Introduction
Gastric cancer accounts for ~10% of annual
cancer-associated mortalities globally in 2011 (1). Gastric cancer is more prevalent in South
Korea, Japan, Eastern Europe and South America, compared with other
geographical regions (2012) (2).
According to cancer statistics in 2013, gastric cancer was the most
frequently diagnosed cancer in males, and the fourth most common
cancer among females in South Korea (3). The risk factors for gastric cancer
include Helicobacter pylori infection, cigarette smoking,
alcohol abuse, obesity and a history of gastritis (2). Surgery is considered to be the most
effective treatment for gastric cancer, while adjuvant therapies,
including chemotherapy and chemoradiation, are additionally used to
improve patient survival by up to 15% (4). Furthermore, owing to the diverse
resources of medicinal plants, previous researchers have focused on
screening natural compounds as molecular targets for cancer
prevention, which has resulted in the discovery of numerous
anticancer agents, including curcumin, ginsenosides, genistein, and
(−)-epigallocatechin-3-gallate (5).
Rheum undulatum L. (R. undulatum),
also known as rhubarb, is a well-established East-Asian traditional
medicine for the treatment of inflammation (6,7), allergies
(8), dental diseases (6) and blood stagnation (7,9). Previous
studies also revealed that R. undulatum has numerous
pharmacological effects, including hepatocyte protective (10), anti-obesity and hypolipidemic
(7,11), anti-cariogenic (12), anti-allergic (8,9),
tyrosinase inhibition (13),
anti-diabetic (14), anti-platelet
aggregation (15),
anti-atherosclerotic (16) and
anticancer (6,17–20)
effects. Among the natural compounds of R. undulatum,
stilbenes, a group of non-flavonoid phenolic compounds, are
potential candidates for cancer chemoprevention (21). Numerous stilbenes isolated from R.
undulatum, including deoxyrhapontigenin (18), piceatannol (19) and pterostilbene (20), exhibit the ability to mediate cancer
cell death.
Programmed cell death is a key mechanism underlying
the pathogenesis of numerous diseases, particularly cancer.
Apoptosis, autophagy and programmed necrosis are the three main
types of programmed cell death (22).
Apoptosis is the principal type of cell death triggered by DNA
damage (22). Apoptosis is induced by
two independent pathways comprising of the death receptor
(extrinsic) pathway and the mitochondrial (intrinsic) pathway. In
the extrinsic pathway, initiator caspases (caspase-8 and −10) are
activated when pro-apoptotic ligands bind to their cognate death
receptors, tumor necrosis factor receptor superfamily member 10a
(TRAIL) and FAS, via adaptor proteins, such as Fas Associated Via
Death Domain (23). Following
activation, these initiator caspases cleave and stimulate effector
caspases (caspase-3 and −7) to induce cell apoptosis (23). The intrinsic pathway is regulated by
the interactions between pro- and anti-apoptotic B-cell lymphoma-2
(BCL-2) family proteins. When DNA damage or metabolic stress
occurs, BCL-2-associated X (BAX) and BCL-2 antagonist/killer (BAK)
are temporarily stimulated by the activation of BH3-only protein,
and BCL-2, BCL-extra large or myeloid cell leukemia 1 are inhibited
(23). BAX and BAK are also activated
by BH3 interacting domain death agonist (BID), which facilitates a
crosstalk between the extrinsic and intrinsic pathways (23). The activation of BAX and BAK
stimulates major outer membrane porin, which results in the release
of second mitochondria-derived activator of caspase (SMAC) and
cytochrome c (23). SMAC
blocks X-linked inhibitor of apoptosis, an inhibitor of caspase-9,
to facilitate the activation of caspase-9 induced by apoptosome
formation when cytochrome c interacts with apoptotic
peptidase activating factor 1 (23).
Finally, active caspase-9 activates caspase-3 and −7 to initiate
apoptotic cell death (23).
Numerous regulatory factors as mentioned above
contribute to the control of apoptotic pathways in multicellular
organisms and any abnormal expression of these factors may result
in cancer (23). Therefore, an
important strategy for cancer chemoprevention is stimulating
apoptotic pathways by inhibiting anti-apoptotic BCL-2 family
proteins or activating TRAIL death receptors and the caspase
cascade (24). The aim of the present
study was to evaluate the effects of extracts and compounds
isolated from R. undulatum on human gastric cancer cell
viability and the stimulation of apoptotic cell death, and to
elucidate their molecular mechanisms of action on the apoptosis
pathway.
