Introduction
Anaplastic lymphoma kinase (ALK)-fusion genes,
including encoding echinoderm microtubule-associated protein-like 4
(EML4)-ALK fusion variant, represent a small but important fraction
of oncogenic driver mutations in non-small cell lung cancer
(NSCLC), accounting for approximately 3–7% in all cases worldwide
(1,2).
Small molecule tyrosine kinase inhibitors (TKIs) are part of the
standard therapy for ALK fusion NSCLC. Resistance to
first-generation TKI crizotinib within the first 2 years following
treatment is mediated by a variety of mechanisms, including
secondary mutations within the ALK tyrosine kinase domain and
activation of alternative signaling pathways, including those
involving the ALK fusion gene or secondary KIT gene
amplification (3).
Single nucleotide polymorphism (SNP) array data on
NSCLC tissues and cell lines were evaluated for copy number
aberrations, and amplification of chromosomal segment 4q12
overlapping the locus of proto-oncogenes PDGFRA and
KIT was observed in 4.2% NSCLC samples (4). Therefore, in the present study it was
also taken into consideration whether there may be an activation of
the c-Kit/PDGFRA pathway in the ALK-fusion tumor at the initial
stage of NSCLC, which may subsequently lead to intrinsic TKI
resistance.
The phosphorylated forms of c-Kit and PDGFRA were
selected as biomarkers because phosphorylated proteins are the
biologically active states that function within the cell. In order
to gain comprehensive understanding of the phosphorylated
functional proteins in the c-Kit/PDGFRA signaling pathway and their
association with clinicopathological characteristics of patients
with ALK fusion, the expression of p-c-Kit and p-PDGFRA were
investigated, along with their association with the clinical
outcomes of patients with advanced stage NSCLC with ALK fusion.
Patients and methods
Patients and samples
Patients with tumors that were ALK-positive, as
detected by immunohistochemical staining (IHC) at The First
Affiliated Hospital of Guangzhou Medical University between January
2012 and March 2017, were selected retrospectively for the present
study. The tumors were staged pathologically according to the 2009
International Association for the Study of Lung Cancer (version 7)
(5). Clinical responses were
evaluated 1 month following the first administration of ALK-TKI
(crizotinib) (250 mg twice daily) and then every 3 months using
computed tomography or magnetic resonance imaging scans. The final
follow-up time point was in May 2017. The objective response rate
(ORR) and disease control rate (DCR) were assessed independently by
the present investigators and one radiologist, according to the
Response Evaluation Criteria In Solid Tumors (RECIST version 1.1)
(6). Progression-free survival (PFS)
was measured from the day of treatment initiation until disease
progression or mortality. Overall survival (OS) time was measured
from the day of initiated treatment until death. Formalin-fixed and
paraffin-embedded (FFPE) primary tumor tissues collected during
bronchoscopic or percutaneous lung biopsies were evaluated by two
pathologists in order to meet the criterion of ≥50% tumor cells.
Specimens of insufficient tissue quantity or quality for molecular
analyses were excluded. The present study was approved by the
Institutional Review Board of The First Affiliated Hospital,
Guangzhou Medical University. Written informed consent was obtained
from all participants prior to the study.
IHC staining
FFPE NSCLC tissue specimens from patients with
metastatic NSCLC were prospectively tested for ALK by IHC using the
Ventana platform (Roche Diagnostics, Basel, Switzerland). The assay
was developed as a system with the Ventana anti-ALK (D5F3) rabbit
monoclonal primary antibody (dilution, 1:100; cat. no., Ref
790–4794; Roche Diagnostics, Basel, Switzerland), according to the
manufacturer's protocols, in combination with the OptiView DAB IHC
detection and OptiView Amplification kits (Ventana Medical Systems,
Inc, Tucson, AZ, USA) for use on a Ventana BenchMark XT automated
staining instrument (Ventana Medical Systems, Inc.).
