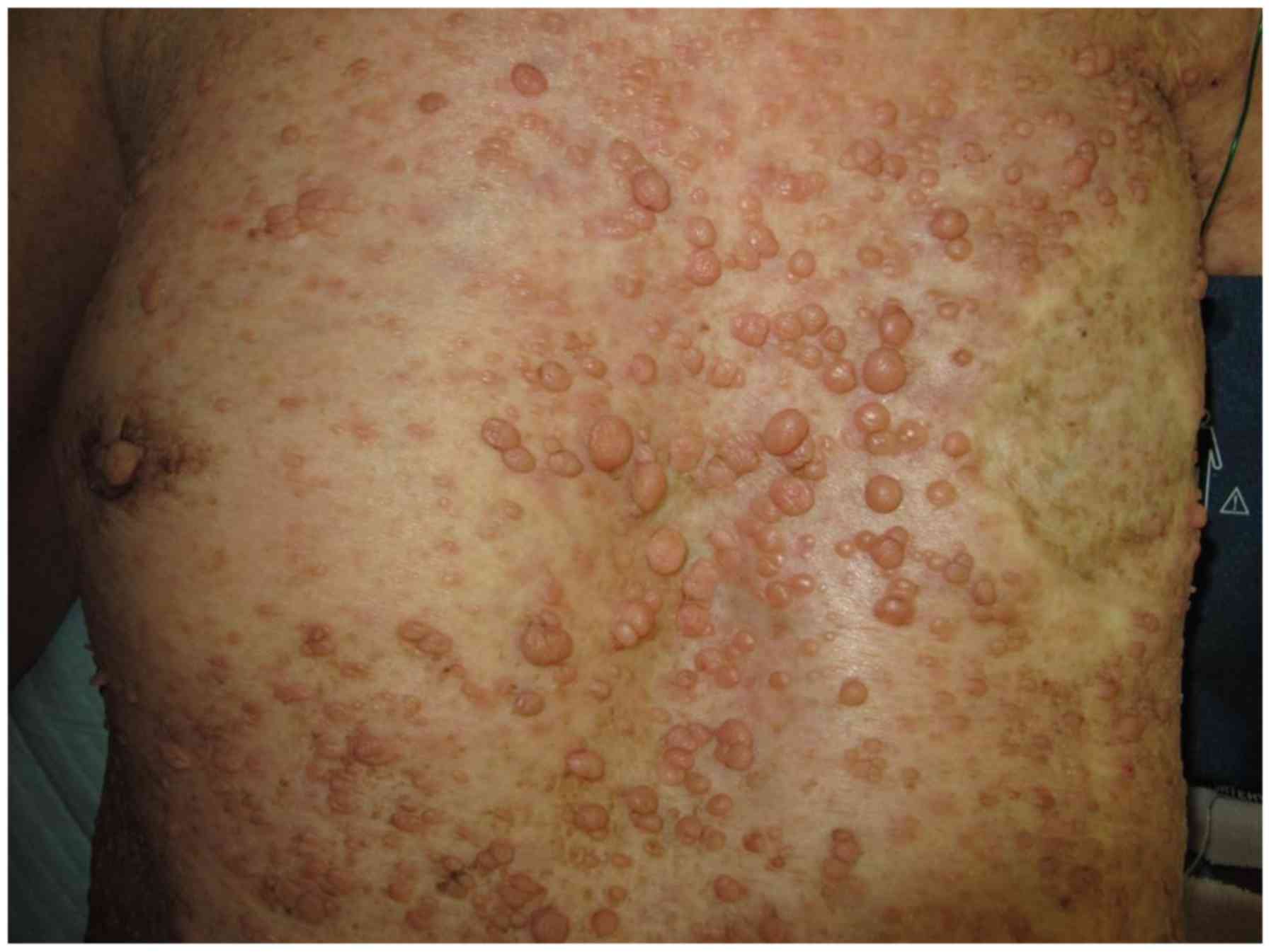
The patient presented with classical NF1, indicated by
neurofibromas over the trunk (Fig.
