Introduction
Primary central nervous system lymphoma (PCNSL) is
an aggressive non-Hodgkin's lymphoma that is confined to the
central nervous system (CNS). It is a rare tumor that commonly
exhibits the morphological and immunophenotypical features of
diffuse large B-cell lymphoma (DLBCL). PCNSL frequently occurs in
the brain, eyes, pia mater and spinal cord, accounting for ~3% of
CNS tumors (1). The disease has a
poor prognosis, with a life expectancy without treatment of 3–5
months.
The only known risk factor for PCNSL is
immunodeficiency, including as a result of acquired
immunodeficiency syndrome (AIDS), transplant and immunosuppressive
therapy, inherited immunodeficiency and other acquired immune
deficiencies (2). Human Epstein-Barr
virus infection may be important in the etiology of PCNSL in
immunocompromised patients (2). Other
etiological possibilities include in situ malignant
lymphocytes, clonal hyperplasia in the CNS, dysplasia of
lymphocytes the CNS and the malignant transformation of lymphocytes
following CNS infection (3). Studies
have reported that PCNSL occurs as a result of genetic alterations,
and the occurrence of lymphoma is heavily associated with microRNAs
(miRNAs) (4–6). Zheng et al (7) demonstrated that the miRNA expression of
primary central nervous system (PCNS)-DLBCL differed from that of
germinal center (GC)-DLBCL and non-GC-DLBCL. miRNA-21, located on
chromosome 17 q23.2, presents in a number of solid and non-solid
tumors. The expression of miRNA-21 is increased in DLBCL,
follicular lymphoma, and transformational DLBCL lymphoid tissue
compared with healthy individuals (8). The present study aimed to determine the
expression and diagnostic value of miRNA-21 in PCNSL. The value of
miRNA-21 in distinguishing glioblastoma, another common tumor in
CNS, was also assessed in addition to predicting the therapeutic
effect of chemotherapy in PCNSL, which has not been reported
previously.
Patients and methods
Clinical information
A total of 25 patients with PCNSL from the First
Affiliated Hospital of Harbin Medical University (Harbin, China)
between December 2011 and December 2017 were enrolled in the
present study (male, 13; female, 12; median age, 56.6 years; range,
36–69 years). The inclusion criteria were as follows: i) Patients
with or without an impaired performance of the CNS as the primary
symptoms (headache, dizziness, nausea, vomiting and other elevated
intracranial pressure symptoms; epilepsy, aphasia, visual
impairment, limb weakness, unsteady gait and other neurological
symptoms). Lymphoma lesions were localized in the CNS and were all
confirmed by histopathological examination; ii) all patients
received bone marrow biopsy and an ultrasound to rule out systemic
lymphoma; iii) following detailed physical and auxiliary
examinations, no systemic lymphoid hematopoietic tissue or other
system involvement was confirmed; iv) human immunodeficiency virus
(HIV) antibody test was negative. The exclusion criteria were as
follows: i) cancer; ii) pregnancy; iii) severe infection; iv)
severe cardiovascular or cerebrovascular diseases; v) heart, liver
or kidney dysfunction; and vi) autoimmune disease. The control
groups were 25 patients with glioblastoma (male = 11, female = 14;
median age, 57 years; range, 44–67 years) and 25 healthy volunteers
(male = 13, female = 12; median age: 57.2 years; range: 43–68
years). The inclusion criteria were as follows: i) Patients
diagnosed with glioblastoma or volunteers without CNS diseases; and
ii) HIV antibody test was negative. The exclusion criteria were the
same as for the PCNSL group.
The Harbin Medical University Ethics Committee
(Harbin, China) approved the present study, and all blood samples
or cerebrospinal fluid (CSF) samples were used with the written
informed consent of the patients.
Imaging and clinical evaluations
Cranial computed tomography or magnetic resonance
imaging was used to indicate the site of intracranial invasion. The
Karnofsky performance status (KPS) score (9) was used to assess the performance of
patients with PCNSL in daily activities.
Laboratory examinations
Lactate dehydrogenase (LDH) in the serum and CSF
were examined prior to treatment at the clinical laboratory of The
First Affiliated Hospital of Harbin Medical University. LDH
was detected using the Beckman LDH kit (Beckman Coulter,
Inc., Brea, CA, USA). Protein and sugar contents in CSF were
measured with dry chemistry method (VITROS; Ortho-Clinical
Diagnostics, Inc., Rochester, NY, USA). Leukocytes were counted
using a light microscope (magnification, ×40).
Plasma and CSF samples
Peripheral blood samples (3 ml) from patients with
PCNSL, with glioblastoma, and healthy volunteers were collected
into EDTA anticoagulant tubes prior to chemotherapy, and processed
within 4 h of collection. All patients with PCNSL received high
dose methotrexate (MTX)-based chemotherapy following admission to
hospital. CSF was taken during lumbar intrathecal chemotherapeutic
injection. The CSF was collected into sterile collection tubes
prior to, following one cycle, and following three cycles of
chemotherapy, and was processed within 4 h of collection. Plasma
and CSF were isolated at 4°C under centrifugation for 10 min (1,500
× g). The supernatant was transferred to a clean tube and the
procedure was repeated prior to storage at −80°C.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from patient samples using a
TRIzol® extraction kit (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), according to the
manufacturer's protocol. Optical density (OD) and RNA concentration
were determined using a UV spectrophotometer at 260 and 280 nm.