ALK-positive tumor FFPE sections (4 mm thick) were
used for IHC using an automated immunostainer (Leica Microsystems,
Germany). Briefly, the slides were heated at 95°C for 10 min for
antigen retrieval, and endogenous biotin was blocked at room
temperature for 10 min using a endogenous biotin blocking kit (cat.
no., ab64212, Abcam, Cambridge, UK), and the assay was performed
according to the manufacturer's protocols. Following incubation at
4°C overnight with anti-c-Kit (phosphor Y703) (dilution, 1:50;
ab62154) or anti-PDGFRA (phosphor Y754) (dilution, 1:100; ab5460)
antibody obtained from Abcam. The sections were subsequently
incubated with biotinylated secondary anti-rabbit antibodies with
1:500 dilution (cat. no., K500711) with LSAB2 system-HRP (DAKO;
Agilent Technologies, Inc, Santa Clara, CA, USA) for 30 min at room
temperature. The stained specimens were analyzed by two independent
pathologists using an fluorescent Olympus BX51 microscope
(magnification ×200-1,000; Olympus Corporation, Tokyo, Japan) and
results were scored according to a semi-quantitative method
(7) to reveal the staining intensity
(0=negative; 1=weak/trace; 2=moderate; and 3=strong) and the
percentage of positive cells (0, ≤10; 1, 11–25; 2, 26–50; 3, 51–75;
and 4, 76–100%). This grading produced a final score of 0–7,
calculated as the sum of intensity and percentage scores.
Therefore, the IHC staining was reported as negative (score 0–1) or
positive (score 2–7), further classified as weak (score 2–3),
moderate (score 4–5) or strong (score 6–7). In c-KIT staining low
protein expression was defined as having negative or weak IHC
staining, and high expression was defined as having moderate or
strong staining.
Statistical analysis
The χ2 or Fisher exact tests were used to
compare categorical variables. The Kaplan-Meier method was used to
calculate the PFS and OS rates, and the log-rank test was performed
to compare the PFS and OS among the groups. Cox multivariate
proportional hazard model was used for survival analysis, and the
hazard ratio (HR) and 95% confidence interval (CI) were calculated
using SPSS 16.0 (IBM Corp., Armonk, NY, USA). P<0.05 was
considered to indicate statistically significant differences in a
2-way analysis.
Results
Patient characteristics
Samples from 64 patients with advanced ALK fusion
NSCLC were selected. The patients had, a median age of 52 years
(range, 25–81 years). Histologically, 62 samples classified as
adenocarcinoma and 2 as adenosquamous carcinoma. Brain metastasis
had occurred in 26 (40.6%) patients, including 20 (76.9%) patients
at initial diagnosis and 6 (23.1%) patients while receiving
crizotinib treatment. Within this cohort, 41 patients had received
crizotinib treatment. The characteristics of the patients are
