Introduction
Colorectal cancer (CRC) is the second most
frequently diagnosed cancer and the third leading cause of
cancer-related death in Croatia. According to the data obtained
from the Croatian National Cancer Registry for 2015, there were
1,890 new cases in the male population, and 1,339 in the female
population. During the same year, 2,056 people succumbed to CRC
(1). Despite the existence of the
Croatian national colorectal screening program, the trends in the
rates of incidence and mortality still display an increase in CRC.
At the time of primary diagnosis, 41% of patients have positive
regional lymph nodes (LN) and 14% of patients have evidence of
distant metastases (1).
Approximately 75–80% of all CRC cases are sporadic,
while approximately 20% may be familial, due to low-penetrance
genes without a clear pattern. Only 5% of CRCs are clearly
inherited (familial adenomatous polyposis, Lynch syndrome,
MUTYH-associated polyposis, Peutz-Jeghers syndrome, juvenile
polyposis, Cowden syndrome and serrated polyposis). Most cases of
hereditary CRC have an autosomal dominant inheritance pattern
(except MUTYH-associated polyposis) (2). Relevant risk factors are as follows: Age
(>50 years), lifestyle (high-fat, low-fiber-diet, obesity,
physical inactivity, smoking and alcohol consumption), colorectal
adenoma, inflammatory bowel disease (Crohn's disease and ulcerative
colitis), and a family history of CRC. Primary tumor location is an
important prognostic factor and has an effect on clinical
presentation. The initial symptoms of left-sided CRC tumors are a
change in bowel habits and bleeding; as these symptoms are more
palpable, therefore they are identified, and CRC is diagnosed, at
an earlier stage. Right-sided CRC tumors grow unnoticed until they
are large, and symptoms are unspecific: Abdominal pain, vomiting
and anemia.
Diagnosis should be confirmed with an
endoscopically-guided biopsy. Diagnostic workup should include a
complete blood count, liver and renal function tests,
carcinoembryonic antigen and cancer antigen 19-9, multi-slice
computed tomography of the chest, abdomen and pelvis, and magnetic
resonance imaging of the pelvis (for rectal cancer). A
multidisciplinary team approach insures that patients receive the
best possible care. The pathology report should include the
histology type, degree of differentiation, depth of bowel wall
infiltration (T status), affected and examined LNs (N status) and
presence of lymphovascular or perineural invasion (PNI). Clinical
and pathological staging should be performed according to the
latest edition of the International Union Against Cancer
(UICC)/American Joint Committee of Cancer (AJCC)
tumor-node-metastasis (TNM) classification for CRC. Clinical
outcomes have improved dramatically over the past 15 years due to
the availability of more active chemotherapeutic agents, and
anti-VEGF and anti-EGFR targeted agents, but also due to the
development of different surgical approaches (including
colon-first, liver-first, two-step or simultaneous resection) and
the possibilities for the local ablative treatment of liver, lung
and peritoneal metastases (including chemoembolization,
radioembolization, stereotactic radiotherapy and radiofrequency
ablation). The median survival rate for patients with metastatic
CRC is currently about 30 months. This improvement is achieved due
to continuum of care, which includes different possible
combinations of patient treatment i.e., combinations of drugs and
different time-frame of therapy. The latest findings are focused on
microsatellite instability high CRC, which may be sensitive to
programmed cell death protein 1 (PD-1) inhibitors.
It is critical to identify the molecular markers of
CRC, which can be used to monitor or predict the progression and
prognosis of patients with CRC, and to investigate these potential
biomarkers as therapeutic targets.
A crucial role in the progression and aggressiveness
of CRC is attributed to epithelial-mesenchymal transition (EMT), a
reversible developmental process that includes the dissolution of
adherens junctions and loss of apicobasolateral polarity, resulting
in the formation of migratory mesenchymal cells with invasive
properties. During the EMT process, cancer cells lose the
expression of cellular adhesion proteins such as epithelial (E-)
cadherin and γ-catenin (3).
E-cadherin is a member of the large cadherins family of
calcium-dependent cell adhesion proteins. This single-pass
transmembrane glycoprotein, encoded by the CDH1 gene on chromosome
16q22.1, has a molecular weight of 120 kDa. The mature protein
comprises a long extracellular domain with five E-cadherin repeats
(EC1-5), a short transmembrane domain, and a cytoplasmic domain
that includes juxtamembranous p120, and γ- and β-catenin binding
sites (4).
Predominantly expressed at the basolateral membrane
of epithelial cells, where its function is primarily cell-cell
adhesion, E-cadherin has been shown to be essential during morula
compaction and the subsequent epithelial tissue organization, which
is achieved through hemophilic interactions between cadherin
molecules, first among adjacent cells (trans-interaction) and then
within the same cell by lateral association (cis-interaction)
(5). Malignant epithelial cells
undermine the function of E-cadherin in several ways, including
gene mutations, epigenetic silencing by promoter hypermethylation,
loss of heterozygosity, transcriptional silencing and microRNAs
that regulate expression, transport and protein turnover at the
cell surface (6–8).
Neural precursor cell-expressed developmentally
downregulated 9 (NEDD9) protein is a member of the non-catalytic
scaffolding proteins family (9),
which also includes CASS1/BCAR1/p130Cas, CASS3/EFS/Sin and
CASS4/HEPL. These proteins show the conservation of similar domain
structures; an N-terminal Src homology 3 (SH3) domain that binds
protein substrates (e.g., FAK, PYK2) and contains polyproline
motifs, and a large substrate domain incorporating multiple YxxP
motifs, which are phosphorylated by the Src family kinases to
create binding sites for proteins with SH2 domains. The serine-rich
region likely folds into a 4-helix bundle and highly conserved
carboxyl-terminal domain that mediates homo- and heterodimerization
with CASS1/BCAR1/p130Cas. Although the protein is mainly
cytoplasmic, small quantities are localized with centrosomes and
the ciliary basal body. The signaling function of NEDD9 is
integrin-dependent, regulated by the phosphorylation of serines,
threonines and tyrosines in the structural domains. PP2A
phosphatases are potential regulators of the NEDD9 phosphorylation
status. NEDD9 has a molecular weight of 93 kDa and oscillates
between a faster migrating form of 105 kDa in G1/S cells and a
slower migrating form of 115 kDa in G2/M cells. Previous studies
have identified the crucial role of NEDD9 in the coordination of
signaling cascades, contributing to changes in cell adhesion,
migration, invasion and EMT (9,10). In
normal human tissue, the highest level of NEDD9 is expressed in the
lungs and kidneys, which are rich in immature lymphoid cells, and
in the fetal brain prior to downregulation in the adult brain
(10). Many cell lines, such as
epithelial tumor-, melanoma-, lymphoma- and glioblastoma-derived
cell lines, express an abundance of NEDD9.
