Introduction
Cervical cancer is the fourth most common type of
cancer in females worldwide (1),
with >52,000 new cases diagnosed annually and a morbidity rate
that is markedly high in the Shanxi Province of China (2). Cervical cancer has a complicated
etiology. Infection with human papillomavirus (HPV), particularly
high-risk HPV, has been implicated in cervical squamous cell
carcinoma (3,4), however, not all females with HPV
infection develop cervical cancer (5,6), which
indicates that other factors are responsible for inducing cervical
carcinogenesis. The HPV oncogenes E2 and E6 exhibit differential
effects during carcinogenesis (7,8). In our
previous study (9), it was
identified that both the expression levels of HPV16 E6 and E2 were
significantly higher in patients with cervical cancer compared with
healthy controls and the expression level of E6 was higher compared
with E2 in each group. The cervix is the narrow portion of the
uterus at the point that joins with the top of the vagina. The
majority of cervical cancer cases are squamous cell carcinomas,
which occur in the squamous epithelial cells that line the cervix
(10,11). This transformation zone, where the
majority of cervical cancer cases arise, is the most
estrogen-sensitive region of the cervix (12). DNA damage by estrogen metabolites may
also contribute to cervical carcinogenesis (12,13).
Furthermore, females taking oral contraceptives are at an increased
risk of developing cervical cancer (14,15),
which suggests an association between hormonal exposure and cancer
risk. Parity has also been demonstrated to be a cofactor, along
with HPV, in increasing the risk of developing cervical cancer
(16). Similarly, lesions have been
observed in mice that were perinatally exposed to
diethylstilbestrol (17,18).
The role of female hormones in cervical
carcinogenesis has traditionally been studied using cervical cell
lines and HPV-16 transgenic mice (19,20),
however, the role of estradiol in the development of precancerous
and cancerous lesions, particularly the association between
estradiol and HPV infection, remains unclear (21). Periodic changes in the levels of
endogenous hormones are under tight regulation, however, this
regulation may be disrupted in certain conditions, including
disease and certain environmental exposure. Therefore, further
studies are required to investigate the association and mechanism
between endogenous hormones and the development of cervical cancer.
Therefore, we conducted a case-control study to examine the
association between estradiol level and the risk of cervical
cancer, and to determine the potential synergistic effect of
estradiol and HPV on the progression of cervical cancer.
Materials and methods
Subjects
The present study was conducted with females that
were engaged in agriculture and mining in Shanxi Province, located
in the north of China. Cervical cancer mortality rates in this
population are relatively high (22). Participants were assigned to either
the case group or the control group according to their pathologic
diagnosis. According to our pilot study, the HPV infective rate
among all normal individuals was 28%, with an odds ratio (OR) of 3.
The following sample size calculation formula was used to determine
that a sample size of 74 for each group was required:
n={Z1-α/2*[(2*ρ*(1-ρ)]1/2+
Zβ* [P1*(1- P1)+ P0
*(1- P0)]1/2}2/ (P1-
P0)2, P1=(OR*P0)/(1-
P0+ OR*P0), ρ=
(P1+P0)/2, where α = 0.05 and β = 0.10 (Z:
critical value of standard normal distribution, P: HPV exposure
rate). Therefore, a total of 74 patients with cervical cancer that
were newly diagnosed by histologic assessment were recruited, and
74 healthy controls were selected at random among 582 healthy
females who were diagnosed without cervical intraepithelial
neoplasia, invasive cancer or other gynecologic diseases during a
routine physical examination between January 2016 and December 2016
at Shanxi Province Tumor Hospital (Taiyuan, China). The controls
were matched to patients according to age, place of residence,
marital status and menopausal status. Pregnant females, females
with ovarian disease or other types of cancer, and those who had
been treated with steroid hormones in the prior six months were
excluded. Written informed consent was obtained from each
participant prior to study initiation. The mean ages of women
included in the case and control groups were 47.32 years (range,
25–75 years) and 47.42 years (range, 29–77 years),
respectively.
Clinical information was obtained by a questionnaire
following ethical institutional approval. All subjects were
interviewed according to the questionnaire, which focused on
demographic characteristics, lifestyle, personal hygiene behavior
and reproductive factors. Cervical tissue specimens were surgically
obtained from all females in the case group, and cells were
obtained from a cervical smear from females in both the case and
control groups. In addition, venous blood was collected during days
5–8 of the menstrual cycle from all participants and the serum was
separated for subsequent analysis of estradiol levels. Informed
consent forms were signed by all individuals who agreed to
participate in the study. The study was approved by the Science
Research Ethics Committee of Shanxi Medical University (Taiyuan,
China).
General polymerase chain reaction
(PCR) analysis
Genomic DNA was extracted from cervical tissues and
cells using the standard phenol-chloroform method (23). HPV DNA was detected by PCR with the
following consensus primers from the L1 regions of HPV
type 16, 18, 33, 6, 11 and 31: Forward,
5′-CGTAAACGTTTTCCCTATTTTTTT-3′ and reverse,
5′-TACCCTAAATACTCTGTATTG-3′ (24).