outlined in Table I.
 | Table I.Clinical characteristics of patients
with non-small cell lung cancer with anaplastic lymphoma kinase
fusion (n=64). |
Table I.
Clinical characteristics of patients
with non-small cell lung cancer with anaplastic lymphoma kinase
fusion (n=64).
|
|
| p-c-Kit level | p-PDGFRA
detection |
|---|
|
|
|
|
|
|---|
| Characteristic | n (%) | High, % | Low, % | P-value | Positive, % | Negative, % | P-value |
|---|
| Age, years |
|
|
| 0.03a |
|
| 0.38 |
| ≥52 | 33 (51.6) | 12, 36.4 | 21, 63.6 |
| 7, 21.2 | 26, 78.8 |
|
|
<52 | 31 (48.4) | 4, 12.9 | 27, 87.1 |
| 4, 12.9 | 27, 87.1 |
|
| Sex |
|
|
| 0.77 |
|
| 0.30 |
| Male | 38 (59.4) | 10, 26.3 | 28, 73.7 |
| 5, 13.2 | 33, 86.8 |
|
|
Female | 26 (40.6) | 6, 23.1 | 20, 76.9 |
| 6, 23.1 | 20, 76.9 |
|
| TKI treatment |
|
|
| 1.0 |
|
| 0.93 |
|
Crizotinib | 41 (64.1) | 11, 26.8 | 30, 73.2 |
| 8, 19.5 | 33, 80.5 |
|
| No TKI
treatment | 23 (35.9) | 5, 21.7 | 18, 78.3 |
| 3, 13.0 | 20, 87.0 |
|
| Line of TKI treatment
(n=41) |
|
|
| 0.38 |
|
| 0.62 |
|
First-line | 25 (61.0) | 5, 20.0 | 20, 80.0 |
| 6, 24.0 | 19, 76.0 |
|
|
Second-line and above | 16 (39.0) | 6, 37.5 | 10, 62.5 |
| 2, 12.5 | 14, 87.5 |
|
| Brain metastasis |
|
|
| 0.77 |
|
| 0.01a |
| Yes | 26 (40.6) | 7, 26.9 | 19, 73.1 |
| 9, 34.6 | 17, 65.4 |
|
| No | 38 (59.4) | 9, 23.7 | 29, 76.3 |
| 2, 5.3 | 36, 94.7 |
|
| Time of brain
metastasis (n=26) |
|
|
| 1.0 |
|
| 0.63 |
|
Incipient | 20 (76.9) | 5, 25.0 | 15, 75.0 |
| 7, 35.0 | 13, 65.0 |
|
| During
TKI treatment | 6 (23.1) | 2, 33.3 | 4, 66.7 |
| 1, 16.7 | 5, 83.3 |
|
| Symptoms of brain
metastasis (n=26) |
|
|
| 1.0 |
|
| 0.67 |
|
Yes | 7 (26.9) | 2, 28.6 | 5, 71.4 |
| 3, 42.9 | 4, 57.1 |
|
| No | 19 (72.1) | 5, 26.3 | 14, 73.7 |
| 6, 31.6 | 13, 68.4 |
|
| Response to
crizotinib (n=41) |
|
|
| 0.99 |
|
| 1.0 |
| Partial
response rate | 28 (68.2) | 7, 25.0 | 21, 75.0 |
| 5, 17.9 | 23, 82.1 |
|
| Stable
disease rate | 9 (22.0) | 2, 22.2 | 7, 77.8 |
| 2, 22.2 | 7, 77.8 |
|
| Disease
progression rate | 4 (9.8) | 2, 50.0 | 2, 50.0 |
| 1, 25.0 | 3, 75.0 |
|
| Overall survival
rate* (n=64) |
| n=16 | n=48 | NA | n=11 | n=53 | NA |
| Percent
of patients alive at one year | 51, 79.6 | 10, 60.0 | 41, 86.0 |
| 8, 73.0 | 43, 81.0 |
|
| Percent
of patients alive at three year | 37, 57.8 | 4, 25.0 | 33, 69.0 |
| 4, 36.0 | 33, 62.0 |
|
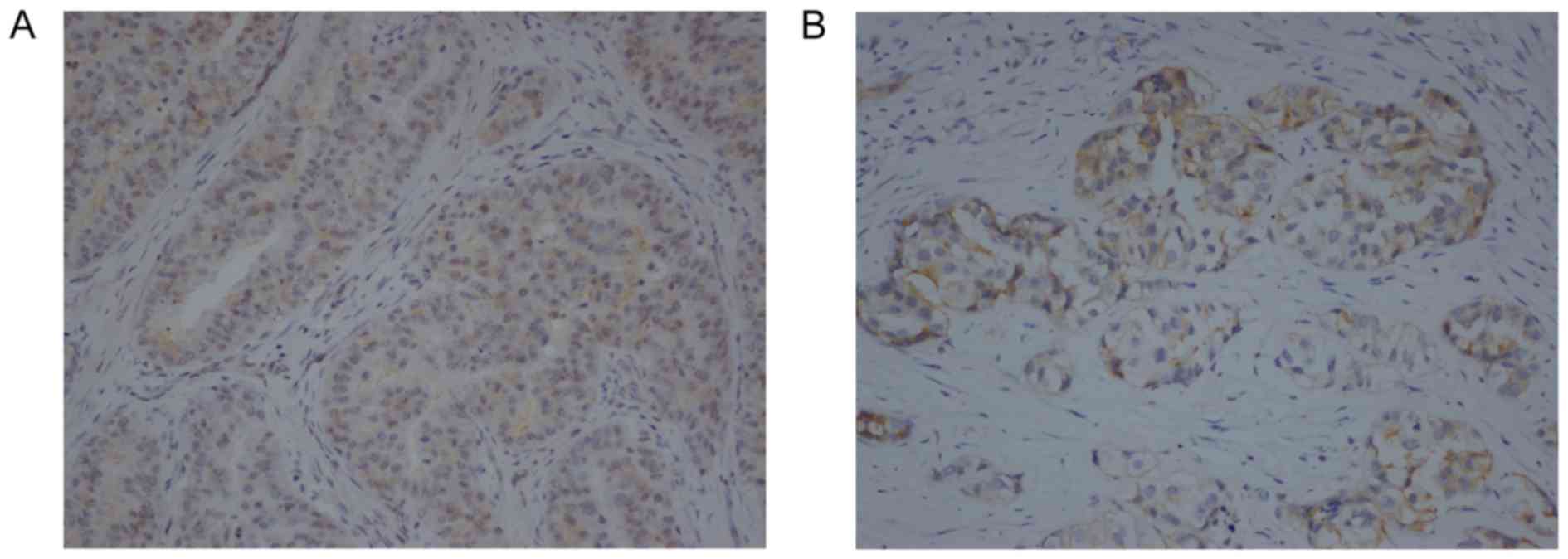
Expression of p-c-Kit and p-PDGFRA in
ALK fusion NSCLC tumor samples
The levels of p-c-Kit and p-PDGFRA were detected in
the samples of tumors with ALK fusion. IHC on the tumor cells
revealed that p-c-Kit was mainly expressed in the membrane and
cytoplasm (Fig. 1A), and p-PDGFRA was
mainly detected in the membrane (Fig.