Due to its pleotropic functions (cell adhesion,
migration, invasion and EMT), the elevated expression of NEDD9 has
emerged as a predictor of poor outcome, metastatic potential and
chemoresistence in multiple cancer types (breast cancer, gastric
cancer, glioblastoma, head and neck squamous cell carcinoma,
hepatocellular carcinoma, non-small cell lung cancer, ovarian
cancer, renal cancer, pancreatic ductal adenocarcinoma, prostate
cancer and T-cell leukemia) (10–19). NEDD9
is a bona fide melanoma metastasis gene that enhances
invasion in vitro and metastasis in vivo of both
normal and transformed melanocytes (14). A growing body of preclinical data
supports the theory that altered NEDD9 function is associated with
other human diseases, such as stroke, Alzheimer's disease and
autosomal dominant polycystic kidney disease.
The data regarding NEDD9 and E-cadherin expression
in CRC are insufficient. However, a study has shown that
overexpression of NEDD9 positively mediates the canonical
Wnt/ß-catenin signaling pathway in CRC and it also negatively
regulates membrane expression of E-cadherin (3). There is also a paper in which NEDD9 is
identified as differentially expressed gene, associated with cyclin
D1, which can be a molecular target for the treatment of CRC,
because it interacts with their corresponding anti-neoplastic drugs
(20). Therefore, our study aimed to
analyze the immunohistochemical NEDD9 and E-cadherin expression in
a tissue microarray of nonmetastatic and metastatic CRC, and to
determine whether their expression is associated with the clinical
behavior and prognosis of CRCs.
Patients and methods
Patient information
Following approval by the Ethical Committee of
Clinical Hospital Center Sestre Milosrdnice (Zagreb, Croatia), a
total of 40 pairs of formalin-fixed, paraffin-embedded (FFPE)
primary CRC and corresponding matched liver metastasis tissue
specimens were retrieved from the tissue bank of the Ljudevit Jurak
Department of Pathology and Cytology. The patients gave their
written informed consent for the use of their biological materials
and data in research. The patients had no history of the familial
aggregation of CRC, had not been previously treated with chemo-
or/and radiotherapy, and had undergone simultaneous colorectal and
hepatic resection between January 1st, 2006 and December 31st,
2013. Tumor staging was performed according to the 7th edition of
the TNM classification for CRC. The follow up deadline was December
31st, 2015. The survival time was calculated from the date of
surgery to the follow up deadline, or the date of death. The
clinicopathological features of patients are summarized in Table I.
 | Table I.Clinical description of the
investigated sample (Dukes D, total n=40). |
Table I.
Clinical description of the
investigated sample (Dukes D, total n=40).
| Parameter | n | % |
|---|
| Sex |
|
Male | 23 | 57.5 |
|
Female | 17 | 42.5 |
| Localization of
primary tumor |
|
Colon | 28 | 70.0 |
|
Rectosigmoid junction | 2 | 5.0 |
|
Rectum | 10 | 25.0 |
| T status |
| T1 | 0 | 0 |
| T2 | 3 | 7.5 |
| T3 | 34 | 85.0 |
| T4 | 3 | 7.5 |
| N status |
| N0 or
Nx | 9 | 22.5 |
| N1 | 18 | 45.0 |
| N2 | 13 | 32.5 |
| Surgical
margins |
|
Negative | 39 | 97.5 |
|
Positive | 1 | 2.5 |
| Microvascular
invasion |
|
Absent | 25 | 62.5 |
|
Present | 15 | 37.5 |
| Perineural
invasion |
|
Absent | 28 | 70.0 |
|
Present | 12 | 30.0 |
| Outcome |
|
Survived | 18 | 45.0 |
|
Succumbed | 22 | 55.0 |
Immunohistochemistry
Immunohistochemical analyses were performed by two
board-certified pathologists who were blinded to the clinical data
of the patients. Paraffin-embedded tissue sections (thickness 3–5
µm) were deparaffinized for 2 h at 60°C and then washed with
distilled water after two and three changes of xylene and ethanol,
respectively. Immunohistochemical staining was performed using the
microwave streptavidin immunoperoxidase (MSIP) protocol, and by use
of the labelled streptavidin-biotin (LSAB) method on a
TechMate™ Horizon automated immunostainer (Dako; Agilent
Technologies, Inc., Santa Clara, CA, USA) (11). Sections were incubated with rabbit
anti-human NEDD9 polyclonal (dilution 1:100, cat. no. ab37161;
Abcam, Cambridge, UK) and mouse anti-human E-cadherin monoclonal
(dilution 1:50, clone NCH-38; Dako; Agilent Technologies, Inc.)
antibodies overnight at 4°C, followed by incubation with
horseradish peroxidase-conjugated secondary goat anti-rabbit
antibody (Abcam) for 1 h at room temperature. Sections were then
washed with PBS and the antigen-antibody complex was
visualized.