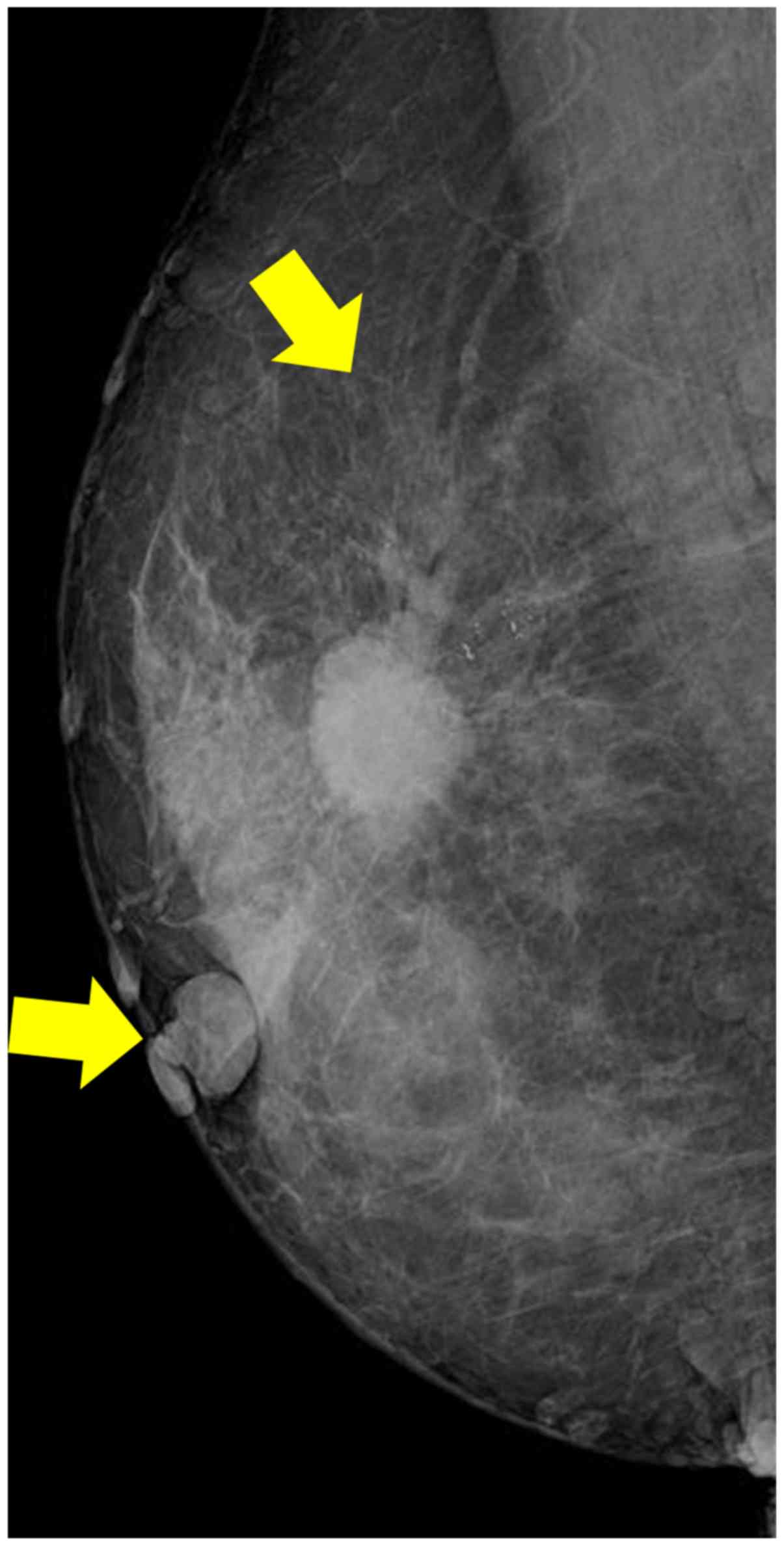
1). A high-density microlobulated mass with microcalcifications
was observed in the right breast on a mammogram (Fig. 2). The mass was 1.8×1.7 cm in size on
ultrasound. Core needle biopsy specimens from the mass revealed
invasive ductal carcinoma. Under the clinical diagnosis of T2N0M0
right-sided breast cancer, the patient underwent a modified radical
mastectomy and sentinel node biopsy. As the sentinel node was
intraoperatively diagnosed as positive for cancer, an axillary
dissection was also performed.
