Introduction
Breast cancer (BC) is a frequently malignant cancer,
which accounts for 30% of all newly diagnosed cancer cases among
women and is a major cause of cancer-associated mortality among
women (1). Currently, treatment of
BC primarily focuses on surgery, chemotherapy and endocrine
therapy. However, the prognosis of BC treatment is not
satisfactory, mainly due to progression and metastasis following
conventional treatment (2,3). The limited current knowledge of the
molecular basis of BC restricts the development of novel treatment
strategies (4). BC develops as a
consequence of cellular alterations that promote cell proliferation
and metastasis, and/or suppress apoptosis (5). These changes result from dysregulation
of key signal transduction pathways within the cell, which transmit
extracellular signals to transcription factors. Therefore, the
mechanisms underlying the unlimited growth of BC cells require
urgent elucidation to identify potential therapeutic
strategies.
Continuous cell division is frequently associated
with the stabilization of telomere length due to the reactivation
of telomerase. Currently, repression of telomerase and shorter
telomeres are considered potential anticancer mechanisms (6). Telomerase consists of several subunits,
including human telomerase reverse transcriptase (hTERT) and human
telomerase RNA. hTERT is a limiting factor for telomerase activity
and high hTERT levels have been observed in ~90% of human cancer
types, including BC (7). Notably,
the expression levels of hTERT are closely correlated with clinical
aggressiveness and poor prognosis in BC (8,9). Shi
et al used a specific inhibitor of telomerase activity and
revealed that telomerase inhibition significantly affects BC cell
growth, cell cycle and apoptosis (10). Additionally, Yu et al
(11) previously demonstrated that
zinc finger E-box binding homeobox 1, a multifunctional cancer
stimulatory factor, promotes BC cell invasiveness, proliferation
and apoptosis by regulating hTERT expression. Therefore, hTERT may
be investigated as a potential anticancer drug target.
Luteolin (39, 49, 5, 7-tetrahydroxyflavone) is a
flavone compound present in a number of medicinal plants. Flavones
are a class of flavonoids, among the most abundant secondary
metabolites in plants, and are widely known to be involved in
various pharmacological activities (12). Luteolin exhibits a range of antitumor
activities by suppressing cell proliferation and invasion, inducing
cell cycle arrest and apoptosis, sensitizing drug resistance and
mitigating metastasis of cancer cells (13,14). In
BC, luteolin has been reported to enhance paclitaxel-induced
apoptosis (15) and to sensitize
drug-resistant BC cells to tamoxifen (16). In addition, luteolin may inhibit cell
migration and invasion, and reverse the epithelial-mesenchymal
transition of MDA-MB-231 cells (17). Although the protective role of
luteolin in BC has been revealed, the underlying mechanism of
action of luteolin on BC cells remains largely unclear.
It has previously been suggested that several
medicinal plants and herbal ingredients, including resveratrol,
crocin and papaverine, could be used as inhibitors of the
telomerase enzyme and the active site of telomerase (18). However, whether luteolin has the
ability to downregulate telomerase activity and hTERT expression
remains unclear. The present study aimed to confirm the effects of
luteolin on cell growth, invasion, cell cycle progression and
apoptosis in the BC cell line MDA-MB-231. The present study
additionally intended to measure the effect of consecutive
treatment with luteolin on telomerase activity and hTERT
expression, as well as to explore the underlying mechanisms.
Materials and methods
Cell culture and treatment
A human BC cell line (MDA-MB-231) was obtained from
the Cell Bank of Chinese Academy of Sciences (Shanghai, China) and
cultured in RPMI-1640 medium (Hyclone; GE Healthcare Life Sciences,
Logan, UT, USA), supplemented with 10% fetal bovine serum (FBS,
Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and 1%
penicillin and streptomycin (Hyclone; GE Healthcare Life Sciences).
All cells were maintained at 37°C in a humidified atmosphere
containing 5% CO2. Luteolin was purchased from Cayman Chemical Co.
(Ann Arbor, MI, USA), and 0.029 g luteolin was dissolved in 200 µl
dimethyl sulfoxide to obtain 0.5 M luteolin and stored at −20°C.
Prior to use, the stock was diluted to 1, 2, 4, 8, 16, 32, 64, 128,
256 µM luteolin in 10% FBS RPMI-1640 medium for MTS assay, and 1,
10 and 30 µM luteolin in FBS-free RPMI-1640 medium for all other
experiments. MDA-MB-231 cell cultures received various
concentrations of luteolin for 24 or 48 h to evaluate its effect on
BC cells.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from cells using
TRIzol® reagent, according to the manufacturer's
protocol (Invitrogen; Thermo Fisher Scientific, Inc.). The total
RNA yield was determined using the NanoDrop ND-8000 UV-Vis
spectrophotometer (NanoDrop Technologies; Thermo Fisher Scientific,
Inc.). cDNA was synthesized using a PrimeScript RT-PCR kit (Takara
Bio, Inc., Otsu, Japan) under the following conditions: 95°C for 15
sec; followed by 30 cycles of 95°C for 5 sec and 60°C for 60 sec.
Quantification was performed using RT Real-Time SYBR Green assays
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) on the ABI PRISM
7900 HT Sequence Detection system (Applied Biosystems; Thermo
Fisher Scientific, Inc.). The reaction conditions were as follows:
94°C for 5 min, followed by 40 cycles at 95°C for 15 sec, 65°C for
30 sec and 72°C for 30 sec, and a final extension step at 72°C for
5 min. Each sample was examined in triplicate and the relative mRNA
expression levels were determined using the 2−ΔΔCq
method (19), normalized for the
housekeeping gene GAPDH. Primers used for RT-qPCR are shown in
Table I.
 | Table I.Primer sequences used for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primer sequences used for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Sequence
(5′-3′) |
|---|
| hTERT | Forward:
CGGAAGAGTGTCTGGAGCAA |
|
| Reverse:
CTCCCACGACGTAGTCCATG |
| c-Myc | Forward:
CAATGTCAAGAGGCGAACACA |
|
| Reverse:
CGTCGTTTCCGCAACAAG |
| GAPDH | Forward:
CTTTGGTATCGTGGAAGGACTC |
|
| Reverse:
GTAGAGGCAGGGGATGATGTTCT |
MTS assay
The MTS assay is a colorimetric assay in which the
amount of color produced is directly proportional to the number of
viable cells. Briefly, 5×104 cells in the log growth phase were
plated on 96-well plates. Following luteolin exposure for 24 h
(17,20), 20 µl MTS (Promega Corporation,
Madison, WI, USA) labeling reagent was added to each well and
incubated for another 4 h. Absorbance at 490 nm was measured using
a microplate reader (Molecular Devices, LLC, Sunnyvale, CA, USA).
The half maximal inhibitory concentration (IC50) values were
determined from the concentration curves generated using GraphPad
Prism software 6.0 (GraphPad Software, Inc., La Jolla, CA,
USA).
Colony formation assays
Cell proliferation was monitored using a colony
formation assay. Briefly, 300 cells/well were grown in 6-cm plates
and maintained in Dulbecco's modified Eagle's medium (Thermo Fisher
Scientific, Inc.) containing 10% FBS with or without various
concentrations of luteolin. After 14 days, cells were fixed with 4%
paraformaldehyde for 15 min at room temperature and stained with 5
g/l crystal violet (Beyotime Institute of Biotechnology, Haimen,
China) for 3 min at room temperature. Visible colonies were counted
manually.