The reactions were determined in epithelial tumor
components, as well as from the epithelial components of metastatic
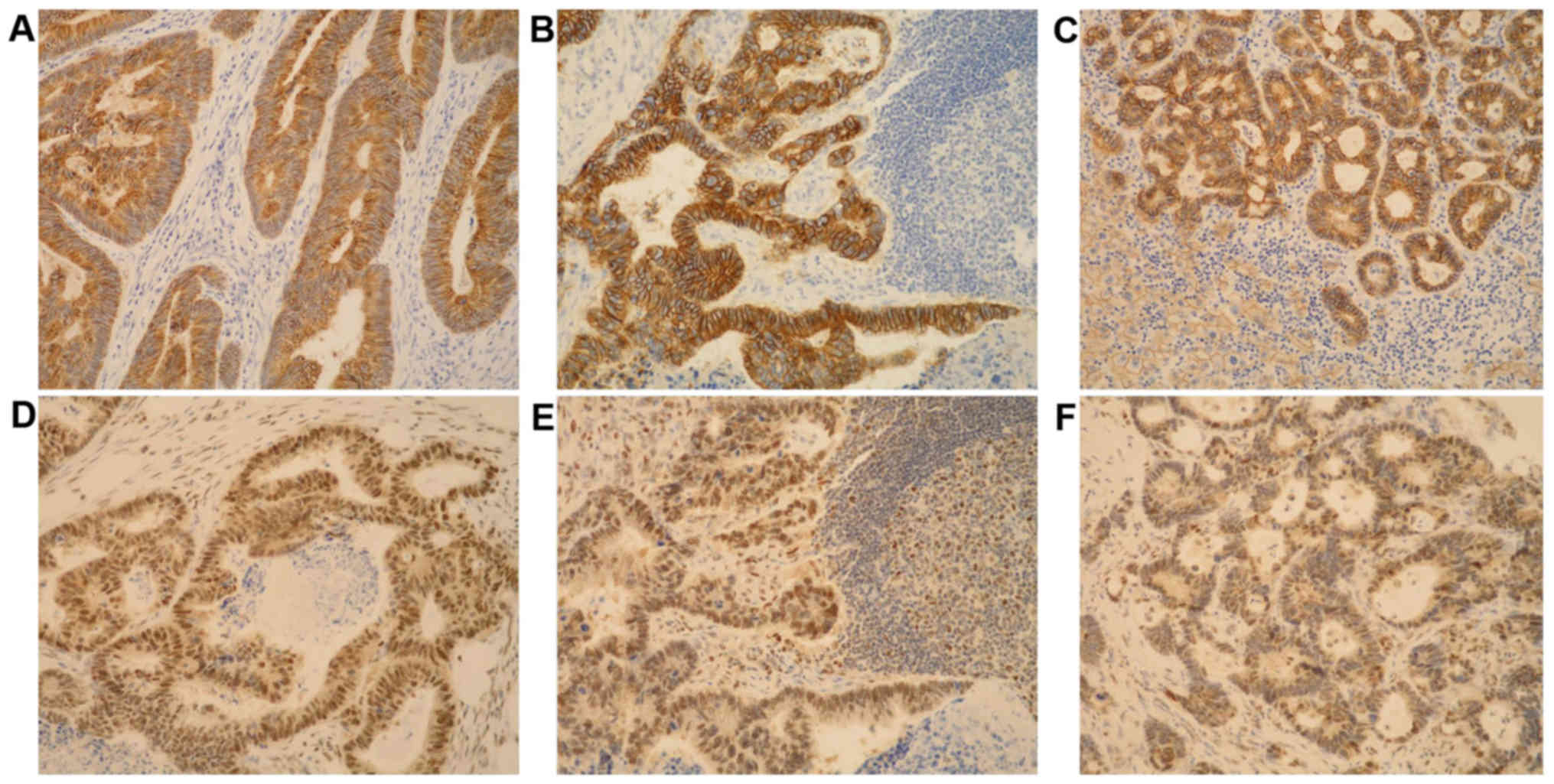
tumors in the LN and liver (Fig. 1).
Positive reactions were determined at the site of strongest
activity (‘hot spot’) under a magnification, ×400 for a total of
1,000 tumor cells. The ‘hot spot’ was established following
inspection of the whole section at a magnification of ×40. The
results for E-cadherin were presented semi-quantitatively using an
immunohistochemical staining index (ISI), obtained by multiplying
the intensity of reaction with the percentage of cells with a
positive reaction. The range of ISI was from 0 to 9: 0, represents
no reaction, 1–4 represents a low E-cadherin reaction, 5–9
represents a high E-cadherin reaction. The intensity of the
reaction was scored as follows: 0, no reaction; 1, weak reaction;
2, moderate reaction; and 3, strong reaction. The percentage of
immunoreactive tumor cells was scored as follows: 0, for no
reaction; 1, 0–10% of positive tumor cells; 2, >10-50% of
positive tumor cells; and 3, >50% of positive tumor cells
(21). The results for NEDD9 were
presented semi-quantitatively and scored in the following way: 0,
no reaction; 1, weak reaction in 0–10% of tumor cells; 2, moderate
reaction in >10-25% of tumor cells; and 3, strong reaction in
>25% of tumor cells (11).
Statistical analysis
The normality of data distribution was assessed with
the Kolmogorov-Smirnov test, and appropriate non-parametric tests
were used in the following statistical analyses. Spearman's ρ and
Kendal's τ-b correlation coefficients for nominal-ordinal
correlation were used to analyze associations between E-cadherin
and NEDD9 expression in the primary tumor, LN and liver with other
clinical variables. A log-rank (Mantel-Cox) test of the equality of
survival distributions was performed for the expression of
E-cadherin and NEDD9 in the primary tumor, LN and liver in relation
to the survival outcome. The outcomes were illustrated with
Kaplan-Meier survival curves. P<0.05 was considered to indicate
a statistically significant difference. The data analysis software
SPSS Statistics, version 23.0 (IBM Corp., Armonk, NY, USA) was used
for statistical analyses and the production of graphical
images.
Results
A clinical description of the investigated sample is
shown in Table I. Of the patients,
57.5% were male. Median (interquartile range, IQR) age was 64.0
(57.3–73.5) years. Of the tumors, 70.0% were localized in the right
colon. Median (IQR) tumor size was 50.0 (36.3–60.0) mm. Among the
patients, 85.0% had T3 stage, and 22.5% had N0 or Nx stage.
Microvascular invasion was positive in 37.5% of patients, and PNI
in 30.0% of patients. Death occurred in 55.0% of patients, with a
median (IQR) survival time of 620.5 (164.3–964.8) days. The median
(IQR) percentage of positive LN was 13.9% (0.0–44.1%).
The E-cadherin and NEDD9 expression scores in the
investigated samples are shown in Table
II. 87.5% of the patients had a strong expression of E-cadherin
in the primary tumor, 67.7% in the LN, and 77.5% in the liver.
Highly positive NEDD9 expression in the primary tumor was
identified in 22.5% of patients, while 35.5% had highly positive
NEDD9 expression in the LN, and 30.0% in the liver.
 | Table II.E-cadherin and NEDD9 expression in
the investigated sample (Dukes D, total n=40). |
Table II.
