Introduction
Cervical cancer has a high incidence rate and poses
a serious health risk to women. Nearly 50 million new patients with
cervical cancer are recorded worldwide annually (1). At present, no effective treatment for
patients with advanced cervical cancer exists. Therefore,
identifying targets for diagnosis and treatment is essential
(2,3).
MicroRNAs (miRs/miRNAs) are small non-coding RNAs
that regulate protein at the mRNA level; they are 18 to 22
nucleotides in length, and are ubiquitous in eukaryotes (4–6). A
number of studies have reported the abnormal expression of miRNAs
in cervical cancer, including increased expression of miR-21 and
miR-218, and reduced expression of miR-34 and miR-145 (7–10).
Alterations in the levels of these miRNAs have been demonstrated to
influence the expression of certain steps in key signaling
pathways, including caspase-8 and caspase-3 in apoptosis, and
phosphatase and tensin homolog (PTEN), an important tumor
suppressor (11,12).
miR-21 acts as an oncogene in cancer (13) by regulating a number of pathways
involved in tumor development. In the present review, the signaling
pathways regulated by miR-21 in cervical cancer were systematically
summarized, including the TNF-α/caspase-3/caspase-8, PI3K/AKT/mTOR
and RAS/MEK/ERK pathways. The impact of miR-21 on GAS5 lncRNA and
TIMP3 was also discussed. miR-21 also regulates other pathways,
including tropomyosin 1, pro-apoptotic FAS ligand, B-cell
translocation gene 2, Sprouty and F-box subfamily 1 (14). The present review provides an overall
analysis of miR-21 and its impact in signaling pathways.
miR-21 expression and gene targeting in
cervical cancer
The ability to influence cell proliferation by
regulating miR-21 expression may prove to be a novel mechanism to
prevent and treat cervical cancer. Upregulation of miR-21
expression has been demonstrated in numerous studies using patient
tissue samples, whole blood and cervical cancer cell lines,
including HeLa, SiHa and HT-3 cells (Table I) (7,11–13,15–18).
These studies identified target genes, including von Hippel-Lindau
tumor suppressor (VHL), tumor necrosis factor α (TNF-α), tissue
inhibitor of metalloproteinases 3 (TIMP3), growth arrest-specific 5
(GAS5), RAS p21 protein activator 1 (RASA1), programmed cell death
4 (PDCD4) and PTEN, as shown in Table
I. The mechanism by which miR-21 regulates TNF-α expression is
unclear. miR-21 may regulate numerous signaling pathways via its
target genes, including TNF-α/caspase-3/caspase-8,
RASA1/mitogen-activated protein kinase kinase (MEK) and protein
kinase B/mammalian target of rapamycin (AKT/mTOR) pathways. Certain
target genes of miR-21 do not participate in these signaling
pathways, including TIMP3 and GAS5 (7,13).
 | Table I.Targets of microRNA-21 in cervical
cancer. |
Table I.
Targets of microRNA-21 in cervical
cancer.