Zero-point adjustment prior to measurement was conducted with
diethylpyrocarbonate-treated water. Each sample was tested twice,
and an OD260/OD280 ratio of 1.8–2.0 was deemed acceptable.
To detect miRNA-21, RT-qPCR was performed, according
to the manufacturer's protocol. Briefly, 20 µl of each sample (100
ng total RNA) was used in the RT reactions (42°C for 50 min, 95°C
for 5 min, followed by 4°C). qPCR was conducted using the following
conditions: 95°C for 5 min, 40 cycles of 30 sec at 95°C, and 40 sec
at 72°C. The miRNA expression levels were calculated as the
quantification cycle (2−ΔΔCq) of miRNA-21 (10). β-actin (forward,
5′-GGCACCCAGCACAATGAAG-3′, and reverse, 5′-CGTCATACTCCTGCTTGCTG-3′)
was used as the internal control. RT reactions and qPCR were
performed using a SuperScript VILO Synthesis kit (Thermo Fisher
Scientific, Inc.) and the SsoAdvanced SYBR® Green
Supermix (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The
primers used were as follows: miRNA-21 forward,
5′-GTGCAGGGTCCGAGGT-3′, and reverse,
5′-GCCGCTAGCTTATCAGACTGATGT-3′.
Statistical analysis
Each experiment was repeated two more times and the
mean value was calculated. Values for miRNA-21 in the plasma and
CSF are displayed as the mean ± standard deviation. Data between
the PCNSL and the control groups were compared using analysis of
variance with Student-Newman-Keuls post hoc test. Correlations
between miRNA-21 expression levels in the plasma and those in the
CSF were assessed using the Pearson's correlation coefficient.
Receiver operating characteristic (ROC) analysis was used to
determine diagnostic values. The Wilcoxon test was used to test the
value changes in CSF miRNA-21 prior to and following chemotherapy,
in patients who responded to chemotherapy and those who did not.
Spearman's rank correlation coefficient was used to test the
reliability and validity of miRNA-21 in predicting therapeutic
effect. Statistical analyses were conducted using SPSS 19.0 (IBM
Corp., Armonk, NY, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Clinical features of patients with
PCNSL
Following donation, 25 plasma samples and 75 CSF
samples for each time point were analyzed (prior to, following one
cycle, and following three cycles of chemotherapy) from 13 male and
12 female patients with PCNSL, and 50 plasma samples from the
control groups (25 patients with glioblastoma and 25 healthy
volunteers). There was no significant difference in sex or age
between different groups (P>0.05). The pathological types of the
patients with PCNSL were all B-cell type lymphoma, in which DLBCL
accounted for 23 cases, and the other 2 cases were unclassified
B-cell type lymphoma. Headache, nausea, vomiting, and other
symptoms of intracranial hypertension were the most common
symptoms. Visual impairment, limb weakness, numbness, paraplegia,
and other physical disabilities were also observed.
Imaging and clinical evaluations
All patients with PCNSL underwent imaging
examinations by cranial computed tomography or magnetic resonance
imaging. Imaging data revealed that the frontal lobe was most
frequently affected (data not shown).
The KPS score was used to assess the performance of
the 25 patients with PCNSL in daily activities. The KPS score was
≥80 in 15 cases, and <80 in 10 cases (the scores of 3 cases were
<60). The majority of the patients remained independent with
relatively mild symptoms (data not shown).
Laboratory examination
Of the 25 patients with PCNSL, LDH in the serum of 6
cases (24%) was aberrantly elevated, while it was normal in 19
cases (76%). CSF was also tested. The normal group was defined by
leukocyte number, protein and sugar content within the normal
limits. Samples outside of these limits were assigned to the
abnormal group. CSF examinations revealed that the majority of
patients with PCNSL had a normal leukocyte count and sugar content,
but abnormal protein content. Patient number and percentage in
normal and abnormal groups are displayed in Table I.
 | Table I.Cerebrospinal fluid analyses of
patients with PCNSL. |
Table I.
Cerebrospinal fluid analyses of
patients with PCNSL.
