Introduction
As one of the most important structures of the human
body, the pelvic cavity has a complex anatomical structure,
including multiple organs, fine vessels and nerves (1). These features suggest that a variety of
diseases, including different types of tumours, may occur in the
pelvic cavity. Pelvic tumours exhibit a wide range of origins and
an abundant blood supply. The anatomical structure of the
tumour-feeding arteries, as well as the collateral circulation and
variation, may be more complex. In addition, a large tumour could
compress the pelvic tissue and organs, leading to deformation and
displacement of the anatomical structure of the pelvis (2). The location of a tumour and the feeding
artery is therefore difficult to confirm (1,3). Due to
these features of the pelvic cavity, tracking the source of
tumour-feeding arteries and determining a treatment programme,
particularly an associated vascular interventional treatment, is
essential.
As one of the main branches of the common iliac
artery, the internal iliac artery (IIA) supplies the majority of
the organs in the pelvic cavity, and it is believed that this
artery is closely associated with the occurrence, development and
treatment of pelvic diseases (3).
With the widespread use of interventional embolization in the
treatment of pelvic neoplasms, it is necessary for physicians to
investigate the IIA and its pattern of division and branching to
ensure any interventional procedure is secure and to avoid
untargeted embolization. Nevertheless, due to numerous variations
that exist in the anatomy of the IIA, for which there are also
significant differences between sex and ethnic groups, there are no
unified IIA classification criteria at present, although a number
of studies have been performed on the classification of the IIA
(4–7). The earliest systematic classification
and description of the IIA was provided in 1928 by the Japanese
scholar Adachi (8). The Adachi
system classifies the distribution pattern of the IIA into 5 types
with 8 groups based on a cadaver specimen study and remains in use
today (8). Subsequently, Yamaki, a
Japanese scholar, provided further research based on Adachi's
classification and put forward Yamaki's classification (7), which is the most reproducible and
simple classification for this complex vascular system, from
clinical and imaging views (3). The
studies by Adachi and Yamaki were performed on cadaver specimens,
and the corresponding imaging evaluations, using different imaging
modalities, are lacking. Although several previous studies have
described the human pelvic vascular anatomy and its frequent
variations from perspective imaging, interventional radiologists
lack a simple model that can facilitate target branch
identification (7,9–13).
At present, digital subtraction angiography (DSA)
remains the gold standard for assessing blood vessels, particularly
for smaller branches or vascular anastomosis. Additionally, DSA is
also the primary route for determining the feeding artery of the
tumour blood supply, which can be used to guide interventional
therapy. However, DSA is an invasive procedure that is subject to
inherent limitations, including vascular embolism, vascular injury
and high cost. With the development of radiology technology,
multislice computed tomography angiography (MSCTA) is an important
technique that is increasingly used in vascular imaging studies for
its characteristic merits, including observing vascular lesions in
various directions and investigating the variation of blood flow at
different stages of vascular lesions through CT post-processing
technology (6,14–16).
Although the diagnostic performance of MSCTA has
been proven to be superior to that of DSA in certain fields
(17,18), there are few reports detailing the
anatomy of the IIA and its branches by MSCTA, as well as
assessments of the diagnostic quality of the feeding arteries in
pelvic tumours. Therefore, the aim of the present study was to
explore the anatomical structure and classification characteristics
of the IIA, and to estimate the diagnostic value for preoperative
assessment of patients with pelvic tumours by MSCTA compared with
DSA.
Materials and methods
Study population
Between January 2013 and August 2017, a total of 43
patients (27 male and 16 female) with pelvic tumours who has been
assessed in The Sixth Affiliated Hospital of Sun Yat-sen University
(Guangzhou, Guangdong, China) were enrolled in the present study.
MSCTA and DSA examinations and intervention therapy were performed
for all patients. The mean age of the patients was 52.7 years
(range, 24–91 years). Radiological imaging and medical records were
reviewed to obtain valuable information, including clinical
characteristics, visualization quality, classification of the IIA
and terminal branches of the pelvic tumour-feeding artery. The
diagnosis of these patients was routinely confirmed by two senior
pathologists at The Sixth Affiliated Hospital of Sun Yat-sen
University by histopathology and immunohistochemistry (IHC) of
biopsy or surgical samples. There were 9 cases of refractory
bladder bleeding and 4 cases of rectal bleeding due to tumour
invasion. The main manifestations of the remaining patients
included abdominal pain and distension, an abdominal mass and
complicated intestinal obstruction.
Patients with the following criteria were excluded:
Iodine allergy, severe coagulation disorders with an evident
bleeding tendency, severe arteriosclerosis, vital organ failure,
tumour cachexia, high fever, infection, female menstrual period and
pregnancy. The study protocol was approved by the Institutional
Ethics Review Board of The Sixth Affiliated Hospital of Sun Yat-Sen
University and complies with the ethical principles of the
Declaration of Helsinki. Informed consent was obtained from all
individual participants included in the study.
IHC staining procedure
Tissue blocks were fixed in 10% formalin and
embedded in paraffin, and were then sliced into several sections at
a thickness of 4 µm prior to IHC staining. Briefly, the sections
were dewaxed in xylene and hydrated with a graded alcohol series,
and were then washed with PBS (pH 7.4) three times, each time for 3
min. The sections were placed in freshly-prepared boiled citrate
buffer (pH 6.0) for antigen retrieval at 95°C from 5 min. The
sections were treated with 3% H2O2 in 0.1 M
PBS to block endogenous peroxidase activity at room temperature for
30 min. Following washing with PBS, each slide was incubated with
the appropriate primary antibody overnight at 4°C. PBS alone was
used as the blank control. Slides were washed three times following
incubation with the appropriate secondary antibody in 0.1 M PBS (pH
7.4) for 30 min at room temperature. The IHC reaction was
visualized with a light microscope (magnification, ×200 or ×400)
following 3,3′-diaminobenzidine chromogen staining for 5–10 min at
room temperature and hematoxylin counterstaining for 5 min at room
temperature.