1B). The associations between p-c-Kit and p-PDGFRA levels and
the clinicopathological features of the patients with advanced
NSCLC are depicted in Table I. Out of
64 ALK fusion tumor samples, 30 (46.9%) were p-c-Kit-positive. High
levels of p-c-Kit were observed in 16 out of 64 (25%) tumors. The
tumors from older patients (≥52 years) exhibited high p-c-Kit
expression significantly more frequently than those from younger
individuals (P=0.03). Regarding p-PDGFRA expression, 11 out of 64
(17.2%) specimens were positive. Positivity for p-PDGFRA was
observed with a higher occurrence in the samples of ALK fusion
patients with brain metastasis than those without (34.6 vs. 5.3%;
P=0.01).
Association between p-c-Kit and
p-PDGFRA expression and the efficacy of crizotinib
In the total study population, the ORR (the sum of
complete and partial response rate) and DCR (the sum of complete,
partial response and stable disease rate) were 68.2 and 90.2%,
respectively (Table I). There was no
difference in either ORR or DCR between high-level and low-level
p-c-Kit groups, as well as the subgroups between p-PDGFRA positive
and negative (P>0.05).
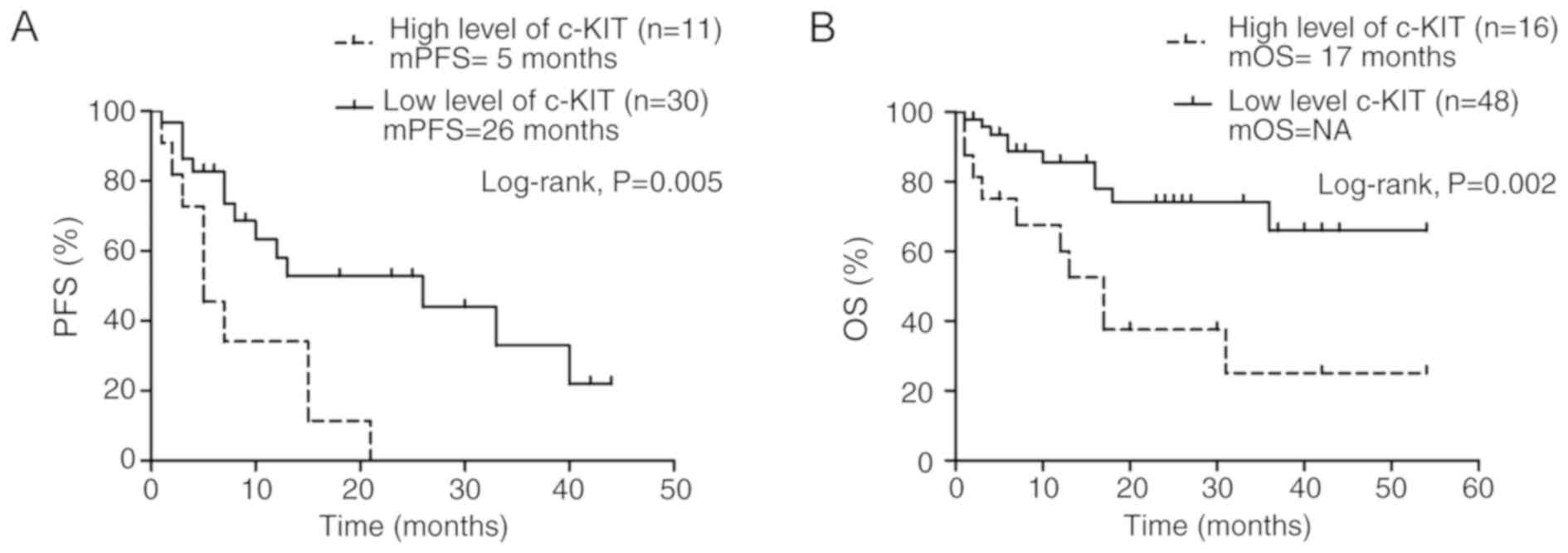
The median PFS time of crizotinib-treated patients
(n=41) with ALK fusion was 7 months, (range, 1–44 months; 95% CI,
5.1–18.5). The median PFS time of the patients with high p-c-Kit
levels was significantly shorter than those with low levels (5 vs.