| Cases of PCNSL | Leukocyte count, n
(%) | Protein content, n
(%) | Sugar content, n
(%) |
|---|
| Within normal
limits | 15 (60.0) | 8
(32.0) | 18 (72.0) |
| Beyond normal
limits | 10 (40.0) | 17 (68.0) | 7
(28.0) |
Expression and diagnostic value of
plasma miRNA-21 in PCNSL
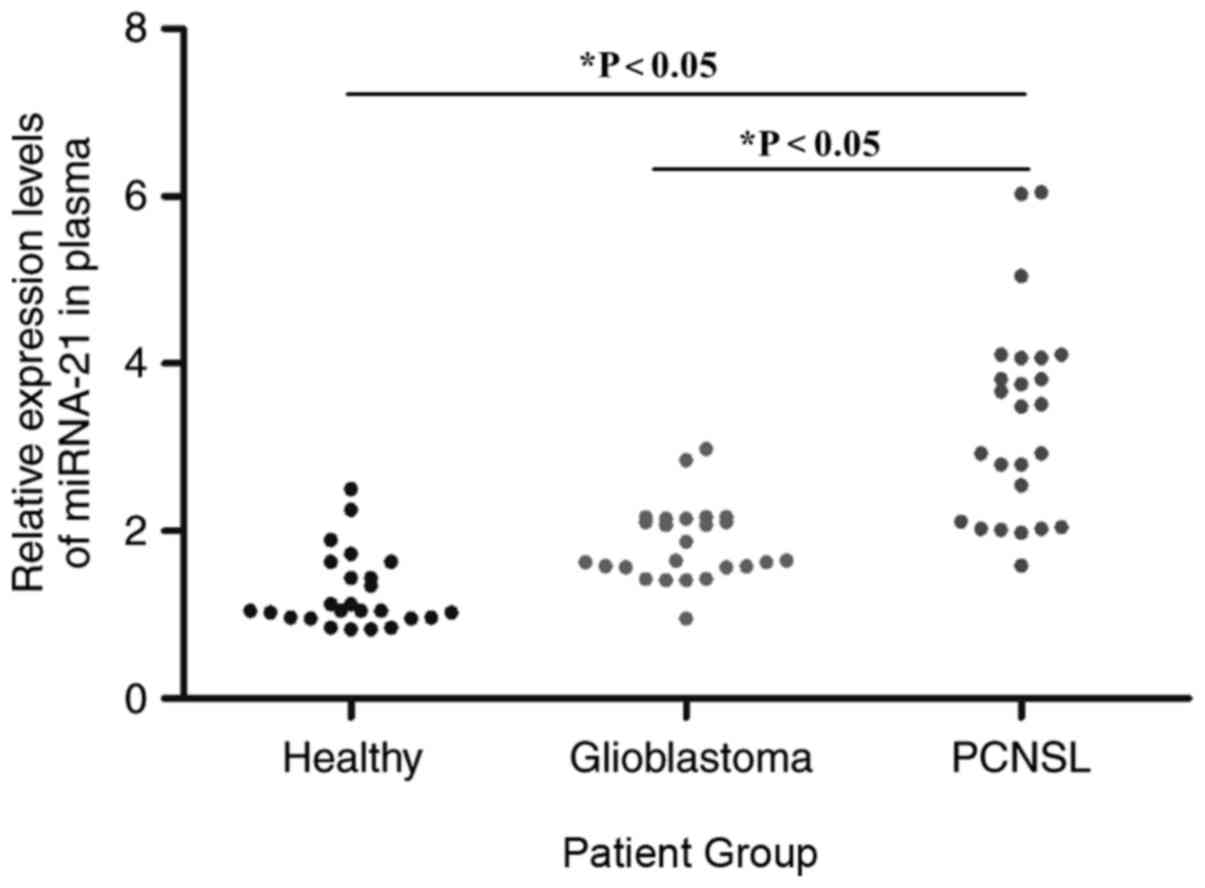
The relative expression levels of miRNA-21 in the
plasma of the PCNSL, glioblastoma, and healthy groups were assessed
using RT-qPCR prior to chemotherapy. The miRNA-21 expression levels
in the plasma of the PCNSL group were significantly higher when
compared with that in the other groups. In addition, miRNA-21
expression in the plasma of the glioblastoma group was also
significantly higher compared with that in the healthy group
(P<0.05). The relative expression levels of miRNA-21 were higher
in the PCNSL group and there was a slight overlap in the