E-cadherin and NEDD9 expression in
the investigated sample (Dukes D, total n=40).
| ISI | n | % |
|---|
| E-cadherin in
primary tumor |
| 0 | 2 | 5.0 |
| 1 | 3 | 7.5 |
| 2 | 35 | 87.5 |
| E-cadherin in lymph
nodes |
| 0 | 4 | 12.9 |
| 1 | 6 | 19.4 |
| 2 | 21 | 67.7 |
| E-cadherin in
liver |
| 0 | 1 | 2.5 |
| 1 | 8 | 20.0 |
| 2 | 31 | 77.5 |
| NEDD9 in primary
tumor |
| 0 | 8 | 20.0 |
| 1 | 9 | 22.5 |
| 2 | 14 | 35.0 |
| 3 | 9 | 22.5 |
| NEDD9 in lymph
nodes |
| 0 | 7 | 22.6 |
| 1 | 5 | 16.1 |
| 2 | 8 | 25.8 |
| 3 | 11 | 35.5 |
| NEDD9 in liver |
| 0 | 3 | 7.5 |
| 1 | 11 | 27.5 |
| 2 | 14 | 35.0 |
| 3 | 12 | 30.0 |
The correlation coefficients for E-cadherin and
NEDD9 expression in the primary tumor, LN and liver with clinical
characteristics are shown in Table
III. Significant positive correlation was noted between the
percentage of positive LN with the ISI for E-cadherin in the LN
(ρ=0.372; P=0.039) and with NEDD9 expression in LN (ρ=0.451;
P=0.011), indicating that higher expression is significantly
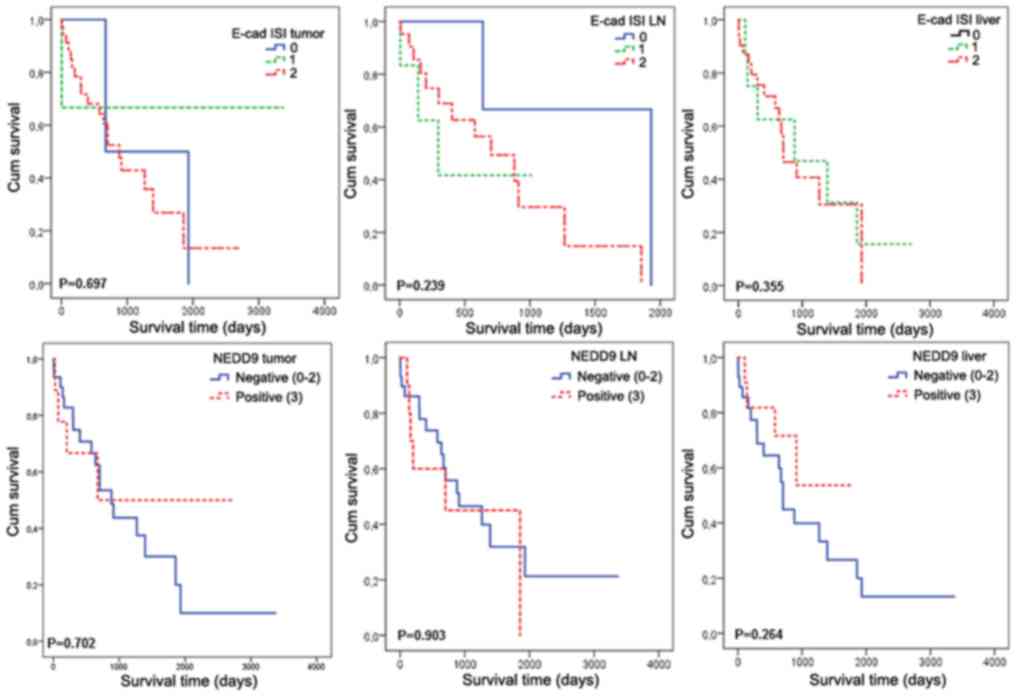
associated with a higher percentage of positive LN. Kaplan-Meier
survival curves with log-rank tests for the expression of
E-cadherin and NEDD9 in the primary tumor, LN and liver in relation
to the survival outcome are shown in Fig.
2. There was no significant prediction of mortality associated
with the expression of E-cadherin and NEDD9 at any location,
indicating that for this tumor stage (Dukes D), other prognostic
markers are likely to be more clinically relevant.
 | Table III.Coefficients for the correlation
between E-cadherin and NEDD9 expression in the primary tumor, lymph
nodes and liver with clinical characteristics. |
Table III.
Coefficients for the correlation
between E-cadherin and NEDD9 expression in the primary tumor, lymph
nodes and liver with clinical characteristics.