26 months; P=0.005; Fig. 2A).
Furthermore, the patients with high levels of p-c-Kit in their
samples exhibited significantly lower OS times than those with
low-level p-c-Kit (median OS time, 17 months vs. NA, not available;
P=0.002; Fig. 2B), whether they had
received TKI or not. The one- and three-year OS rates in the
patients with high p-c-Kit levels were 60 and 25%, respectively,
and in those with low levels they were 86 and 69% respectively
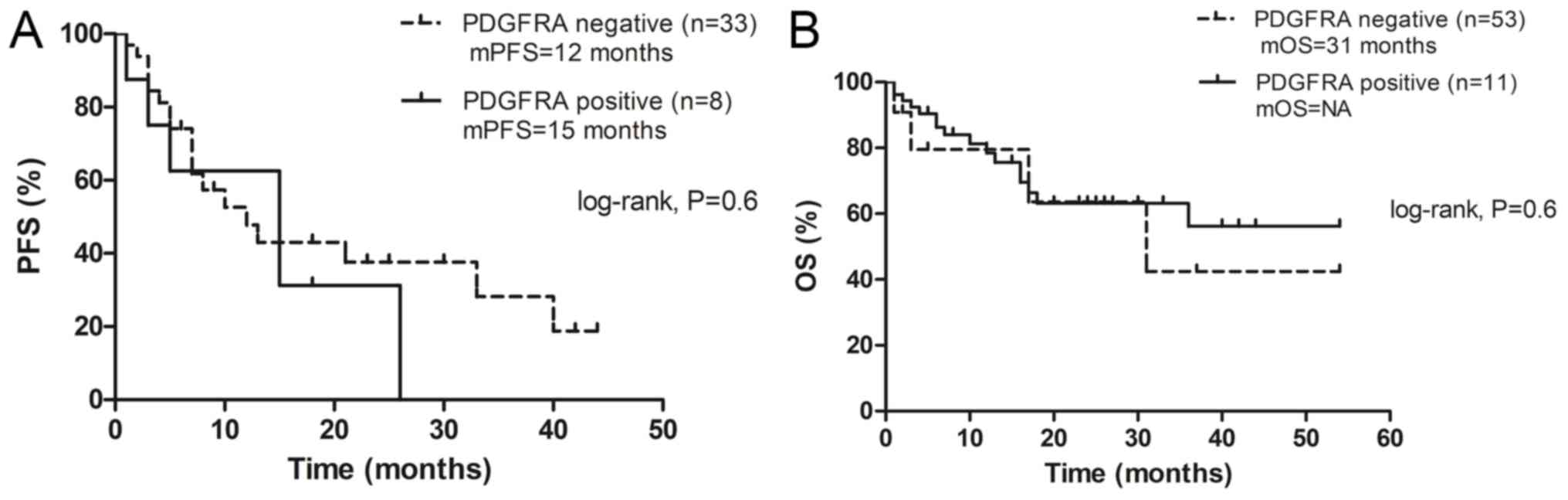
(Table I). Notably, the results
revealed no significant difference in PFS (Fig. 3A) of crizotinib and OS (Fig. 3B) between patients with
p-PDGFRA-positive tumors and those with p-PDGFRA-negative tumors
(P>0.05).
