Introduction
Gastric cancer is one of the most common malignant
tumors in the digestive system. In 2015, the American Cancer
Society reported 24,590 new cases of gastric cancer in the United
States, of which 63.20% were male (1). Of the 1 million new cases of gastric
cancer in the world each year, Asians account for 74% (2). Incidence of gastric cancer has racial
and regional characteristics (3).
Gastric adenocarcinoma as the most common type of gastric cancer
originates from the gastric glandular epithelial cells accounting
for ~95% of gastric cancer (4).
Pathogenesis of gastric cancer is not yet clear, and may be closely
related to dietary habits and Helicobacter pylori (5). Oncogenes and suppressor genes, changes
in epigenetics and changes in chromosome stability, are likely to
alter cell proliferation, apoptosis, and invasion, and thus
participate in the development of gastric cancer (6).
Numerous findings have shown that a variety of
lncRNAs and miRNAs are involved in the occurrence of gastric cancer
(7). miRNAs (~22 bp) are endogenous
RNAs that do not encode protein, but play a regulatory role at the
level of transcription and translation (8). miRNAs can bind to the 3′-untranslated
region sequence of the target mRNA through base pairing, thereby
degrading target mRNA or preventing translation of target mRNA, and
play an important role in tumor cell proliferation,
differentiation, and malignant transformation (9). It has been shown that miR-124 is
downregulated in many cancer tissues such as liver cancer and can
inhibit tumor growth (5,10). Liu et al (11) found that miR-124 may be a predictor of
LNM and tumor stage in gastric cancer. In order to understand the
role of miR-124 in gastric cancer, the present study investigated
the expression of miR-124 in gastric cancer SGC-7901 cells and its
effect on biological function. Our study provides a new potential
molecular target for the treatment of gastric cancer.
Materials and methods
Cells and reagents
Gastric adenocarcinoma SGC-7901 cell line, human
normal gastric mucosal epithelial GES-1 cells (Shanghai Guandao
Bio-engineering Co., Ltd., Shanghai, China); miR-124 agomir and
negative control sequence (Shanghai GenePharma Co., Ltd., Shanghai,
China); fetal bovine serum and RPMI-1640 medium (Shanghai Hengyuan
Biotechnology Co., Ltd., Shanghai, China); EasyPure miRNA kit,
TransScript Green miRNA Two-Step qRT-PCR SuperMix kit (Beijing
TransGen Biotech Co., Ltd., Beijing, China);
Lipofectamine® 3000 transfection reagent and TRIzol
reagent (Thermo Fisher Scientific, Inc., Waltham, MA, USA); qPCR
primers (Sangon Biotech Co., Ltd., Shanghai, China); MTT (Yeasen
Biotechnology, Co., Ltd., Shanghai, China); Transwell cell (Beijing
Yiming Fuxing Biotechnology, Beijing, China).
The study was approved by the Ethics Committee of
The First Hospital of Jilin University (Changchun, China). Signed
informed consents were obtained from the patients or the
guardians.
RT-qPCR detection of miR-124 expression in cancer
and adjacent tissues. Fifty cases of identified gastric
adenocarcinoma and paracancerous tissues were obtained in The First
Hospital of Jilin University. RT-qPCR was used to detect the
expression of miR-124 in cancer and adjacent tissues. TRIzol
reagent was used to extract total RNA from cancer and paracancerous
tissues. After purification using an EasyPure miRNA kit, the
concentration and purity of RNA were detected by UV
spectrophotometer. RNA samples with a A260/A280 ratio of 1.8–2.0
were subjected to reverse transcription in accordance with the
reverse transcription kit instructions: 37°C for 1 h and 85°C 5
sec. qPCR reaction system was prepared strictly in accordance with
the qPCR kit instructions. Reaction conditions of PCR reaction:
94°C for 30 sec, followed by 40 cycles of 94°C for 5 sec and 60°C
for 30 sec. GAPDH was used as the endogenous control and the primer
sequences are listed in Table I. The
2−ΔCq method was used to process qRT-PCR results
(12).
 | Table I.qPCR primer sequences. |
Table I.
qPCR primer sequences.
| Gene | Forward | Reverse |
|---|
| GAPDH |
5′-GGTGGTGCTAAGCGTGTTA-3′ |
5′-CCCTCCACAATGCCAA-3′ |
| miR-124 |
5′-TAAGGCACGCGGTGAATG-3′ |
5′-TGGTGTCGTGGAGTCG-3′ |
Gastric adenocarcinoma tissue inclusion criteria
(13): i) pathological examination
confirmed gastric adenocarcinoma; ii) intended for surgical
treatment; iii) patients with normal functions of important organs
such as heart and lung and patients were able to withstand surgical
resections; iv) signed informed consent. Exclusion criteria were:
i) patients with postoperative recurrence of gastric cancer and
recurrent gastric cancer; ii) history of abdominal surgery,
abdominal abscess, and peritoneal inflammation; iii) patients with
important organ dysfunction; iv) recent use of hormone drugs, blood
products and immunosuppressive agents; v) received preoperative
radiation therapy, chemotherapy and other antitumor therapy.