MSCTA imaging and image reconstruction
protocol
Patients fasted for 4–6 h prior to MSCTA
examination. A 128- or 640-row multidetector spiral CT scanner
(Toshiba Aquilion ONE; Canon, Inc., Tokyo, Japan) was used. The
patient was placed in a supine position on the scanner table. The
range of the scan was from the lower edge of the fourth lumbar
spine to the upper femur. The scan parameters were 120 kV and 450
mAs. Each patient received a single CT scan. Power injection
settings were as follows: Iodine contrast agent volume, 100 ml
(iopromide injection, 370 mg/ml); and injection rate, 4 ml/sec,
injected by the elbow vein, followed by an injection of 20 ml
physiological saline by the same method. The bolus injection was
used to measure the CT value in the abdominal aorta (above the
renal arteries), which acted as the region of interest (ROI). The
MSCTA scan (arterial phase, venous phase and delay period)
automatically started when the CT value in the ROI reached 100
Houndsfield units and was then delayed 6 sec. The acquired data was
interpreted by reading the axial reformats at ≤1.0 mm in thickness,
which allowed study of the vascular anatomy. Post-processing was
performed using StartVitrea 6.6 post-processing software (Canon,
Inc.). Multi-planner reformation, maximum intensity projections
(MIPs) and volume rendering were used with three-dimensional (3D)
reconstructions. Satisfactory MSCTA images were reconstructed
according to these 3 reconstruction techniques by adjusting the
different thresholds and patterns.
DSA protocol
A DSA instrument (INNOVA 3100; GE Healthcare,
Chicago, IL, USA) was used. A right transfemoral approach was used
for artery access. Initially, the 2 pelvic common iliac arteries
were visualised by performing digital angiography in the abdominal
aorta (injection volume, 20 ml; injection rate, 15 ml/sec).