Materials and methods
Plant material
The rhizomes of R. undulatum were purchased
from Kyung-dong Herbal Market (Seoul, South Korea) in 2015. The
plant material was authenticated by Dr Rack-Seon Seong, the
director of Center of Natural Resources Research, Jeonnam
Bioindustry Foundation (Jeonnam, South Korea). The voucher specimen
of this plant material (RU201506) was deposited in the Herbarium of
College of Pharmacy, Yonsei Institute of Pharmaceutical Sciences,
Yonsei University (Incheon, South Korea).
Chemicals and reagents
The Ez-Cytox Cell Viability assay kit was acquired
from Daeil Lab Service Co., Ltd. (Seoul, South Korea). The primary
antibodies against BID (cat. no. 2002; 1:1,000 dilution), BAX (cat.
no. 2772; 1:1,000 dilution), BCL-2 (cat. no. 2876; 1:1,000
dilution), cleaved caspase-3 (cat. no. 9661; 1:1,000 dilution),
cleaved caspase-8 (cat. no. 9496; 1:1,000 dilution), poly
(ADP-ribose) polymerase (PARP) (cat. no. 9542; 1:1,000 dilution),
and β-actin (cat. no. 4967; 1:2,000 dilution), the horseradish
peroxidase (HRP)-conjugated anti-rabbit secondary antibodies (cat.
no. 7074; 1:3,000 dilution), and the radioimmunoprecipitation assay
(RIPA) buffer were purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA). The Pierce™ BCA Protein assay kit
was obtained from Thermo Fisher Scientific, Inc. (Waltham, MA,
USA).
Sample preparation for cell viability
assay
The dried rhizomes of R. undulatum were
extracted using a Soxhlet extractor with 3 different methods to
prepare samples for the cell viability assay. A total of 10 g dried
rhizomes of R. undulatum was extracted with 100 ml 100%
ethanol (EtOH) at 60°C for 24 h to yield the RU1 extract (4.96 g),
whereas 10 g dried rhizomes was extracted with 100 ml 50% EtOH at
100°C for 24 h to yield the RU2 extract (5.65 g). For the last
sample, 10 g dried rhizomes was extracted with 100 ml of 50% EtOH
at 60°C for 13 h to yield the RU3 extract (5.85 g). The 0.5%
DMSO-treated group was used as a control.
Extraction and isolation of stilbene
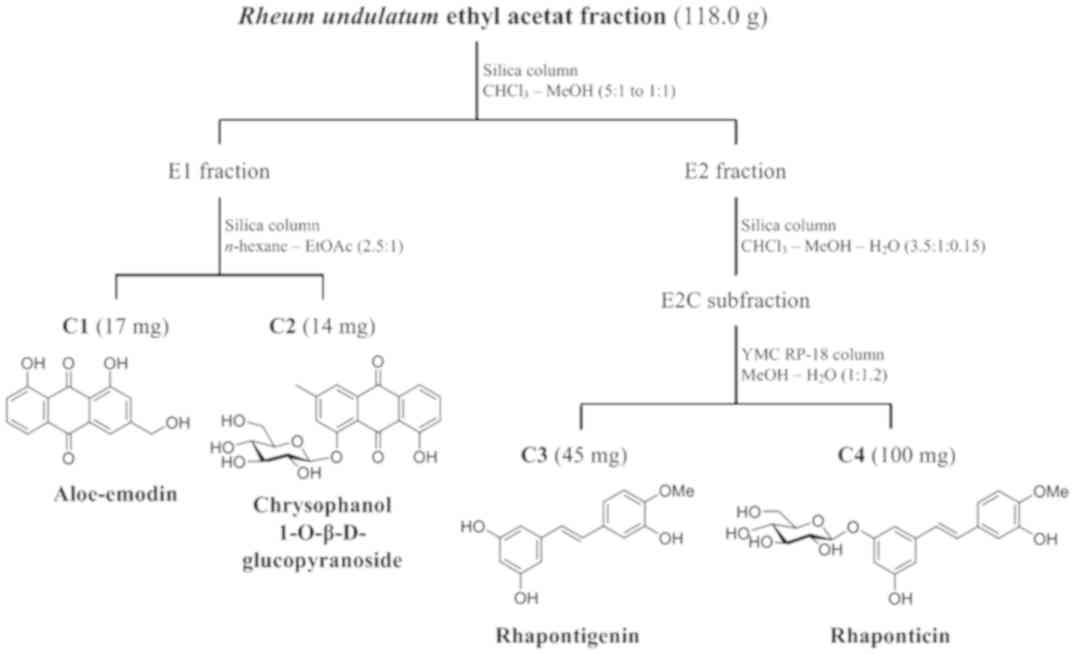
compounds
The dried rhizomes of R. undulatum (6.0 kg)
were sonicated thrice for 4 h at 65°C with 5.0 l of 100% methanol.