Cell transfection
Cells were divided into miR-124 agomir group, NC
group and blank group. Cells were incubated with RPMI-1640 medium
containing 10% FBS at 37°C in a 5% CO2 incubator.
Transfection was performed when the cell confluence reached 80% and
transfection was performed strictly in accordance with the
instructions of Lipofectamine® 3000. Cells in miR-124
group were transfected with miR-124 agomir, cells in NC group were
transfected with agomir negative control sequence and cells in
control group were not transfected. Cells were incubated for 5 min
at room temperature and plasmid DNA and liposome complexes were
mixed with the cells. Cultured cells were incubated at 37°C with 5%
CO2 for 2 days and transfection was checked. Then, the
cell was collected for subsequent experimentation.
RT-qPCR detection of miR-124
expression in different groups of cells
RT-qPCR was used to detect the expression of miR-124
in each group. Specific methods used were the same as in ‘Cells and
reagents’.
MTT assay to detect cell
proliferation
When the cell confluency reached 80%, cell
suspensions were prepared with a cell density of 2×104
cells/ml. Then, 200 µl cell suspension was added into each well of
a 96-well plate. Cell viability was measured by MTT assay. A total
of 20 µl of 5 mg/ml MTT solution was added into each well at 12,
24, 48, 72, and 96 h after the initiation of cell culture for color
development. After incubation for another 4 h, 150 µl Formanzan
Lysing Solution was added into each well, followed by shaking at
room temperature for 10 min. When crystals were completely
dissolved, absorbance (OD) at the wavelength of 490 nm was measured
using a microplate reader. The measurement was repeated 3
times.
Transwell invasion assay to detect
cell invasion ability
Serum-free DMEM medium was used to starve cultured
cells for 12 h to remove the effects from serum. When cell
confluence reached 80%, cells were resuspended with the invasion