Subsequently, the two IIAs were catheterised for imaging. The
contralateral (usually the left) IIA was initially catheterised and
visualised by performing digital angiography in the artery origin
in the neutral position and repeating in the contralateral oblique
and ipsilateral oblique positions (injection volume, 10 ml;
injection rate, 5 ml/sec). The right IIA was visualised by the same
method following catheterisation.
Image evaluation
All MSCTA and DSA images were assessed by 2 senior
radiologists with 5 and 3 years of experience in the field of CT
vascular imaging and angiography, respectively. The 2 readers were
blinded to the diagnostic patient information and to the results of
each other's analysis. The cases were randomly reviewed and the
reading interval between MSCTA and DSA images was 7–10 days. The
pelvic arterial 3D vascular network was constructed, and the
anatomical structures of the IIA were contained in the evaluation
and assessed by the classification criteria of Yamaki et al
(7). The mode of branching of the
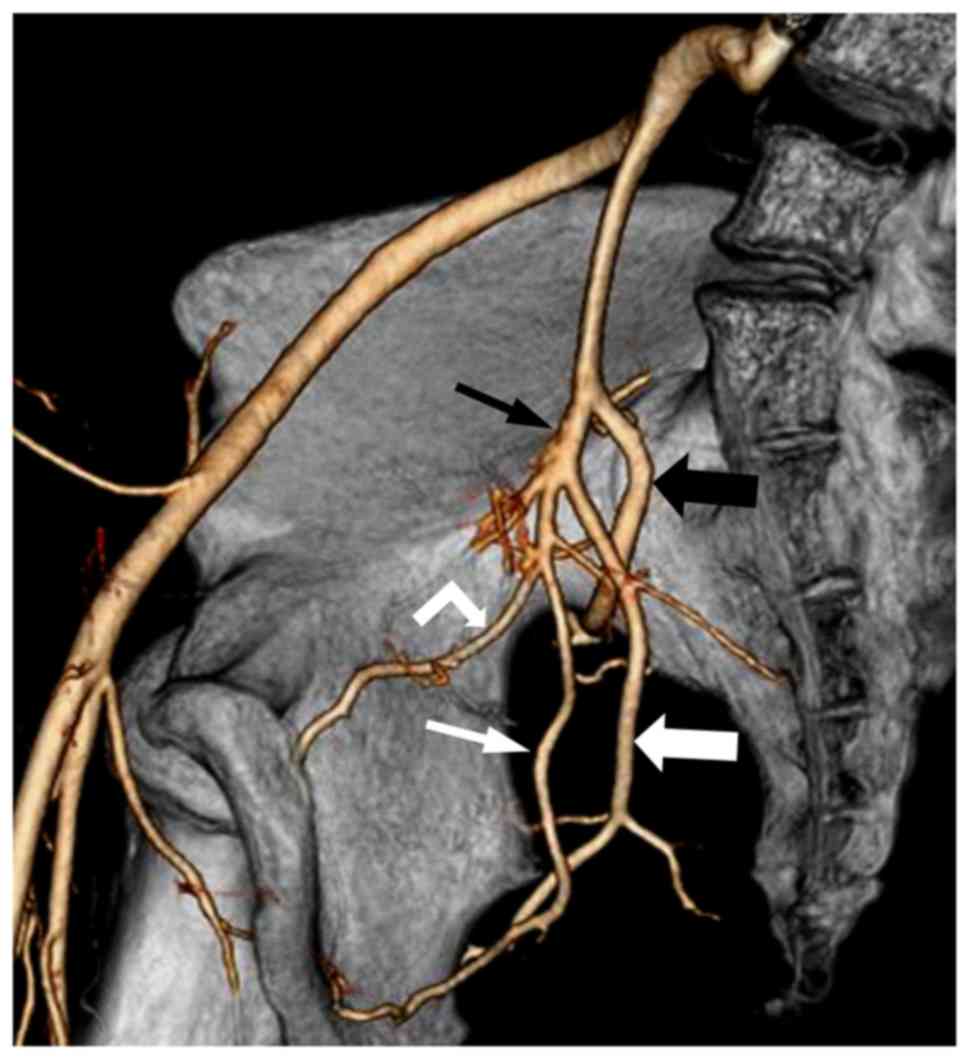
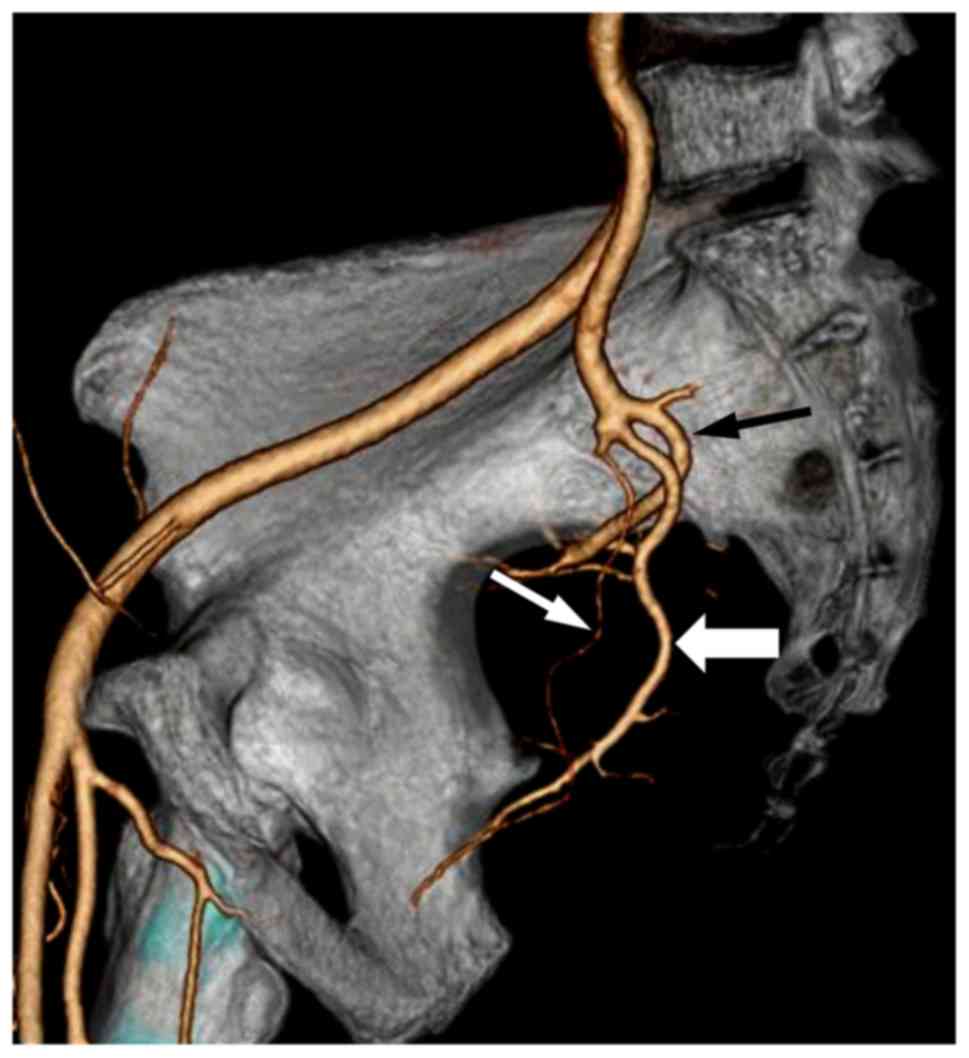
IIA was classified into 4 groups. Group A: The IIA divided into 2
branches, the superior gluteal artery, (posterior division) and the
common trunk of the inferior gluteal and internal pudendal arteries
(anterior division). Group B: The IIA divided into 2 branches, the
common gluteal trunk (posterior division) of the superior gluteal
and inferior gluteal arteries, and the internal pudendal artery
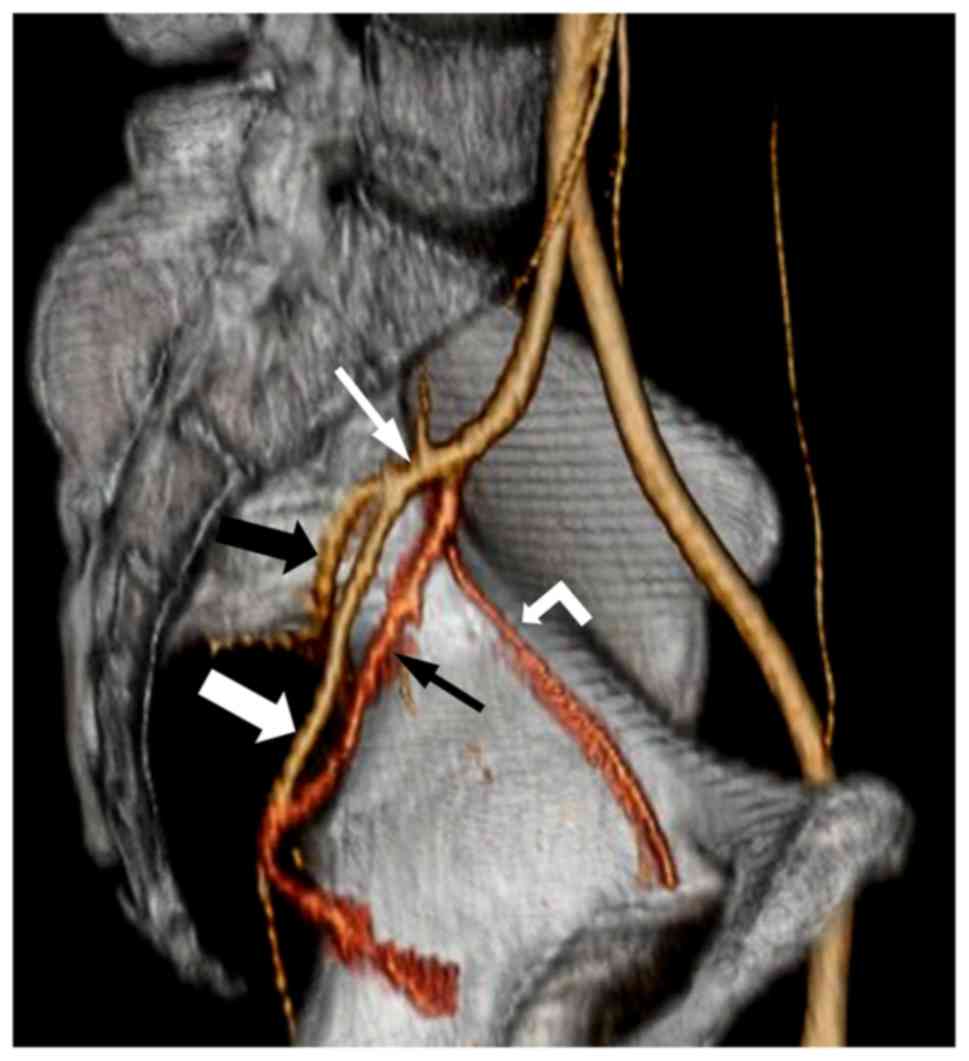
(anterior division). Group C: The IIA simultaneously divided into 3
major branches, the internal pudendal, inferior gluteal and
superior gluteal arteries. Group D: The IIA divided into the
inferior gluteal artery (posterior division), the common trunk of
the superior gluteal and internal pudendal arteries (anterior
division).