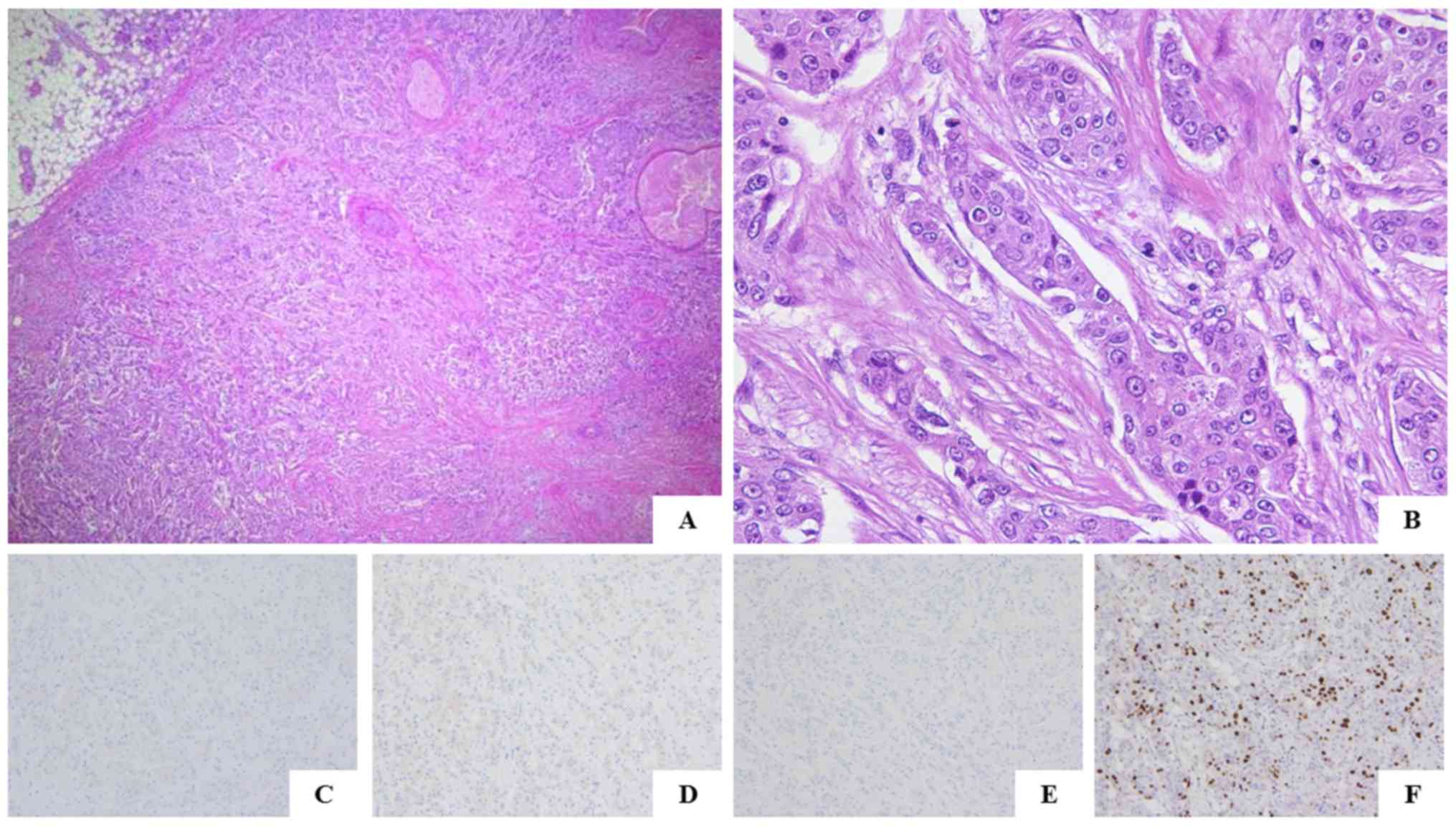
The histopathological diagnosis of the
surgically-resected specimens was invasive ductal carcinoma of no
special type, nuclear grade 3 (Fig. 3A
and B). The right-sided breast tumor was negative for ER, PgR
and HER2. The Ki-67 labeling index was 26.5% (Fig. 3C-F). On histological review of the
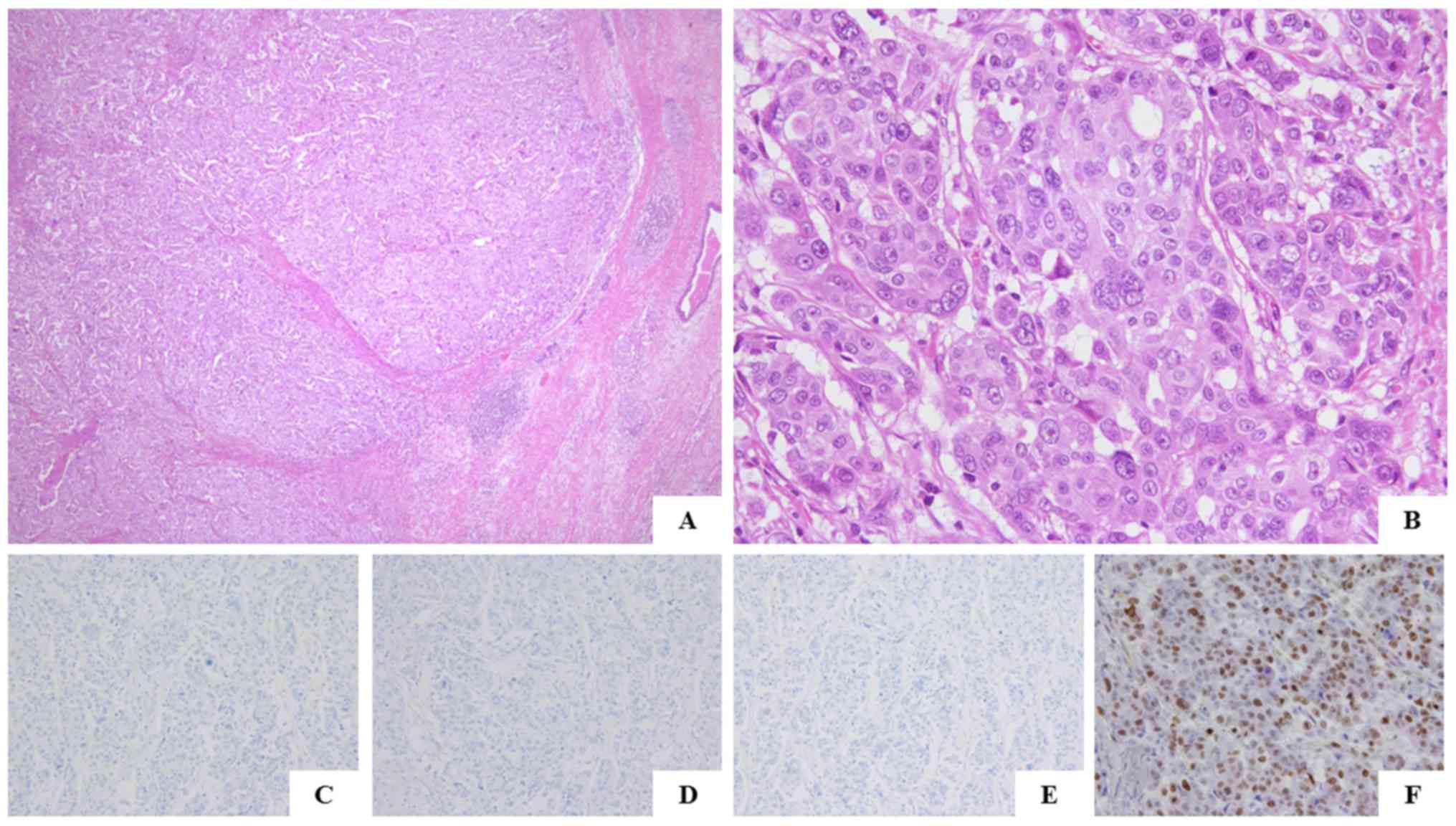
surgical specimens from the left-sided breast cancer obtained 34
years ago, the invasion range of the tumor was 5.0 cm, and the
tumor was diagnosed as an invasive, ductal, solid-tubular
carcinoma, nuclear grade 3 (Fig. 4A and
B). The pathological features of these bilateral types of
breast cancer were similar. The left-sided breast cancer was also
negative for ER, PgR and HER2, with a Ki-67 labeling index of 74.0%
(Fig. 4C-F). The tumors were positive
for the basal-like markers epidermal growth factor receptor (EGFR)
and cytokeratin 5/6, and partially positive for vimentin. The
patient is currently undergoing adjuvant therapy with epirubicin
and cyclophosphamide, and treatment with docetaxel, with no
evidence of recurrence in the 20 months following the most recent
surgical treatment, according to the patient's 3 month
follow-ups.
Materials and methods
Histological examination and
immunohistochemistry
Histological examination was performed on the
surgically-resected specimens fixed with 10% formalin for 24 h at
room temperature. Paraffin-embedded sections (thickness, 4-µm) was