buffer. Cells were counted and cell density was adjusted to
5×104/ml. A total of 200 µl of cell suspension was
evenly spread over 6-well Transwell upper chamber (cat. no. 354480;
BD Biosciences, Franklin Lakes, NJ, USA) polycarbonate membrane and
600 µl of DMEM medium containing 20% fetal bovine serum was added
into the lower chamber. The cells were cultured at 37°C with 5%
CO2 for 24 h. A cotton swab was used to gently wipe off
the uninvaded cells on polycarbonate membrane. After washing with
PBS 3 times, the membrane was fixed with 4% paraformaldehyde for 10
min, followed by crystal violet staining for 10 min. After washing
with PBS 3 times, 5 visual fields (×400) were selected under a
microscope (Olympus Corporation, Tokyo, Japan) to count cells and
calculate the average value.
Statistical analysis
SPSS20.0 [AsiaAnalytics (formerly SPSS China),
Shanghai, China] statistical software package was used to test and
analyze the data. Measured data were expressed as mean ± standard
deviation. ANOVA followed by LSD test was used for comparisons
among three groups. Comparisons of data at different time-points in
the same group of MTT assay were performed by repeated measures
analysis of variance. Significance level was α=0.05.
Results
Expression of miR-124 in gastric
adenocarcinoma tissues and adjacent tissues
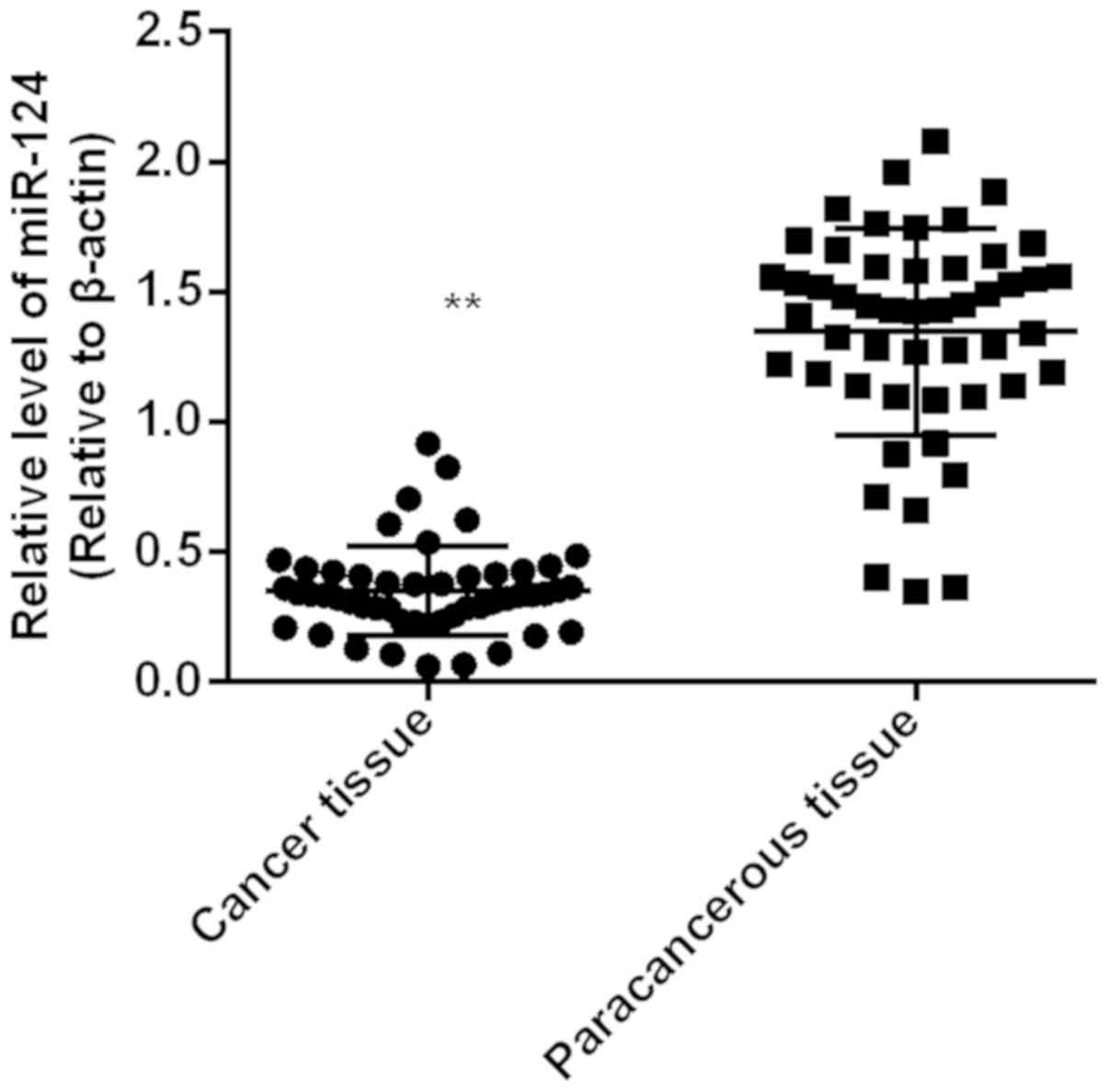
Expression level of miR-124 in gastric
adenocarcinoma tissue (0.35±0.02) was significantly lower than that
in paracancerous tissue (1.34±0.06) (P<0.01). Expression level
of miR-124 in cancer tissue was lower than that in corresponding
paracarcinoma tissues in 45 cases, and the downregulation rate was
90% (Fig. 1).
Expression of miR-124 in GES-1 and
SGC-7901 before transfection
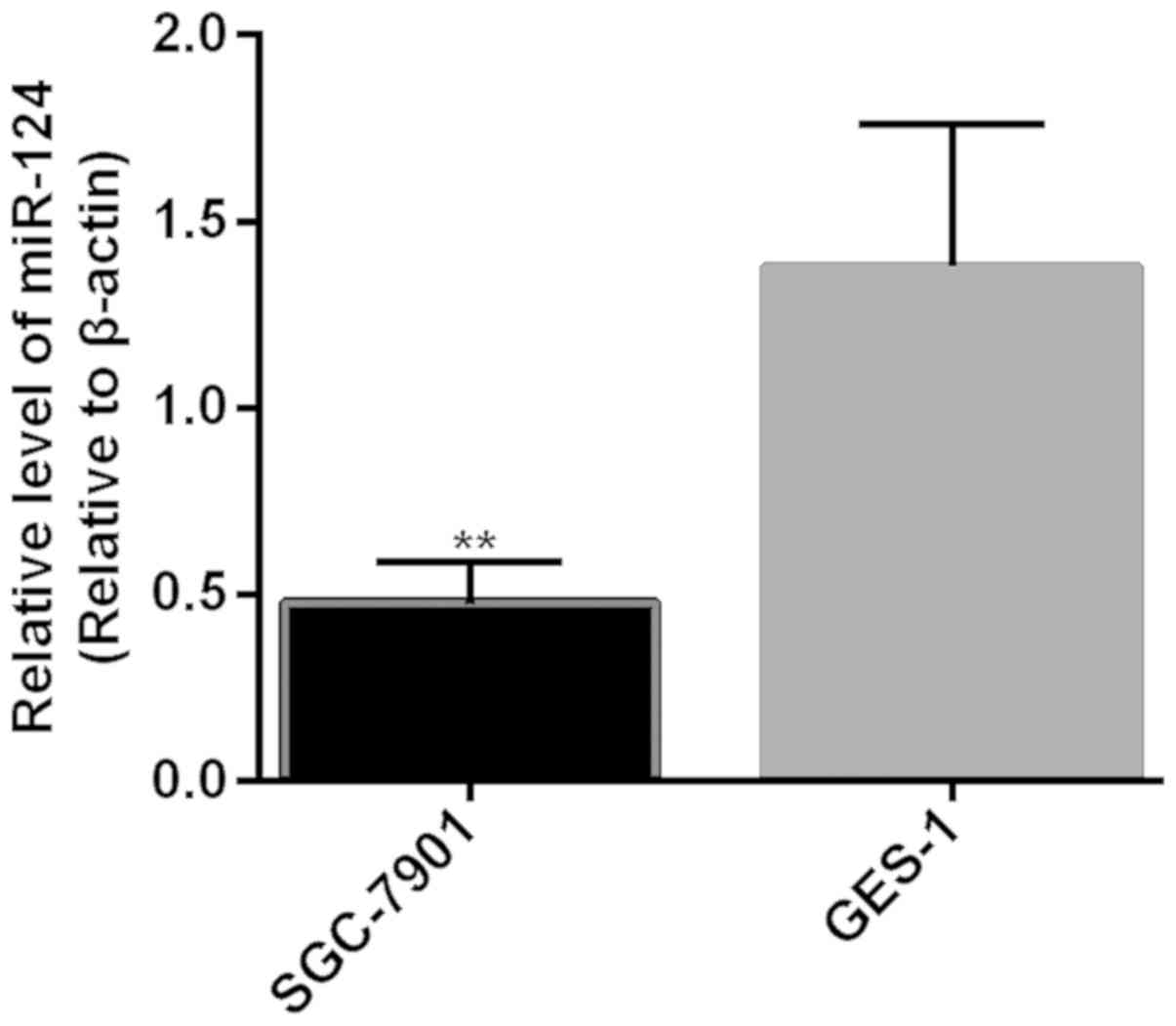
Expression level of miR-124 in cells of the SGC-7901
cell line (0.48±0.11) was significantly lower than that in cells of
the GES-1 cell line (1.38±0.38) (P<0.01) (Fig. 2).