Regarding the quality evaluation of IIA and its
branches, 4 main branches were counted as 4 anatomical segments,
according to Yamaki's classification, and they were separately
evaluated on MSCTA and DSA images.
The readers evaluated the images according to a
5-point scoring system, which was modified from Danias et al
(19) and Pfeil et al
(20) as follows: 1 point,
non-diagnostic; 2 points, poor quality, vessel border was
suspected, but not clearly visible, and vessel segments definable,
but with significant blurring or artefacts; 3 points, moderate
quality, sharpness of the vessel border was insufficient, but
vessel segments clearly definable with moderate blurring or
artefacts; 4 points, good quality, good sharpness of the vessel
border and diagnostic information available with minimal blurring
or artefacts; and 5 points, excellent diagnostic quality without
blurring or artefacts, and with sharply defined vessel borders.
In addition, the 2 readers tracked the feeding
arteries of the pelvic tumours and assessed the imaging quality of
the trunk and terminal branches of the tumour-feeding artery
according to the 5-point scoring system. Due to the complexity of
the type of tumour and the branches of the blood supply artery, it
was difficult to define the terminal branch of the feeding artery.
To accurately evaluate the imaging quality between the two
examinations, the 2 readers defined the terminal branch of the
feeding artery as the vascular segment that contacted or entered
the tumour subsequent to the feeding artery.
The existence of pelvic tumours may cause
compression or stenosis of the feeding arteries. Changes in the
arterial diameter caused by the tumours were evaluated with source
images and reconstructed images (mainly MIP images) of each tumour
in the imaging modes by the 2 readers. The stenosis was recorded as
follows: 0, Artery free from tumour; 1, artery displaced, but not
narrowed by tumour; 2, tumour narrowing artery <50%; and 3,
tumour narrowing artery ≥50%.
Statistical analysis
Data were processed using SPSS 20.0 software (IBM
Corp., Armonk, NY, USA). Measurement data are expressed as the mean
± standard deviation and count data are expressed as a percentage.
The 2 sample rates were compared using the χ2 test for a
2×4 data table. The differences in the vascular image quality of
the IIA and the visualization quality of the tumour-feeding
arteries between the two imaging modes were determined by the
Wilcoxon signed-rank test. A κ test was used to analyse the
conformity between the two imaging modes. κ coefficient values were
interpreted as excellent consistency ≥0.80, good consistency
0.61–0.80, medium 0.41–0.60 and poor ≤0.40 (21). P<0.05 was considered to indicate
that a difference was statistically significant.
Results
The 3D digital models of pelvic arteries in all 43
pelvic tumour patients were successfully constructed. These tumours
included rectal cancer (n=15), cervical cancer (n=6), prostate
cancer (n=4), gastrointestinal stromal tumours in the rectum (n=3),
liposarcoma (n=3), malignant solitary fibrous tumours (n=2), Ewing
sarcoma (n=2), neurofibrosarcoma (n=1), sigmoid colon cancer (n=1),
anal canal carcinoma (n=1), uterine leiomyosarcoma (n=1), ovarian
mucinous cystadenocarcinoma (n=1), bladder cancer (n=1), a
sacrococcygeal neurogenic spindle cell tumour (n=1) and a gastric
cancer pelvic metastatic tumour (n=1). The branching patterns of
the IIA of 86 pelvic sides were reviewed with MSCTA and DSA, based
on the 4 groups of Yamaki's classification. No other special types
were identified in the present study. Based on images acquired by
MSCTA, 64 pelvic sides were classified as Group A (74.4%) (Fig. 1), 8 as Group B (9.3%) (Fig. 2) and 14 as Group C (16.3%) (Fig. 3), with no cases in Group D. Of the 43
patients, 26 had the same type of bilateral IIA. Additionally, no
significant difference was observed between the right and left
sides in the classification of the IIA (P=0.73; Table I). The classifications of 86 pelvic
sides of the IIA were also assessed in images acquired by DSA. A
total of 63 pelvic sides were classified as Group A (73.3%), 7 as
Group B (8.1%) and 16 as Group C (18.6%), with no cases of Group D.
Of the 43 patients, 23 had the same type of bilateral IIA, and no
significant difference was observed between the right and left
sides in the classification of the IIA (P=0.95; Table II). These results demonstrated an
excellent consistency between MSCTA and DSA with regard to the
classification of the IIA (κ=0.81) (Table III).
 | Table I.Classification of the internal iliac
arteries in the left and right sides by multislice computed
tomography angiography. |
Table I.
Classification of the internal iliac
arteries in the left and right sides by multislice computed
tomography angiography.
| Side | Total, n | Group A, n (%) | Group B, n (%) | Group C, n (%) | Group D, n (%) | χ2 | P-value |
|---|
| Right | 43 | 33 (76.8) | 5 (11.6) | 5 (11.6) | 0 (0.0) |
|
|
| Left | 43 | 31 (72.1) | 3 (7.0) | 9 (20.9) | 0 (0.0) |
|
|
| Total | 86 | 64 (74.4) | 8 (9.3) | 14 (16.3) | 0 (0.0) | 2.0 | 0.73 |
 | Table II.Classification of the internal iliac
arteries in the left and right sides by digital subtraction
angiography. |
Table II.