|
| E-cadherin | NEDD9 |
|---|
|
|
|
|
|---|
| Expression of
site | Primary tumor | Lymph nodes | Liver | Primary tumor | Lymph nodes | Liver |
|---|
| Age (years) |
| ρ | 0.245 | −0.048 | 0.305 | −0.084 | −0.139 | 0.052 |
| P | 0.127 | 0.799 | 0.055 | 0.605 | 0.457 | 0.748 |
| n | 40 | 31 | 40 | 40 | 31 | 40 |
| Tumor size
(cm) |
| ρ | −0.166 | −0.281 | −0.265 | −0.025 | 0.08 | 0.175 |
| P | 0.305 | 0.126 | 0.098 | 0.88 | 0.669 | 0.281 |
| n | 40 | 31 | 40 | 40 | 31 | 40 |
| T status |
| ρ |
| −0.191 |
|
| 0.047 | 0.299 |
| P |
| 0.304 |
|
| 0.803 | 0.061 |
| n | 40 | 31 | 40 | 40 | 31 | 40 |
| N status |
| ρ | 0.263 | 0.345 | 0.084 | 0.106 | 0.261 | 0.195 |
| P | 0.102 | 0.057 | 0.604 | 0.515 | 0.156 | 0.227 |
| n | 40 | 31 | 40 | 40 | 31 | 40 |
| M status |
| ρ |
| P |
| n | 40 | 31 | 40 | 40 | 31 | 40 |
| Surgical
margins |
| τB | 0.06 | −0.334 | 0.086 | −0.108 | −0.255 | 0.015 |
| P | 0.711 | 0.066 | 0.598 | 0.507 | 0.166 | 0.929 |
| n | 40 | 31 | 40 | 40 | 31 | 40 |
| Microvascular
invasion |
| ρ | 0.292 | −0.022 | 0.176 | −0.053 | −0.041 | 0.108 |
| P | 0.067 | 0.907 | 0.278 | 0.743 | 0.825 | 0.508 |
| n | 40 | 31 | 40 | 40 | 31 | 40 |
| Perineural
invasion |
| ρ | 0.07 | 0.041 | 0.225 | 0.039 | 0.192 | −0.094 |
| P | 0.668 | 0.826 | 0.163 | 0.81 | 0.3 | 0.563 |
| n | 40 | 31 | 40 | 40 | 31 | 40 |
| Positive lymph
nodes (%) |
| ρ | 0.2 | 0.372 | −0.016 | 0.026 | 0.451 | 0.175 |
| P | 0.217 | 0.039a | 0.922 | 0.872 | 0.011a | 0.281 |
| n | 40 | 31 | 40 | 40 | 31 | 40 |
| Sex |
| τB | 0.177 | 0.249 | 0.107 | 0.129 | 0.207 | 0.217 |
| P | 0.263 | 0.157 | 0.497 | 0.378 | 0.217 | 0.143 |
| n | 40 | 31 | 40 | 40 | 31 | 40 |
| Survival time
(days) |
| ρ | −0.137 | −0.075 | −0.292 | −0.035 | −0.229 | 0.068 |
| P | 0.398 | 0.688 | 0.067 | 0.829 | 0.215 | 0.676 |
| n | 40 | 31 | 40 | 40 | 31 | 40 |
Discussion
Left- and right-sided CRCs differ with respect to
biology, epidemiology, pathology and clinical presentation. It is
expected that most patients with synchronous liver metastases have
right-sided CRC.
Various studies have shown controversial results
regarding the expression levels of E-cadherin in CRC. Yun et
al (22) studied stage III CRC,
and found positive expression in 98.3% of samples. Dorudi et
al (23) found that 81.2% of well
and moderately differentiated tumors expressed strong positivity
for E-cadherin, while 85.7% of poorly differentiated tumors were
E-cadherin-negative. Three studies (Miladi-Abdennadher et al
(24), Palaghia et al
(25) and Elzagheid et al
(26) identified a marginally lower
expression (74.3, 67.69 and 59%, respectively). We attribute the
results of Elzagheid et al (26) to the inclusion of all CRC stages in
their study. In contrast to the aforementioned studies, Gu et
al (27) identified that only 20%
of patients with metastatic CRC had positive E-cadherin expression.
The limitation of our study is reflected in the fact that we
assessed only the membranous expression of E-cadherin. Elzagheid
et al (28) assessed the
cytoplasmic expression of E-cadherin. Tóth et al (29) used a scale based only on the
percentage of immunopositive cells. Palaghia et al (25) used two scoring systems that were
initially established for gastric carcinoma.
Our results show concordance with the results of
Elzagheid et al (26),
Khoursheed et al (30) and
Roca et al (31), who reported
that E-cadherin expression was not associated with tumor stage.
Nevertheless, Ghadimi et al (32) reported a significant association
between reduced E-cadherin and lower tumor grade, but did not
identify a clear correlation between the loss of E-cadherin
expression and the depth of tumor infiltration into the intestinal
wall. Kwak et al (33) showed
that the reduced expression of E-cadherin was associated with
advanced stage tumors (P=0.029), while Lugli et al (34) demonstrated that the loss of membranous
E-cadherin was associated with a higher T-stage (P=0.03). Similar
results were reported by Miladi-Abdennadher et al (24), who reported that E-cadherin expression
was correlated with tumor size (P=0.02). Although the stage of the
tumor was determined for every patient, the relatively small size
of the group represented a limitation of the afore-mentioned
study.
However, Roca et al (31) found no association between E-cadherin
expression and LN metastasis. Similar to the present study, Kwak
et al (33) demonstrated an
association between E-cadherin expression and LN metastasis
(P=0.004). Lugli et al (34)
reported that in mismatch repair-proficient CRC, the loss of
membranous E-cadherin was independently associated with a higher N
stage (P<0.0001). In MLH1− CRC, the loss of
membranous E-cadherin was associated with a higher N stage (P=0.05)
(34). Node-positive cancers
exhibited significant loss of E-cadherin (P<0.001) according to
Karamitopoulou et al (35).
Ozgüven et al (36) found that
reduced E-cadherin expression was significantly associated with LN
metastasis (P=0.01). A borderline association of E-cadherin
expression and LN metastasis (P=0.09) was reported by Elzagheid
et al (26). Kim et al
(37) reported that E-cadherin
expression may serve as a predictive marker for tumor invasion and
LN metastasis.
E-cadherin expression was increased in up to 40% of
liver metastases, compared with only 17% of metastatic LNs that
were studied by Ikeguchi et al (38), whose results are consistent with those
of the present study. The results of Kim et al (39), who analyzed patients that had
undergone curative surgery for primary CRC and liver metastases,
showed that E-cadherin expression in the tumor center was greater
than that of the tumor margin, in the primary tumor and liver
metastases (P<0.001, P=0.006, respectively). A likely
explanation is the possibility that tumor cells regain epithelial
features in distant metastases. Dorudi et al (23) and Mohri (40) postulated that negative E-cadherin
expression was associated with liver metastasis. Nanashima et
al (41) reported that negative
E-cadherin expression tended to be associated with a poor
prognosis. Kaihara et al (42)
reported that LN metastasis and the decreased expression of
E-cadherin were associated with liver metastasis. Elzagheid et
al (28) reported that the
E-cadherin membranous (MI) and cytoplasmic index (CI) were
significantly higher in liver metastases compared to other anatomic
sites (MI, P=0.034; CI, P=0.022). Truant et al (43) demonstrated that the expression of
E-cadherin significantly increased in metastases compared with
normal liver tissue. Gagliardi et al (44) compared liver metastases with their
corresponding primary tumors, and found a complete loss of
E-cadherin expression in 50% of liver metastases, while 86% of the
primary tumors associated with the liver metastases exhibited
strong expression.