Expression of miR-124 in cells of
miR-124, NC and control groups after transfection
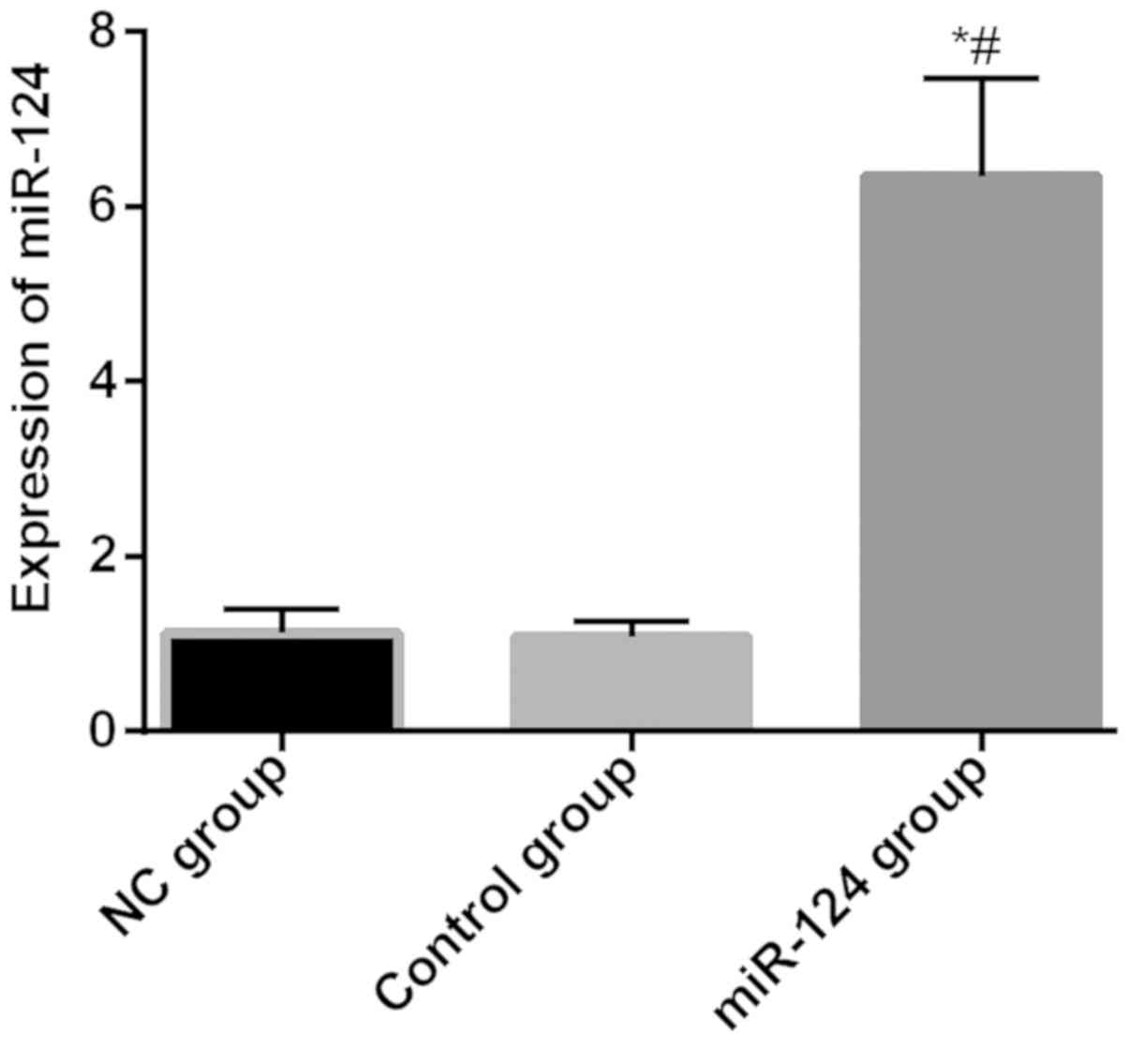
Relative expression levels of miR-124 in NC group,
control group and miR-12 group were 1.12±0.28, 1.09±0.17,
6.35±1.11, respectively. Relative expression level of miR-124 in
miR-12 group was significantly higher than that in the NC and
control groups (P<0.05), and there was no difference between the
NC and control groups (P>0.05) (Fig.