Classification of the internal iliac
arteries in the left and right sides by digital subtraction
angiography.
| Side | Total, n | Group A, n (%) | Group B, n (%) | Group C, n (%) | Group D, n (%) | χ2 | P-value |
|---|
| Right | 43 | 33 (76.8) | 5 (11.6) | 5 (11.6) | 0 (0.0) |
|
|
| Left | 43 | 30 (72.1) | 2 (4.7) | 11 (23.2) | 0 (0.0) |
|
|
| Total | 86 | 63 (73.3) | 7 (8.1) | 16 (18.6) | 0 (0.0) | 0.71 | 0.95 |
 | Table III.Consistency analysis between
classification of the internal iliac arteries by MSCTA and DSA. |
Table III.
Consistency analysis between
classification of the internal iliac arteries by MSCTA and DSA.
| Technique | Group A, n | Group B, n | Group C, n | Group D, n | Total, n |
|---|
| MSCTA | 64 | 8 | 14 | 0 | 86 |
| DSA | 63 | 7 | 16 | 0 | 86 |
Regarding the evaluation of the quality of images of
the IIA and its branches, a total of 430 vascular anatomical
segments of the IIAs and their branches were separately evaluated
on MSCTA and DSA images. The 2 readers carefully analysed each
arterial segment and scored and summarized the imaging quality
(Table IV). The results indicated
that the overall imaging quality of DSA was slightly higher
compared with that of MSCTA, but the difference between the means
was not statistically significant (DSA, 4.14±0.94; MSCTA,
4.13±0.95; P=0.09). In addition, an excellent image quality was
shown between the two imaging modes (κ=0.94±0.06) and the 2 readers
achieved excellent consistency in the assessment of the vascular
imaging quality in the MSCTA and DSA images
(κMSCTA=0.87±0.10, κDSA=0.88±0.09).
 | Table IV.Imaging quality of 430 vascular
anatomical segments of the IIAs evaluated on CTA and DSA
images. |
Table IV.
Imaging quality of 430 vascular
anatomical segments of the IIAs evaluated on CTA and DSA
images.
|
| CTA | DSA |
|---|
|
|
|
|
|---|
| Vessel segment | Reader 1 | Reader 2 | κ | Reader 1 | Reader 2 | κ |
|---|
| IIA | 5.00±0.00 | 5.00±0.00 | 1.00 | 5.00±0.00 | 5.00±0.00 | 1.00 |