Transwell assays
In total, ~1×105 MDA-MB-231 cells were seeded at the
top of Matrigel-coated invasion chambers (24-well plates, 8 mm pore
size; BD Biosciences, Franklin Lakes, NJ, USA) with serum-free
medium containing various concentrations of luteolin. Medium
containing 10% FBS was used as a chemoattractant in the lower
chambers. After 24 h of incubation at 37°C in a 5% CO2 atmosphere,
the media were removed from the wells and washed twice with PBS.
The cells in the upper chambers were removed using a cotton swab.
Migratory cells were fixed with 4% paraformaldehyde at room
temperature for 5 min, and stained with 5 g/l crystal violet for 3
min at room temperature. Cells were counted in six representative
fields using an inverted light microscope at ×200 magnification
(Olympus BX53; Olympus Corporation, Tokyo, Japan).
Western blotting
Cells were lysed using ice-cold lysis buffer (25 mM
HEPES, 1.5% Triton X-100, 0.1% SDS, 0.5 M NaCl, 5 mM EDTA and 0.1
mM sodium deoxycholate). The protein concentration of each sample
was quantified using a bicinchoninic acid protein assay kit (Thermo
Fisher Scientific, Inc.). Equal quantities (20 µg) of protein/lane
were separated via SDS-PAGE on a 10–12% gel and were then
transferred to polyvinylidene difluoride membranes. The membranes
were blocked with 5% skimmed milk in Tris-buffered saline with 0.1%
Tween-20 (TBST) for 1 h at room temperature. After washing with
TBST, the membranes were hybridized with primary antibodies against
p21 (1:1,000; cat. no. ab109520; Abcam, Cambridge, UK), Survivin
(1:1,000; cat. no. #2808; CST Biological Reagents Co., Ltd.,
Shanghai, China), cyclin D1 (1:1,000; cat. no. #2978; CST
Biological Reagents Co., Ltd.), B-cell lymphoma 2 (BCL-2; 1:1,000;
cat. no. ab32124; Abcam), Bcl-2-associated X protein (Bax; 1:1,000;
cat. no. #5023; CST Biological Reagents Co., Ltd.), caspase-3
(1:500; cat. no. #9662; CST Biological Reagents Co., Ltd.), hTERT
(1:1,000; cat. no. ab32020; Abcam), c-Myc (1:1,000; cat. no. #2276;
CST Biological Reagents Co., Ltd.), phosphorylated
phosphorylated-nuclear factor κB (NF-κB) inhibitor α (pIκBα;
1:1,000; cat. no. #2859; CST Biological Reagents Co., Ltd.), IκBα
(1:1,000; cat. no. #4814; CST Biological Reagents Co., Ltd.) and
GAPDH (1:1,000; cat. no. ab9485; Abcam) overnight at 4°C. After
washing in TBST, the membranes were incubated with the appropriate
horseradish peroxidase (HRP)-conjugated secondary antibodies (cat.
nos. A21010 and A21020; 1:5,000; Abbkine Scientific Co., Ltd.,
Wuhan, China) at room temperature for 1 h. The protein bands were
visualized using Immobilon Western Chemiluminescent HRP substrate
(EMD Millipore, Billerica, MA, USA) and imaged by LAS-4000 mini
luminescent image analyzer (Fujifilm, Life Science, New Haven, CT,
USA). ImageJ software (version 1.8.0; National Institutes of
Health, Bethesda, MD, USA) was used for densitometric
semi-quantification of the blots.
Apoptosis analysis
The Annexin-fluorescein isothiocyanate apoptosis
detection kit (eBioscience; Thermo Fisher Scientific, Inc.) was
used to evaluate apoptosis. After treatment, cells in the 0, 10, 30
µM luteolin-treated groups were harvested and centrifuged for 5 min
at 1,680 × g at room temperature, and were then suspended at a
density of 1×106 cells/ml. Subsequently, the cells were diluted in
buffer, and 10 µl Annexin V and 10 µl propidium iodide (PI) were
added to 100 µl cell suspension. Subsequently, they were incubated
for 15 min at room temperature in the dark. Samples were analyzed
by flow cytometry using NovoExpress software (ACEA BioSciences
Inc., San Diego, CA, USA). Each experiment was performed in
triplicate at least. A terminal
deoxynucleotidyl-transferase-mediated dUTP nick end labeling
(TUNEL) apoptosis detection kit (Yeasen, Shanghai, China) was used
to determine cell apoptosis. Briefly, MDA-MB-231 cells were fixed
with 4% paraformaldehyde for 5 min at room temperature,
permeabilized with 0.1% Triton X-100 for 5 min at room temperature
and labeled with terminal deoxynucleotidyl transferase reaction mix
for 60 min at 37°C. DAPI was used to stain nuclei. A Nikon Eclipse
Ti-U fluorescence microscope (Nikon Corporation, Tokyo, Japan) was
used to observe morphological nuclear DNA fragmentation in the
stained BC cells (magnification, ×200).
Cell cycle analysis
MDA-MB-231 cells were cultivated in 6-well plates.
After treatment, cells were harvested and centrifuged at 1,680 × g
for 5 min at room temperature. The supernatant was discarded and
the cell pellet was washed twice with 3 ml PBS and centrifuged
again. Subsequently, ice-cold 70% ethanol was added to fix the
cells at 4°C for 1 h. After washing with PBS, 1 ml PI solution (50
µg/ml) containing 50 µg/ml RNase A (DNase free) was added to the
cells for staining in the dark at room temperature for 15 min. The
distribution of cells in each cell cycle phase was measured using a
flow cytometer at 488 nm excitation wavelength and over 630 nm
emission wavelength. The cell cycle distribution was evaluated by
calculating the proportion of cells in the G0/G1, S and G2/M stages
using NovoExpress software (BioSciences Inc.).
Telomeric repeat amplification
protocol (TRAP)-ELISA analysis
Telomerase activity was determined by TRAP assay
using the TeloTAGGG Telomerase PCR ELISA PLUS kit (Roche
Diagnostics GmbH, Mannheim, Germany), according to the
manufacturer's protocol. Treated cells were collected and lysed in
ice-cold lysis buffer (Beyotime Institute of Biotechnology,
Shanghai, China) for 30 min. Following centrifugation (10,000 × g
for 10 min at 4°C), the supernatants were collected and stored at
−80°C, and ~5 µl of each supernatant was added to the PCR reaction
mixture provided in the kit. PCR was then carried out under the
following reaction conditions: Primer elongation (25°C for 30 min),
telomerase inactivation (94°C for 5 min), 30 cycles of
amplification (94°C for 30 sec, 50°C for 30 sec, 72°C for 90 sec)
and extension (72°C for 5 min). The PCR product was denatured and
hybridized with the digoxigenin (DIG)-labeled telomeric
repeat-specific probe. Finally, the PCR product was detected using
the anti-DIG-POD antibody (1:5,000, incubated at 37°C for 60 min)
and measured at 450 nm on a microplate reader.