3).
Cell proliferation after miR-124
transfection
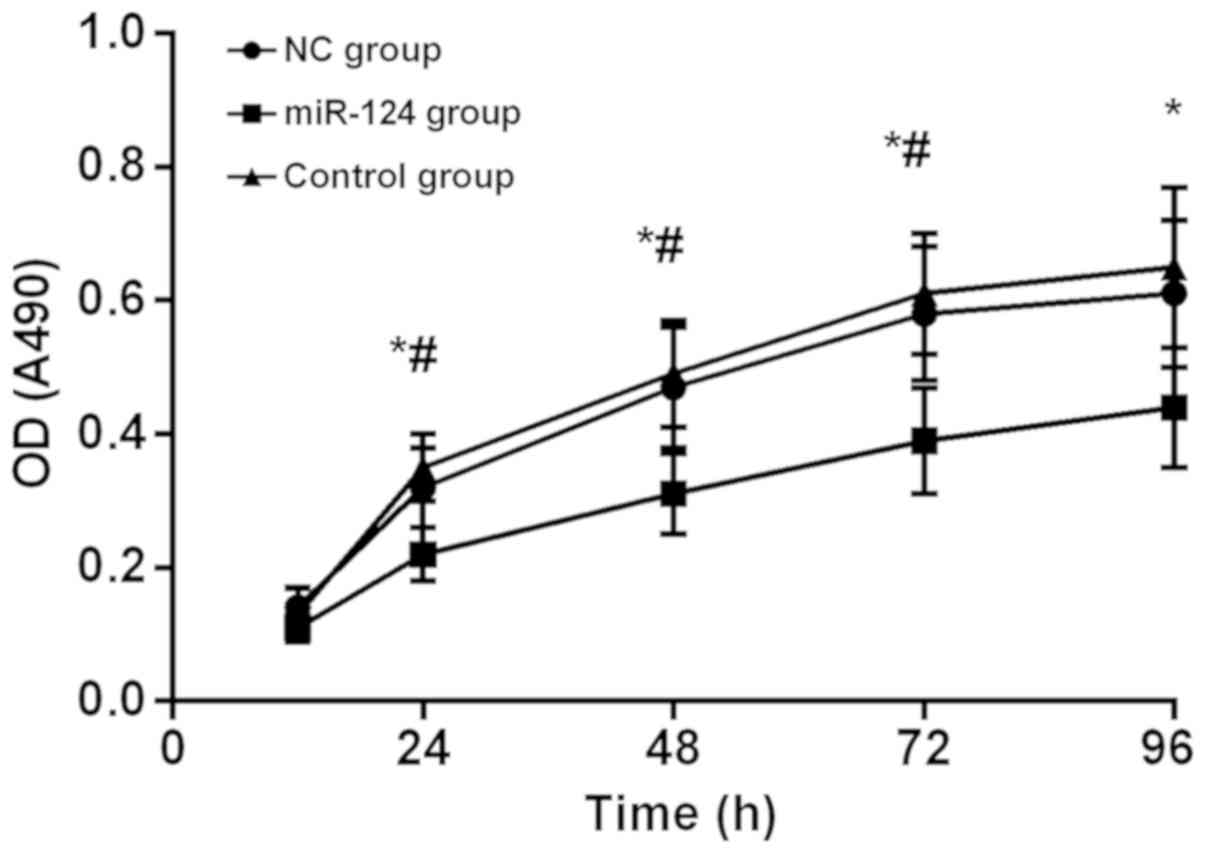
At 12 h after transfection, there was no significant
difference in OD at 490 nm among the 3 groups (P>0.05). OD (490)
of miR-124 group, NC group and control group at 24 h were
0.22±0.04, 0.32±0.06 and 0.35±0.05, respectively, OD (490) and
miR-124 group was significantly lower than that in NC group and
control group (P<0.01). The difference between NC group and
control group was not statistically significant (P>0.05). OD
(490) in all three groups showed a gradual upward trend, but at 24
h, OD (490) in miR-124 group was significantly lower than that in
NC group and control group (P<0.01), but there was no difference
between NC group and control group (P>0.05). OD values of all 3
groups at 24, 48, and 72 h were all higher than those at the
previous time-point (P<0.01), and there was no significant
difference between 96 h and 72 h (P>0.05). OD values at
different time-points in each group are listed in Table II, and the MTT proliferation curve is
shown in Fig. 4.
 | Table II.OD values of each group. |
Table II.