| First author | Year | Sample type | Target gene | Target region | Expression
change | (Refs.) |
|---|
| Cai et al | 2018 | Cervical carcinoma
cell lines: SiHa, HeLa, CaSki, C-4-1 and C-33-A | VHL | 3′-UTR | Downregulated | (32) |
| Xu et al | 2017 | HeLa Cells | TNF-α | Unknown | Upregulated | (11) |
| Zhang et al | 2018 | Cervical carcinoma
tissues, HeLa and SiHa cell lines | TIMP3 | 3′-UTR | Downregulated | (7) |
| Wen et al | 2017 | Cervical cancer
tissue | GAS5 | 3′-UTR | Downregulated | (13) |
| Zhang et al | 2016 | Whole-blood, HeLa and
HT-3 cells | RasA1 | 3′-UTR | Downregulated | (15) |
| Chen et al | 2015 | HeLa cell | PDCD4 and PTEN | 3′-UTR | Upregulated | (12) |
| Yao et al | 2009 | HeLa cells | PDCD4 | 3′-UTR | Downregulated | (18) |
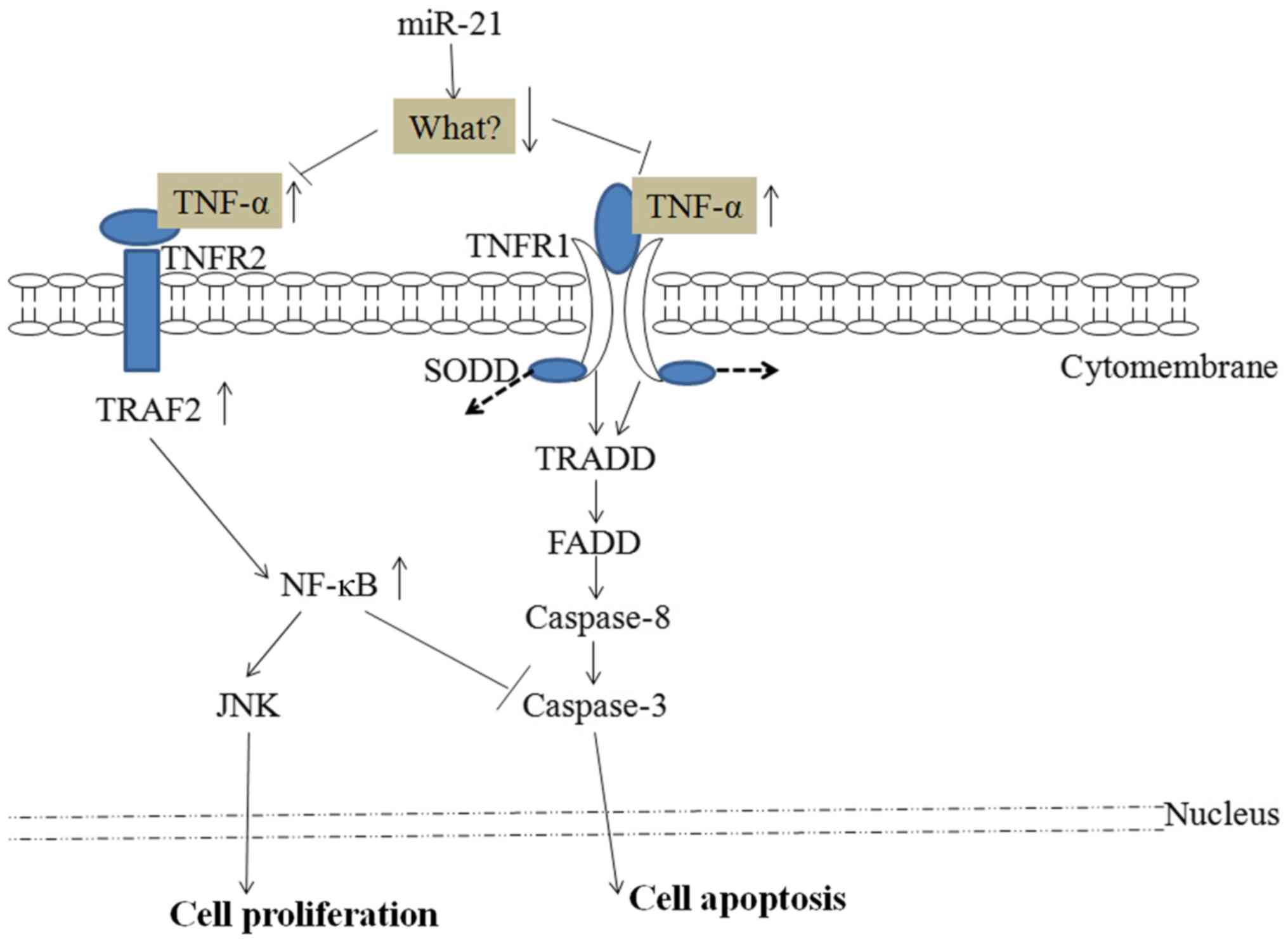
miR-21 and the TNF-α/caspase-3/caspase-8
signaling pathway
Cell apoptosis is a complex, multistage process that
involves numerous genes. Apoptosis can be induced by endoplasmic
reticulum stress, the mitochondrial pathway and death receptor
pathways. The expression and function of TNF-α, caspase-3 and
caspase-8 have been extensively studied in cancer apoptosis. These
genes appear to serve important roles in disease pathogenesis and
can be used as important prognostic markers. miR-21 has been shown
to upregulate mRNA and protein expression levels of TNF-α through
an unknown target in HeLa cells, thus impacting their proliferation
(11). TNF-α has two receptors
(TNFR1 and TNFR2) (19). As shown in
Fig. 1, the cellular apoptosis
program is activated when TNF-α, which is upregulated by miR-21,
binds and activates the TNFR1 receptor in HeLa cells (20). The proliferation capability of the
cells is activated when TNF-α binds to TNFR2, upregulating nuclear
factor κB (NF-κB), and thus inhibiting caspase-3 and activating
c-Jun N-terminal kinase (JNK) (11).