Statistical analysis
All values are expressed as the means ± standard
deviation. Statistical analysis was performed using SPSS version
19.0 (SPSS; IBM Corp., Armonk, NY, USA). One-way analysis of
variance (ANOVA) and an unpaired Student's t-test were performed to
compare means among all measured variables. When ANOVA results were
significant, multiple comparisons of means were applied with Tukey
HSD post hoc analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
Luteolin inhibits the proliferation
and invasion of BC cells
Firstly, the cytotoxicity of luteolin on MDA-MB-231
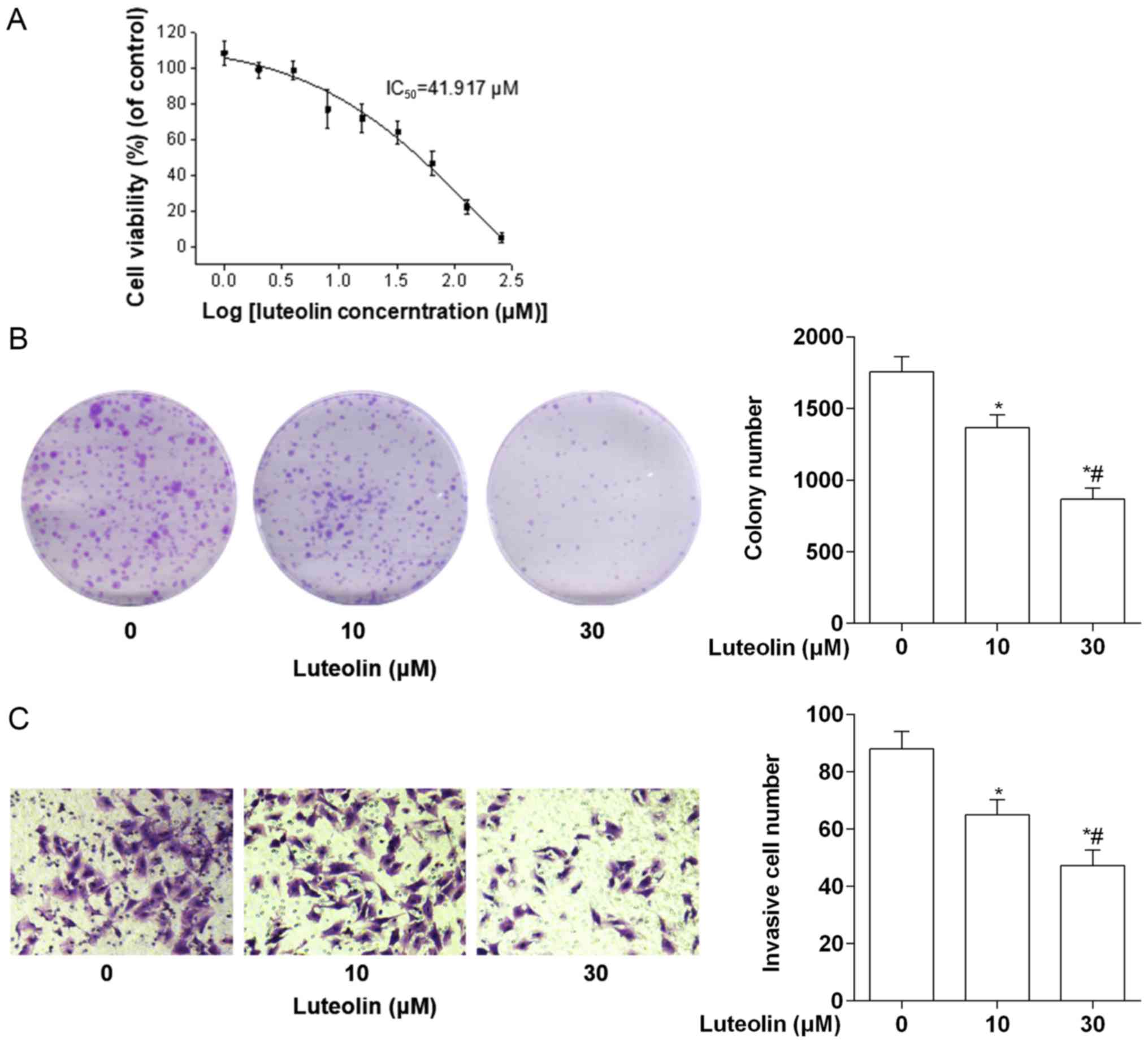
cells was assessed using an MTS assay. As shown in Fig. 1A, luteolin demonstrated a
concentration-dependent effect on the viability of MDA-MB-231
cells. The IC50 for luteolin on MDA-MB-231 cells was 41.917 µM. To
further assess the long-term effects of luteolin treatment on cell
proliferation, a colony formation assay was performed. As expected,
luteolin inhibited the colony-forming potential of MDA-MB-231 cells
in a dose-dependent manner (Fig.
1B). In addition, cell invasion assay results revealed that the
invasion of MDA-MB-231 cells treated with 10 µM luteolin was
significantly inhibited compared with the control, and the
inhibition was more significant when assessing 30 µM luteolin
(Fig. 1C).
Luteolin blocks cell cycle progression
and modulates cell cycle regulatory protein expression
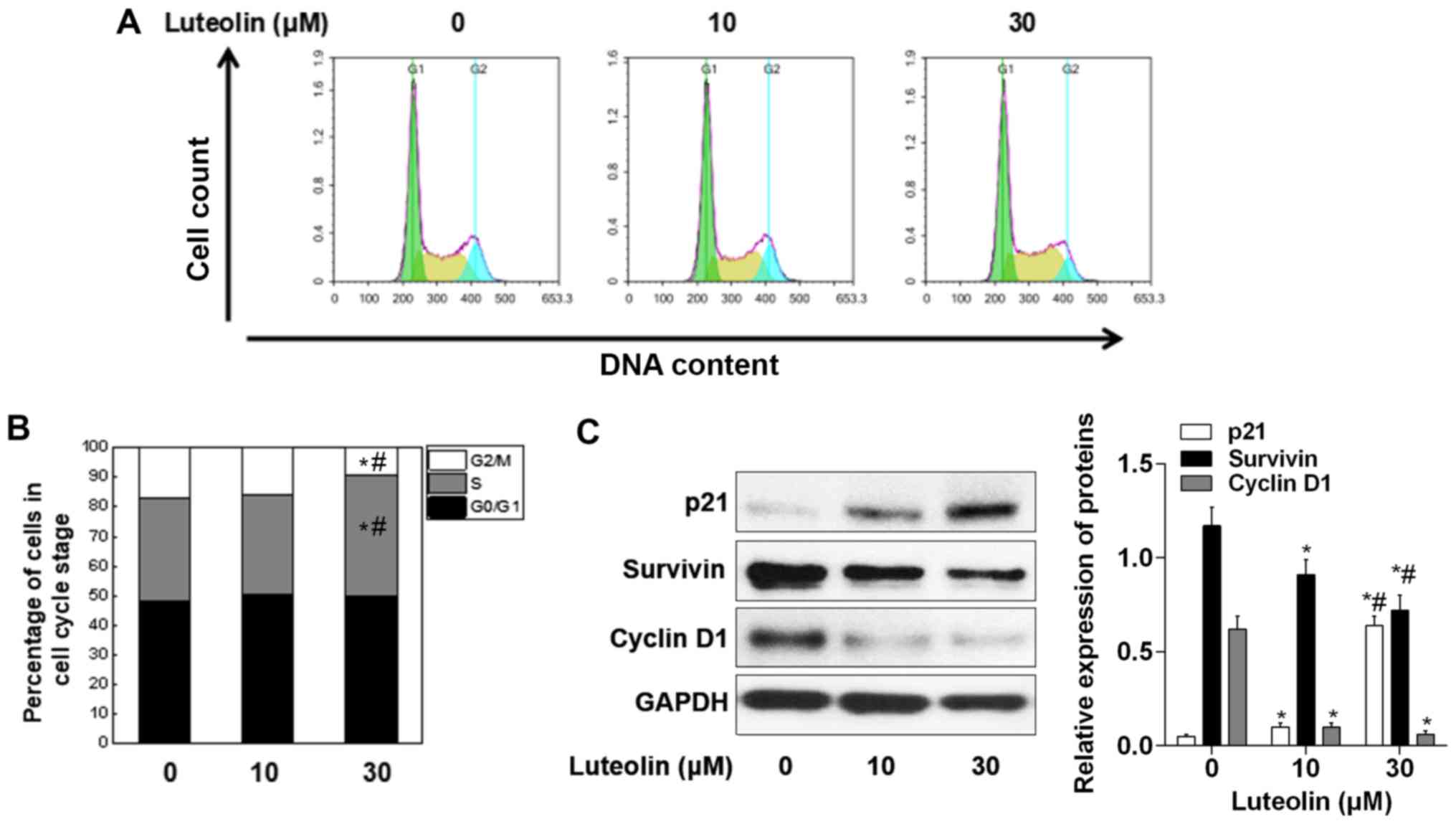
The present study investigated whether
luteolin-mediated growth suppression was due to cell cycle