OD values of each group.
| Time-points (h) | NC group | miR-124 group | Control group | F-value | P-value |
|---|
| 12 | 0.14±0.03 | 0.11±0.02 | 0.13±0.04 | 2.17 | 0.14 |
| 24 | 0.32±0.06 |
0.22±0.04a,b | 0.35±0.05 | 16.25 | <0.001 |
| 48 | 0.47±0.09 |
0.31±0.06a,b | 0.49±0.08 | 14.52 | <0.001 |
| 72 | 0.58±0.10 |
0.39±0.08a,b | 0.61±0.09 | 15.69 | <0.001 |
| 96 | 0.61±0.11 |
0.44±0.09a | 0.65±0.12 | 9.70 | <0.001 |
| F-value | 49.45 | 39.25 | 61.20 |
|
|
| P-value | <0.001 | <0.001 | <0.001 |
|
|
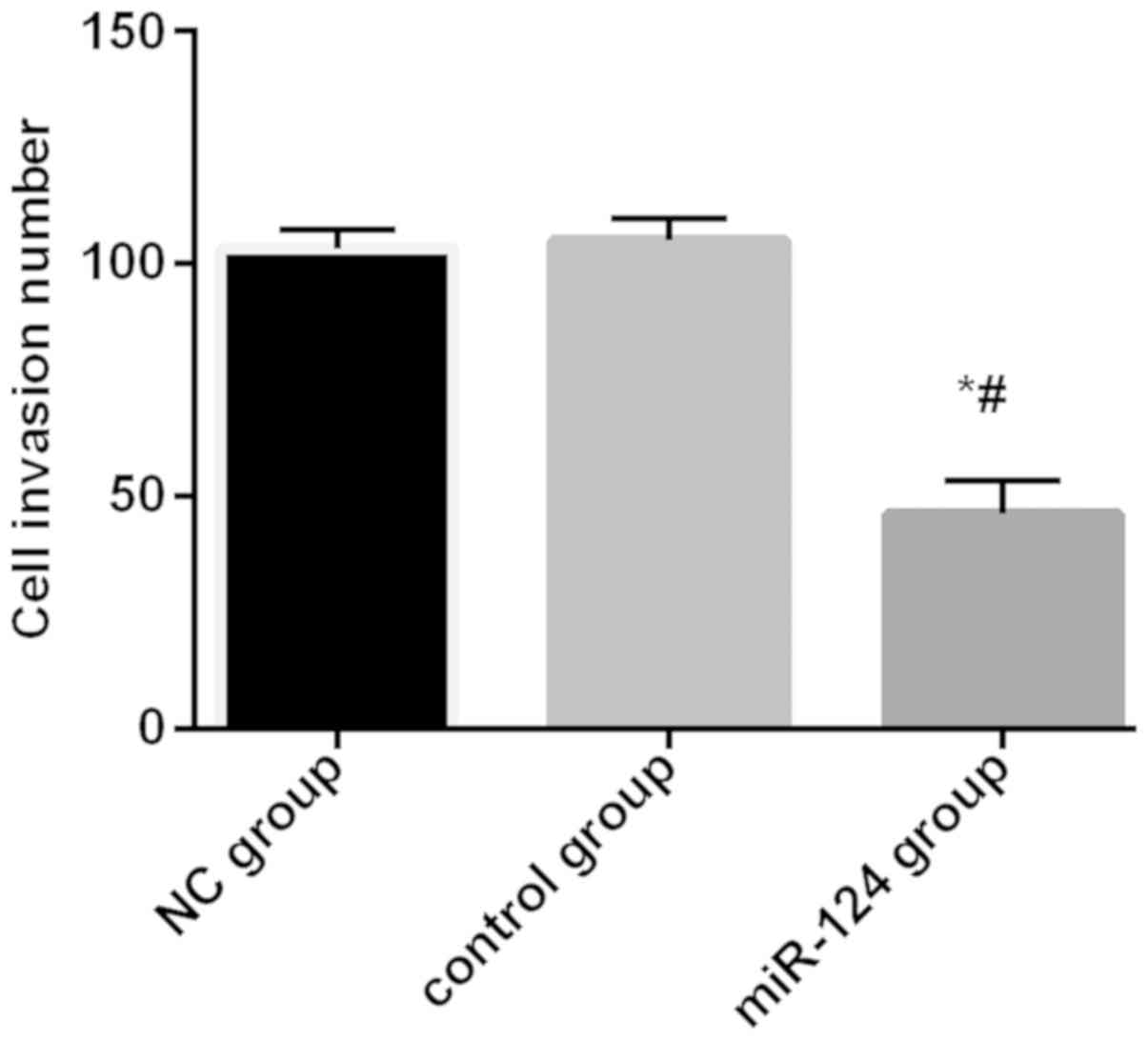
Invasion ability of cells after
miR-124 transfection
The number of invading cells in miR-124 group, NC
group, and control group was 46.17±7.13, 103.13±4.14, and
105.11±4.62, respectively. The number of invading cells in miR-124
group was significantly lower than that of the NC group and control
group (P<0.01). There was no significant difference in the
number of invading cells between NC group and control group
(Fig. 5).