distribution of miRNA-21 in the PCNSL group and the glioblastoma
group, while there was a greater overlap between the glioblastoma
group and the healthy group (Fig. 1).
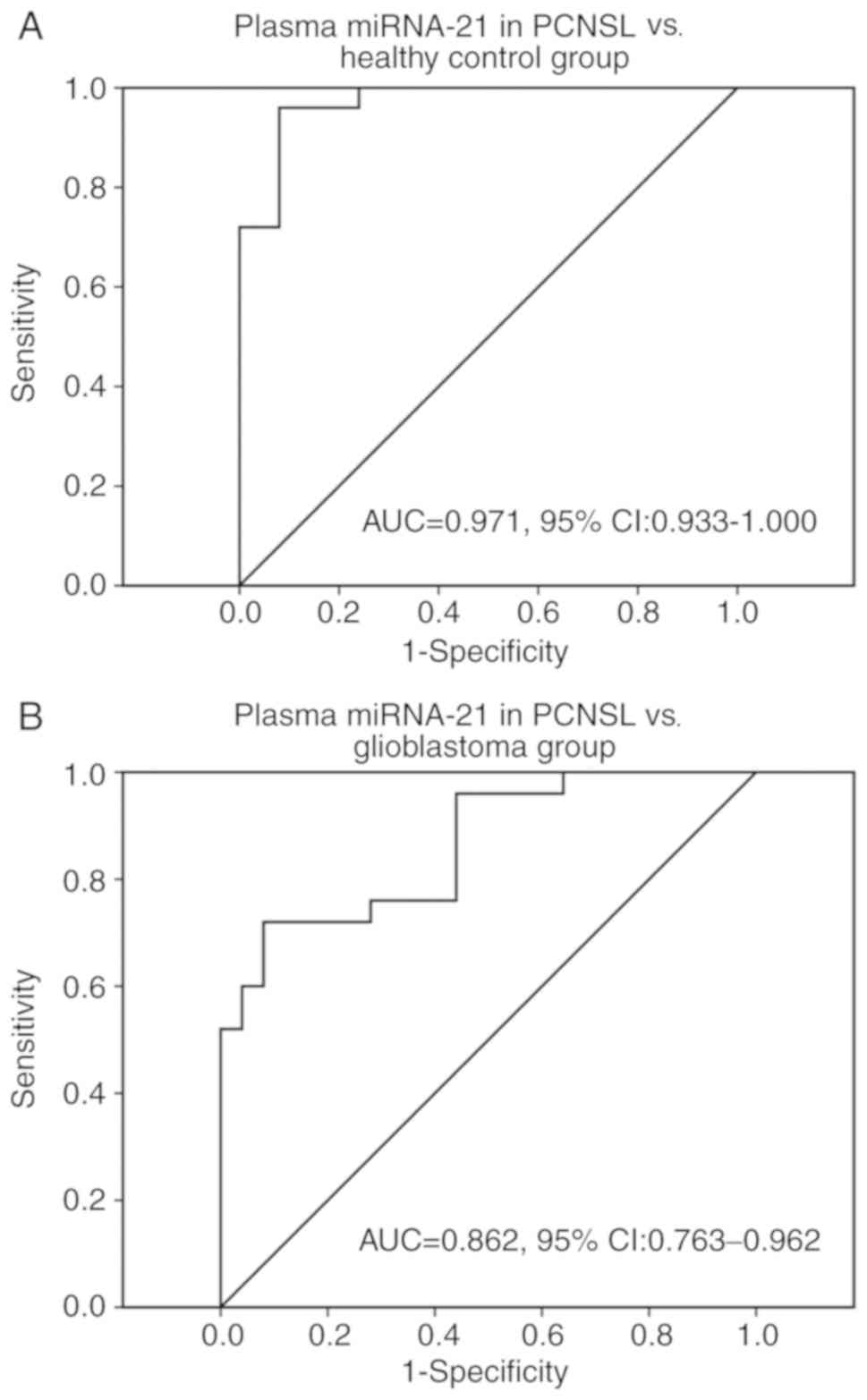
The ROC curve illustrates that miRNA-21 in the plasma had a high
specificity for distinguishing PCNSL from the healthy control group
[area under the curve (AUC), 0.971; 95% confidence interval (CI),
0.933–1.000] (Fig. 2A) and
glioblastoma group (AUC, 0.862; 95% CI, 0.763–0.962) (Fig. 2B).
Correlation of miRNA-21 expression in
CSF and plasma in PCNSL
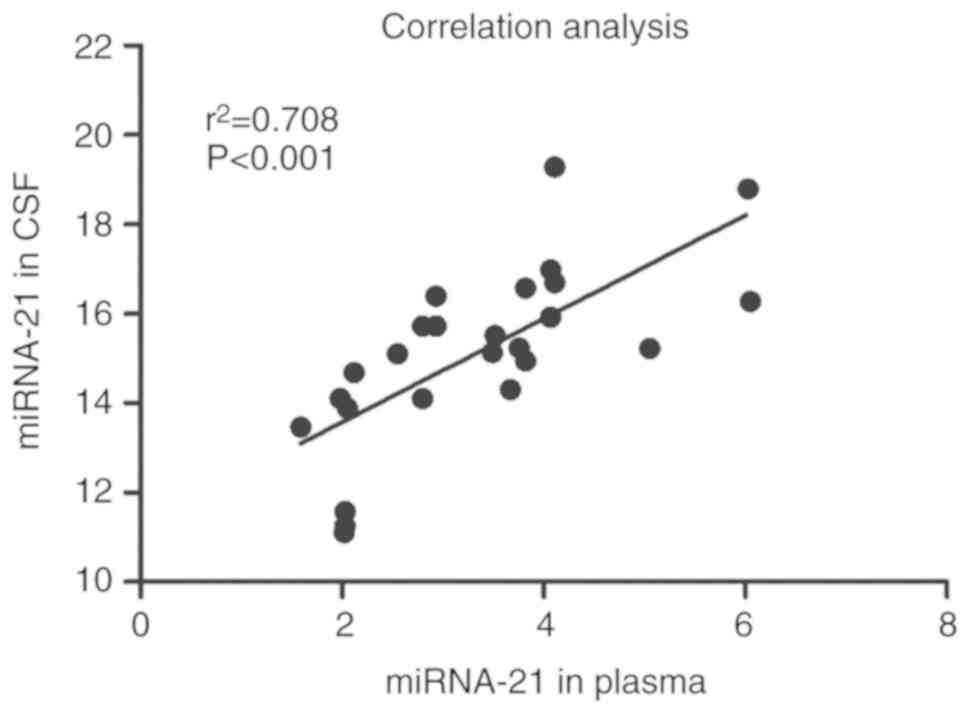
By comparing the expression levels of miRNA-21 in
plasma and CSF prior to chemotherapy in patients with PCNSL, the
correlation analysis revealed a significantly positive correlation
(Pearson's correlation coefficient: r2=0.708,
P<0.001) (Fig. 3).