A connection between survival rate and the reduced
expression of E-cadherin was found by Kwak et al (33) and Kang et al (45), but was without statistical
significance in a multivariate analysis; Lee et al (46) identified that the aberrant expression
of E-cadherin in the invasive margin was a significant and
independent risk factor for disease-free and overall survival in
multivariate analysis, while Yun et al (22) reported that decreasing E-cadherin
expression was associated with a poor outcome in terms of overall
survival in univariate (P=0.016), but not multivariate (P=0.303,
risk ratio=1.984, 95% confidence interval=0.539–7.296), analysis
(46). The present study did not
identify any statistically significant association between the
survival rate and E-cadherin expression. Regarding the
controversial results of E-cadherin expression, it should be noted
that in various papers that we have mentioned, the research
procedures were performed using various monoclonal antibodies,
devices (instruments), classifications, and scoring systems
(cut-off values). Therefore, there are many points that could have
affected the difference in the results.
Studies of the immunohistochemical expression of
NEDD9 in human tissue samples are few in the literature. Xia et
al (47) found that NEDD9
expression is increased in ~50% of CRC samples, compared with
normal colorectal tissue. Li et al (48) noted the high expression of NEDD9 in 68
of 92 CRC samples, compared with 12 of 92 in normal tissues
(P<0.01). It was found that NEDD9 was significantly associated
with an advanced TNM stage (P=0.014), pT grade (P=0.009), pN
(P=0.013) and pM status (P=0.047). Patients with a higher NEDD9
expression had a significantly shorter overall survival rate
(P<0.01) (48).
The present study identified a strong expression of
NEDD9 in 22.5% of primary CRC tumors, 35.5% of LNs and 30% of liver
metastases. As did Li et al (48), we found a significant positive
correlation between positive LN and NEDD9 expression. The
difference in the expression of NEDD9 was also noted in cell lines
(primary cell line SW480, and LN metastatic cell line SW620,
derived from the same patient) (49).
To the best of our knowledge, there are no published data on the
expression of NEDD9 in CRC liver metastasis. The expression is
similar to that of LN. Further studies of the expression of NEDD9
in liver metastases are needed. Potentially due to the small study
cohort, no connection between the expression of NEDD9 in the
primary tumor, LNs or liver metastases with survival rate was
identified in the present study. Limitations of our study mostly
arise from the small sample size. However, the registry of Croatian
patients that had a resection of their CRC does not exist.
Furthermore, there are no universally accepted guidelines for the
treatment of CRC, i.e., a similar case will be treated rather
differently in various institutions. This situation poses
unsurmountable challenges for the accruement of a larger Dukes D
patients' group. Since 2014, the EGFR testing has become a standard
part of pathological reports. If we were performing the research
now, we would include the results of EGFR as a factor determining
the sampling of our groups. Clinical significance/relevance lies in
the possibility of an improved distinction among patients who will
experience more benefits of anti-EGFR therapy. Similar studies
about the expression of E-cadherin and benefit of anti-EGFR therapy
were published in settings of patients with lung adenocarcinoma
(50,51). However, the patients included from
2014 still would not have a sufficient follow-up data at this
point. Due to our objectively limited resources and the
impossibility to use CEA-controlled oncolytic adenovirus (it is not
available in Croatia), CEA was not used, although we recognize it
as ‘one of the most important factors’. Additional limitations to
our study arise from the absence of data on E-cadherin and NEDD9
expression in CRC cell lines and animal models of CRC. In the
future diagnostic procedures, if the equipment, samples, and
experienced, professional staff would be provided to our
institution, we intend to use this method (52).
Acknowledgements
The authors would like to thank Mrs. Smole from the
Clinical Hospital Center Sestre Milosrdnice institutional library,
for helping us obtain the necessary references, which would not
have otherwise been available to us.
Funding
No funding was received.
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
PJ designed and performed the research, wrote the
manuscript and analyzed the clinical data. PR and MPB collected and
analyzed the samples. MM performed the statistical analysis. BK
contributed to the design of the research, supervised the
experiments and wrote the manuscript.
Ethics approval and consent to
participate
All of the samples were collected at the time of
diagnosis and after obtaining written informed consent. All
experiments were performed in accordance with the Declaration of
Helsinki and were approved by the Ethics Committee of Clinical
Hospital Center Sestre Milosrdnice.
Patient consent for publication
The patients provided written informed consent for
the publication of any associated data and accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Croatian National Cancer Registry: Cancer
incidence in Croatia 2015. Bulletin No. 40. Zagreb, Croatian
National Institute of Public Health. 2018.
|
|
2
|
Balmaňa J, Balanguer F, Cervantes A and
Arnold D: Familial risk-colorectal cancer: ESMO clinical practice
guidelines. Ann Oncol. 24 (Suppl 6):vi73–vi80. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tikhmyanova N and Golemis EA: NEDD9 and
BCAR1 negatively regulate E-cadherin membrane localization, and
promote E-cadherin degradation. PLoS One. 6:e221022011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Huntsman DG and Caldas C: Assignment of
the E-cadherin gene (CDH1) to chromosome 16q22.1 by radiation
hybrid mapping. Cytogenet Cell Genet. 83:82–83. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fleming TP, Javed Q and Hay M: Epithelial
differentiation and intercellular junction formation in the mouse
early embryo. Dev Suppl. 105–112. 1992.PubMed/NCBI
|
|
6
|
Paschos KA, Canovas D and Bird NC: The
role of cell adhesion molecules in the progression of colorectal
cancer and the development of liver metastasis. Cell Signal.