A multivariate Cox proportional hazard model was
used to analyze the significance of p-c-Kit expression in the PFS
time of patients. The factors included in the analysis were age,
brain metastasis, crizotinib line of treatment and p-c-Kit status
of the tumor. The results revealed that a high p-c-Kit level was
the only significant predictive factor for poor PFS time in the
patients treated by crizotinib (HR=2.7; 95% CI, 1.0–7.4; P=0.048).
This was also the only significant prognostic factor for poor OS
time (HR=5.3; 95% CI, 1.5–18.5; P=0.01; data not shown).
Discussion
To date, a limited number of biomarkers have been
proven to be associated with survival in patients with ALK fusion
NSCLC (8,9). c-Kit is a transmembrane tyrosine kinase,
with SCF as a ligand, serving an important role in inducing a
number of signal transduction pathways, including those of
mitogen-activated protein kinase and phosphatidyl inositol
3-kinase/protein kinase B (10,11). To
the best of our knowledge, the present study is the first to
investigate the association between the phosphorylated functional
proteins of the c-Kit/PDGFRA signaling pathway, and the efficacy of
crizotinib and prognosis in patients with advanced ALK fusion
NSCLC. The present data revealed that high levels of p-c-Kit
occurred more frequently in older patients (≥52 years) and
p-c-PDGFRA expression was associated with metastasis to the brain
(P<0.05). In the present study, p-PDGFRA expression was detected
in primary tumor samples prior to initial treatment in patients
with brain metastasis. PDGF serves an important role as a driver of
tumor growth in glioblastoma multiforme and other malignant brain
tumors, including malignant peripheral nerve sheath tumors and
seminoma, and chromosomal region 4q12, containing the PDGFRA
and KIT genes, is often amplified in these tumors (12). It is therefore hypothesized that the
PDGFRA pathway is associated with a subset of brain metastases
occurring in patients with ALK fusion NSCLC, as in the case of the
aforementioned patient with PDGFRA amplification in the
metastatic brain tumor. However, the intratumoral heterogeneity may
be caused by one clone harboring two gene aberrations or two
distinctive clones occurring in these patients (13).
Currently, first or second generation TKIs are
prescribed to patients with ALK fusion NSCLC as a first-line
treatment. The second-generation TKIs could achieve improved PFS
(but not OS) times and control of metastatic brain tumors as
first-line treatment, compared with crizotinib (14). However, it is unknown which patients
would benefit more from the second-generation TKIs. To date, only
BIM deletion polymorphism and EML4-ALK variants 3a/b
have been found to be associated with poor clinical response of
patients with ALK fusion NSCLC to crizotinib (8,9).
Therefore, further studies of the intrinsic mechanism of resistance
to crizotinib would be beneficial for improved decision-making in
TKI selection for first-line treatment.
In the present study, although no association was
observed between the levels of p-c-Kit or p-PDGFRA and the response
rate of the tumor following crizotinib treatment, the patients with
high p-c-Kit levels exhibited a lower PFS time than those with low
levels (5 vs. 26 months, P=0.005). Additionally, high levels of
p-c-Kit was the only independent prognostic factor for poor PFS
time following crizotinib treatment (HR=2.7, P<0.05). Although
the study has some limitations due to small sample numbers and
uncertainty of interpretation of the immunochemical staining
results, patients with low levels of p-c-Kit responded
significantly better to crizotinib treatment compared with the
patients treated by crizotinib reported in the previous clinical
trials in which the median PFS was 11 months (15,16).
Previous studies identified that the production of high levels of
c-Kit in NSCLC tumors is mediated by KIT gene amplification,
and not a gene mutation, which stimulates the activation of
downstream signaling molecules in the KIT signaling pathway
(17). This form of intrinsic
crizotinib resistance could be overcome with a combination of ALK
and c-Kit inhibitors. However, KIT gene amplification has
been reported to occur in ≤5% of NSCLC cases (4). Therefore, it was speculated that if high
p-c-Kit expression is caused by overamplification of the KIT
gene in only a small subset of ALK fusion tumors,
post-transcriptional or post-translational regulation may also
contribute to the final majority of p-c-Kit levels. For ALK fusion
NSCLC with de novo bypass pathway activation, it is unknown
if the next-generation ALK TKIs will be superior to crizotinib.