intervention. Following treatment of MDA-MB-231 cells with 10 and
30 µM luteolin for 24 h, the treated cells were subjected to cell
cycle progression analysis. The results revealed that although
there were significant differences between the 10 µM
luteolin-treated group and the control group, following 30 µM
luteolin treatment, MDA-MB-231 cells exhibited a 1.21-fold
(34.05–41.07%) increase in the number of cells in S phase, as
compared with the control (Fig. 2A and
B). Therefore, the expression levels of cell cycle-regulated
proteins in MDA-MB-231 cells were subsequently investigated. A
dose-dependent decrease in the levels of cyclin D1 and Survivin was
identified, whereas the protein expression levels of p21 were
increased in MDA-MB-231 cells following luteolin treatment
(Fig. 2C).
Luteolin induces apoptosis of BC
cells
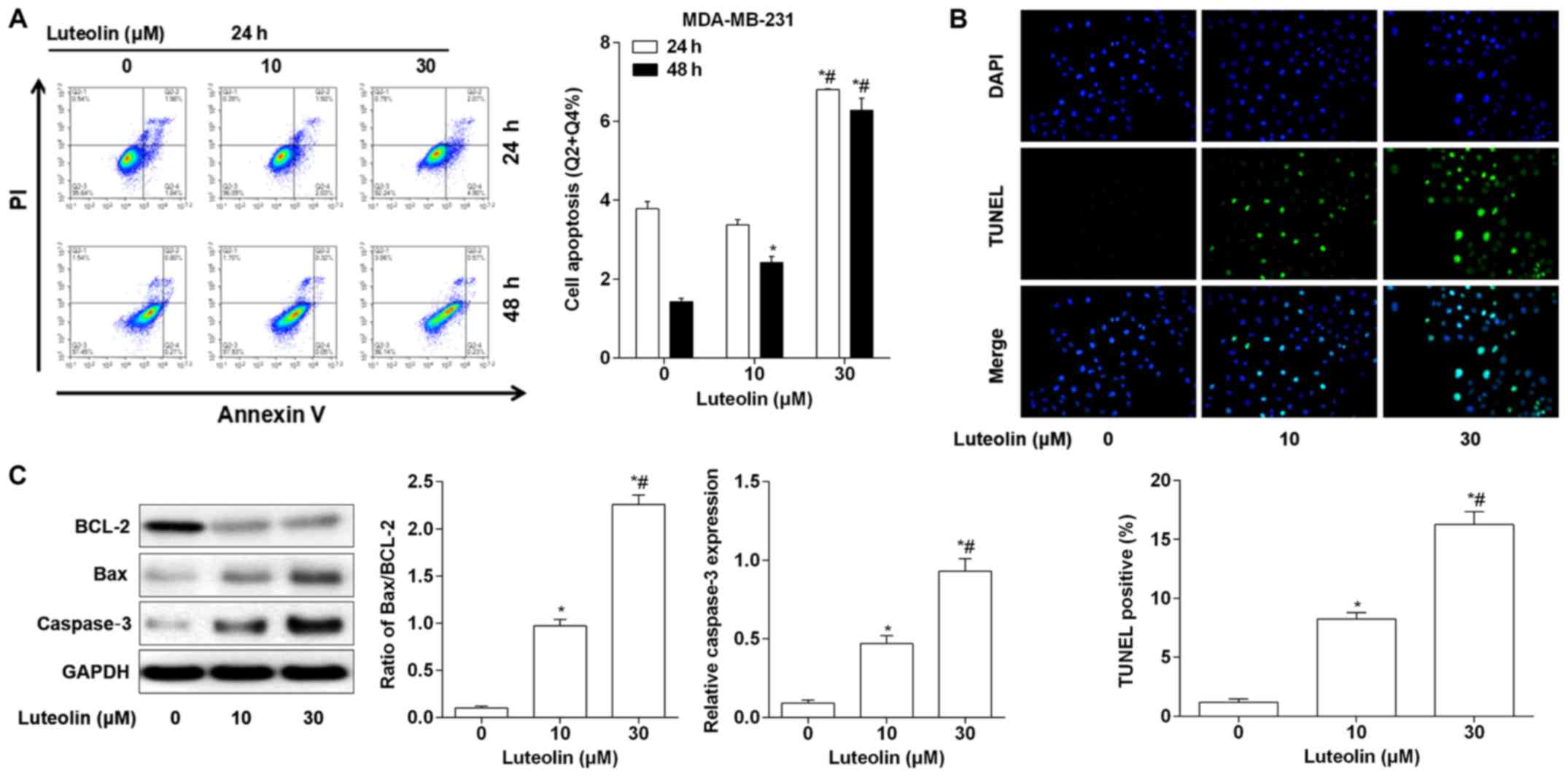
As cell apoptosis is one of the leading mechanisms
in BC formation, a cell apoptosis assay using flow cytometry was
carried out. As shown in Fig. 3A, no
significant differences were identified between the group of cells
treated with 10 µM luteolin for 24 h and the control group, with
respect to the percentage of apoptotic cells. MDA-MB-231 cells
treated with 30 µM luteolin for 24 h exhibited potent cell
apoptosis. In addition, luteolin treatment for 48 h induced cell
apoptosis in a dose-dependent manner in MDA-MB-231 cells. To
further validate the effects of luteolin on cell apoptosis, a TUNEL
assay was performed. The results (Fig.