performed using hematoxylin-eosin (H&E) staining. For H&E
staining, sections were stained with 0.12 g/v% hematoxylin solution
produced by Hematoxylin (C.I. 75290) cryst. (cat. no. 104302; Merck
KGaA, Darmstadt, Germany) three times for 3 min/stain at room
temperature, and with eosin Y (cat. no. 058-00062; Wako Pure
Chemical Industries, Ltd., Osaka, Japan) two times for 1.5
min/stain at room temperature. The sections were treated with 5%
hydrogen peroxide for 5 min, followed by incubation with 0.4% block
ace (cat. no. UK-B80; DS pharma, Japan) for 10 min at room
temperature. The following commercially available primary
antibodies were used: ER (cat. no. IR084; dilution, 1:100); PgR
(cat. no. IR068; dilution, 1:100); HER2 (cat. no. A0485; dilution,
1:100); Ki-67 (cat. no. M7240); Cytokeratin 5/6 (CK5/6; cat. no.
M7237; dilution, 1:50) and Vimentin (cat. no. M0725; dilution,
1:500; all Dako; Agilent Technologies, Inc., Santa Clara, CA, USA)
at 4°C overnight, and subsequently incubated with Histofine
Simplestain Max PO (cat. no. 424151; Nichirei Biosciences, Inc.
Tokyo, Japan) as the secondary antibody for 1 h at room
temperature. Immunohistochemical staining for EGFR was performed
using the EGFR pharmDx™ kit (Dako; Agilent Technologies, Inc.,
Santa Clara, CA, USA). Sections were deparaffinized in three
sequential xylene baths for 5 min at room temperature, 100% ethanol
for 3 min and 95% ethanol for 3 min, followed by a 5-min single
wash in wash-buffer solution (Dako; Agilent Technologies, Inc.).