| Superior gluteal
artery | 4.53±0.57 | 4.52±0.63 | 0.80 | 4.57±0.56 | 4.57±0.61 | 0.84 |
| Inferior gluteal
artery | 4.27±0.71 | 4.27±0.71 | 0.84 | 4.26±0.69 | 4.28±0.70 | 0.79 |
| Obturator
artery | 3.10±1.01 | 3.06±1.01 | 0.79 | 3.09±0.98 | 3.09±0.98 | 0.82 |
| Internal pudendal
artery | 3.76±0.67 | 3.78±0.68 | 0.80 | 3.80±0.67 | 3.78±0.66 | 0.80 |
| Mean ± SD | 4.13±0.94 | 4.13±0.96 | 0.87±0.10 | 4.14±0.93 | 4.14±0.94 | 0.88±0.09 |
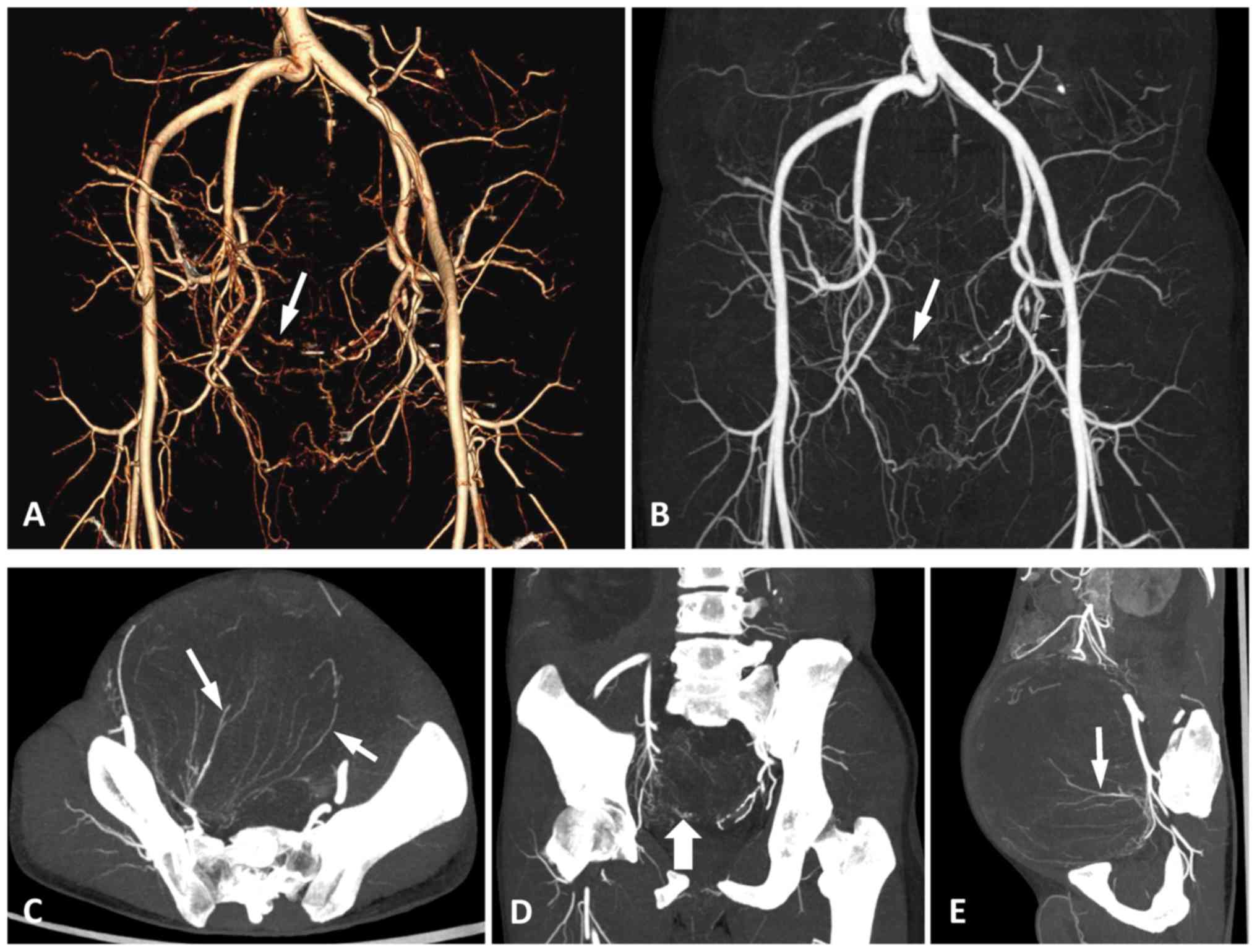
As several different types of pelvic neoplasms were
included in the present study, the arteries supplying blood to the
tumours were tracked and identified on DSA and MSCTA. There was no
difference in the types of feeding arteries between the two
examinations, and the accuracy of MSCTA in diagnosing the type and
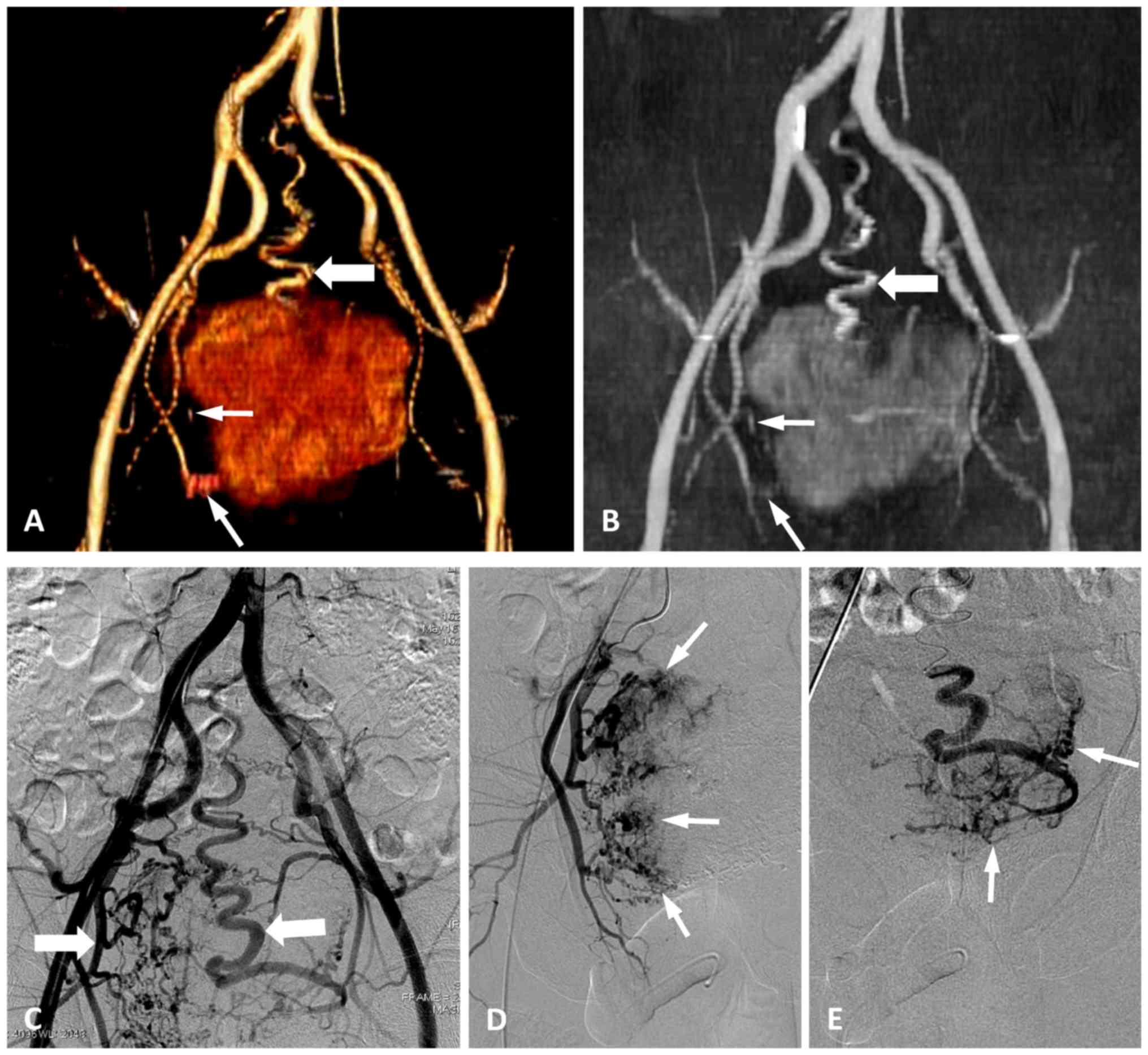
quantity of feeding arteries was 100% (Fig. 4), which is consistent with the
results using DSA.
To evaluate the ability of MSCTA and DSA to display
the terminal branches of the feeding artery, the 2 readers tracked
the feeding arteries of the pelvic tumours and assessed the imaging
quality of the trunk and terminal branches of the tumour-feeding
artery according to the 5-point scoring system. The results
demonstrated that the two imaging modes had good consistency in
displaying the main trunk of the feeding artery (κ=0.67). However,
the consistency of MSCTA and DSA was poor in displaying the
terminal small branches of the feeding arteries (κ=0.14;
χ2=14.29; P=0.027). Therefore, the difference was
statistically significant between the MSCTA and DSA images for
showing the terminal branches of feeding arteries in pelvic tumours
(Fig. 5).
Finally, the degree of arterial stenosis caused by
pelvic tumours on the imaging modes was summarized by the 2
readers. The internal consistency of MSCTA and DSA was excellent
for each of the 2 readers (reader 1, κ=0.87; reader 2, κ=0.81).