The solvent was removed in vacuo to yield 700.0 g of crude
extract. Following dispersion in 2 l of water, the crude extract
was solvent-partitioned sequentially with 2 l of chloroform
(CHCl3) and 2 l of ethyl acetate (EtOAc) to give
CHCl3 (7.7 g), EtOAc (118.0 g), and H2O
(335.0 g) fractions. The EtOAc fraction (118.0 g) was separated by
silica gel open column chromatography (4.5 cm diameter x
60 cm long; Kieselgel 60, 70–230 mesh and 230–400 mesh, Merck, NJ,
USA) with a CHCl3-MeOH (5:1 to 1:1, v/v, SK Chemicals,
South Korea) gradient solvent system to yield E1, E2 and E3
fractions. Compounds C1 (17 mg) and C2 (14 mg) were acquired from
the E1 (5.4 g) fraction using silica gel open column chromatography
(3.5 cm diameter * 30 cm long) with an n-hexane-EtOAc
(2.5:1, v/v, SK Chemicals, Gyeonggi-do, South Korea) isocratic
solvent system. The E2 fraction (10.6 g) was separated on a silica
open gel column (3.5 cm diameter × 35 cm long) eluted with a
CHCl3-MeOH-H2O (3.5:1:0.15, v/v/v) isocratic
solvent system to obtain E2A (2.3 g), E2B (1.8 g), and E2C (2.0 g)
subfractions. Compounds C3 (45 mg) and C4 (100 mg) were isolated
from the E2C subfraction using YMC RP-18 open column chromatography
(150 µm, Fuji Silysia Chemical Ltd., Japan; 2.5 cm diameter * 30 cm
long) with a MeOH-H2O (1:1.2, v/v) isocratic solvent
system. All the open column chromatography was performed at the
room temperature at a flow rate of 3 ml/min. The isolated compounds
were identified as aloe-emodin (C1), chrysophanol
1-O-β-D-glucopyranoside (C2), rhapontigenin (C3) and rhaponticin
(C4), by high-resolution electrospray ionization mass spectrometry
(HR-ESI-MS), 1D and 2D NMR spectroscopic analysis. The process of
isolation is summarized in Fig.
2.
Cell culture
The human gastric cancer cell line AGS was purchased
from the American Type Culture Collection (Manassas, VA, USA). The
cells were cultured with RPMI-1640 medium (Cellgro; Corning
Incorporated, Corning, NY, USA) containing 10% fetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc.), and supplemented with 100
U/ml penicillin, 100 mg/ml streptomycin and 1% sodium pyruvate. The
cultured cells were incubated at 37°C in a humidified atmosphere
containing 5% CO2.
Cell viability assay
AGS cells were seeded in 96-well plates at a density
of 1×104 cells/well for 24 h at 37°C, and then treated
with 25, 50, 100 and 200 µg/ml of the R. undulatum extracts.
Following 24 h of treatment, the cultured cells were incubated with
an Ez-Cytox Cell Viability assay kit for 1 h at 37°C, according to
the manufacturer's protocol. Cell viability was evaluated by
measuring the optical density change at 450 nm using a microplate
reader (PowerWave XS; BioTek Instruments, Inc., Winooski, VT,
USA).
Image-based cytometry assay
AGS cells were seeded on 6-well plates at a density
of 4×105 cells/well for 24 h at 37°C in order to allow
the cells to adhere to the bottom of the well, and were then
treated with the isolated compounds of R. undulatum at
concentrations of 100 µM. Following a 12 h treatment, the cells
were collected and stained in Annexin Binding Buffer (Thermo Fisher
Scientific, Inc.) with Annexin V Alexa Fluor 488 (Invitrogen;
Thermo Fisher Scientific, Inc.) for 30 min in dark at the room
temperature. Annexin V-positive cells were determined with a Tali
image-based cytometer (Invitrogen; Thermo Fisher Scientific, Inc.)
and analyzed by TaliPCApp version 1.0 software (Invitrogen; Thermo
Fisher Scientific, Inc.).