Overexpression of miR-21 has been shown to activate the NF-κB/JNK
signaling pathway in certain studies (21–23).
miR-21 and the AKT/mTOR signaling
pathway
Signaling through phosphoinositide3-kinase (PI3K)
and its downstream targets, AKT and mTOR, serves a key role in
differentiation, proliferation and cell survival (24). Tumor cell proliferation is increased
by activating the PI3K/AKT/mTOR signaling pathways (Fig. 2). The tumor suppressor PTEN is a
negative regulator of the PI3K/PTEN/AKT signaling pathways, and it
targets AKT to regulate cellular functions, including cell growth,
differentiation, proliferation and migration (25). PTEN is significantly upregulated in
miR-21-knockout cells, indicating that miR-21 downregulates PTEN
and reduces its negative regulation of the PI3K/PTEN/AKT signaling
pathway (12).
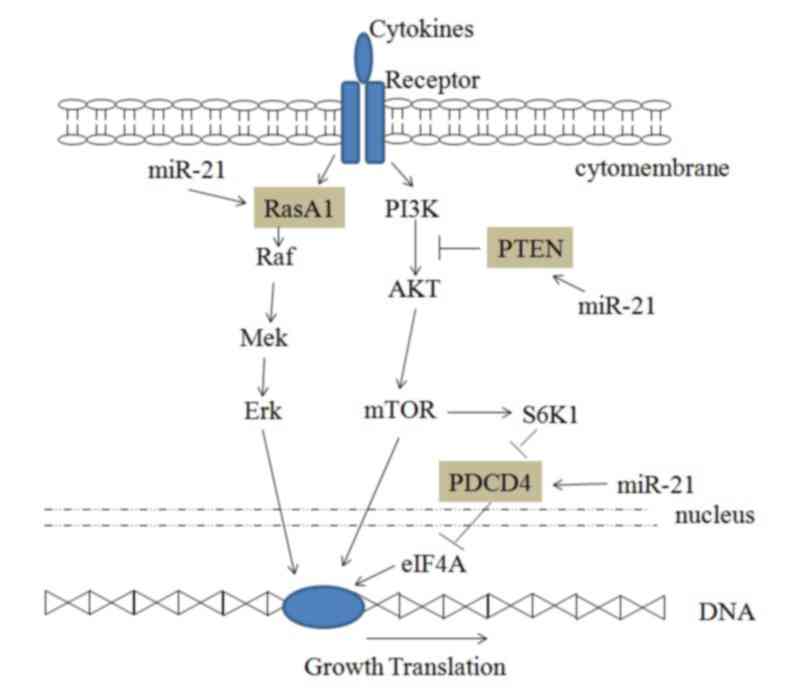 | Figure 2.miR-21 and the AKT/mTOR and RasA1
signaling pathways. miR-21, microRNA-21; RasA1, RAS p21 protein
activator 1; Raf, Raf proto-oncogene; Mek, mitogen-activated
protein kinase kinase; Erk, extracellular signal-regulated kinase;
PI3K, phosphoinositide 3-kinase; AKT, protein kinase B; mTOR,
mammalian target of rapamycin; PTEN, phosphatase and tensin
homolog; S6K1, ribosomal protein S6 kinase-1; PDCD4, programmed
cell death 4; eIF4A, eukaryotic initiation factor 4A. |
PDCD4 is a tumor suppressor that inhibits
translation initiation by binding to eukaryotic initiation factor
4A (eIF4A) (26). A number of
experiments have shown that PDCD4 is an important target of miR-21
and can regulate the proliferation of cancer cells (12,18).