3B) illustrated that treatment with luteolin for 48 h triggered
cell apoptosis in a dose-dependent manner. Following luteolin
treatment, there was a decrease in BCL-2 expression and an increase
in Bax protein expression in MDA-MB-231 cells. Luteolin treatment
also markedly increased the ratio of Bax/BCL-2 in a
concentration-dependent manner. Furthermore, caspase-3 protein
expression was also increased following luteolin treatment
(Fig. 3C).
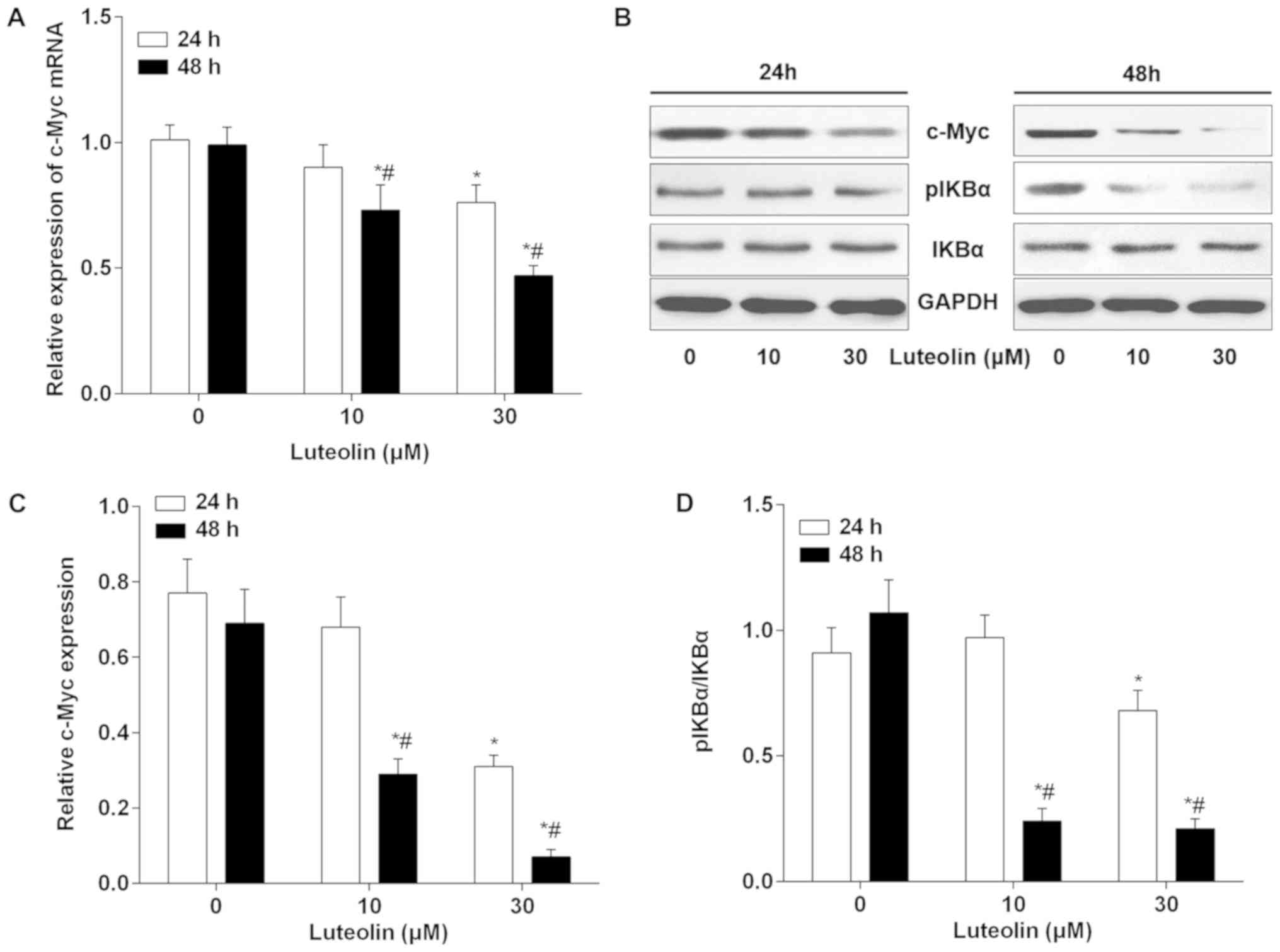
Luteolin downregulates hTERT and c-Myc
expression in BC cells
Previous studies have suggested that hTERT is
upregulated in BC and serves a role in regulating cell cycle,
proliferation and tumorigenesis (21,22). The
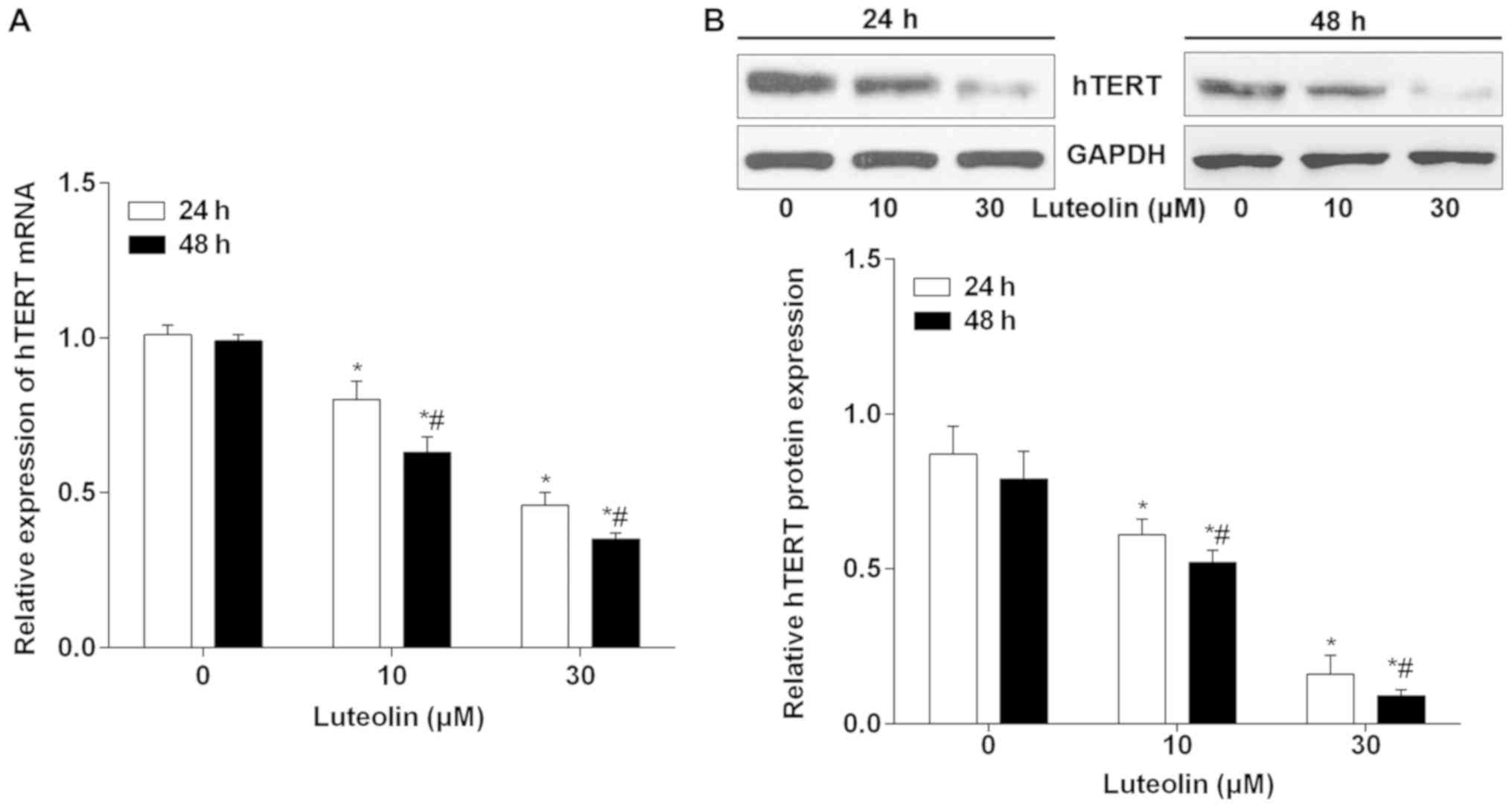
hTERT levels were measured to reveal any potential correlative or
mechanistic effects that luteolin may have on BC cells. As shown in
Fig. 4A, the mRNA expression levels
of hTERT were significantly downregulated following 24 h treatment
with 10 and 30 µM luteolin. Additionally, 10 and 30 µM luteolin
treatment for 48 h led to the downregulation of hTERT mRNA in
MDA-MB-231 cells. A similar change in hTERT protein caused by
luteolin treatment was detected (Fig.