Western blot analysis
AGS cells were seeded on 6-well plates at a density
of 4×105 cells/well and treated with aloe-emodin and
chrysophanol 1-O-β-D-glucopyranoside at a concentration of 100 µM.
At 24 h following treatment, the cells were collected and lysed in
RIPA buffer supplemented with 1 mM phenylmethylsulfonyl fluoride to
obtain whole-cell extracts. Proteins in the whole-cell extracts of
each test sample were quantified by using the Pierce™
BCA Protein assay kit (Thermo Fisher Scientific, Inc.). Equal
amounts of proteins (20 µg/lane) were separated by 10% SDS-PAGE and
transferred onto polyvinylidene difluoride (PVDF) membranes. The
membranes were blocked with 5% skim milk for 1 h at room
temperature. The separated proteins were identified by incubation
at 20°C with epitope-specific primary and secondary antibodies (as
described previously) prior to visualization with ECL Advance
Western Blotting Detection reagents (GE Healthcare Life Sciences,
Little Chalfont, UK) and a FUSION Solo Chemiluminescence system
(PEQLAB Biotechnologie GmbH, Erlangen, Germany).
Statistical analysis
The data are presented as the mean ± standard
deviation. Statistical significance was determined by one-way
analysis of variance with a Bonferroni correction for multiple
comparisons using SigmaStat version 4.0 software (Systat Software,
Inc., San Jose, CA, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Treatment with R. undulatum extracts
reduces cell viability
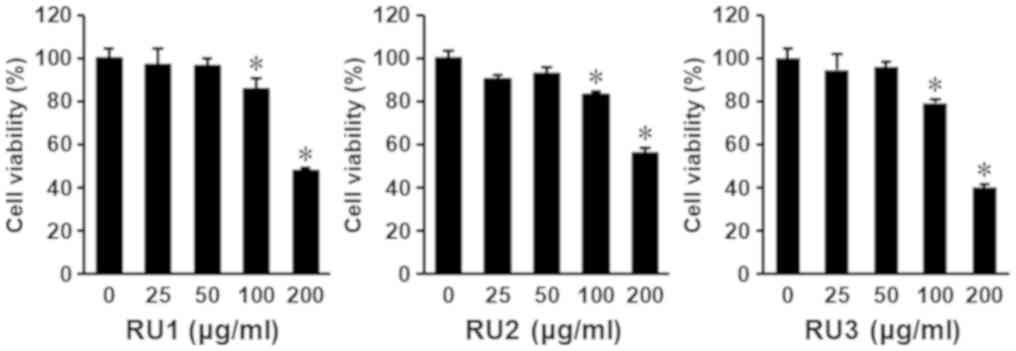
The viability of AGS cells was determined using cell
viability assays to evaluate the cytotoxic effects of the three
extracts from the dried rhizomes of R. undulatum. The
extracts, designated RU1, RU2 and RU3, were prepared using
different extraction methods. AGS cells were seeded on 96-well
plates at a density of 1×104 cells/well and then treated
with the three extracts at concentrations (25, 50, 100 and 200
µg/ml). Following 24 h of treatment, cell viability was evaluated
using the Exactor Cell Viability assay kit. As depicted in Fig. 1, all extracts exhibited
antiproliferative effects on AGS cells in a dose-dependent manner
at concentrations of 100 and 200 µg/ml. The IC50 values
of RU1, RU2, and RU3 extracts were 208.02±2.99, 222.67±2.10, and
175.89±1.40 µg/ml, respectively (Fig.
1). The RU3 extract, which was extracted using 50% EtOH as a
solvent and heated at 60°C for 13 h, had the strongest
antiproliferative effect on AGS cells. A number of previous studies
focused on the anticancer effects of the methanolic extract of
R. undulatum (6,18,20). In
the present study, the ethanolic extract of this medicinal plant
also exhibited inhibitory effects on cancer cell proliferation.
Collectively, these results demonstrate that the differences in
ethanol concentration, and temperature and time of extraction
process, affected the pharmacological effect of extracts.