Subsequently, at room temperature, the section was rinsed in
wash-buffer for 5 min, incubated in proteinase K solution (Dako;
Agilent Technologies, Inc.) for 5 min, rinsed again in the
wash-buffer for 5 min, incubated in peroxidase blocking agent for 5
min at room temperature, rinsed, incubated with the primary EGFR
antibody (clone 2-18C9, mouse monoclonal, prediluted) for 30 min at
room temperature, rinsed, incubated with visualization reagent for
30 min, rinsed twice with the buffer, incubated with
3,3′-Diaminobenzidine for 5 min at room temperature and finally
rinsed again with the buffer. Slides were counterstained with 0.12
g/v% hematoxylin solution produced by Hematoxylin cryst. (C.I.
75290; cat. no. 104302; Merck KGaA, Darmstadt, Germany) three times
for 3 min/stain at room temperature, and rinsed gently in reagent
quality water. Slides were observed under a fluorescent microscope
(magnification, ×40, ×200, and ×400).
Histological study
ER and PgR were assessed by immunohistochemistry and
defined as positive when ≥1% of constituent carcinoma cells were
immunoreactive (7). Judgment of HER2
was made according to the American Society of Clinical
Oncology/College of American Pathologists guideline 2013 (8). Ki-67 was evaluated according to the
recommendation of Breast Cancer Working Group (9). Histological diagnosis and pathological
stage were determined by the clinical and pathological recording of
breast cancer by Union for International Cancer Control (UICC)
(10).
Statistical analysis
A total of 90 previously reported cases of breast
cancer in 84 Japanese patients with NF1 were assessed. Furthermore,
the 90 cases of breast cancer associated with NF1 were compared
with the 86,478 cases of the Breast Cancer Registry of the Japanese
Breast Cancer Society in 2015 as a reference population (11). The compared parameters were
laterality, age, UICC pStage, histological type, ER, PgR, HER2. A
total of 82,842 patients without distant metastasis, histological
type, ER, PgR, and HER2 were examined. The χ2 test or
Fisher's exact test was used to determine the association between
patients with NF1 and the general population, for bilateral and
unilateral breast cancer, and other parameters, in patients with
NF1. Differences were considered statistically significant when
P<0.05. All statistical analyses were performed using
JMP® Pro 12 (SAS Institute Inc., Cary, NC, USA). Results
are discussed in Tables I and
II and the Discussion section.
 | Table I.Comparison of clinicopathological
features between patients with breast cancer with neurofibromatosis
type 1 (11) and patients with breast
cancer surgically resected in Japan in 2015. |
Table I.
Comparison of clinicopathological
features between patients with breast cancer with neurofibromatosis
type 1 (11) and patients with breast
cancer surgically resected in Japan in 2015.
| Parameter | NF1 patients (n=84;
n=90 affected breasts) | General population
(n=86,478; n=86,478 breasts) | P-value |
|---|
| Laterality, n
(%) |
|
| 0.8532 |
|
Unilateral | 77 (91.7) | 78,173 (90.4) |
|
|
Bilateral | 7 (8.3) | 8,305 (9.6) |
|
| Age, years |
|
| <0.0001 |
| Median,
range | 53, 25–88 | 60, unknown |
|
| <35, n
(%) | 10 (11.9) | 1,579 (1.8) |
|
| ≥35, n
(%) | 72 (85.7) | 84,775 (98.0) |
|
| Unknown,
n (%) | 2 (2.4) | 124 (0.2) |
|
| UICC pStage, n (%)
(10) |
|
| <0.0001 |
| 0, I | 15 (16.7) | 46,662 (53.9) |
|
| II, III,
IV | 61 (67.8) | 37,334 (43.2) |
|
|
Unknown | 14 (15.6) | 2,482 (2.9) |
|
| Histological type, n
(%) |
|
| <0.0001 |
| DCIS | 4 (4.4) | 10,908
(13.2)a |
|
| IDC | 58 (64.4) | 59,941
(72.3)a |
|
| Special
type | 18 (20.0) | 10,212
(12.3)a |
|
|
Unknown | 10 (11.1) | 1,781
(2.2)a |
|
| ER, n (%) |
|
| <0.0001 |
|
Positive | 14 (15.6) | 60,768
(73.4)a |
|
|
Negative | 22 (24.4) | 12,021
(14.5)a |
|
|
Unknown | 54 (60.0) | 10,053
(12.1)a |
|
| PgR, n (%) |
|
|
|
|
Positive | 11 (12.2) | 52,997
(64.0)a | <0.0001 |
|
Negative | 23 (25.6) | 19,330
(23.3)a |
|
|
Unknown | 56 (62.2) | 10,515
(12.7)a |
|
| HER2, n (%) |
|
| >0.999 |
|
Positive | 1 (1.1) | 9,817
(11.9)a |
|
|
Negative | 5 (5.6) | 54,702
(66.0)a |
|
|
Unknown | 84 (93.3) | 18,323
(22.1)a |
|
 | Table II.Comparison of clinicopathological
factors between bilateral breast cancer and unilateral breast
cancer in patients with neurofibromatosis type 1. |
Table II.