High-risk HPV16 DNA was detected using multiple PCR to amplify the
E6 and E2 gene sequences with the following primers: HPV16 E2
forward, 5′-AAGGGCGTAACCGAAATCGGT-3′ and reverse,
5′-CATATACCTCACGTCGCAG-3′); and HPV16 E6 forward,
5′-CTTGGGCACCGAAGAAACC-3′ and reverse, 5′-TTGGTCACGTTGCCATTCAC-3′
(25). PCR was performed using 50-µl
samples, containing 100 ng template DNA, 25 mM MgCl2, 10
mM each of deoxyadenosine triphosphate, deoxythymidine
triphosphate, deoxycytidine triphosphate and deoxyguanosine
triphosphate, 25 pM of each consensus forward and reverse primer,
and 1 U of Jump-Start Taq DNA polymerase (cat no. D0089: Bio Basic
Inc., Markham, ON, Canada). The following conditions were used: 30
cycles at 95°C for 30 sec, 55°C for 60 sec, 72°C for 60 sec and a
final extension at 72°C for 10 min. 10 µl PCR products and 2 µl
ethidium bromide were mixed and then subjected to electrophoresis
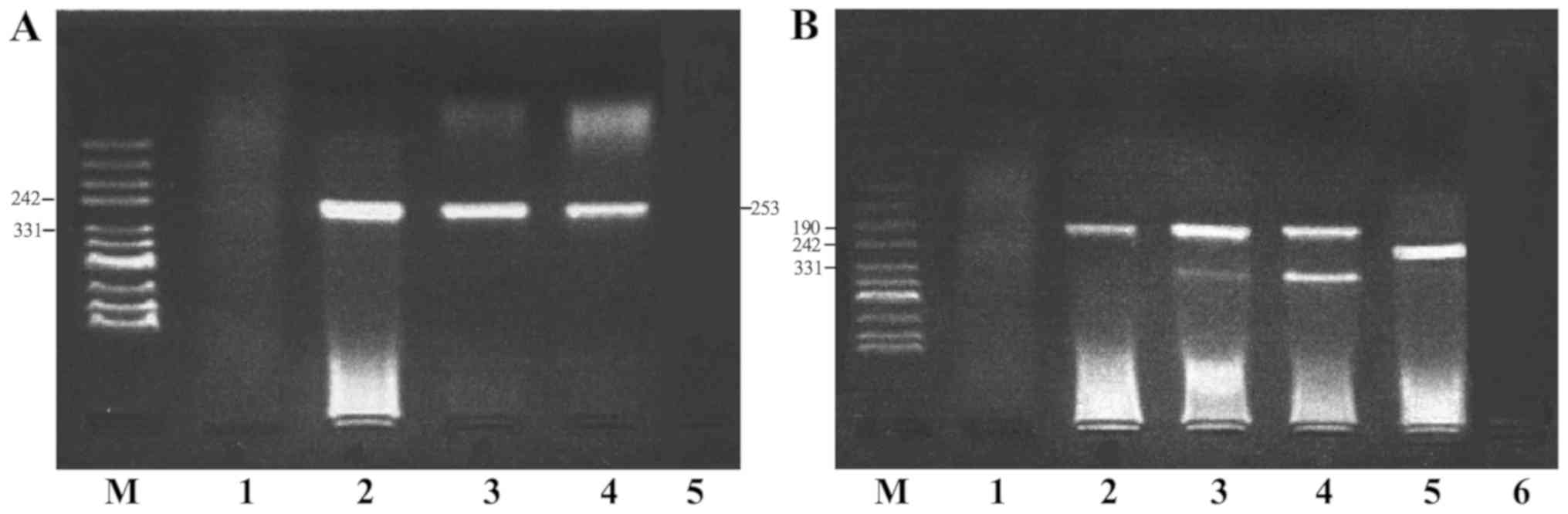
on 2% agarose gels. A 253-base pair (bp) fragment represented
HPV-positive cases, and two bands of 351 and 208 bp, which were
produced by the HPV16 E2 and E6 genes, respectively, represented
HPV16-positive cases.
Enzyme-linked immunosorbent assay
(ELISA)
Estradiol levels in the serum were measured using an
Estradiol enzyme immunoassay (EIA) kit (cat no. BC-1111; Biocheck
Inc., San Francisco, CA, USA), according to the manufacturer's
protocol.