Compounds isolated from R. undulatum
reduce cell viability in a dose-dependent manner
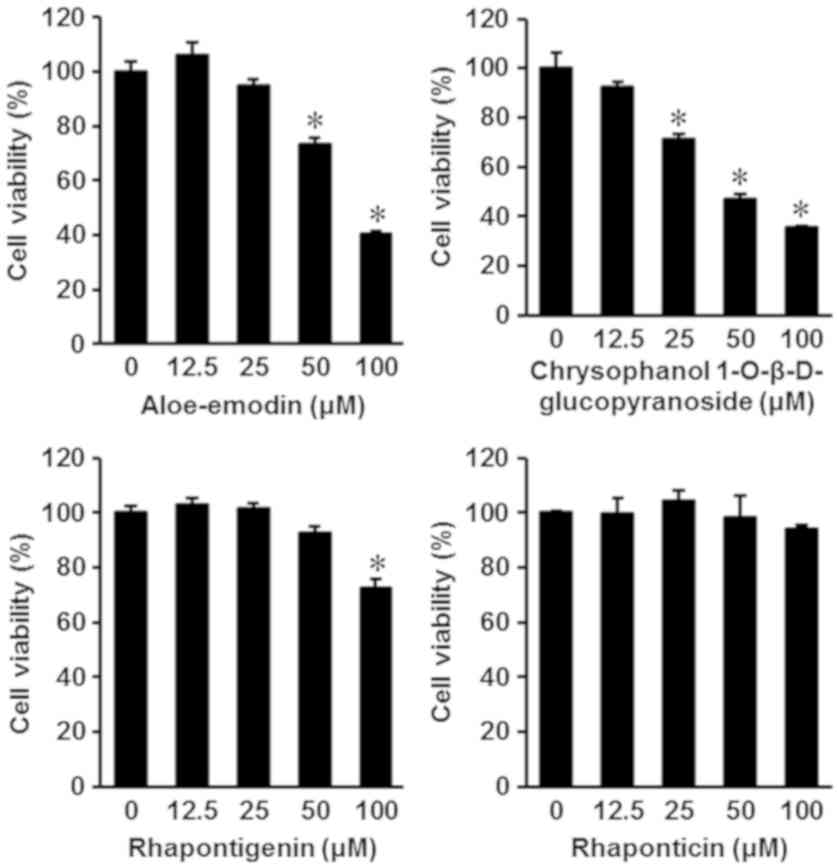
The isolated compounds were also evaluated for
cytotoxic effects on the human gastric carcinoma cell line AGS. The
results are depicted in Fig. 3. Among
the four isolated compounds, aloe-emodin and chrysophanol
1-O-β-D-glucopyranoside exhibited an increased potential for
anti-proliferative effects on AGS cells. The IC50 values
of aloe-emodin and chrysophanol 1-O-β-D-glucopyranoside were
84.66±0.44 and 68.28±0.29 µM, respectively (Fig. 3). To confirm whether the anticancer
effects of aloe-emodin and chrysophanol 1-O-β-D-glucopyranoside on
AGS cells were mediated by apoptotic pathway activation,
image-based cytometry was performed. Following treatment with
aloe-emodin and chrysophanol 1-O-β-D-glucopyranoside at a
concentration of 100 µM, the percentage of apoptotic cells labeled
with Annexin V significantly increased (P<0.05) to 52.66±5.50
and 54.33±6.65%, respectively, compared with 3.33±1.52% in the
control group (Fig. 4).
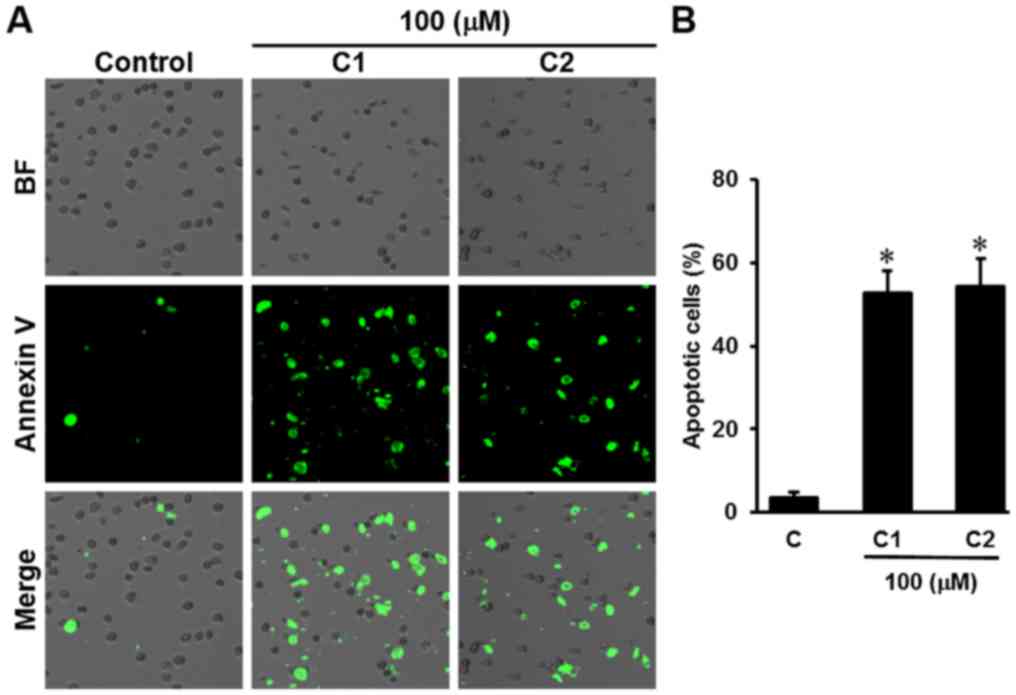 | Figure 4.Effect of Rheum undulatum
compounds on the induction of apoptotic cell death. (A) Microscopic
pictures from image-based cytometry assay. Cells were treated with
aloe-emodin or chrysophanol 1-O-β-D-glucopyranoside at a
concentration of 100 µM. Following treatment with these compounds
for 12 h, the cells were stained with Annexin V-Alexa Fluor 488 to
identify the apoptotic cells. The magnification cells: from left to
right, AGS cells were treated with 0.5% DMSO (Control), aloe-emodin