Comparison of clinicopathological
factors between bilateral breast cancer and unilateral breast
cancer in patients with neurofibromatosis type 1.
| Parameter | Bilateral breast
cancer (n=7; n=13 affected breasts) | Unilateral breast
cancer (n=77; n=77 affected breasts) | P-value |
|---|
| Age at first breast
cancer, years |
|
| 0.1542 |
| Median,
range | 45, unknown | 53, unknown |
|
| <35,
n (%) | 2 (28.6) | 8 (10.4) |
|
| ≥35, n
(%) | 4 (57.1) | 68 (88.3) |
|
|
Unknown, n (%) | 1 (14.3) | 1 (1.3) |
|
| UICC pStage, n (%)
(10) |
|
| 0.1178 |
| 0,
I | 5 (38.5) | 10 (13.0) |
|
| II,
III, IV | 8 (61.5) | 53 (68.8) |
|
|
Unknown | 0 (0) | 14 (18.2) |
|
| Histological type,
n (%) |
|
| 0.1030 |
|
DCIS | 2 (15.4) | 2 (2.6) |
|
|
IDC | 9 (69.2) | 49 (63.6) |
|
| Special
type | 1 (7.7) | 17 (22.1) |
|
|
Unknown | 1 (7.7) | 9 (11.7) |
|
| ER, n (%) |
|
| 0.0625 |
|
Positive | 0 (0) | 14 (18.2) |
|
|
Negative | 6 (46.2) | 16 (20.8) |
|
|
Unknown | 7 (53.8) | 47 (61.0) |
|
| PgR, n (%) |
|
| 0.6379 |
|
Positive | 1 (7.7) | 10 (13.0) |
|
|
Negative | 5 (38.5) | 18 (23.4) |
|
|
Unknown | 7 (53.8) | 49 (63.6) |
|
| HER2, n (%) |
|
| >0.9999 |
|
Positive | 0 (0) | 1 (1.3) |
|
|
Negative | 2 (15.4) | 3 (3.9) |
|
|
Unknown | 11 (84.6) | 73 (94.8) |
|
Discussion
NF1 is an autosomal dominant genetic disorder caused
by germline mutation of the NF1 gene, with an estimated
birth incidence of 1 in 2,000–5,000 individuals (12). In total, 40,000 people are estimated
to be affected in Japan (1). The
diagnosis of NF1 is made based on the presence of >6
café-au-lait spots and multiple neurofibromas. In patients with
NF1, half do not present with a family history, and therefore, the
disease is considered to be caused by a de novo germline
NF1 mutation (13).
Patients with NF1 have an increased risk of
developing malignant peripheral nerve sheath tumors, optic and
other gliomas, including cerebellar astrocytomas, ependymomas
third-ventricle astrocytomas, cerebral astrocytomas, brain stem
gliomas, and spinal cord tumors, and leukemia (4). Epidemiological studies have demonstrated
that patients with NF1 are 4-times more likely to develop malignant
tumors compared with the general population (14,15).
Furthermore, the risk of breast cancer in younger patients with NF1
(<50 years old) has been reported to be 2.68- to 4.9-times
higher compared with that in the general population (16–18).
Screening mammography is recommended for women ≥40 years old who
are affected by NF1 (16). The number
of ductal carcinoma in situ (DCIS) cases has risen with the
recent increase in breast cancer screening. Uusitalo et al
(5) reported that 32 out of 1,404
patients with NF1 were diagnosed with breast cancer. In this study,
the characteristics of patients with breast cancer with NF1
included negative ER, negative PgR and amplified HER2 results,
higher tumor grade and a low 5-year survival rate (68.1% in
patients with NF1 vs. 82.0% in the control group).
A total of 90 previously reported cases of breast
cancer in 84 Japanese patients with NF1 were assessed (Table I) (11).
In one case of bilateral breast cancer, the details of the initial
breast cancer were unknown. The mean age of these patients with
breast cancer was 52.6 years (range, 25–88 years). Only in one case
was the patient male. The rate of bilateral breast cancer was 8.3%
(7/84). When the 90 cases of breast cancer associated with NF1 were
compared with the 86,478 cases of breast cancer resected in Japan
in 2015 as a reference population, the NF1-associated breast
cancers were characterized by a younger age (P<0.0001), a more
advanced clinical stage (P<0.0001), and ER and PgR negativity
(P<0.0001). Among the patients with breast cancer associated
with NF1, the percentage of patients <35 years was 11.9%, which
was higher compared with that in the reference group (1.8%).