Downregulation of PDCD4 by miR-21 increases HeLa cell growth
(18).
miR-21 and the RasA1 signaling pathway
KRAS is an oncogene that is frequently mutated in
cancer, leading to constitutive activation of the
RAS/MEK/extracellular signal-regulated kinase (ERK) signaling
pathway, which promotes cell proliferation, anti-apoptosis
signaling and malignant transformation (27). RasA1 is a member of the
Ras-GTPase-activating family that inactivates KRAS. Luciferase
activity assay in 293T cells demonstrated the miR-21 targets the
3′-untranslated region of RasA1 mRNA (15). Furthermore, cell proliferation is
increased by miR-21 targeting RasA1 and activating the RAS/MEK/ERK
signaling pathway (Fig. 2) (28).
miR-21 and other target molecules
Long non-coding RNAs (lncRNAs) serve an important
regulatory role in the biological activities of tumor cells. The
accumulation of lncRNA GAS5, a tumor suppressor, in growth-arrested
cells is regulated by the mTOR and nonsense-mediated mRNA decay
pathways (29). Wen et al
(13) demonstrated that miR-21
directly targets GAS5 lncRNA, which can be used to diagnose the
clinical stage of cervical cancer.
Deregulation of extracellular matrix homeostasis in
cancer contributes to tumor growth and metastasis (30). This process is mediated by matrix
metalloproteinases (MMPs) and their inhibitors, including TIMP3, an
independent promising biomarker in different cancer types. TIMP3
inhibits MMP activity to reduce the migration and invasion of
cancer cells (30,31). Zhang et al (7) found that miR-21 directly targets TIMP3
causing cervical cancer cells to become increasingly invasive and
proliferative, and increasing their viability.
Conclusions and perspectives
The present review provides insight into the effect
of miR-21 on cervical cancer cells, thus supporting novel concepts
for the diagnosis of the disease. As shown in Table I, miR-21 binds different target genes
and regulates numerous signaling pathways, which alter cancer
cells. miR-21 can be used as a biomarker of diagnosis and
potentially as a therapeutic target.
The proliferation and apoptosis of cervical cancer
cells requires the involvement and co-operation of numerous
signaling molecules. The TNFR1/caspase signaling pathway via
caspase-8/-3 can induce widespread cancer cell apoptosis upon
binding to TNF-α, which is regulated by miR-21 targeting of an
as-yet-unknown intermediate (Fig.
1). Transcribed miR-21 can also upregulate cervical cancer cell
proliferation via TNFR2 signaling by activating JNK and inhibiting
caspase-3.
miR-21 can regulate other signaling pathways as
shown in Fig. 2. Cervical cancer
cell proliferation increases due to miR-21 binding and the
inhibition of PTEN, thus inducing the PI3K/AKT/mTOR signaling
pathway activity. Moreover, cell proliferation increases subsequent
to miR-21 binding to RasA1, which inhibits the RAS/MEK/ERK
signaling pathway. Furthermore, miR-21 can reduce the inhibition of
eIF4A by PDCD4 and promote cell proliferation.
miR-21 has potential as a biomarker for the
diagnosis and prognosis of cervical cancer, or as a treatment
target in combination with other drugs to reduce metastasis. More
research is essential to uncover the targets of miR-21 and its role
in signaling pathways in cervical cancer, and to understand the
mechanisms behind its activity.
Acknowledgements
Not applicable.
Funding
The authors were supported by the Technology
Development Project Plan of Shandong Education Department
(Shandong, China) (grant nos. J15LM63 and J14LM54).