(C1), or chrysophanol 1-O-β-D-glucopyranoside (C2); from top to
bottom, the same magnification cells were taken pictures under
bright field (BF), fluorescence to detect Annexin V-stained cells
(Annexin V), or the merge form of bright field and fluorescence
(Merge). (B) The comparative graph illustrates the percentage of
apoptotic cells in each group. *P<0.05 vs. the 0.5% DMSO-treated
(control) group. C: 0.5% DMSO-treated (control) group; C1,
aloe-emodin; C2, chrysophanol 1-O-β-D-glucopyranoside; and BF,
bright field. |
Aloe-emodin and chrysophanol
1-O-β-D-glucopyranoside increase the expression of
apoptosis-associated proteins in AGS cells
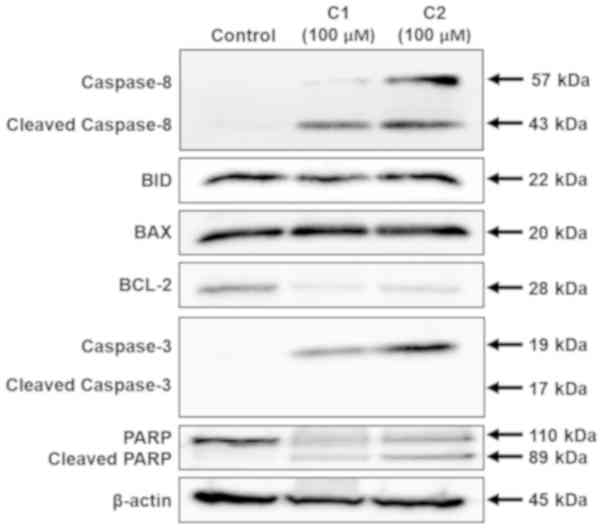
Western blot analysis was performed to identify the
mechanisms by which aloe-emodin and chrysophanol
1-O-β-D-glucopyranoside induced apoptosis. Western blot analysis
results demonstrated that aloe-emodin and chrysophanol
1-O-β-D-glucopyranoside stimulated the expression of
apoptosis-associated proteins in AGS cells. As depicted in Fig. 5, full-length and cleaved caspase-8,
and full-length caspase-3 expression markedly increased following
treatment with aloe-emodin or chrysophanol 1-O-β-D-glucopyranoside
at a concentration of 100 µM. Treatment with these compounds also
increased PARP cleavage in AGS cells. Additionally, aloe-emodin and
chrysophanol 1-O-β-D-glucopyranoside induced the downregulation of
the anti-apoptotic protein, BCL-2. The pro-apoptotic BCL-2 family
proteins, BID and BAX, were slightly induced when exposed to
chrysophanol 1-O-β-D-glucopyranoside.