21:665–674. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
El-Bahrawy MA, Poulsom R, Jeffery R,
Talbot I and Alison MR: The expression of E-cadherin and catenins
in sporadic colorectal carcinoma. Hum Pathol. 32:1216–1224. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pećina-Slaus N: Tumor suppressor gene
E-cadherin and its role in normal and malignant cells. Cancer Cell
Int. 3:172003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tikhmyanova N, Little JL and Golemis EA:
CAS proteins in normal and pathological cell growth control. Cell
Mol Life Sci. 67:1025–1048. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kong C, Wang C, Wang L, Ma M, Niu C, Sun
X, Du J, Dong Z, Zhu S, Lu J and Huang B: NEDD9 is a positive
regulator of epithelial-mesenchymal transition and promotes
invasion in aggressive breast cancer. PLoS One. 6:e226662011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Štajduhar E, Sedić M, Leniček T, Radulović
P, Kerenji A, Krušlin B, Pavelić K and Kraljević Pavelić A:
Expression of growth hormone receptor, plakoglobin and NEDD9
protein in association with tumour progression and metastasis in
human breast cancer. Tumor Biol. 35:6425–6434. 2014. View Article : Google Scholar
|
|
12
|
Zhang SS, Wu LH, Liu Q, Chen KS and Zhang
XF: Elevated expression of NEDD9 is associated with metastatic
activity in gastric cancer. Onco Targets Ther. 8:633–640. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chang JX, Gao F, Zhao GQ and Zhang GJ:
Expression and clinical significance of NEDD9 in lung tissue. Med
Oncol. 29:2654–2660. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim M, Gans JD, Nogueira C, Wang A, Paik
JH, Feng B, Brennan C, Hahn WC, Cordon-Cardo C, Wagner SN, et al:
Comparative oncogenomics identifies NEDD9 as a melanoma metastasis
gene. Cell. 125:1269–1281. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang H, Mu X, Zhou S, Zhang J, Dai J, Tang
L, Xiao L, Duan Z, Jia L and Chen S: NEDD9 overexpression is
associated with the progression of and an unfavorable prognosis in
epithelial ovarian cancer. Hum Pathol. 45:401–408. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang Z, Shen M, Lu P, Li X, Zhu S and Yue
S: NEDD9 may regulate hepatocellular carcinoma cell metastasis by
promoting epithelial-mesenchymal-transition and stemness via
repressing Smad7. Oncotarget. 8:1714–1724. 2017.PubMed/NCBI
|
|
17
|
Xue YZ, Sheng YY, Liu ZL, Wei ZQ, Cao HY,
Wu YM, Lu YF, Yu LH, Li JP and Li ZS: Expression of NEDD9 in
pancreatic ductal adenocarcinoma and its clinical significance.
Tumour Biol. 34:895–899. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Morimoto K, Tanaka T, Nitta Y, Ohnishi K,
Kawashima H and Nakatani T: NEDD9 crucially regulates
TGF-β-triggered epithelial-mesenchymal transition and cell invasion
in prostate cancer cells: Involvement in cancer progressiveness.
Prostate. 74:901–910. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang J, Wang S, Luan Y, Zhang W, Sun C,
Cheng G, Li K, Xin Q, Lin Z, Qi T and Kong F: Overexpression of
NEDD9 in renal cell carcinoma is associated with tumor migration
and invasion. Oncol Lett. 14:8021–8027. 2017.PubMed/NCBI
|
|
20
|
Cui X, Shen K, Xie Z, Liu T and Zhang H:
Identification of key genes in colorectal cancer using random walk
with restart. Mol Med Rep. 15:867–872. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hong SM, Li A, Olino K, Wolfgang CL,
Herman JM, Schulick RD, Iacobuzio-Donahue C, Hruban RH and Goggins
M: Loss of E-cadherin expression and outcome among patients with
resectable pancreatic adenocarcinomas. Mod Pathol. 24:1237–1247.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yun JA, Kim SH, Hong HK, Yun SH, Kim HC,
Chun HK, Cho YB and Lee WY: Loss of E-Cadherin expression is
associated with a poor prognosis in stage III colorectal cancer.
Oncology. 86:318–328. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dorudi S, Sheffield JP, Poulsom R,
Northover JM and Hart IR: E-cadherin expression in colorectal
cancer. An immunocytochemical and in situ hybridization study. Am J
Pathol. 142:981–986. 1993.PubMed/NCBI
|
|
24
|
Miladi-Abdennadher I, Abdelmaksoud-Dammak
R, Ayed-Guerfali DB, Ayadi L, Khabir A, Amouri A, Frikha F, Tahri
N, Ellouz S, Frikha M, et al: Expression of COX-2 and E-cadherin in
Tunisian patients with colorectal adenocarcinoma. Acta Histochem.
114:577–581. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Palaghia M, Mihai C, Lozneanu L, Ciobanu
D, Trofin AM, Rotariu A, Târcoveanu F and Cijevschi Prelipcean C:
E-cadherin expression in primary colorectal cancer and metastatic
lymph nodes. Rom J Morphol Embryol. 57:205–209. 2016.PubMed/NCBI
|
|
26
|
Elzagheid A, Buhmeida A, Laato M,
El-Faitori O, Syrjänen K, Collan Y and Pyrhönen S: Loss of
E-cadherin expression predicts disease recurrence and shorter
survival in colorectal carcinoma. APMIS. 120:539–548. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gu J, Zhu X, Ye Y, Qu J, Huang L, Li R, Yu
Y and Leng X: The level of expression of adhesion molecules CD44v6
and E-cadherin in colorectal cancer and analysis of correlates with
metastasis. Zhonghua Wai Ke Za Zhi. 37:108–109. 4–1999.(In
Chinese). PubMed/NCBI
|
|
28
|
Elzagheid A, Algars A, Bendardaf R, Lamlum
H, Ristamaki R, Collan Y, Syrjanen K and Pyrhonen S: E-cadherin
expression pattern in primary colorectal carcinomas and their
metastases reflects disease outcome. World J Gastroenterol.
12:4304–4309. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tóth L, András C, Molnár C, Tanyi M, Csiki
Z, Molnár P and Szántó J: Investigation of β-catenin and E-cadherin
expression in Dukes B2 stage colorectal cancer with tissue
microarray method. Is it a marker of metastatic potential in rectal
cancer? Pathol Oncol Res. 18:429–437. 2012.PubMed/NCBI
|
|
30
|
Khoursheed MA, Mathew TC, Makar RR, Louis
S, Asfar SK, Al-Sayer HM, Dashti HM and Al-Bader A: Expression of
E-cadherin in human colorectal cancer. Surgeon. 1:86–91. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Roca F, Mauro LV, Morandi A, Bonadeo F,
Vaccaro C, Quintana GO, Specterman S, de Kier Joffé EB, Pallotta
MG, Puricelli LI and Lastiri J: Prognostic value of E-cadherin,
beta-catenin, MMPs (7 and 9), and TIMPs (1 and 2) in patients with
colorectal carcinoma. J Surg Oncol. 93:151–160. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ghadimi BM, Behrens J, Hoffmann I, Haensch
W, Birchmeier W and Schlag PM: Immunohistological analysis of
E-cadherin, alpha-, beta- and gamma-catenin expression in
colorectal cancer: Implications for cell adhesion and signaling.