miRNA-21 expression levels in the CSF
of patients with PCNSL prior to and following chemotherapy
Patients admitted to the hospital were predominantly
treated with high dose MTX-based chemotherapy. The treatment
regimen consisted of chemotherapy alone or followed by whole brain
radiation therapy. The effects of treatment were evaluated
following three cycles of chemotherapy. The complete remission rate
was 52%, partial remission was 36%, stability was 8% and disease
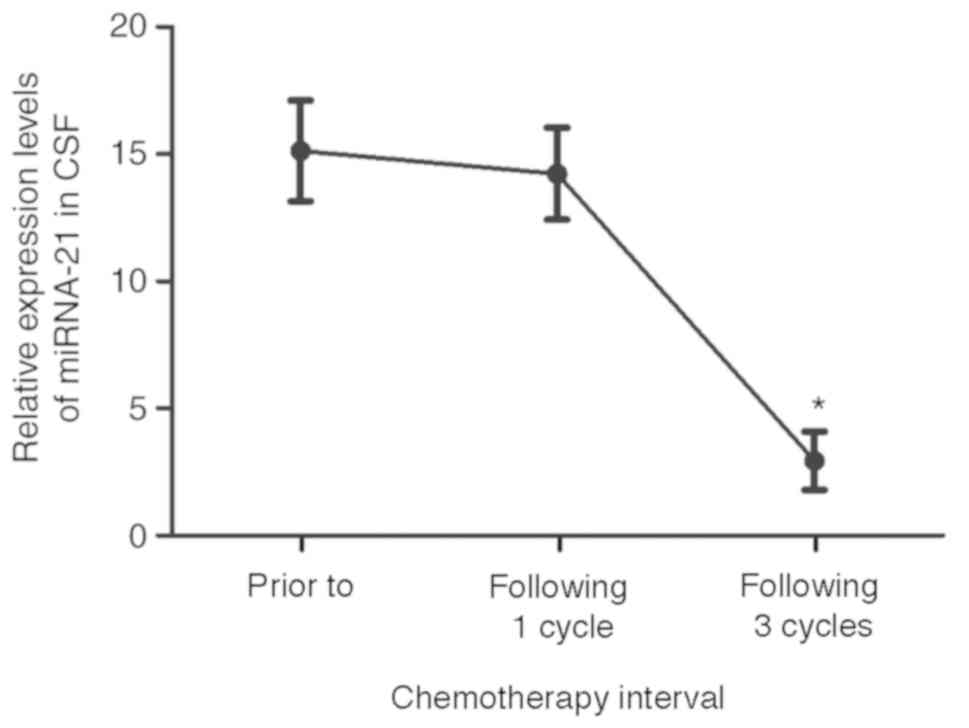
progression was 4%. The total response rate was 88%. miRNA-21 was
detected in patients with PCNSL prior to, following one cycle, and
following three cycles of chemotherapy. miRNA-21 in patients with
PCNSL had a relatively high expression level of 15.12±1.98 prior to
and 14.23±1.81 following one cycle of chemotherapy (P>0.05), and
as low as 2.94±1.15 following 3 courses of chemotherapy (of the 22
cases that responded; P<0.001, Fig.
4). However, there was no significant difference between the
expression levels of miRNA-21 in patients prior to treatment
compared with those in patients following one course of
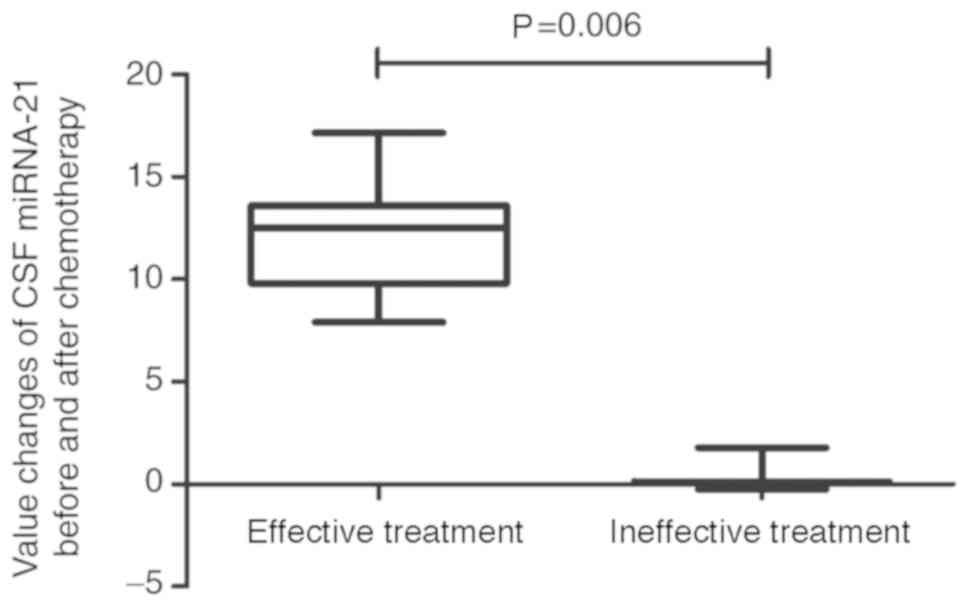
chemotherapy. The CSF miRNA-21 value prior to treatment minus the
value following chemotherapy was calculated. Variations in CSF
miRNA-21 were much higher prior to and following chemotherapy in
patients who responded to chemotherapy compared with those in cases
who had no response (P=0.006; Fig.
5). Spearman's rank correlation coefficient confirmed the
reliability and validity of this biomarker (Spearman's rho=0.563,
P=0.003; data not shown).
Discussion
PCNSL is a type of non-Hodgkin's lymphoma with a
high proliferative tumor index of >50%. However, the incidence
of PCNSL in the general population is low, and accounts for ~3% of
all CNS tumors (1). Patients'
specimens are difficult to collect; in the present study, plasma
and CSF were collected from 25 patients with PCNSL, with an average
age of 56.6 years, and 92% of PCNSL cases were positive for DLBCL.