4B). Furthermore, c-Myc has been identified as an inducer of
hTERT transcription and may bind the hTERT promoter. To explore the
possibility that luteolin downregulated hTERT via the c-Myc
pathway, the effects of luteolin on c-Myc expression were
evaluated. As expected, luteolin treatment downregulated the
expression of c-Myc in a time- and dose-dependent manner at the
mRNA and protein levels in MDA-MB-231 cells (Fig. 5A-C). NF-κB signaling is one of the
major upstream targets of c-Myc and a number of studies have
demonstrated that luteolin targets NF-κB (23–25).
NF-κB normally binds to IκBα and is localized in the cytoplasm.
Following activation, IκBα is phosphorylated and dissociates from
NF-κB, which allows NF-κB to translocate from the cytoplasm to the
nucleus to target downstream genes. Therefore, the alteration of
IκBα and pIκBα were evaluated by western blotting following
luteolin treatment. As shown in Fig. 5B
and D, although no significant change in pIκBα was identified
following 10 µM luteolin treatment for 24 h, the levels of pIκBα
were significantly decreased following treatment with luteolin for
48 h, in a dose-dependent manner.
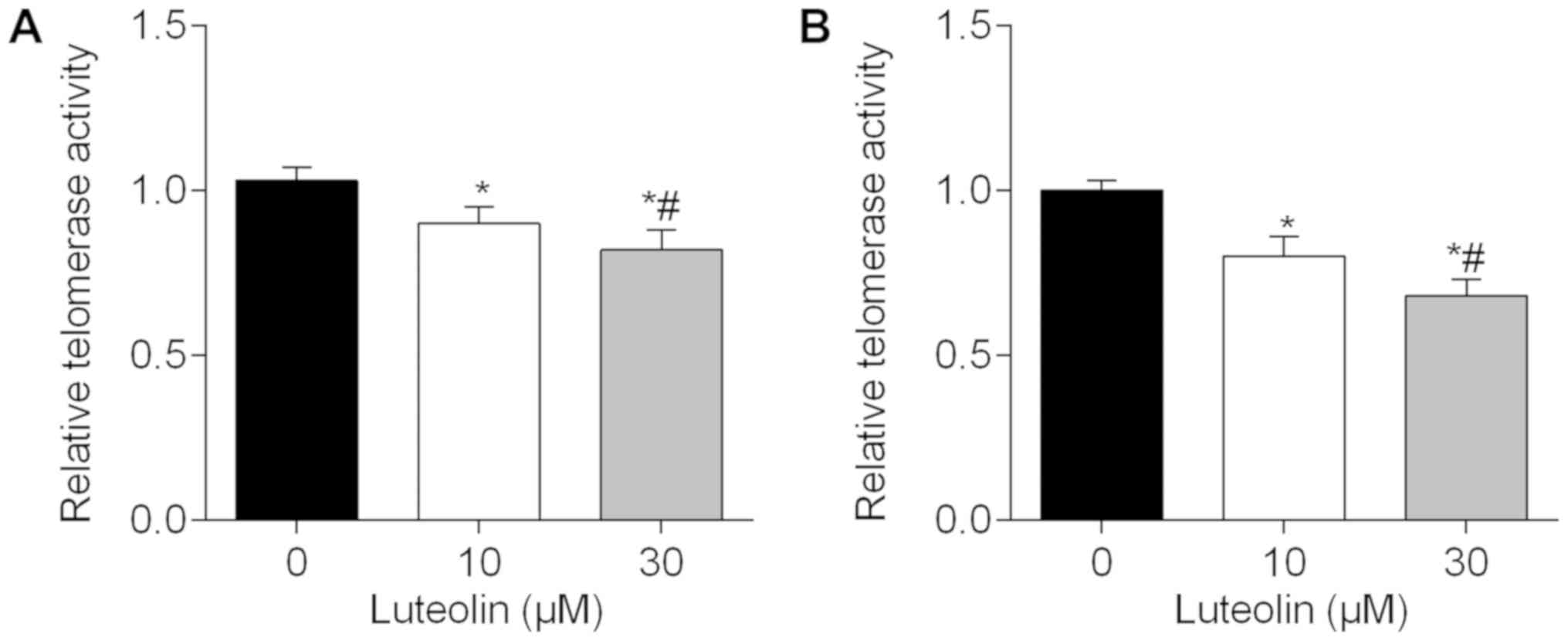
Luteolin reduces telomerase activity
in BC cells
Since hTERT is the catalytic and limiting component
of telomerase, telomerase activity in MDA-MB-231 cells was measured
to fully examine the luteolin-mediated repression of hTERT. As
illustrated in Fig. 6A, telomerase
activity was reduced in a dose-dependent manner following treatment
with luteolin for 48 h. In addition, as shown in Fig. 6B, treatment with luteolin for 72 h
also reduced the activity levels of telomerase in a dose-dependent
manner.
Discussion
BC is the most common type of cancer and the second
most common cause of cancer-associated mortality in women.
Therefore, investigating the molecular mechanisms underlying the
initiation and development of BC, and searching for more efficient
therapeutic agents and strategies are of important clinical value
(26). Traditional herbal medicines
have long been utilized to prevent and treat neoplasms. Searching
for natural products directed at suppressing proliferation and
inducing apoptosis of cancer cells may be a novel strategy for BC
treatment (27). Luteolin is a
natural flavonoid that exists in several types of vegetables,
fruits and medicinal herbs, and inhibits tumorigenesis in various
types of cancer, including BC (17,28,29). The
present study validated the effect of luteolin on cell
proliferation, invasion, cell cycle progression and apoptosis in BC
cells in vitro. Luteolin significantly inhibited cell
growth, invasion and cell cycle progression, and effectively
induced apoptosis of MDA-MB-231 BC cells. In the present study, it
was identified that luteolin reduced telomerase levels in a
dose-dependent manner. The mechanism for this may include
downregulation of hTERT expression via inhibition of the
NF-κB-c-Myc axis. The results provided evidence for a novel
mechanism via which luteolin exerts its anticancer properties.