Excellent consistency between the 2 readers was also obtained on
MSCTA and DSA (MSCTA, κ=0.81; DSA, κ=0.97).
Discussion
As a non-invasive and comprehensive method for the
imaging of the majority of the major vessels in the body, MSCTA may
provide visualiszation of the 3D spatial association between the
tumour, tumour-feeding arteries, and adjacent organs and large
blood vessels by using post-processing reconstruction technology
(14,22). As the gold standard for vascular
display, DSA can visually display and observe the shape of blood
vessels and the location of lesions. The technique has a large
advantage in tracking the vascular path and showing the image in
real time, and associated treatments can be performed while
conducting a diagnosis. In addition, the radiation dose of the
minimally invasive examination of DSA may be slightly higher
compared with that of MSCTA, but remains in the safe range. Several
studies reported that the sensitivity and specificity of MSCTA in
the diagnosis of certain vascular diseases was approximately the
same as DSA (23,24). However, the absence of data on the
non-invasive examination of vascular anatomy of complex pelvic
tumours, particularly data on the preoperative assessment of the
vascular and haemodynamic parameters, may complicate the treatment
and prognosis of tumours.
In the present study, MSCTA and DSA were used to
investigate the classification of the IIA, which is the main
feeding artery of the pelvic cavity with dense branches. Although
there is no uniform classification of the IIA at present (4), Yamaki's classification is the most
commonly used classification criteria. In the study by Yamaki et
al (7), 645 pelvic sides of the
IIA were typed by autopsy, and Group A was considered the basic
branching pattern of the IIA, as it was the most frequent (79.5%),
followed by Group B (15.0%), while Group C and Group D occurred at
rates of 5.3 and 0.2%, respectively. In the present study, the main
classification of the IIA was also Group A, but Group B was less
frequent compared with Group C, and no patients were classified as
Group D. The relatively small number of samples in the present
study may be the cause of these unexpected results. In general, the
dominant classification was Group A, and there was no significant
difference in the classification of the IIA between the left and
right sides, in MSCTA and DSA images (P=0.95), which was consistent
with the study by Yamaki et al (7).
In addition, the two imaging modes had excellent
consistency in the IIA classification (κ=0.81). In the present
study, the total imaging quality of the IIA and its branches on
MSCTA appeared slightly lower compared with that on DSA, but the
difference between them was not statistically significant
(P=0.09).
Regarding the assessment of the supply artery and
small terminal branches in the pelvic tumour, images acquired by
MSCTA clearly revealed the type and number of feeding arteries in
the pelvic tumours, with no difference between the two
examinations. Additionally, the accuracy of MSCTA in diagnosing the
type and imaging quantity of the feeding arteries was 100%, which
was consistent with DSA. MSCTA and DSA demonstrated good
consistency in visualising the main trunk of the feeding artery
(κ=0.67). However, the two imaging modes exhibited poor consistency
and a significant difference in the assessment of the small
terminal branches of the feeding arteries (κ=0.14; P=0.027). These
results may indicate that DSA is more useful compared with MSCTA in
the evaluation of the small branches, a finding that has been
reflected in a previous study (3).
In addition, the extent of arterial invasion by pelvic tumours is
of interest to clinicians, particularly oncologists. The internal
consistency was excellent between the two imaging modes and the 2
readers. MSCTA appears to be an ideal alternative to DSA in
assessing arterial invasion caused by pelvic tumours.
The rapid development of pelvic vascular
interventional therapy based on the arterial system has led to its
widespread use in women with hysteromyoma and postpartum
haemorrhage associated with pelvic vascular injury, in men with
benign prostatic hyperplasia and in patients with advanced pelvic
tumours that were not suitable for surgery, as well as in other
candidates (25–27). Selective arterial cannulation is
necessary for vascular interventional therapy. In addition, the IIA
and its branches are the main blood supply for pelvic tumours, so
it is important to understand the classification and systematic
anatomical structure of the IIA, and to confirm the arterial source
of the blood supply prior to treatment (28,29).
Imaging of the IIA in 3D using MSCTA and
post-processing software demonstrated that MSCTA may replace DSA in
the evaluation of the iliac vascular classification to a certain
extent (3). For pelvic intervention,
the introduction of high-quality non-invasive imaging techniques,
including MSCTA, could facilitate the study of the classification
and branches of the IIA by interventional radiologists in more
detail, with the aim of positioning the target vessel correctly,
improving the accuracy of selective cannulation and greatly
reducing the procedure time. In addition, for surgery and minimally
invasive surgery, the construction of the 3D anatomy of the pelvic
vessels prior to surgery may improve the accuracy of the surgery
and reduce intraoperative and postoperative bleeding, and other
serious complications.
Additionally, MSCTA can successfully evaluate the
types of arteries feeding the tumours. The present study revealed
that there were no significant differences between MSCTA and DSA in
visualising the main trunk of the feeding arteries in pelvic
tumours. However, MSCTA was less sensitive compared with DSA in
displaying the terminal small branches of tumour-feeding arteries.
DSA is an invasive procedure, which is usually used prior to pelvic
selective arterial embolization; the bilateral iliac vessels cannot
be clearly displayed simultaneously on it, which means that DSA
cannot be used as a routine assessment for identifying the feeding
arteries of pelvic tumours. Preoperative MSCTA enables
interventional radiologists to achieve more information about the
feeding arteries in pelvic tumours, including origin, form and
branches, particularly in older patients where iliac
atherosclerotic lesions may prohibit vascular access.
The present study had several limitations. Firstly,
the data was analysed retrospectively and, as such, the study was
subject to the inherent limitations of retrospective studies.
Secondly, the classification of the IIA was divided based on the
main branch of the IIA, ignoring the influence of its small
branches for classification. Finally, the present single-centre
preliminary study had a small sample size, and the imaging quality
of the IIA and the feeding arteries was assessed by 2 radiologists,
which may cause bias to a certain degree. Therefore, a multicentre
study or a systematic meta-analysis may be required to conquer
these limitations.