Discussion
The present study is not the first to report the
anticancer effects of R. undulatum on gastric cancer cell
lines. According to the study by Hong et al (6), the methanolic extract of R.
undulatum induced apoptotic cell death through activation of
caspase cascade and inhibition of BCL-2 protein expression. The
present study focused on screening the anti-apoptotic effects of
the major phytochemical components in R. undulatum methanol
extracts and the results demonstrated that aloe-emodin and
chrysophanol 1-O-β-D-glucopyranoside significantly induced
apoptotic cell death, as observed by image-based cytometry.
Aloe-emodin is a well established drug candidate for cancer therapy
(25). Chen et al (25) reported that aloe-emodin at a
concentration of 150 µM induced apoptotic cell death in human
gastric carcinoma cells by stimulating the release of
apoptosis-inducing factor and cytochrome c, as well as by
activating caspase-3. In the present study, aloe-emodin at a
concentration of 100 µM also induced apoptosis in AGS cells via the
mitochondrial pathway and death receptor pathway, with the
activation of caspase-8. Additionally, aloe-emodin was observed to
regulate the intrinsic apoptotic pathway by downregulating the
expression of BCL-2. Furthermore, chrysophanol
1-O-β-D-glucopyranoside exhibited similar anticancer effects to
aloe-emodin, and therefore could be suitable for chemotherapeutic
application.
In conclusion, the initial phytochemical analysis of
the methanolic extract of dried rhizomes from R. undulatum
resulted in the isolation of four stilbenes. Among these compounds,
aloe-emodin and chrysophanol 1-O-β-D-glucopyranoside effectively
suppressed the viability of AGS cells, with IC50 values
of 84.66±0.44 and 68.28±0.29 µM, respectively. In a previous study,
various stilbenes, including resveratrol, pterostilbene,
piceatannol and pinosylvin, have been observed to increase cancer
cell death (21). The results of
western blot analysis in the present study demonstrated that
aloe-emodin and chrysophanol 1-O-β-D-glucopyranoside activated the
caspase cascade in apoptosis pathway and downregulated the
anti-apoptotic protein, BCL-2.
Acknowledgements
The authors would like to thank Dr Rack-Seon Seong,
the director of Center of Natural Resources Research, Jeonnam
Bioindustry Foundation (Jeonnam, South Korea), for authenticating
the plant material used in the present study.
Funding
This study was funded by the Gachon University
Research Fund, 2017 (grant no. GCU-2017-0132) and the Basic Science
Research Program through the National Research Foundation of Korea
(NRF) funded by the Ministry of Science, ICT & Future Planning
(grant no. NRF-2017R1A2B2011807).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JHK and KSK conceived and designed the experiments;
TAT, DL, SP, SHK, and JGP performed the experiments; TAT, DL and
SHK analyzed the data; JHK and KSK contributed the
reagents/materials/analysis tools; TAT, JHK and KSK wrote the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Conflict of interest
The authors declare that they have no competing
interests.
References
|
1
|
Guggenheim DE and Shah MA: Gastric cancer
epidemiology and risk factors. J Surg Oncol. 107:230–236. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Griffin-Sobel JP: Gastrointestinal
cancers: Screening and early detection. Semin Oncol Nurs.
33:165–171. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Oh CM, Won YJ, Jung KW, Kong HJ, Cho H,
Lee JK, Lee DH and Lee KH; Community of Population-Based Regional
Cancer Registries, : Cancer statistics in korea: Incidence,
mortality, survival, and prevalence in, 2013. Cancer Res Treat.
48:436–450. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Orditura M, Galizia G, Sforza V,
Gambardella V, Fabozzi A, Laterza MM, Andreozzi F, Ventriglia J,
Savastano B, Mabilia A, et al: Treatment of gastric cancer. World J
Gastroenterol. 20:1635–1649. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Park JM, Lee HJ, Yoo JH, Ko WJ, Cho JY and
Hahm KB: Overview of gastrointestinal cancer prevention in Asia.
Best Pract Res Clin Gastroenterol. 29:855–867. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hong NR, Park HS, Ahn TS, Jung MH and Kim
BJ: Association of a methanol extract of Rheum undulatum L.