Statistical analysis
SPSS 19.0 statistical software (IBM Corp., Armonk,
NY, USA) was used to analyze the data. Demographic and clinical
variables are presented as means ± standard deviations, with ranges
for continuous variables, and frequencies and percentages for
categorical variables. Each variable was compared between the case
group and the control group. For continuous variables, a two-sample
t-test was used, while categorical variables were compared with a
chi-square test to identify significant differences in HPV/HPV16
infection and estradiol levels between the two groups, and to
obtain maximum-likelihood estimates of the OR and corresponding 95%
confidence intervals (CIs). Effect modification between estradiol
and HPV/HPV16 positivity in cervical cancer cases was measured
according to additive interaction patterns (26), Additive interaction is a type of
biological interaction which means the effect of two (or more)
factors acting on a disease is not equal to the sum of the
independent effects of these factors separately (27). Furthermore, the extent of additive
interaction with the relative excess risk of interaction (RERI),
the attributable proportion of interaction (API) and synergy index
(S) were estimated. If there was no association, RERI and API were
equal to 0 and S was equal to 1. P<0.05 was considered to
indicate a statistically significant difference.
Results
Demographic characteristics of the
subjects
There was no significant difference between the ages
of the case and control groups (t=0.06; P=0.952). In addition, no
significant differences were identified between the groups with
respect to education level, frequency of vaginal cleaning, marital
status and menopausal status (all P>0.05). However, a
significantly higher number of women in the case group were
identified to have an occupation as a farmer compared with the
control group (P<0.05). The full demographic characteristics are
summarized in Table I.
 | Table I.Logistic regression analysis
comparing characteristics of individuals in the case group and
control group. |
Table I.
Logistic regression analysis
comparing characteristics of individuals in the case group and
control group.
| Variables | Patients, n
(%) | Controls, n
(%) | χ2 | P-value | OR (95%CI) |
|---|
| Education level
(high school or above) | 25 (33.78) | 29 (39.19) | 2.77 | 0.096 | 0.47
(0.19–1.15) |
| Occupation
(farmer) | 70 (94.59) | 62 (83.78) | 3.98 | 0.046 | 3.67
(1.02–13.14) |
| Frequency of
vaginal cleaning (≥3 times a week) | 21 (28.38) | 29 (39.19) | 2.14 | 0.144 | 0.46
(0.16–1.31) |
| Marital status
(married) | 49 (66.22) | 45 (60.81) | 1.02 | 0.312 | 0.77
(0.47–1.28) |
| Menopausal status
(post-menopause) | 29 (39.19) | 19 (25.68) | 3.20 | 0.074 | 0.50
(0.23–1.07) |
| Number of parity
(≥3 times) | 26 (35.14) | 12 (16.22) | 6.96 | 0.008 | 2.22
(1.23–4.03) |
HPV and HPV16 infection in the case
and control groups
HPV and HPV16 E6/E2 DNA were detected by
semi-quantitative PCR (Fig. 1). The
HPV16 E2 gene was absent in certain HPV16-positive samples,
therefore, HPV16 E6-positive samples were selected to represent
HPV16-positive samples. The positivity rates of HPV and HPV16 in
the case group (77.03 and 52.70%, respectively) were significantly
higher compared with those in the control group (47.30 and 21.62%,
respectively), with OR values of 3.74 (95% CI, 1.84–7.59) and 4.04
(95% CI, 1.97–8.28), respectively (Table II). To prevent any deviation caused
by differences in tissue and cell specimens, both types of
specimens were analyzed consistently for HPV and HPV16 in 24
cervical cancer cases. The results demonstrated that the
consistency among the percentage of HPV- and HPV16-positive cases
was 91.67% (κ=0.78) and 87.50% (κ=0.75), respectively (Table III).
 | Table II.HPV and HPV16 infection in the case
group and control group. |
Table II.
HPV and HPV16 infection in the case
group and control group.
| Infection | Case group (n=74)
Positive, n (%) | Control group
(n=74) Positive, n (%) | χ2 | P-value | OR (95% CI) |
|---|
| HPV | 57 (77.03) | 35 (47.30) | 13.904 | <0.001 | 3.74
(1.84–7.59) |
| HPV16 E6 | 39 (52.70) | 16 (21.62) | 15.306 | <0.001 | 4.04
(1.97–8.28) |
| HPV16 E2 | 31 (41.90) | 13 (17.60) | 10.479 |
0.001 | 3.38
(1.59–7.21) |
 | Table III.Consistency analysis of tissue and
cell specimens for HPV and HPV16. |
Table III.