Availability of data and materials
The datasets used or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YW was a major contributor in writing the
manuscript. YW and CJ were responsible for the collection of the
relevant literature. SZ and KF revised the manuscript critically
for important intellectual content. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hoeben A, Polak J, Van De Voorde L,
Hoebers F, Grabsch HI and de Vos-Geelen J: Cervical esophageal
cancer: A gap in cancer knowledge. Ann Oncol. 27:1664–1674. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Buda A, Elisei F, Palazzi S, De Ponti E,
Arosio M, Vecchione F, Dell'Anna T, Cuzzocrea M, Bussi B, Giuliani
D, et al: Quality of care for cervical and endometrial cancer
patients: The impact of different techniques of sentinel lymph node
mapping on patient satisfaction. Ann Surg Oncol. 23:2975–2981.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Meier T and Kharofa J: Magnetic resonance
imaging-guided high-dose rate brachytherapy for cervical cancer.
Semin Roentgenol. 51:106–111. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jiang XI, Luo Y, Zhao S, Chen Q, Jiang C,
Dai Y, Chen Y and Cao Z: Clinical significance and expression of
microRNA in diabetic patients with erectile dysfunction. Exp Ther
Med. 10:213–218. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jia W, Wu Y, Zhang Q, Gao GE, Zhang C and
Xiang Y: Expression profile of circulating microRNAs as a promising
fingerprint for cervical cancer diagnosis and monitoring. Mol Clin
Oncol. 3:851–858. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Graziano A, Lo Monte G, Piva I, Caserta D,
Karner M, Engl B and Marci R: Diagnostic findings in adenomyosis: A
pictorial review on the major concerns. Eur Rev Med Pharmacol Sci.
19:1146–1154. 2015.PubMed/NCBI
|
|
7
|
Zhang Z, Wang J, Wang X, Song W, Shi Y and
Zhang L: MicroRNA-21 promotes proliferation, migration, and
invasion of cervical cancer through targeting TIMP3. Arch Gynecol
Obstet. 297:433–442. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang SF, Liu YF, Cheng CW, Yang WE, Lin
WL, Ko JL and Wang PH: Impact of microRNA-34a and polymorphism of
its target gene CA9 on susceptibility to uterine cervical cancer.
Oncotarget. 8:77860–77871. 2017.PubMed/NCBI
|
|
9
|
Zhou X, Yue Y, Wang R, Gong B and Duan Z:
MicroRNA-145 inhibits tumorigenesis and invasion of cervical cancer
stem cells. Int J Oncol. 50:853–862. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jiang Z, Song Q, Zeng R, Li J, Li J, Lin
X, Chen X, Zhang J and Zheng Y: MicroRNA-218 inhibits EMT,
migration and invasion by targeting SFMBT1 and DCUN1D1 in cervical
cancer. Oncotarget. 7:45622–45636. 2016.PubMed/NCBI
|
|
11
|
Xu L, Xu Q, Li X and Zhang X: MicroRNA-21
regulates the proliferation and apoptosis of cervical cancer cells
via tumor necrosis factor-α. Mol Med Rep. 16:4659–4663. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen B, Chen X, Wu X, Wang X, Wang Y, Lin
TY, Kurata J, Wu J, Vonderfecht S, Sun G, et al: Disruption of
microRNA-21 by TALEN leads to diminished cell transformation and
increased expression of cell-environment interaction genes. Cancer
Lett. 356:506–516. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wen Q, Liu Y, Lyu H, Xu X, Wu Q, Liu N,
Yin Q, Li J and Sheng X: Long noncoding RNA GAS5, which acts as a
tumor suppressor via microRNA 21, regulates cisplatin resistance
expression in cervical cancer. Int J Gynecol Cancer. 27:1096–1108.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shi J: Considering exosomal miR-21 as a
biomarker for cancer. J Clin Med. 5(pii): E422016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang L, Zhan X, Yan D and Wang Z:
Circulating MicroRNA-21 is involved in lymph node metastasis in
cervical cancer by targeting RASA1. Int J Gynecol Cancer.