Eur J Cancer. 35:60–65. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kwak JM, Min BW, Lee JH, Choi JS, Lee SI,
Park SS, Kim J, Um JW, Kim SH and Moon HY: The prognostic
significance of E-cadherin and liver intestine-cadherin expression
in colorectal cancer. Dis Colon Rectum. 50:1873–1880. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lugli A, Zlobec I, Minoo P, Baker K,
Tornillo L, Terracciano L and Jass JR: Prognostic significance of
the wnt signalling pathway molecules APC, beta-catenin and
E-cadherin in colorectal cancer: A tissue microarray-based
analysis. Histopathology. 50:453–464. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Karamitopoulou E, Zlobec I, Patsouris E,
Peros G and Lugli A: Loss of E-cadherin independently predicts the
lymph node status in colorectal cancer. Pathology. 43:133–137.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ozgüven BY, Karaçetin D, Kabukçuoğlu F,
Taşkin T and Yener Ş: Immunohistochemical study of E-cadherin and
β-catenin expression in colorectal carcinomas. Pol J Pathol.
62:19–24. 2011.PubMed/NCBI
|
|
37
|
Kim SA, Inamura K, Yamauchi M, Nishihara
R, Mima K, Sukawa Y, Li T, Yasunari M, Morikawa T, Fitzgerald KC,
et al: Loss of CDH1 (E-cadherin) expression is associated with
infiltrative tumour growth and lymph node metastasis. Br J Cancer.
114:199–206. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ikeguchi M, Taniguchi T, Makino M and
Kaibara N: Reduced E-cadherin expression and enlargement of cancer
nuclei strongly correlate with hematogenic metastasis in colorectal
adenocarcinoma. Scand J Gastroenterol. 35:839–846. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kim JC, Roh SA, Kim HC, Koo KH, Cho YK, Yu
CS, Kwon YM and Kim JS: Coexpression of carcinoembryonic antigen
and E-cadherin in colorectal adenocarcinoma with liver metastasis.
J Gastrointest Surg. 7:931–938. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mohri Y: Prognostic significance of
E-cadherin expression in human colorectal cancer tissue. Surg
Today. 27:606–612. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nanashima A, Yamaguchi H, Sawai T,
Yamaguchi E, Kidogawa H, Matsuo S, Yasutake T, Tsuji T, Jibiki M,
Nakagoe T and Ayabe H: Prognostic factors in hepatic metastases of
colorectal carcinoma: Immunohistochemical analysis of tumor
biological factors. Dig Dis Sci. 46:1623–1628. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kaihara T, Kusaka T, Nishi M, Kawamata H,
Imura J, Kitajima K, Itoh-Minami R, Aoyama N, Kasuga M, Oda Y, et
al: Dedifferentiation and decreased expression of adhesion
molecules, E-cadherin and ZO-1, in colorectal cancer are closely
related to liver metastasis. J Exp Clin Cancer Res. 22:117–123.
2003.PubMed/NCBI
|
|
43
|
Truant SC, Gouyer VP, Leteurtre EA,
Zerimech F, Huet GM and Pruvot FR: E-cadherin and beta-catenin mRNA
levels throughout colon cancer progression. J Surg Res.
150:212–218. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gagliardi G, Kandemir O, Liu D, Guida M,
Benvestito S, Ruers TG, Benjamin IS, Northover JM, Stamp GW, Talbot
IC, et al: Changes in E-cadherin immunoreactivity in the
adenoma-carcinoma sequence of the large bowel. Virchows Arch.
426:149–154. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kang H, Min BS, Lee KY, Kim NK, Kim SN,
Choi J and Kim H: Loss of E-cadherin and MUC2 expressions
correlated with poor survival in patients with stages II and III
colorectal carcinoma. Ann Surg Oncol. 18:711–719. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lee SJ, Choi SY, Kim WJ, Ji M, Lee TG, Son
BR, Yoon SM, Sung R, Lee EJ, Youn SJ and Park SM: Combined aberrant
expression of E-cadherin and S100A4, but not β-catenin is
associated with disease-free survival and overall survival in
colorectal cancer patients. Diagn Pathol. 8:992013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Xia D, Holla VR, Wang D, Menter DG and
DuBois RN: HEF1 is a crucial mediator of the proliferative effects
of prostaglandin E(2) on colon cancer cells. Cancer Res.
70:824–831. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Li P, Zhou H, Zhu X, Ma G, Liu C, Lin B
and Mao W: High expression of NEDD9 predicts adverse outcomes of
colorectal cancer patients. Int J Clin Exp Pathol. 7:2565–2570.
2014.PubMed/NCBI
|
|
49
|
Li Y, Bavarva JH, Wang Z, Guo J, Qian C,
Thibodeau SN, Golemis EA and Liu W: HEF1, a novel target of Wnt
signaling, promotes colonic cell migration and cancer progression.
Oncogene. 30:2633–2643. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Awasthi S, Maity T, Oyler BL, Qi Y, Zhang
X, Goodlett DR and Guha U: Quantitative targeted proteomic analysis
of potential markers of tyrosine kinase inhibitor (TKI) sensitivity
in EGFR mutated lung adenocarcinoma. J Proteomics. 189:48–59. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Miao Y, Li AL, Wang L, Fan CF, Zhang XP,
Xu HT, Yang LH, Liu Y and Wang EH: Overexpression of NEDD9 is
associated with altered expression of E-Cadherin, β-Catenin and
N-Cadherin and predictive of poor prognosis in non-small cell lung
cancer. Pathol Oncol Res. 19:281–286. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhang R, Zhang X, Ma B, Xiao B, Huang F,
Huang P, Ying C, Liu T and Wang Y: Enhanced antitumor effect of
combining TRAIL and MnSOD mediated by CEA-controlled oncolytic
adenovirus in lung cancer. Cancer Gene Ther. 23:168–177. 2016.
View Article : Google Scholar : PubMed/NCBI
|