Consistency analysis of tissue and
cell specimens for HPV and HPV16.
| Variables | HPV, n (%) | HPV16, n (%) |
|---|
| Tissue
specimens/Cell specimens |
|
+/+ | 17 (70.83) | 10 (41.67) |
|
+/− | 1 (4.17) | 2 (8.33) |
|
−/+ | 1 (4.17) | 1 (4.17) |
|
−/− | 5 (20.83) | 11 (45.83) |
| Total | 24 (100.00) | 24 (100.00) |
| Consistency rate
(%) | 91.67 | 87.50 |
| κ | 0.78 | 0.75 |
Estradiol levels in the case and
control groups
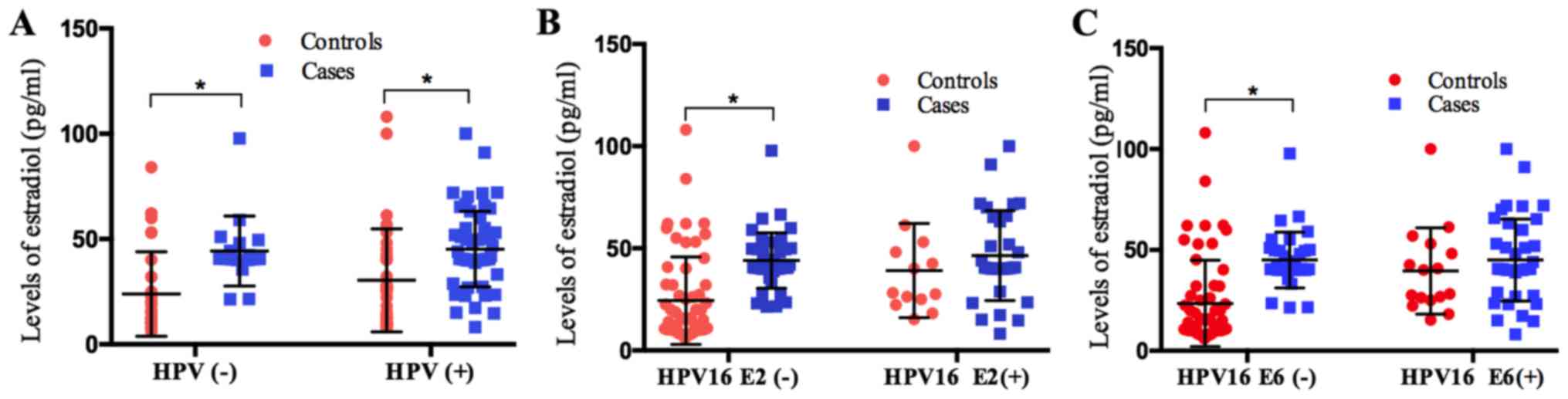
Estradiol expression levels in the case group
(45.98±11.48 pg/ml) were significantly higher compared with the
control group (26.96±8.31 pg/ml; Table
III). This significant difference between the case and control
groups was maintained regardless of the HPV status (Fig. 2A). However, it was identified that
estradiol expression level was only significantly higher in the
case group compared with the control when the HPV16 E2 or E6 status
was negative (Fig. 2B and C).
The lowest concentration of estradiol (40 pg/ml)
detected in healthy females during the follicular period was
defined as the cut-off value; therefore, estradiol levels >40
pg/ml were considered as abnormally high. Accordingly, estradiol
levels in 78.38% (58/74) of the cervical cancer cases were revealed
to be abnormally high, whereas the estradiol level was identified
as abnormally high in only 27.03% (20/74) of the controls, which
indicates a significant difference between the two groups (Table IV).
 | Table IV.Association between estradiol level
and cervical cancer occurrence. |
Table IV.
Association between estradiol level
and cervical cancer occurrence.
| Group | n | Mean ± SD,
pg/ml | Estradiol >40
pg/ml, n (%) | χ2 | P-value | OR (95% CI) |
|---|
| Case | 74 | 45.98±11.48 | 58 (78.38) | 39.14 | <0.001 | 9.79
(4.60–20.82) |
| Control | 74 | 26.96±8.31 | 20 (27.03) |
|
|
|
Association between estradiol level
and HPV or HPV16 infection
The risk of cervical cancer in HPV- or
HPV16-positive females with abnormally high levels of estradiol was
higher compared with females who were either HPV/HPV16-positive or
exhibited abnormally high levels of estradiol. According to a
method previously described by Knol and VanderWeele (26), the present study quantitatively
analyzed the association between estradiol and HPV/HPV16 infection.
and estimated the parameters RERI, API and S (Table V). The results revealed an additive
interaction pattern between estradiol levels and HPV/HPV16
infection.
 | Table V.Additive interaction between
estradiol level and HPV or HPV16 infection. |
Table V.
Additive interaction between
estradiol level and HPV or HPV16 infection.