Nakamura et al (19) similarly
reported that the rate of patients with breast cancer and NF1 who
were ≤35 years was 18.5%. Among the 90 cases of breast cancer, only
16.7% (15/90) were diagnosed as early-stage breast cancer (UICC;
stage 0 or I). Therefore, the majority of cases were diagnosed at
stage II or at a more advanced stage. Murayama et al
(20) considered that breast tumors
were overlooked until they increased in size due to the symptoms of
systemic neurofibromatosis. In the present case, only following an
extensive amount of time (2 years) did the patient realize that the
breast tumor may be different from a skin tumor. In addition, the
present case verified that another characteristic of NF1-associated
breast cancer is ER (22/36, 61.1%) and PgR (23/34, 67.6%)
negativity. With regard to HER2, Uusitalo et al (5) reported that HER2 was positive in 30.8%
of NF1-associated breast cancers. However, in the present
literature review, the HER2-positive rate was only 16.7% (1/6).
Following the division of the 84 patients into two
groups representing unilateral and bilateral cancer, it was
observed that the clinicopathological characteristics slightly
differed. The rate of ER-negative tumors was higher among the
bilateral NF1 cases compared with that among the unilateral NF1
cases (100% vs. 53%; P=0.063), although there was no statistical
difference. Likewise, bilateral cases had a higher percentage of
stage 0 or I breast cancer, DCIS and patients <35 years old
compared with unilateral cases, although these results were not
statistically significant (Table
II). There was no significant difference in PgR or HER2 status
between the unilateral and bilateral groups.
The NF1 gene is located on the
pericentromeric region of the long arm of chromosome 17. This gene
encodes neurofibromin, which is composed of 59 exons, spanning 350
kD of genomic DNA; it inhibits Ras activity by facilitating the
conversion of active Ras-GTP to inactive Ras-GDP (21).
The NF1 and BRCA1 genes are located on
the long arm of chromosome 17, and are topologically relatively
close to each other with a genetic distance of 20 cM. Ceccaroni
et al (6) reported that two
linked mutations at NF1 and BRCA1 caused concurrent
NF1, and hereditary breast and ovarian cancer (HBOC) in the
same family. In addition, previous reports have examined single
germline mutations in NF1 and BRCA1 genes in the same
individual (22,23). In these reports, examination of a
BRCA1 gene mutation as an early screening method for
patients with NF1 was indicated to aid the early detection of
breast cancer, thus reducing morbidity and mortality rates.
In the National Comprehensive Cancer Network
guidelines for genetic/familial high-risk assessment of breast and
ovarian cancer, detailed genetic risk assessment is recommended for
patients ≤50 years old, for patients ≤60 years old with
triple-negative breast cancer and for patients with a history of
two primary types of breast cancer (24). Based on the aforementioned criteria,
the present patient had a high genetic risk for HBOC, and genetic
testing for BRCA1/2 may have been beneficial for early
detection of the second breast cancer.
In summary, the present study reports the case of an
NF1 patient with metachronous bilateral breast cancer. The two
breast cancers were diagnosed as triple-negative and basal-like
subtype by immunohistochemistry. Based on a literature review of 84
patients, a younger age onset, an advanced clinical stage and
hormone receptor negativity were indicated to be characteristic
features of breast cancer in patients with NF1.
Acknowledgements
Not applicable.
Funding
The present study was supported in part by a
grant-in-aid for defense medicine from the Ministry of Defense and
a grant-in-aid from the Foundation for Promotion of Defense
Medicine.
Availability of data and materials
The findings used and/or analyzed during this
published article are available from the corresponding author on
reasonable request.
Authors' contributions
YY, TE and HT collaborated in the conception and
design of the study. YY, TY, ToK, MH, MF, and TaK acquired the
data. YY, TE, TY, ToK, MH, MF, TaK, HU, JY and HT performed data
analysis and interpretation. All authors were involved in writing
the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was provided by the patient
for publication of this study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yoshida Y, Kuramochi A, Ohta A, Furumura
M, Imafuku S, Matsuo M, Chikuda H, Funasaki H, Saito K, Saya H, et
al: Diagnostic criteria and treatment guidelines for nerve fibrosis
type 1 (Recklinghausen's disease). Jpn J Dermatol. 118:1657–1666.
2008.(In Japanese).
|
|
2
|
Wallace MR, Marchuk DA, Andersen LB,
Letcher R, Odeh HM, Saulino AM, Fountain AM, Brereton A, Nicholson
J and Mitchell AL: Type 1 neurofibromatosis gene: Identification of
a large transcript disrupted in three NF1 patients. Science.
249:181–186. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gottfried ON, Viskochil DH and Couldwell
WT: Neurofibromatosis Type 1 and tumorigenesis: Molecular
mechanisms and therapeutic implications. Neurosurg Focus.