Notably, the dose applied in the present study was
determined by an MTS assay. The results of the present study
suggested that high concentrations of luteolin (>42 µM)
exhibited cytotoxic effects on MDA-MB-231 cells. This differed from
the study performed by Park et al (28), which identified an IC50 value of 27
µM in MDA-MB-231 cells. This discrepancy may be due to different
manufacturers of luteolin. Consistent with previous studies
(17,29,30), the
antiproliferative activity of luteolin on BC cells was confirmed
using a colony formation assay. In addition, the present study
revealed that luteolin significantly inhibited invasion of BC
cells. A previous study indicated that luteolin exerts antitumor
activities by interfering with the cell cycle (31). Park et al demonstrated that
luteolin induces cell cycle arrest in MCF-7 BC cells (28). In the present study, it was
identified that, although luteolin treatment caused abnormal cell
cycle arrest in MDA-MB-231 cells, it decreased the essential cell
cycle regulators cyclin D1 and Survivin in a dose-dependent manner,
which partly explained the mechanism through which luteolin affects
the cell cycle of BC cells.
Notably, it was also revealed that luteolin
decreased the expression levels of another novel cell cycle
participator, hTERT. hTERT is critical to the survival and
progression of cancer cells, due to being involved in the
maintenance of the telomeric ends of chromosomes following each
replication, which solves the end replication problem. Notably,
hTERT is particularly valuable as a target for the prevention or
treatment of unlimited cell cycle progression that sustains cancer
(32). Bai et al (33). identified that decreased hTERT
transcription was indirectly caused by a significant decrease in
activation of the upstream NF-κB pathway, which resulted from
decreased phosphorylation of IκBα. In addition, a previous study
also demonstrated that activated NF-κB may bind to the c-Myc
promoter to activate its expression, and that c-Myc preferentially
binds to the hTERT promoter, increasing hTERT mRNA expression
(34). In the present study it was
identified that luteolin treatment in BC cells inhibited the NF-κB
pathway, and subsequent c-Myc expression, to significantly decrease
hTERT expression levels. Although this was not the first time that
luteolin was shown to inhibit activation of the NF-κB signaling
pathway (12), to the best of our
knowledge, the present study was the first to demonstrate that
luteolin may reduce telomerase levels in BC cells, providing
evidence for a potential mechanism for the antitumor effects of
luteolin.
Using flow cytometry and western blotting, the
present study also confirmed that luteolin may induce significant
apoptosis of BC cells. Notably, luteolin upregulated the
pro-apoptotic factor Bax and downregulated the survival factor
BCL-2, leading to a prominent increase in the ratio of Bax/BCL-2 in
BC cells, suggesting that luteolin modulated mitochondrial function
to mediate cell death. It has also been suggested that luteolin may
be suitable to treat the resistance of human BC cells (16,35).
Notably, Tu et al previously identified that luteolin may
sensitize drug-resistant human BC cells to tamoxifen (16). Sabzichi et al suggested that
luteolin could sensitize MDA-MB 231 cells to doxorubicin by
suppressing nuclear factor erythroid 2-related factor 2 mediated
signaling (35). The association
between apoptosis and telomerase activity has been firmly
established by researchers, since numerous anticancer agents induce
apoptosis through downregulation of telomerase activity (36,37).
Moon et al reported that gefitinib could induce cell
apoptosis by decreasing telomerase activity in MDA-MB-231 cells
(38). Similarly, Moradzadeh et
al demonstrated that epigallocatechin-3-gallate treatment
significantly increased apoptosis of T47D BC cells and decreased
expression of hTERT mRNA, indicating that it may serve as a novel
agent which decreases resistance to chemotherapy (39). The regulation of hTERT by luteolin
treatment involved in chemotherapy resistance during BC treatment
should be investigated in vitro and in vivo.
In conclusion, the present study demonstrated that
luteolin inhibited BC proliferation, invasion and cell cycle
progression, and induced cell apoptosis, through suppressing
activation of NF-κB/c-Myc and subsequent inhibition of hTERT
transcription. These results suggested that luteolin may be a
useful, natural and chemopreventive agent for the treatment of
breast cancer.
Acknowledgements
Not applicable.
Funding
This work was supported by Zhejiang Provincial
Science and Technology Projects of Traditional Chinese Medicine
(grant no. 2017ZB089), the National Natural Science Foundation of
China (grant nos. 81772537 and 81374014), Zhejiang Provincial
Medical and Healthy Science and Technology Projects (grants nos.
2018ZD047 and 2016ZDB013), and Zhejiang Provincial Science and
Technology Projects (grant nos. LGF18H160041, 2017C33212,
2017C33213 and 2015C33264).
Availability of data and materials
All data generated or analyzed during this study are
included in the published article.
Authors' contributions
KJ designed the study and performed the statistical
analysis. LH and HL performed all experiments and data correction.
KJ and HL wrote the manuscript. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
BC
|
breast cancer
|
|
hTERT
|
human telomerase reverse
transcriptase
|
|
NF-κB
|
nuclear factor-κB
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
DeSantis CE, Fedewa SA, Goding Sauer A,
Kramer JL, Smith RA and Jemal A: Breast cancer statistics, 2015:
Convergence of incidence rates between black and white women. CA
Cancer J Clin. 66:31–42. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nagini S: Breast cancer: Current molecular
therapeutic targets and new players. Anticancer Agents Med Chem.
17:152–163. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Perou CM, Sørlie T, Eisen MB, van de Rijn
M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA,
et al: Molecular portraits of human breast tumours. Nature.
406:747–752. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lin CH, Chang CY, Lee KR, Lin HJ, Chen TH
and Wan L: Flavones inhibit breast cancer proliferation through the
Akt/FOXO3a signaling pathway. BMC Cancer. 15:9582015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shay JW: Role of telomeres and telomerase
in aging and cancer. Cancer Discov. 6:584–593. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu B, Peng M and Song Q: The co-expression
of telomerase and ALT pathway in human breast cancer tissues.
Tumour Biol. 35:4087–4093. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kulić A, Plavetić ND, Gamulin S,
Jakić-Razumović J, Vrbanec D and Sirotković-Skerlev M: Telomerase
activity in breast cancer patients: Association with poor prognosis
and more aggressive phenotype. Med Oncol. 33:232016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lu L, Zhang C, Zhu G, Irwin M, Risch H,
Menato G, Mitidieri M, Katsaros D and Yu H: Telomerase expression
and telomere length in breast cancer and their associations with
adjuvant treatment and disease outcome. Breast Cancer Res.