This is consistent with earlier published data indicating that the
median age of onset for PCNSL in patients without other potential
immune diseases is between 50 and 65 years (11). Owing to the involvement of the immune
response, T cells and macrophages are frequently present in PCNSL
lesions. Long-term immunosuppressive therapy or underlying disease
is a considerable risk factor for PCNSL. PCNSL is more common in
patients suffering from chronic inflammatory diseases, including
systemic lupus erythematosus, tuberculosis, vasculitis, serious
congenital immunodeficiency, AIDS, or those who have undergone
immunosuppressive treatment as a result of transplantation
(2). However, the discovery of the
etiology of nervous system symptoms and brain lesions in patients
with PCNSL remains a challenge. Immunosuppression is also the cause
of other common, lethal brain tumors including glioblastoma, and it
is difficult to distinguish between PCNSL and glioblastoma with
imaging methods alone (12). In
patients with suspected PCNSL, brain tumor stereotactic biopsy is
the most effective diagnostic method. However, CNS biopsy carries a
risk of bleeding and brain tissue damage. Therefore, an improved
diagnostic approach is required.
The symptoms of PCNSL are also observable in other
CNS disorders. In the majority of cases of DLBCL lymphoma with poor
outcome, LDH is elevated (13).
However, in the present study, LDH was normal in 76% of PCNSL
cases. In the majority of cases, routine CSF assessment, leukocyte
count and sugar content were within the normal limits, as was the
protein content in 32% of cases. As a number of the patients
displayed no obvious signs of disease, PCNSL cannot be diagnosed by
the routine examination of CSF and biochemistry.
There is growing evidence to suggest that an
imbalance in miRNA expression is evident in various types of
cancer, and that miRNAs may act as oncogenes and tumor suppressor
genes (5,14). Alterations in miRNA expression levels
are apparent in various malignant tumors, including leukemia and
lymphoma, thus miRNAs are increasingly used as diagnostic and
prognostic markers (15–17). The direct extraction of miRNAs from
tumor samples (18) and simple
detection of circulating miRNAs in human serum or plasma is also
advantageous. Furthermore, it has been reported that miRNAs predict
the clinical course of malignant tumors, including chronic
lymphocytic leukemia and acute myeloid leukemia, in addition to
certain organic diseases, including pancreatic tumors (19,20).
Therefore, a miRNA biomarker may be useful in the detection of
PCNSL.
It has also been reported that the CSF expression
levels of miRNA-21, miRNA-19, and miRNA-92a are increased in
patients with PCNSL (21). Baraniskin
et al (22) detected miRNA-15b
and miRNA-21 expression levels in CSF through a comparative study
of 23 patients with PCNSL, 10 patients with glioblastoma, 7
patients with brain metastases, and 10 control patients with
various neurological disorders. The inclusion of miRNA-15b and
miRNA-21 in combined expression analyses resulted in a diagnostic
accuracy of 90% sensitivity and 100% specificity to distinguish
patients with glioblastoma from those with PCNSL. Baraniskin et
al (23) later confirmed their
previous findings in an enlarged PCNSL cohort (n=39; sensitivity
97.4%). In combined analyses of miRNA-21, miRNA-19b, and miRNA-92
in CSF, it was possible to differentiate PCNSL from other
neurological disorders.
There is a certain degree of miRNA-21 expression in
normal human plasma, where it is involved in the regulation of cell
growth and differentiation (14).
Therefore, miRNA-21 may contribute to the development and
progression of disease, though only a small fraction of the
biological functions of miRNA have been elucidated. Conti et
al (24) and Wei et al
(25) demonstrated that the
expression level of miRNA-21 increased 7–11-fold in patients with
glioblastoma compared with the normal control group, suggesting
that miRNA-21 may be used for tumor diagnosis and staging.
Although obtaining CSF through a lumbar puncture is
less invasive compared with a brain tumor biopsy, blood examination
is a yet more favorable option. Lawrie et al (26) were able to detect miRNAs in the serum
of patients with DLBCL. However, Baraniskin et al (23) revealed that miRNA levels in the serum
of patients with PCNSL (n=14) were not elevated compared with those
of the control groups, including those with various neurological
disorders (headache, seizures, syncope and stroke); this suggested
that the blood-brain barrier was responsible for the differences
between CSF and blood circulation in patients with PCNSL, and the
small sample size may have been a further hindrance. In the present
study, Pearson's correlation analysis revealed that miRNA-21
expression levels in the plasma were significantly positively
correlated with those in the CSF. These results and those of a
previous study (27) confirmed that
it was possible to detect miRNA-21 in the plasma, as opposed to the
CSF, of patients with PCNSL; Mao et al (27) revealed that expression levels of serum
miRNA-21 did not differ significantly among CNS inflammation,
metastases and healthy control groups. Thus, this was not repeated
in the present study, and only glioblastoma (another common CNS
tumor), and the healthy control groups were designated. RT-qPCR was
used to confirm that miRNA-21 expression levels were higher in the
plasma of patients with PCNSL, compared with those in the
glioblastoma and healthy control groups. miRNA-21 expression in the
plasma of patients with glioblastoma was also significantly higher
compared with those in the control group. These results advocate
plasma miRNA-21 to be a biomarker for the diagnosis of glioblastoma
and PCNSL; lower miRNA-21 expression levels suggest glioblastoma,
and higher levels suggest PCNSL. The results were consistent with
an aforementioned previous study (28), in which the authors proposed that
serum miRNA-21 expression levels were of value in identifying and
prognostically stratifying patients with PCNSL, though they did not
conclude whether miRNA-21 may be used to monitor the efficacy of
chemotherapy.