Discussion
In China, with the gradual increase of economic
level, incidence and mortality of gastric cancer have also
increased year by year (14), and the
onset age of disease is becoming younger and younger (15). Symptoms of early gastric cancer are
not obvious. Most patients were in advanced or late metastasis
stage at the time of diagnosis. More than half of the patients have
recurrence after surgery. Chemotherapy is also ineffective in some
cases due to multiple drug resistance (16). miRNAs play an important role in the
development of tumors and have been the focus of research in recent
years (17). Studies have shown that
miR-124 can inhibit the progression of cancer such as liver cancer
and cervical cancer, promote the differentiation of CD133 tumors,
and induce tumor cell apoptosis (18). Studies have shown that miR-124 is
downregulated in gastric cancer, but the mechanism of action of
miR-124 in gastric cancer remains unclear. Therefore, the
expression of miR-124 in gastric cancer SGC-7901 cells and its
effect on biological function were explored in this study and the
role of miR-124 in gastric cancer was evaluated.
Expression level of miR-124 in gastric
adenocarcinoma tissues was significantly lower than that in
paracancerous tissues and expression level of miR-124 in SGC-7901
cells was significantly lower than that in GES-1 cells, indicating
that miR-124 was downregulated in gastric cancer tissues. Hu et
al (19) also found that miR-124
expression level was significantly reduced in gastric
adenocarcinoma tissue samples and cell lines. After transfection,
although proliferation curves of all three groups showed an upward
trend, cell proliferation rate in miR-124 group was significantly
lower than that in NC group and control group, indicating inhibited
cell proliferation after miR-124 transfection. Pan et al
(20) found that lentiviral vectors
that stably express miR124 can inhibit the growth, migration, and
invasion of gastric cancer cells. Li et al (21) found that miR-124 can inhibit cell
invasion and epithelial mesenchymal transformation by inhibiting
Snail2 expression.
In this study, the downregulated expression of
miR-124 in gastric adenocarcinoma was verified at both tissue and
cell levels. We conclude that miR-124 is downregulated in gastric
adenocarcinoma tissue and miR-124 can inhibit the proliferation and
invasion of gastric adenocarcinoma cells and inhibit the
development of gastric adenocarcinoma. However, only one cell line
was used and conclusions may be biased. We will include more cell
lines in our future studies. Although MTT and Transwell invasion
experiments have shown that miR-124 can inhibit the proliferation
and invasion of gastric adenocarcinoma cells, the downstream target
genes and specific signaling pathways remain to be further
studied.
Through literature investigations, we found that
downstream target genes of miR-124 are SPHK1 and ROCK. These target
genes will be verified in our subsequent studies and relevant
pathways will also be identified. Xia et al (22) found that miR-124 can downregulate
SPHK1. The expression of miR-124 in gastric cancer tissues is
reduced and the silencing effect on SPHK1 is relieved. SPHK1
expression is increased in gastric cancer and closely related to
the shortened survival time of patients. Hu et al (19) found that miR-124 overexpression
inhibits ROCK1 and miR-124 functions as a tumor suppressor by
targeting ROCK1. Jiang et al (23) found that miR-124 negatively regulates
Notch1 signaling by targeting JAG1 to inhibit GC cell growth,
migration, invasion and induce cell cycle arrest. In conclusion,
miR-124 can inhibit the proliferation and invasion of gastric
adenocarcinoma cells. Downregulation of miR-124 expression in
gastric adenocarcinoma may be closely related to the development of
gastric adenocarcinoma.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JM and HW drafted the manuscript. JM, HW and XW were
mainly devoted to collecting and interpreting the data. XW and PS
were responsible for PCR and MTT assays. All authors read and
approved the final study.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
The First Hospital of Jilin University (Changchun, China). Signed
informed consents were obtained from the patients or the
guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Li H, Xie S, Liu M, Chen Z, Liu X, Wang L,
Li D and Zhou Y: The clinical significance of downregulation of
mir-124-3p, mir-146a-5p, mir-155-5p and mir-335-5p in gastric
cancer tumorigenesis. Int J Oncol. 45:197–208. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Maiarù M, Morgan OB, Tochiki KK, Hobbiger
EJ, Rajani K, Overington DW and Géranton SM: Complex regulation of
the regulator of synaptic plasticity histone deacetylase 2 in the
rodent dorsal horn after peripheral injury. J Neurochem.