13:R562011. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shi Y, Sun L, Chen G, Zheng D, Li L and
Wei W: A combination of the telomerase inhibitor, BIBR1532, and
paclitaxel synergistically inhibit cell proliferation in breast
cancer cell lines. Target Oncol. 10:565–573. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yu P, Shen X, Yang W, Zhang Y, Liu C and
Huang T: ZEB1 stimulates breast cancer growth by up-regulating
hTERT expression. Biochem Biophys Res Commun. 495:2505–2511. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Aziz N, Kim MY and Cho JY:
Anti-inflammatory effects of luteolin: A review of in vitro, in
vivo, and in silico studies. J Ethnopharmacol. 225:342–358. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ma L, Peng H, Li K, Zhao R, Li L, Yu Y,
Wang X and Han Z: Luteolin exerts an anticancer effect on NCI-H460
human non-small cell lung cancer cells through the induction of
Sirt1-mediated apoptosis. Mol Med Rep. 12:4196–4202. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lin Y, Shi R, Wang X and Shen HM:
Luteolin, a flavonoid with potential for cancer prevention and
therapy. Curr Cancer Drug Targets. 8:634–646. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang MY, Wang CJ, Chen NF, Ho WH, Lu FJ
and Tseng TH: Luteolin enhances paclitaxel-induced apoptosis in
human breast cancer MDA-MB-231 cells by blocking STAT3. Chem Biol
Interact. 213:60–68. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tu SH, Ho CT, Liu MF, Huang CS, Chang HW,
Chang CH, Wu CH and Ho YS: Luteolin sensitises drug-resistant human
breast cancer cells to tamoxifen via the inhibition of cyclin E2
expression. Food Chem. 141:1553–1561. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lin D, Kuang G, Wan J, Zhang X and Li H,
Gong X and Li H: Luteolin suppresses the metastasis of
triple-negative breast cancer by reversing
epithelial-to-mesenchymal transition via downregulation of
β-catenin expression. Oncol Rep. 37:895–902. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ganesan K and Xu B: Telomerase inhibitors
from natural products and their anticancer potential. Int J Mol
Sci. 19(pii): E132017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun DW, Zhang HD, Mao L, Mao CF, Chen W,
Cui M, Ma R, Cao HX, Jing CW, Wang Z, et al: Luteolin inhibits
breast cancer development and progression in vitro and in vivo by
suppressing notch signaling and regulating MiRNAs. Cell Physiol
Biochem. 37:1693–1711. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kyo S, Takakura M, Fujiwara T and Inoue M:
Understanding and exploiting hTERT promoter regulation for
diagnosis and treatment of human cancers. Cancer Sci. 99:1528–1538.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vageli D, Ioannou MG and Koukoulis GK:
Transcriptional activation of hTERT in breast carcinomas by the
Her2-ER81-related pathway. Oncol Res. 17:413–423. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lou L, Liu Y, Zhou J, Wei Y, Deng J, Dong
B and Chai L: Chlorogenic acid and luteolin synergistically inhibit
the proliferation of interleukin-1β-induced fibroblast-like
synoviocytes through regulating the activation of NF-κB and
JAK/STAT-signaling pathways. Immunopharmacol Immunotoxicol.
37:499–507. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang X, Du Q, Yang Y, Wang J, Dou S, Liu
C and Duan J: The protective effect of Luteolin on myocardial
ischemia/reperfusion (I/R) injury through TLR4/NF-κB/NLRP3
inflammasome pathway. Biomed Pharmacother. 91:1042–1052. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen T, Li B, Xu Y, Meng S, Wang Y and
Jiang Y: Luteolin reduces cancer-induced skeletal and cardiac
muscle atrophy in a Lewis lung cancer mouse model. Oncol Rep.
40:1129–1137. 2018.PubMed/NCBI
|
|
26
|
Goel S, Wang Q, Watt AC, Tolaney SM,
Dillon DA, Li W, Ramm S, Palmer AC, Yuzugullu H, Varadan V, et al:
Overcoming therapeutic resistance in HER2-positive breast cancers
with CDK4/6 inhibitors. Cancer Cell. 29:255–269. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hasanpourghadi M, Pandurangan AK and
Mustafa MR: Modulation of oncogenic transcription factors by
bioactive natural products in breast cancer. Pharmacol Res.
128:376–388. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Park SH, Ham S, Kwon TH, Kim MS, Lee DH,
Kang JW, Oh SR and Yoon DY: Luteolin induces cell cycle arrest and
apoptosis through extrinsic and intrinsic signaling pathways in
MCF-7 breast cancer cells. J Environ Pathol Toxicol Oncol.
33:219–231. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cook MT, Liang Y, Besch-Williford C,
Goyette S, Mafuvadze B and Hyder SM: Luteolin inhibits
progestin-dependent angiogenesis, stem cell-like characteristics,
and growth of human breast cancer xenografts. Springerplus.
4:4442015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cook MT, Liang Y, Besch-Williford C and
Hyder SM: Luteolin inhibits lung metastasis, cell migration, and
viability of triple-negative breast cancer cells. Breast Cancer
(Dove Med Press). 9:9–19. 2016.PubMed/NCBI
|
|
31
|
Ding S, Hu A, Hu Y, Ma J, Weng P and Dai
J: Anti-hepatoma cells function of luteolin through inducing
apoptosis and cell cycle arrest. Tumour Biol. 35:3053–3060. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ren X, Zhang Z, Tian J, Wang H, Song G,
Guo Q, Tian J, Han Y, Liao Q, Liu G, et al: The downregulation of
c-Myc and its target gene hTERT is associated with the
antiproliferative effects of baicalin on HL-60 cells. Oncol Lett.
14:6833–6840. 2017.PubMed/NCBI
|
|
33
|
Bai D, Ueno L and Vogt PK: Akt-mediated
regulation of NFkappaB and the essentialness of NFkappaB for the
oncogenicity of PI3K and Akt. Int J Cancer. 125:2863–2870. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Papanikolaou V, Athanassiou E, Dubos S,
Dimou I, Papathanasiou I, Kitsiou-Tzeli S, Kappas C and Tsezou A:
hTERT regulation by NF-κB and c-myc in irradiated HER2-positive
breast cancer cells. Int J Radiat Biol. 87:609–621. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sabzichi M, Hamishehkar H, Ramezani F,
Sharifi S, Tabasinezhad M, Pirouzpanah M, Ghanbari P and Samadi N:
Luteolin-loaded phytosomes sensitize human breast carcinoma MDA-MB
231 cells to doxorubicin by suppressing Nrf2 mediated signalling.
Asian Pac J Cancer Prev. 15:5311–5316. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jagadeesh S, Kyo S and Banerjee PP:
Genistein represses telomerase activity via both transcriptional
and posttranslational mechanisms in human prostate cancer cells.
Cancer Res. 66:2107–2115. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yeo M, Rha SY, Jeung HC, Hu SX, Yang SH,
Kim YS, An SW and Chung HC: Attenuation of telomerase activity by
hammerhead ribozyme targeting human telomerase RNA induces growth
retardation and apoptosis in human breast tumor cells. Int J
Cancer. 114:484–489. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Moon DO, Kim MO, Heo MS, Lee JD, Choi YH
and Kim GY: Gefitinib induces apoptosis and decreases telomerase
activity in MDA-MB-231 human breast cancer cells. Arch Pharm Res.
32:1351–1360. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Moradzadeh M, Hosseini A, Erfanian S and
Rezaei H: Epigallocatechin-3-gallate promotes apoptosis in human
breast cancer T47D cells through down-regulation of PI3K/AKT and
Telomerase. Pharmacol Rep. 69:924–928. 2017. View Article : Google Scholar : PubMed/NCBI
|