Studies have demonstrated that aberrantly expressed
miRNAs are associated with disease stage, drug resistance and
survival in a large proportion of patients with cancer. Therefore,
targeting these specific miRNAs may provide an efficient and
optimal approach to the treatment of these types of cancer
(29–32). The study by Baraniskin et al
(23) provided evidence that CSF
miRNAs have great potential as biomarkers for monitoring the
treatment and disease follow-up of patients with PCNSL.
In the present study, patients were predominantly
treated with high dose MTX-based chemotherapy combined with
intrathecal injection, and only one patient with paraplegia was
first administered radiotherapy (6,28,33); these patients had a high response rate
to treatment (88%). To investigate the variation in miRNA-21
expression level in the CSF, miRNA-21 was subsequently detected
prior to and following chemotherapy. Although the level of miRNA-21
expression decreased following one course of chemotherapy, there
was no significant difference prior to and following treatment.
However, the miRNA-21 expression level decreased significantly
following three cycles of chemotherapy in patients responsive to
treatment, while there were no obvious alterations in expression
level in patients who did not respond to treatment (n=3). These
results indicated that certain chemotherapeutic agents may exhibit
anti-cancerous activity through the regulation of miRNA expression,
which may influence cellular processes including DNA repair, cell
cycle arrest, and apoptosis (34).
This also suggests that miRNA-21 in the CSF may serve an important
role in the evaluation of therapeutic effect and prognostic
assessment in PCNSL.
In brief, miRNA-21 used as a diagnostic and
therapeutic evaluation biomarker of PCNSL, is not only safe and
convenient, but may also improve the accuracy of PCNSL diagnosis
and prognostic estimation. The use of miRNA-21 as a tumor marker
may also have potential in the clinical diagnosis and treatment of
PCNSL.
Acknowledgements
The authors would like to thank Professor Li Ying
for her guidance and help with the experiment.
Funding
The present study was supported by the National Key
Basic Research Development Plan Corpus (grant no. 2012CBA01303),
Harbin Medical University Scientific Research Innovation Projects
(grant no. 2016LCZX61), and Health and Family Planning Commission
Research Project in Heilongjiang province (grant no. 2016-018).
Availability of data and materials
The datasets used or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SW designed the study and analyzed the data. KY
wrote the manuscript and analyzed the data. YC, YT and JH performed
the laboratory work. YC and YT also analyzed the data. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Harbin Medical
University (Harbin, China) Ethics Committee, and all blood samples
or cerebrospinal fluid (CSF) samples were used with the written
informed consent of the patients.
Patient consent for publication
Both patients and volunteers provided written
informed consent for the publication of this study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dahiya S, Murphy ES, Chao ST, Stevens GH,
Peereboom DM and Ahluwalia MS: Recurrent or refractory primary
central nervous lymphoma: Therapeutic considerations. Expert Rev
Anticancer Ther. 13:1109–1119. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kleinschmidt-DeMasters BK, Damek DM,
Lillehei KO, Dogan A and Giannini C: Epstein Barr virus-associated
primary CNS lymphomas in elderly patients on immunosuppressive
medications. J Neuropathol Exp Neurol. 67:1103–1111. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Löw S and Batchelor TT: Primary central
nervous system lymphoma. Semin Neurol. 38:86–94. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zorofchian S, El-Achi H, Yan Y, Esquenazi
Y and Ballester LY: Characterization of genomic alterations in
primary central nervous system lymphomas. J Neurooncol.
140:509–517. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yu X, Li Z, Shen J, Chan MT and Wu WK:
Role of microRNAs in primary central nervous system lymphomas. Cell
Prolif. 49:147–153. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Royer-Perron L, Hoang-Xuan K and Alentorn
A: Primary central nervous system lymphoma: Time for diagnostic
biomarkers and biotherapies? Curr Opin Neurol. 30:669–676. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zheng J, Xu J, Ma S, Sun X, Geng M and
Wang L: Clinicopathological study of gene rearrangement and
microRNA expression of primary central nervous system diffuse large
B-cell lymphomas. Int J Clin Exp Pathol. 6:2048–2055.
2013.PubMed/NCBI
|
|
8
|
Musilova K and Mraz M: MicroRNAs in B-cell
lymphomas: How a complex biology gets more complex. Leukemia.
29:1004–1017. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Crooks V, Waller S, Smith T and Hahn TJ:
The use of the karnofsky performance scale in determining outcomes
and risk in geriatric outpatients. J Gerontol. 46:M139–M144. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Villano JL, Koshy M, Shaikh H, Dolecek TA
and McCarthy BJ: Age, gender, and racial differences in incidence
and survival in primary CNS lymphoma. Br J Cancer. 105:1414–1418.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu X, Nerisho S, Dastidar P, Ryymin P,
Järvenpää R, Pertovaara H, Eskola H and Kellokumpu-Lehtinen PL:
Comparison of different MRI sequences in lesion detection and early
response evaluation of diffuse large B-cell lymphoma - a whole-body
MRI and diffusion-weighted imaging study. NMR Biomed. 26:1186–1194.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ichiki A, Carreras J, Miyaoka M, Kikuti
YY, Jibiki T, Tazume K, Watanabe S, Sasao T, Obayashi Y, Onizuka M,
et al: Clinicopathological analysis of 320 cases of diffuse large
B-cell lymphoma using the hans classifier. J Clin Exp Hematop.