| A, Additive
interaction between estradiol level and HPV infection |
|---|
|
|---|
| HPV | Estradiol,
pg/ml | Case group, n | Control group,
n | OR (95% CI) | RERI | API | S |
|---|
| + | ≥40 | 44 | 11 | 41.33
(10.64–160.53) | 18.67 | 0.45 | 1.86 |
| + | <40 | 13 | 24 | 5.60
(1.06–8.56) |
|
|
|
| − | ≥40 | 14 | 8 | 18.08
(4.16–78.60) |
|
|
|
| − | <40 | 3 | 31 | 1.00 |
|
|
|
|
| B, Additive
interaction between estradiol level and HPV16 infection |
|
| HPV16 | Estradiol,
pg/ml | Case group,
n | Control group,
n | OR (95%
CI) | RERI | API | S |
|
| + | ≥40 | 28 | 8 | 32.90
(9.80–110.48) | 5.20 | −0.16 | 0.86 |
| + | <40 | 11 | 8 | 12.93
(3.54–47.23) |
|
|
|
| − | ≥40 | 30 | 11 | 25.64
(8.10–81.13) |
|
|
|
| − | <40 | 5 | 47 | 1.00 |
|
|
|
Discussion
HPV infection is relatively common among females
(28,29). Persistent HPV infection may cause
intraepithelial pre-neoplastic lesions and invasive cervical cancer
(30,31). The present study revealed that the
positivity rates of HPV and HPV16 in patients with cervical cancer
were 77.03 and 52.07%, respectively, which were significantly
higher compared with the controls. Among the HPV-positive cases,
>68% were HPV16-positive. The results of previous studies, which
also investigated HPV infection and cervical cancer in females of
Shanxi Province (32,33), in combination with the present
results suggest that cervical cancer is closely associated with HPV
infection, particularly the high-risk type HPV16, which is
considered to be a primary cause of cervical cancer.
To the best of our knowledge, the etiological role
of HPV in the development of uterine cervix squamous cell carcinoma
remains unclear. Cervical carcinogenesis is a multistep process
initiated by HPV, however, infection alone is insufficient to
induce malignancy (34). HPV leads
to the development of intraepithelial lesions in the presence of
other cofactors (35). One such
cofactor that has been reported to initiate neoplasia in cervical
cancer is prolonged exposure to sex hormones (14,15).
Therefore, the potential association between uterine cervix
squamous cell carcinoma and sex hormones has been extensively
studied (36,37). A meta-analysis has demonstrated that
controlled ovarian hyperstimulation (COH) for in vitro
fertilization (IVF) could elevate the levels of hormones, however
an increased risk of cervical cancer was not identified, which
indicates a protective role of IVF (38). However, females undergoing IVF are
considered to have stable sexual relationships and a high
socioeconomic status, and may be treated for cervical lesions prior
to IVF. In addition, HPV infection has been revealed to be
significantly lower in females undergoing IVF compared with
controls (39). All these factors
may reduce the risk of hormone levels on cervical cancer
development. By contrast, long-term use of oral contraceptives has
been demonstrated to increase the risk of cervical cancer (40). Elevated hormone levels were revealed
to induce the proliferation and apoptosis of cervical
adenocarcinoma HeLa cells in vitro (19). The biological effects of COH or oral
contraceptives in the human body may be influenced by numerous
factors, such as drug type, dosage, time of taking medicin and so
on (40). A number of studies
investigating endogenous steroid hormones have demonstrated an
association between endogenous hormone levels and cervical cancer
based on the general population (41–43).
Estradiol is the most important steroid hormone in the normal
female endocrine system (44); the
present study identified that endogenous estradiol levels in
patients with cervical cancer were higher compared with controls,
which suggests that high endogenous estradiol levels are associated
with an elevated risk of cervical cancer. Estrogen receptor-α binds
to the forkhead box P3 promoter and modulates regulatory T-cell
function in human cervical cancer (45). Therefore, sex hormones may have an
influence on the immune system. In summary, this evidence suggests
estrogen exposure may be associated with hyperplasia and squamous
differentiation of reserve cells.
Notably, the present study obtained different
conclusions according to the expression of the HPV16 oncogene. A
significant difference in HPV positivity was identified between
patients and controls, however, estradiol levels in patients were
significantly higher compared with controls only when HPV16 E2 or
E6 status was negative. HPV16 E6 protein is required for cell
transformation, while the HPV E2 protein is required for viral
replication and gene expression (46). Estrogen has been demonstrated to
upregulate the transcription of HPV E6 oncogenes in vitro
(47). A number of studies involving
HPV-positive transgenic mice that expressed HPV16 oncogenes in
their basal keratinocytes have demonstrated that chronic exposure
to estradiol is required for the induction of cervical tumors
(47–49). Furthermore, based on biological
interaction analysis, the present study revealed an additive
interaction between endogenous estradiol level and HPV/HPV16
occurrence. In addition, it was identified that the risk of
cervical cancer in females with both a high endogenous estradiol
level and HPV infection was 18.67-fold greater compared with the
risk associated with other factors, accounting for 45% of the risk,
and was 1.86-fold greater compared with the sum of risks associated
with endogenous estradiol level and HPV infection. A similar
interaction was also observed between the endogenous estradiol
level and HPV16 infection, with an RERI of −5.20, API of −0.16 and
S of 0.86. These results indicate that the additive effect of
HPV/HPV16 infection and high estradiol level is greater compared
with their individual effects on cancer risk.
The present study possessed a number of limitations.