As the present findings revealed that p-c-Kit levels
are a predictive marker for TKI treatment, it was hypothesized that
they may also be a prognostic factor for survival in patients with
ALK fusion NSCLC. The results identified that high levels of
p-c-Kit predicted poor survival of the patients whether they had
received TKI treatment or not.
KIT gene functional mutations may be driver
mutations in certain malignant tumors, including gastrointestinal
stromal tumors, leukemia, lymphoma and mast cell tumors (17). In lung cancer, 69.2% of small cell
lung cancer (SCLC) was reported to express c-Kit, as detected by
IHC (18). Conflicting results have
been reported on the association between c-Kit expression and
prognosis in SCLC (19,20). Studies on c-Kit in NSCLC have focused
more on the early stages of the cancer, and the expression of c-Kit
in NSCLC tumors has been associated with an increased mortality
rate (21). Inconsistent results from
previous studies regarding the association between c-Kit expression
and prognosis may be due to differences in samples and IHC staining
measurements (10,21). In the present study, c-Kit protein was
expressed in 46.9% of advanced NSCLC with ALK fusion, in agreement
with a previous study on early stage NSCLC (8). The patients were further divided into
two groups (high vs. low levels of c-Kit). The results demonstrated
that patients with high levels of p-c-Kit exhibited significantly
lower survival times than those with low levels (P<0.05).
Furthermore, high levels of p-c-Kit was the only significant
indicator of poor survival in patients with ALK fusion NSCLC
(HR=5.3, P<0.05).
In conclusion, the present study suggests that
p-PDGFRA may be expressed more often in ALK-positive cases of NSCLC
with brain metastasis, and that c-Kit signaling activation may
serve as a predictor of crizotinib efficacy and as a prognostic
indicator for advanced stage ALK fusion NSCLC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
HY, FW and DH were responsible for collecting and
analyzed the data. QD and DX were responsible for
immunohistochemical staining. PH and XL analyzed the stained
specimens. All authors including HY, FW, DH, QD, DX, PH and XL have
read and approved the final version of this manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board of The First Affiliated Hospital, Guangzhou Medical
University. Written informed consent was obtained from all
participants prior to the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhou JX, Yang H, Deng Q, Gu X, He P, Lin
Y, Zhao M, Jiang J, Chen H, Lin Y, et al: Oncogenic driver
mutations in patients with non-small-cell lung cancer at various
clinical stages. Ann Oncol. 24:1319–1325. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Auliac JB, Monnet I, Dubos-Arvis C,
Chiappa AM, Baize N, Bota S, Vergnenegre A, Doubre H, Locher C,
Bizieux A, et al: Non-small-cell lung cancer (NSCLC) harboring ALK
translocations: Clinical characteristics and management in a
real-life setting: A French retrospective analysis (GFPC 02–14
study). Target Oncol. 12:833–838. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Katayama R, Shaw AT, Khan TM,
Mino-Kenudson M, Solomon BJ, Halmos B, Jessop NA, Wain JC, Yeo AT,
Benes C, et al: Mechanisms of acquired crizotinib resistance in
ALK-rearranged lung Cancers. Sci Transl Med. 4:120ra172012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ramos AH, Dutt A, Mermel C, Perner S, Cho
J, Lafargue CJ, Johnson LA, Stiedl AC, Tanaka KE, Bass AJ, et al:
Amplification of chromosomal segment 4q12 in non-small cell lung
cancer. Cancer Biol Ther. 8:2042–2050. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kassis ES, Vaporciyan AA, Swisher SG,
Correa AM, Bekele BN, Erasmus JJ, Hofstetter WL, Komaki R, Mehran
RJ, Moran CA, et al: Application of the revised lung cancer staging
system (IASLC Staging Project) to a cancer center population. J
Thorac Cardiovasc Surg. 38:412–418.e1-e2. 2009. View Article : Google Scholar
|
|
6
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwarts LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang H, Wang W, Zhang Y, Zhao J, Lin E,
Gao J and He J: The role of NF-E2-related factor 2 in predicting
chemoresistance and prognosis in advanced non-small cell lung
cancer. Clin Lung Cancer. 12:166–171. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang L, Jiang T, Li X, Wang Y, Zhao C,
Zhao S, Xi L, Zhang S, Liu X, Jia Y, et al: Clinical features of
Bim deletion polymorphism and its relation with crizotinib primary
resistance in Chinese patients with ALK/ROS1 fusion-positive
non-small cell lung cancer. Cancer. 123:2927–2935. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Woo CG, Seo S, Kim SW, Jang SJ, Park KS,
Song JY, Lee B, Richards MW, Bayliss R, Lee DH and Choi J:
Differential protein stability and clinical responses of EML4-ALK
fusion variants to various ALK inhibitors in advanced
ALK-rearranged non-small cell lung cancer. Ann Oncol. 28:791–797.