57:54–63. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu W, Sun M, Zou GM and Chen J: MicroRNA
and cancer: Current status and prospective. Int J Cancer.
120:953–960. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang Z, Han J, Cui Y, Fan K and Zhou X:
Circulating microRNA-21 as noninvasive predictive biomarker for
response in cancer immunotherapy. Med Hypotheses. 81:41–43. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ivo D'Urso P, Fernando D'Urso O, Damiano
Gianfreda C, Mezzolla V, Storelli C and Marsigliante S: miR-15b and
miR-21 as circulating biomarkers for diagnosis of glioma. Curr
Genomics. 16:304–311. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Roth P, Keller A, Hoheisel JD, Codo P,
Bauer AS, Backes C, Leidinger P, Meese E, Thiel E, Korfel A and
Weller M: Differentially regulated miRNAs as prognostic biomarkers
in the blood of primary CNS lymphoma patients. Eur J Cancer.
51:382–390. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Calin GA, Sevignani C, Dumitru CD, Hyslop
T, Noch E, Yendamuri S, Shimizu M, Rattan S, Bullrich F, Negrini M
and Croce CM: Human microRNA genes are frequently located at
fragile sites and genomic regions involved in cancers. Proc Natl
Acad Sci USA. 101:2999–3004. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gao HY, Wang W, Luo XG, Jiang YF, He X, Xu
P, Chen X and Li XY: Screening of prognostic risk microRNAs for
acute myeloid leukemia. Hematology. 23:747–755. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Greither T, Grochola LF, Udelnow A,
Lautenschlager C, Würl P and Taubert H: Elevated expression of
microRNAs 155, 203, 210 and 222 in pancreatic tumors is associated
with poorer survival. Int J Cancer. 126:73–80. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Baraniskin A, Kuhnhenn J, Schlegel U, Chan
A, Deckert M, Gold R, Maghnouj A, Zöllner H, Reinacher-Schick A,
Schmiegel W, et al: Identification of microRNAs in the
cerebrospinal fluid as marker for primary diffuse large B-cell
lymphoma of the central nervous system. Blood. 117:3140–3146. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Baraniskin A, Kuhnhenn J, Schlegel U,
Maghnouj A, Zöllner H, Schmiegel W, Hahn S and Schroers R:
Identification of microRNAs in the cerebrospinal fluid as biomarker
for the diagnosis of glioma. Neuro Oncol. 14:29–33. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Baraniskin A, Kuhnhenn J, Schlegel U,
Schmiegel W, Hahn S and Schroers R: MicroRNAs in cerebrospinal
fluid as biomarker for disease course monitoring in primary central
nervous system lymphoma. J Neurooncol. 109:239–244. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Conti A, Aguennouz M, La Torre D,
Tomasello C, Cardali S, Angileri FF, Maio F, Cama A, Germanò A,
Vita G and Tomasello F: miR-21 and 221 upregulation and miR-181b
downregulation in human grade II–IV astrocytic tumors. J
Neurooncol. 93:325–332. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wei D, Wan Q, Li L, Jin H, Liu Y, Wang Y
and Zhang G: MicroRNAs as potential biomarkers for diagnosing
cancers of central nervous system: A meta-analysis. Mol Neurobiol.
51:1452–1461. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lawrie CH, Gal S, Dunlop HM, Pushkaran B,
Liggins AP, Pulford K, Banham AH, Pezzella F, Boultwood J,
Wainscoat JS, et al: Detection of elevated levels of
tumour-associated microRNAs in serum of patients with diffuse large
B-cell lymphoma. Br J Haematol. 141:672–675. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mao X, Sun Y and Tang J: Serum miR-21 is a
diagnostic and prognostic marker of primary central nervous system
lymphoma. Neurol Sci. 35:233–238. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rubenstein JL, Gupta NK, Mannis GN,
Lamarre AK and Treseler P: How I treat CNS lymphomas. Blood.
122:2318–2330. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li Y and Sarkar FH: MicroRNA targeted
therapeutic approach for pancreatic cancer. Int J Biol Sci.
12:326–337. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gasparini P, Cascione L, Landi L, Carasi
S, Lovat F, Tibaldi C, Alì G, D'Incecco A, Minuti G, Chella A, et
al: microRNA classifiers are powerful diagnostic/prognostic tools
in ALK-, EGFR-, and KRAS-driven lung cancers. Proc Natl Acad Sci
USA. 112:14924–14929. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Winther M, Alsner J, Tramm T, Baeksgaard
L, Holtved E and Nordsmark M: Evaluation of miR-21 and miR-375 as
prognostic biomarkers in esophageal cancer. Acta Oncol.
54:1582–1591. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Koutova L, Sterbova M, Pazourkova E,
Pospisilova S, Svobodova I, Horinek A, Lysak D and Korabecna M: The
impact of standard chemotherapy on miRNA signature in plasma in AML
patients. Leuk Res. 39:1389–1395. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang CC, Carnevale J and Rubenstein JL:
Progress in central nervous system Lymphomas. Br J Haematol.
166:311–325. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chakraborty C, Doss CG, Sarin R, Hsu MJ
and Agoramoorthy G: Can the chemotherapeutic agents perform
anticancer activity through miRNA expression regulation? Proposing
a new hypothesis [corrected]. Protoplasma. 252:1603–1610. 2015.
View Article : Google Scholar : PubMed/NCBI
|