Firstly, analysis of HPV positivity and high endogenous estradiol
levels at a single point in a female's lifetime are difficult to
interpret as these could represent either a recent change of a
long-term status. Secondly, a case-control study can only suggest
associations and cannot determine causal associations. In addition,
given the complicated association between endogenous estradiol
level and HPV infection in cervical cancer, there are numerous
other influencing factors that should be investigated.
Despite these limitations, the cervix epithelium is
a hormone-dependent epithelium, and the present study provides a
strong indication that abnormally high endogenous estradiol levels
may be associated with increased risk of cervical cancer and
estradiol may serve as a cofactor along with HPV infection in the
development of cervical cancer. Further studies, including a
prospective cohort study should be performed to provide etiological
evidence that may clarify the mechanism of the association between
endogenous hormone level and HPV infection in cervical
carcinogenesis. In addition, further studies are required to
evaluate whether an increased risk of cervical cancer is associated
with other factors. Furthermore, the results of the present study
suggest that estradiol may represent a new treatment strategy in
cases of cervical cancer due to HPV infection, however, this
requires further investigation.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81703313, 81473060
and 81702583).
Availability of data and materials
The datasets used or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
LD performed data analyses, drafted the manuscript,
finalized and submitted the manuscript. LD, and MF contributed to
perform the DNA extraction, PCR experiments and ELISA experiments.
CL and QZ contributed to the data collection and quality control.
JW and LD conceived the idea, designed and led the project. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Science Research
Ethics Committee of Shanxi Medical University (Taiyuan, China) and
all participants provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Skinner RS, Wheeler CM, Romanowski B,
Castellsagué X, Lazcano-Ponce E, Del Rosario-Raymundo RM, Vallejos
C, Minkina G, Pereira Da Silva D, McNeil S, et al: Progression of
HPV infection to detectable cervical lesions or clearance in adult
women: Analysis of the control arm of the VIVIANE study. Int J
Cancer. 138:2428–2438. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Madeleine MM, Anttila T, Schwartz SM,
Saikku P, Leinonen M, Carter JJ, Wurscher M, Johnson LG, Galloway
DA and Daling JR: Risk of cervical cancer associated with Chlamydia
trachomatis antibodies by histology, HPV type and HPV cofactors.
Int J Cancer. 120:650–655. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Choi YJ and Park JS: Clinical significance
of human papillomavirus genotyping. J Gynecol Oncol. 7:e212016.
View Article : Google Scholar
|
|
6
|
Egawa N, Egawa K, Griffin H and Doorbar J:
Human papillomaviruses: epithelial tropisms, and the development of
neoplasia. Viruses. 7:3863–3890. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bellanger S, Tan CL, Xue YZ, Teissier S
and Thierry F: Tumor suppressor or oncogene? A critical role of the
human papillomavirus (HPV) E2 protein in cervical cancer
progression. Am J Cancer Res. 1:373–389. 2011.PubMed/NCBI
|
|
8
|
Doorbar J, Egawa N, Griffin H, Kranjec C
and Murakami I: Human papillomavirus molecular biology and disease
association. Rev Med Virol. 25 (Suppl 1):S2–S23. 2015. View Article : Google Scholar
|
|
9
|
Wang JT, Ding L, Gao ES and Cheng YY:
Analysis on the expression of human papillomavirus type 16 E2 and
E6 oncogenes and disruption of E2 in cervical cancer. Zhonghua Liu
Xing Bing Xue Za Zhi. 28:968–971. 2007.(In Chinese). PubMed/NCBI
|
|
10
|
Missaoui N, Trabelsi A, Landolsi H,
Jaidaine L, Mokni M, Korbi S and Hmissa S: Cervical adenocarcinoma
and squamous cell carcinoma incidence trends among Tunisian women.
Asian Pac J Cancer Prev. 11:777–780. 2010.PubMed/NCBI
|
|
11
|
Wang SS, Sherman ME, Hildesheim A, Lacey
JV Jr and Devesa S: Cervical adenocarcinoma and squamous cell
carcinoma incidence trends among white women and black women in the
United States for 1976–2000. Cancer. 100:1035–1044. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Auborn KJ, Woodworth C, DiPaolo JA and
Bradlow HL: The interaction between HPV infection and estrogen
metabolism in cervical carcinogenesis. Int J Cancer. 49:867–869.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Newfield L, Bradlow HL, Sepkovic DW and
Auborn K: Estrogen metabolism and the malignant potential of human
papillomavirus immortalized keratinocytes. Proc Soc Exp Biol Med.
217:322–326. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Moodley M, Moodley J, Chetty R and
Herrington CS: The role of steroid contraceptive hormones in the
pathogenesis of invasive cervical cancer: A review. Int J Gynecol
Cancer. 13:103–110. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Salazar EL, Sojo-Aranda I, Lopez R and
Salcedo M: The evidence for an etiological relationship between
oral contraceptive use and dysplastic change in cervical tissue.