26:810–816. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu J, Sun H, Wang X, Yu Q, Li S, Yu X and
Gong W: Increased exosomal microRNA-21 and microRNA-146a levels in
the cervicovaginal lavage specimens of patients with cervical
cancer. Int J Mol Sci. 15:758–773. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gocze K, Gombos K, Juhasz K, Kovacs K,
Kajtar B, Benczik M, Gocze P, Patczai B, Arany I and Ember I:
Unique microRNA expression profiles in cervical cancer. Anticancer
Res. 33:2561–2567. 2013.PubMed/NCBI
|
|
18
|
Yao Q, Xu H, Zhang QQ, Zhou H and Qu LH:
MicroRNA-21 promotes cell proliferation and down-regulates the
expression of programmed cell death 4 (PDCD4) in HeLa cervical
carcinoma cells. Biochem Biophys Res Commun. 388:539–542. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Qiu C, Hou G and Huang D: Molecular
mechanism of TNF-α signal transduction. Chin J Biochemistry Mol
Biol. 23:430–435. 2007.
|
|
20
|
Micheau O and Tschopp J: Induction of TNF
receptor I-mediated apoptosis via two sequential signaling
complexes. Cell. 114:181–190. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang Y and Wang JK: The functional
analysis of MicroRNAs involved in NF-kappaB signaling. Eur Rev Med
Pharmacol Sci. 20:1764–1774. 2016.PubMed/NCBI
|
|
22
|
Gao Q, Xu L, Yang Q and Guan TJ:
MicroRNA-21 contributes to high glucose-induced fibrosis in
peritoneal mesothelial cells in rat models by activation of the
Ras-MAPK signaling pathway via Sprouty-1. J Cell Physiol. Dec
4–2018.(Epub ahead of print).
|
|
23
|
Zhang H, Wang Y, Lv Q, Gao J, Hu L and He
Z: MicroRNA-21 overexpression promotes the neuroprotective efficacy
of mesenchymal stem cells for treatment of intracerebral
hemorrhage. Front Neurol. 9:9312018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang Z, Chen Q, Zhang J, Wang Y, Hu X,
Yin S, He M, Guan S, Qin W, Xiao Q, et al: Associations of genetic
polymorphisms in pTEN/AKT/mTOR signaling pathway genes with cancer
risk: A meta-analysis in Asian population. Sci Rep. 7:178442017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hu SL, Chang AC, Huang CC, Tsai CH, Lin CC
and Tang CH: Myostatin promotes interleukin-1β expression in
rheumatoid arthritis synovial fibroblasts through inhibition of
miR-21-5p. Front Immunol. 8:17472017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Suzuki C, Garces RG, Edmonds KA, Hiller S,
Hyberts SG, Marintchev A and Wagner G: PDCD4 inhibits translation
initiation by binding to eIF4A using both its MA3 domains. Proc
Natl Acad Sci USA. 105:3274–3279. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gong B, Liu WW, Nie WJ, Li DF, Xie ZJ, Liu
C, Liu YH, Mei P and Li ZJ: MiR-21/RASA1 axis affects malignancy of
colon cancer cells via RAS pathways. World J Gastroenterol.
21:1488–1497. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tidyman WE and Rauen KA: The RASopathies:
Developmental syndromes of Ras/MAPK pathway dysregulation. Curr
Opin Genet Dev. 19:230–236. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mourtada-Maarabouni M and Williams GT:
Growth arrest on inhibition of nonsense-mediated decay is mediated
by noncoding RNA GAS5. Biomed Res Int. 2013:3580152013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Martin del Campo SE, Latchana N, Levine
KM, Grignol VP, Fairchild ET, Jaime-Ramirez AC, Dao TV, Karpa VI,
Carson M, Ganju A, et al: MiR-21 enhances melanoma invasiveness via
inhibition of tissue inhibitor of metalloproteinases 3 expression:
In vivo effects of MiR-21 inhibitor. PLoS One. 10:e01159192015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nagao Y, Hisaoka M, Matsuyama A, Kanemitsu
S, Hamada T, Fukuyama T, Nakano R, Uchiyama A, Kawamoto M,
Yamaguchi K and Hashimoto H: Association of microRNA-21 expression
with its targets, PDCD4 and TIMP3, in pancreatic ductal
adenocarcinoma. Mod Pathol. 25:112–121. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cai L, Wang W, Li X, Dong T, Zhang Q, Zhu
B, Zhao H and Wu S: MicroRNA-21-5p induces the metastatic phenotype
of human cervical carcinoma cells in vitro by targeting the von
Hippel-Lindau tumor suppressor. Oncol Lett. 15:5213–5219.
2018.PubMed/NCBI
|