138:222–232. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang SE, Ko SY, Jo S, Choi M, Lee SH, Jo
HR, Seo JY, Lee SH, Kim YS, Jung SJ, et al: TRPV1 regulates stress
responses through HDAC2. Cell Rep. 19:401–412. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang X, Li Y, Dai Y, Liu Q, Ning S, Liu J,
Shen Z, Zhu D, Jiang F, Zhang J, et al: Sulforaphane improves
chemotherapy efficacy by targeting cancer stem cell-like properties
via the miR-124/IL-6R/STAT3 axis. Sci Rep. 6:367962016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang Y, Li H, Han J and Zhang Y:
Down-regulation of microRNA-124 is correlated with tumor metastasis
and poor prognosis in patients with lung cancer. Int J Clin Exp
Pathol. 8:1967–1972. 2015.PubMed/NCBI
|
|
6
|
Wang M, Gu H, Wang S, Qian H, Zhu W, Zhang
L, Zhao C, Tao Y and Xu W: Circulating miR-17-5p and miR-20a:
Molecular markers for gastric cancer. Mol Med Rep. 5:1514–1520.
2012.PubMed/NCBI
|
|
7
|
Pei L, Xia JZ, Huang HY, Zhang RR, Yao LB,
Zheng L and Hong B: Role of miR-124a methylation in patients with
gastric cancer. Zhonghua Wei Chang Wai Ke Za Zhi. 14:136–139.
2011.(In Chinese). PubMed/NCBI
|
|
8
|
Xie L, Zhang Z, Tan Z, He R, Zeng X, Xie
Y, Li S, Tang G, Tang H and He X: MicroRNA-124 inhibits
proliferation and induces apoptosis by directly repressing EZH2 in
gastric cancer. Mol Cell Biochem. 392:153–159. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xiao HJ, Ji Q, Yang L, Li RT, Zhang C and
Hou JM: In vivo and in vitro effects of microRNA-124 on human
gastric cancer by targeting JAG1 through the Notch signaling
pathway. J Cell Biochem. 119:2520–2534. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang TH, Liang LZ, Liu XL, Wu JN, Su K,
Chen JY, Zheng QY, Huang HZ and Liao GQ: Long non-coding RNA MALAT1
interacts with miR-124 and modulates tongue cancer growth by
targeting JAG1. Oncol Rep. 37:2087–2094. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu L, Ye JX, Qin YZ, Chen QH and Ge LY:
Evaluation of miR-29c, miR-124, miR-135a and miR-148a in predicting
lymph node metastasis and tumor stage of gastric cancer. Int J Clin
Exp Med. 8:22227–22236. 2015.PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Costa WL Jr, Coimbra FJ, Fogaroli RC,
Ribeiro HS, Diniz AL, Begnami MD, Mello CA, Fanelli MF, Silva MJ,
Fregnani JH, et al: Adjuvant chemoradiotherapy after
d2-lymphadenectomy for gastric cancer: The role of n-ratio in
patient selection. results of a single cancer center. Radiat Oncol.
7:1692012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou J, Zhou J, Wang W, Li W, Wu L, Li G,
Shi J and Zhou S: The polymorphism in miR-25 attenuated the
oncogenic function in gastric cancer. Tumour Biol. 37:5515–5520.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Maeda M, Yamashita S, Shimazu T, Iida N,
Takeshima H, Nakajima T, Oda I, Nanjo S, Kusano C, Mori A, et al:
Novel epigenetic markers for gastric cancer risk stratification in
individuals after Helicobacter pylori eradication. Gastric Cancer
1113. 1–11. 2018.
|
|
17
|
Yuan Q, Sun T, Ye F, Kong W and Jin H:
MicroRNA-124-3p affects proliferation, migration and apoptosis of
bladder cancer cells through targeting AURKA. Cancer Biomark.
19:93–101. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang T, Zhou Y, Zhang J, Wong CC, Li W,
Kwan JSH, Yang R, Chan AKY, Dong Y, Wu F, et al: SRGAP1, a crucial
target of miR-340 and miR-124, functions as a potential oncogene in
gastric tumorigenesis. Oncogene. 37:1159–1174. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hu CB, Li QL, Hu JF, Zhang Q, Xie JP and
Deng L: miR-124 inhibits growth and invasion of gastric cancer by
targeting ROCK1. Asian Pac J Cancer Prev. 15:6543–6546. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pan Y, Wu A, Xu F, Chen C, Jiang L and Jin
R: Lentivirus-mediated overexpression of miR-124 suppresses growth
and invasion by targeting JAG1 and EZH2 in gastric cancer. Oncol
Lett. 15:7450–7458. 2018.PubMed/NCBI
|
|
21
|
Li SL, Gao HL, Lv XK, Hei YR, Li PZ, Zhang
JX and Lu N: MicroRNA-124 inhibits cell invasion and
epithelial-mesenchymal transition by directly repressing Snail2 in
gastric cancer. Eur Rev Med Pharmacol Sci. 21:3389–3396.
2017.PubMed/NCBI
|
|
22
|
Xia J, Wu Z, Yu C, He W, Zheng H, He Y,
Jian W, Chen L, Zhang L and Li W: miR-124 inhibits cell
proliferation in gastric cancer through down-regulation of SPHK1. J
Pathol. 227:470–480. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jiang L, Lin T, Xu C, Hu S, Pan Y and Jin
R: miR-124 interacts with the Notch1 signalling pathway and has
therapeutic potential against gastric cancer. J Cell Mol Med.
20:313–322. 2016. View Article : Google Scholar : PubMed/NCBI
|