2017.PubMed/NCBI
|
|
10
|
Herpel E, Jensen K, Muley T, Warth A,
Schnabel PA, Meister M, Herth FJ, Dienemann H, Thomas M and
Gottschling S: The cancer stem cell antigens CD133, BCRP1/ABCG2 and
CD117/c-KIT are not associated with prognosis in resected
early-stage non-small cell lung cancer. Anticancer Res.
31:4491–4500. 2011.PubMed/NCBI
|
|
11
|
Marech I, Gadaleta CD and Ranieri G:
Possible prognostic and therapeutic significance of c-Kit
expression, mast cell count and microvessel density in renal cell
carcinoma. Int J Mol Sci. 15:13060–13076. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Holtkamp N, Ziegenhagen N, Malzer E,
Hartmann C, Giese A and von Deimling A: Characterization of the
amplicon on chromosomal segment 4q12 in glioblastoma multiforme.
Neuro Oncol. 9:291–297. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cai W, Lin D, Wu C, Li X, Zhao C, Zheng L,
Chuai S, Fei K, Zhou C and Hirsch FR: Intratumoral heterogeneity of
ALK-rearranged and ALK/EGFR coaltered lung adenocarcinoma. J Clin
Oncol. 33:3701–3709. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qin A and Gadgeel S: The current landscape
of anaplastic lymphoma kinase (ALK) in non-small cell lung cancer:
Emerging3 treatment paradigms and future directions. Target Oncol.
12:709–718. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Solomon BJ, Mok T, Kim DW, Wu YL, Nakagawa
K, Mekhail T, Felip E, Cappuzzo F, Paolini J, Usari T, et al:
First-line crizotinib versus chemotherapy in ALK-positive lung
cancer. N Engl J Med. 371:2167–2177. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Solomon BJ, Cappuzzo F, Felip E, Blackhall
FH, Costa DB, Kim DW, Nakagawa K, Wu YL, Mekhail T, Paolini J, et
al: Intracranial efficacy of crizotinib versus chemotherapy in
patients with advanced ALK-positive non-small-cell lung cancer:
Results from PROFILE 1014. J Clin Oncol. 34:2858–2865. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liang J, Wu YL, Chen BJ, Zhang W, Tanaka Y
and Sugiyama H: The C-kit receptor-mediated signal transduction and
tumor-related diseases. Int J Biol Sci. 9:435–443. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li AS, Sun LN and Zhan ZL: Expression and
mutational analysis of c-kit gene in small cell lung cancer. Chin J
Cancer Prev Treat. 14:917–919. 2007.
|
|
19
|
Yokouchi H, Nishihara H, Harada T, Ishida
T, Yamazaki S, Kikuchi H, Oizumi S, Uramoto H, Tanaka F, Harada M,
et al: Immunohistochemical profiling of receptor tyrosine kinases,
MED12, and TGF-βRII of surgically resected small cell lung cancer,
and the potential of c-kit as a prognostic marker. Oncotarget.
8:39711–39726. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Terada T: An immunohistochemical and
molecular genetic analysis of KIT and PDGFRA in small cell lung
carcinoma in Japanese. Int J Clin Exp Pathol. 5:331–338.
2012.PubMed/NCBI
|
|
21
|
Xiao H, Wang J, Liu Y and Li L: Relative
influence of c-Kit expression and epidermal growth factor receptor
gene amplification on survival in patients with non-small cell lung
cancer. Oncol Lett. 8:582–588. 2014. View Article : Google Scholar : PubMed/NCBI
|