Gynecol Endocrinol. 15:23–28. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Muñoz N, Franceschi S, Bosetti C, Moreno
V, Herrero R, Smith JS, Shah KV, Meijer CJ and Bosch FX;:
International Agency for Research on Cancer (IARC) Multicentric
Cervical Cancer Study Group: Role of parity and human
papillomavirus in cervical cancer: The IARC multicentric
case-control study. Lancet. 359:1093–1101. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Plapinger L and Bern HA: Adenosis-like
lesions and other cervicovaginal abnormalities in mice treated
perinatally with estrogen. J Natl Cancer Inst. 63:507–518.
1979.PubMed/NCBI
|
|
18
|
McLachlan JA, Newbold RR and Bullock BC:
Long-term effects on the female mouse genital tract associated with
prenatal exposure to diethylstilbestrol. Cancer Res. 40:3988–3999.
1980.PubMed/NCBI
|
|
19
|
Liu Y, Tian LB, Yang HY and Zhang HP:
Effects of estradiol and progesterone on the growth of HeLa
cervical cancer cells. Eur Rev Med Pharmacol Sci. 21:3959–3965.
2017.PubMed/NCBI
|
|
20
|
Brake T and Lambert PF: Estrogen
contributes to the onset, persistence, and malignant progression of
cervical cancer in a human papillomavirus-transgenic mouse model.
Proc Natl Acad Sci USA. 102:2490–2495. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
den Boon JA, Pyeon D, Wang SS, Horswill M,
Schiffman M, Sherman M, Zuna RE, Wang Z, Hewitt SM, Pearson R, et
al: Molecular transitions from papillomavirus infection to cervical
precancer and cancer: Role of stromal estrogen receptor signaling.
Proc Natl Acad Sci USA. 112:E3255–E3264. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang SK, Kang LN, Chang IJ, Zhao FH, Hu
SY, Chen W, Shi JF, Zhang X, Pan QJ, Li SM, et al: The natural
history of cervical cancer in Chinese women: results from an
11-year follow-up study in china using a multistate model. Cancer
Epidemiol Biomarkers Prev. 23:1298–1305. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sambrook J and Russell DW: Purification of
nucleic acids by extraction with phenol:chloroform. CSH Protoc.
2006(pii): pdb.prot4455. 2006.
|
|
24
|
Yoshikawa H, Kawana T, Kitagawa K, Mizuno
M, Yoshikura H and Iwamoto A: Detection and typing of multiple
genital human papillomaviruses by DNA amplification with consensus
primers. Jpn J Cancer Res. 82:524–531. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Badaracco G, Venuti A, Sedati A and
Marcante ML: HPV16 and HPV18 in genital tumors: Significantly
different levels of viral integration and correlation to tumor
invasiveness. J Med Virol. 67:574–582. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Knol MJ and VanderWeele TJ:
Recommendations for presenting analyses of effect modification and
interaction. Int J Epidemiol. 41:514–520. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rothman KJ, Greenland S and Lash TL:
Chapter 5 Concepts of Interaction. In: Modern epidemiology.
Lippincott Williams & Wilkins. 2008.
|
|
28
|
Goodman MT, Shvetsov YB, McDuffie K,
Wilkens LR, Zhu X, Ning L, Killeen J, Kamemoto L and Hernandez BY:
Acquisition of anal human papillomavirus (HPV) infection in women:
The Hawaii HPV Cohort study. J Infect Dis. 197:957–966. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shvetsov YB, Hernandez BY, McDuffie K,
Wilkens LR, Zhu X, Ning L, Killeen J, Kamemoto L and Goodman MT:
Duration and clearance of anal human papillomavirus (HPV) infection
among women: The Hawaii HPV cohort study. Clin Infect Dis.
48:536–546. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen HC, Schiffman M, Lin CY, Pan MH, You
SL, Chuang LC, Hsieh CY, Liaw KL, Hsing AW, Chen CJ, et al:
Persistence of type-specific human papillomavirus infection and
increased long-term risk of cervical cancer. J Natl Cancer Inst.
103:1387–1396. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Byun JM, Jeong DH, Kim YN, Jung EJ, Lee
KB, Sung MS and Kim KT: Persistent HPV-16 infection leads to
recurrence of high-grade cervical intraepithelial neoplasia.
Medicine (Baltimore). 97:e136062018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dai M, Bao YP, Li N, Clifford GM,
Vaccarella S, Snijders PJ, Huang RD, Sun LX, Meijer CJ, Qiao YL and
Franceschi S: Human papillomavirus infection in Shanxi Province,
People's Republic of China: A population-based study. Br J Cancer.
95:96–101. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Guo J, Zhao F, Liu R and Mu Y: Prevalence
and type distribution of human papillomavirus infection in women
from Datong, China. Scand J Infect Dis. 42:72–75. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Briolat J, Dalstein V, Saunier M, Joseph
K, Caudroy S, Prétet JL, Birembaut P and Clavel C: HPV prevalence,
viral load and physical state of HPV-16 in cervical smears of
patients with different grades of CIN. Int J Cancer. 121:2198–2204.