Mediated cell death in AGS cells with an intrinsic apoptotic
pathway. J Pharmacopuncture. 18:26–32. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee W, Yoon G, Hwang YR, Kim YK and Kim
SN: Anti-obesity and hypolipidemic effects of Rheum
undulatum in high-fat diet-fed C57BL/6 mice through protein
tyrosine phosphatase 1B inhibition. BMB Rep. 45:141–146. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Matsuda H, Tomohiro N, Hiraba K, Harima S,
Ko S, Matsuo K, Yoshikawa M and Kubo M: Study on anti-Oketsu
activity of rhubarb II. Anti-allergic effects of stilbene
components from Rhei undulati Rhizoma (dried rhizome of Rheum
undulatum cultivated in Korea). Biol Pharm Bull. 24:264–267.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Matsuda H, Tewtrakul S, Morikawa T and
Yoshikawa M: Anti-allergic activity of stilbenes from Korean
rhubarb (Rheum undulatum L.): Structure requirements for
inhibition of antigen-induced degranulation and their effects on
the release of TNF-alpha and IL-4 in RBL-2H3 cells. Bioorg Med
Chem. 12:4871–4876. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dong G, Lee Y, Jeong JH, Zhao HY, Jeon R,
Lee HJ and Ryu JH: Stilbenoids from Rheum undulatum protect
hepatocytes against oxidative stress through AMPK activation.
Phytother Res. 29:1605–1609. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jo SP, Kim JK and Lim YH:
Antihyperlipidemic effects of rhapontin and rhapontigenin from
Rheum undulatum in rats fed a high-cholesterol diet. Planta
Med. 80:1067–1071. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Song JH, Yang TC, Chang KW, Han SK, Yi HK
and Jeon JG: In vitro anti-cariogenic activity of dichloromethane
fraction from Rheum undulatum L. Root. Arch Pharm Res.
29:490–496. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee HS, Kim JK, Park KT and Lim YH:
Rhapontigenin converted from rhapontin purified from Rheum
undulatum enhances the inhibition of melanin synthesis. Biosci
Biotechnol Biochem. 76:2307–2309. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Choi SZ, Lee SO, Jang KU, Chung SH, Park
SH, Kang HC, Yang EY, Cho HJ and Lee KR: Antidiabetic stilbene and
anthraquinone derivatives from Rheum undulatum. Arch Pharm
Res. 28:1027–1030. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ko SK, Lee SM and Whang WK: Anti-platelet
aggregation activity of stilbene derivatives from Rheum
undulatum. Arch Pharm Res. 22:401–403. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zeng Y, Song JX and Shen XC: Herbal
remedies supply a novel prospect for the treatment of
atherosclerosis: A review of current mechanism studies. Phytother
Res. 26:159–167. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Choi ES, Cho SD, Jeon JG and Cho NP: The
apoptotic effect of the hexane extract of Rheum undulatum L.
In oral cancer cells through the down-regulation of specificity
protein 1 and survivin. Lab Anim Res. 27:19–24. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Venkatesan T, Jeong MJ, Choi YW, Park EJ,
EI-Desouky SK and Kim YK: Deoxyrhapontigenin, a natural stilbene
derivative isolated from Rheum undulatum L. Induces
endoplasmic reticulum stress-mediated apoptosis in human breast
cancer cells. Integr Cancer Ther. 15:NP44–NP52. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kwon GT, Jung JI, Song HR, Woo EY, Jun JG,
Kim JK, Her S and Park JH: Piceatannol inhibits migration and
invasion of prostate cancer cells: Possible mediation by decreased
interleukin-6 signaling. J Nutr Biochem. 23:228–238. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee H, Kim Y, Jeong JH, Ryu JH and Kim WY:
ATM/CHK/p53 pathway dependent chemopreventive and therapeutic
activity on lung cancer by pterostilbene. PLoS One.
11:e01623352016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sirerol JA, Rodríguez ML, Mena S, Asensi
MA, Estrela JM and Ortega AL: Role of natural stilbenes in the
prevention of cancer. Oxid Med Cell Longev. 2016:31289512016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ouyang L, Shi Z, Zhao S, Wang FT, Zhou TT,
Liu B and Bao JK: Programmed cell death pathways in cancer: A
review of apoptosis, autophagy and programmed necrosis. Cell
Prolif. 45:487–498. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ichim G and Tait SW: A fate worse than
death: Apoptosis as an oncogenic process. Nat Rev Cancer.
16:539–548. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hotchkiss RS, Strasser A, McDunn JE and
Swanson PE: Cell death. N Engl J Med. 361:1570–1583. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen SH, Lin KY, Chang CC, Fang CL and Lin
CP: Aloe-emodin-induced apoptosis in human gastric carcinoma cells.
Food Chem Toxicol. 45:2296–2303. 2007. View Article : Google Scholar : PubMed/NCBI
|