28:E82010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Korf BR: Malignancy in neurofibromatosis
type 1. Oncologist. 5:477–485. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Uusitalo E, Kallionpää RA, Kurki S,
Rantanen M, Pitkäniemi J, Kronqvist P, Härkönen P, Huovinen R,
Carpen O, Pöyhönen M, et al: Breast cancer in neurofibromatosis
type 1: Overrepresentation of unfavourable prognostic factors. Br J
Cancer. 116:211–217. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ceccaroni M, Genuardi M, Legge F,
Lucci-Cordisco E, Carrara S, D'Amico F, Greggi S and Scambia G:
BRCA1-related malignancies in a family presenting with von
recklinghausen's disease. Gynecol Oncol. 86:375–378. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hammond ME, Hayes DF, Dowsett M, Allred
DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS,
Hayes M, et al: American society of clinical oncology/college of
american pathologists guideline recommendations for
immunohistochemical testing of estrogen and progesterone receptors
in breast cancer. Arch Pathol Lab Med. 134:907–922. 2010.PubMed/NCBI
|
|
8
|
Wolff AC, Hammond ME, Hicks DG, Dowsett M,
McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M,
Fitzgibbons P, et al: Recommendations for human epidermal growth
factor receptor 2 testing in breast cancer: American Society of
Clinical Oncology/College of American Pathologists clinical
practice guideline update. J Clin Oncol. 31:3997–4013. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dowsett M, Nielsen TO, A'Hern R, Bartlett
J, Coombes RC, Cuzick J, Ellis M, Henry NL, Hugh JC, Lively T, et
al: Assessment of Ki67 in breast cancer: Recommendations from the
international Ki67 in breast cancer working group. J Natl Cancer
Inst. 103:1656–1664. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brierley JD, Gospodarowicz MK and
Wittekind C: TNM Classification of Malignant Tumours. 8th.
Wiley-Blackwell; Chichester: 2017
|
|
11
|
Salemis NS, Nakos G, Sambaziotis D and
Gourgiotis S: Breast cancer associated with type 1
neurofibromatosis. Breast Cancer. 17:306–309. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rasmussen SA and Friedman JM: NF1 gene and
neurofibromatosis 1. Am J Epidemiol. 151:33–40. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Takano T, Kawashima T, Yamanouchi Y,
Kitayama K, Ueno K and Hamaguchi H: Genetics of neurofibromatosis 1
in Japan: Mutation rate and paternal age effect. Hum Genetics.
89:281–286. 1992. View Article : Google Scholar
|
|
14
|
Sorensen SA, Mulvihill JJ and Nielsen A:
Long-term follow-up of von Recklinghausen neurofibromatosis.
Survival and malignant neoplasms. N Engl J Med. 314:1010–1015.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zoller ME, Rembeck B, Oden A, Samuelsson M
and Angervall L: Malignant and benign tumors in patients with
neurofibromatosis type 1 in a defined Swedish population. Cancer.
79:2125–2131. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sharif S, Moran A, Huson SM, Iddenden R,
Shenton A, Howard E and Evans DG: Women with neurofibromatosis 1
are at a moderately increased risk of developing breast cancer and
should be considered for early screening. J Med Genet. 44:481–484.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Walker L, Thompson D, Easton D, Ponder B,
Ponder M, Frayling I and Baralle D: A prospective study of
neurofibromatosis type 1 cancer incidence in the UK. Br J Cancer.
95:233–238. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Madanikia SA, Bergner A, Ye X and Blakeley
JO: Increased risk of breast cancer in women with NF1. Am J Med
Genet A. 158a:3056–3060. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nakamura M, Tangoku A, Kusanagi H, Oka M
and Suzuki T: Breast cancer associated with Recklinghausen's
disease: Report of a case. Nihon Geka Hokan. 67:3–9.
1998.PubMed/NCBI
|
|
20
|
Murayama Y, Yamamoto Y, Shimojima N,
Takahara T, Kikuchi K, Iida S and Kondo Y: T1 Breast cancer
associated with von recklinghausen's neurofibromatosis. Breast
Cancer. 6:227–230. 1999. View Article : Google Scholar
|
|
21
|
Xu GF, Lin B, Tanaka K, Dunn D, Wood D,
Gesteland R, White R, Weiss R and Tamanoi F: The catalytic domain
of the neurofibromatosis type 1 gene product stimulates ras GTPase
and complements ira mutants of S. cerevisiae. Cell. 63:835–841.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jeon YW, Kim RM, Lim ST, Choi HJ and Suh
YJ: Early-onset breast cancer in a family with neurofibromatosis
type 1 associated with a germline mutation in BRCA1. J Breast
Cancer. 18:97–100. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Campos B, Balmana J, Gardenyes J,
Valenzuela I, Abad O, Fàbregas P, Volpini V and Díez O: Germline
mutations in NF1 and BRCA1 in a family with neurofibromatosis type
1 and early-onset breast cancer. Breast Cancer Res Treat.
139:597–602. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Daly MB, Pilarski R, Berry M, Buys SS,
Farmer M, Friedman S, Garber JE, Kauff ND, Khan S, Klein C, et al:
NCCN Guidelines insights: Genetic/familial high-risk assessment:
Breast and ovarian, version 2.2017. J Natl Compr Canc Netw.
15:9–20. 2017. View Article : Google Scholar : PubMed/NCBI
|