2010. View Article : Google Scholar
|
|
35
|
Luhn P, Walker J, Schiffman M, Zuna RE,
Dunn ST, Gold MA, Smith K, Mathews C, Allen RA, Zhang R, et al: The
role of co-factors in the progression from human papillomavirus
infection to cervical cancer. Gynecol Oncol. 128:265–270. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Moreno V, Bosch FX, Munoz N, Meijer CJ,
Shah KV, Walboomers JM, Herrero R and Franceschi S;: International
Agency for Research on Cancer. Multicentric Cervical Cancer Study
Group: Effect of oral contraceptives on risk of cervical cancer in
women with human papillomavirus infection: The IARC multicentric
case-control study. Lancet. 359:1085–1092. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Santos Filho MV, Gurgel AP, Lobo CD,
Freitas AC, Silva-Neto JC and Silva LA: Prevalence of human
papillomavirus (HPV), distribution of HPV types, and risk factors
for infection in HPV-positive women. Genet Mol Res. 15:2016.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Siristatidis C, Sergentanis TN, Kanavidis
P, Trivella M, Sotiraki M, Mavromatis I, Psaltopoulou T, Skalkidou
A and Petridou ET: Controlled ovarian hyperstimulation for IVF:
Impact on ovarian, endometrial and cervical cancer-a systematic
review and meta-analysis. Hum Reprod Update. 19:105–123. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lundqvist M, Westin C, Lundkvist O,
Simberg N, Strand A, Andersson S and Wilander E: Cytologic
screening and human papilloma virus test in women undergoing
artificial fertilization. Acta Obstet Gynecol Scand Oct.
81:949–953. 2002. View Article : Google Scholar
|
|
40
|
International Collaboration of
Epidemiological Studies of Cervical Cancer, ; Appleby P, Beral V,
Berrington de González A, Colin D, Franceschi S, Goodhill A, Green
J, Peto J, Plummer M and Sweetland S: Cervical cancer and hormonal
contraceptives: collaborative reanalysis of individual data for
16,573 women with cervical cancer and 35,509 women without cervical
cancer from 24 epidemiological studies. Lancet. 370:1609–1621.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rinaldi S, Plummer M, Biessy C,
Castellsagué X, Overvad K, Krüger Kjær S, Tjønneland A,
Clavel-Chapelon F, Chabbert-Buffet N, Mesrine S, et al: Endogenous
sex steroids and risk of cervical carcinoma: results from the EPIC
study. Cancer Epidemiol Biomarkers Prev. 20:2532–2540. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shields TS, Falk RT, Herrero R, Schiffman
M, Weiss NS, Bratti C, Rodriguez AC, Sherman ME, Burk RD and
Hildesheim A: A case-control study of endogenous hormones and
cervical cancer. Br J Cancer. 90:146–152. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Spurgeon ME, den Boon JA, Horswill M,
Barthakur S, Forouzan O, Rader JS, Beebe DJ, Roopra A, Ahlquist P
and Lambert PF: Human papillomavirus oncogenes reprogram the
cervical cancer microenvironment independently of and
synergistically with estrogen. Proc Natl Acad Sci USA.
114:E9076–E9085. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kuhl H: Pharmacology of estrogens and
progestogens: Influence of different routes of administration.
Climacteric. 8 (Suppl 1):S3–S63. 2005. View Article : Google Scholar
|
|
45
|
Adurthi S, Kumar MM, Vinodkumar HS,
Mukherjee G, Krishnamurthy H, Acharya KK, Bafna UD, Uma DK,
Abhishekh B, Krishna S, et al: Oestrogen receptor-α binds the FOXP3
promoter and modulates regulatory T-cell function in human cervical
cancer. Sci Rep. 7:172892017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Blachon S and Demeret C: The regulatory E2
proteins of human genital papillomaviruses are pro-apoptotic.
Biochimie. 85:813–819. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Riley RR, Duensing S, Brake T, Münger K,
Lambert PF and Arbeit JM: Dissection of human papillomavirus E6 and
E7 function in transgenic mouse models of cervical carcinogenesis.
Cancer Res. 63:4862–4871. 2003.PubMed/NCBI
|
|
48
|
Shai A, Brake T, Somoza C and Lambert PF:
The human papillomavirus E6 oncogene dysregulates the cell cycle
and contributes to cervical carcinogenesis through two independent
activities. Cancer Res. 67:1626–1635. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Arbeit JM, Howley PM and Hanahan D:
Chronic estrogen-induced cervical and vaginal squamous
carcinogenesis in human papillomavirus type 16 transgenic mice.
Proc Natl Acad Sci USA. 93:2930–2935. 1996. View Article : Google Scholar : PubMed/NCBI
|