In conclusion, MSCTA demonstrated excellent
consistency with DSA in the classification of the IIA, the imaging
quality evaluation of the IIA and its branches, and in the
evaluation of the pelvic tumour-feeding artery, which is expected
to provide an anatomical basis for pelvic interventional therapy
and surgical treatment. However, in the visualisation of the
terminal arterial branches of the pelvic tumour, DSA remains
irreplaceable, particularly in cases of interventional
embolization.
Acknowledgements
Not applicable.
Funding
This study was supported by grants from the National
Natural Science Foundation of China (no. 81301978), the Ph.D.
Programs Foundation of Ministry of Education of China (no.
20130171120105) and the Young Teacher Cultivation Project of Sun
Yat-Sen University (no. 13YKPY39).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
LL and BZ drafted this manuscript. LL, KW and BZ
analyzed the imaging data and collected the data. YL, HL and ZZ
assisted with statistical analysis. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the Institutional
Ethics Review Board of The Sixth Affiliated Hospital of Sun Yat-Sen
University (Guangzhou, China). Written informed consent for this
study was obtained from each patient.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ding HM, Yin ZX, Zhou XB, Li YB, Tang ML,
Chen SH, Xu DC and Zhong SZ: Three-dimensional visualization of
pelvic vascularity. Surg Radiol Anat. 30:437–442. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Levy AD, Manning MA, Al-Refaie WB and
Miettinen MM: Soft-tissue sarcomas of the abdomen and pelvis:
Radiologic-pathologic features, part 1-common sarcomas: From the
radiologic pathology archives. Radiographics. 37:462–483. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bilhim T, Casal D, Furtado A, Pais D,
O'Neill JE and Pisco JM: Branching patterns of the male internal
iliac artery: Imaging findings. Surg Radiol Anat. 33:151–159. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chantalat E, Merigot O, Chaynes P, Lauwers
F, Delchier MC and Rimailho J: Radiological anatomical study of the
origin of the uterine artery. Surg Radiol Anat. 36:1093–1099. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Naguib NN, Nour-Eldin NE, Hammerstingl RM,
Lehnert T, Floeter J, Zangos S and Vogl TJ: Three-dimensional
reconstructed contrast-enhanced MR angiography for internal iliac
artery branch visualization before uterine artery embolization. J
Vasc Interv Radiol. 19:1569–1575. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Selvaraj L and Sundaramurthi I: Study of
normal branching pattern of the coeliac trunk and its variations
using CT angiography. J Clin Diagn Res. 9:AC01–AC04.
2015.PubMed/NCBI
|
|
7
|
Yamaki K, Saga T, Doi Y, Aida K and
Yoshizuka M: A statistical study of the branching of the human
internal iliac artery. Kurume Med J. 45:333–340. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sakthivelavan S, Aristotle S, Sivanandan
A, Sendiladibban S and Felicia Jebakani C: Variability in the
branching pattern of the internal iliac artery in Indian population
and its clinical importance. Anat Res Int.
2014:5971032014.PubMed/NCBI
|
|
9
|
Bilhim T, Pereira JA, Fernandes L, Rio
Tinto H and Pisco JM: Angiographic anatomy of the male pelvic
arteries. AJR Am J Roentgenol. 203:W373–W382. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mori K, Saida T, Shibuya Y, Takahashi N,
Shiigai M, Osada K, Tanaka N and Minami M: Assessment of uterine
and ovarian arteries before uterine artery embolization: Advantages
conferred by unenhanced MR angiography. Radiology. 255:467–475.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rott G and Boecker F: The extremely rare
vascular variant of a segmental duplicated uterine artery and its
relevance for the interventionist and gynecologist: A case report.
J Med Case Rep. 10:1622016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
de Assis AM, Moreira AM, de Paula
Rodrigues VC, Harward SH, Antunes AA, Srougi M and Carnevale FC:
Pelvic arterial anatomy relevant to prostatic artery embolisation
and proposal for angiographic classification. Cardiovasc Intervent
Radiol. 38:855–861. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bilhim T, Pisco JM, Rio Tinto H, Fernandes
L, Pinheiro LC, Furtado A, Casal D, Duarte M, Pereira J, Oliveira
AG and O'Neill JE: Prostatic arterial supply: Anatomic and imaging
findings relevant for selective arterial embolization. J Vasc
Interv Radiol. 23:1403–1415. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hu HJ, Huang YW and Zhu YC: Tumor feeding
artery reconstruction with multislice spiral CT in the diagnosis of
pelvic tumors of unknown origin. Diagn Interv Radiol. 20:9–16.
2014.PubMed/NCBI
|
|
15
|
Wang MQ, Duan F, Yuan K, Zhang GD, Yan J
and Wang Y: Benign prostatic hyperplasia: Cone-beam CT in
conjunction with DSA for identifying prostatic arterial anatomy.
Radiology. 282:271–280. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee JH, Jeong YK, Park JK and Hwang JC:
‘Ovarian vascular pedicle’ sign revealing organ of origin of a
pelvic mass lesion on helical CT. AJR Am J Roentgenol. 181:131–137.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ota H, Takase K, Igarashi K, Chiba Y, Haga
K, Saito H and Takahashi S: MDCT compared with digital subtraction
angiography for assessment of lower extremity arterial occlusive
disease: Importance of reviewing cross-sectional images. AJR Am J
Roentgenol. 182:201–209. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Duffis EJ, Jethwa P, Gupta G, Bonello K,
Gandhi CD and Prestigiacomo CJ: Accuracy of computed tomographic
angiography compared to digital subtraction angiography in the
diagnosis of intracranial stenosis and its impact on clinical
decision-making. J Stroke Cerebrovasc Dis. 22:1013–1017. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Danias PG, McConnell MV, Khasgiwala VC,
Chuang ML, Edelman RR and Manning WJ: Prospective navigator
correction of image position for coronary MR angiography.
Radiology. 203:733–736. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pfeil A, Betge S, Poehlmann G, Boettcher
J, Drescher R, Malich A, Wolf G, Mentzel HJ and Hansch A: Magnetic
resonance VIBE venography using the blood pool contrast agent
gadofosveset trisodium-An interrater reliability study. Eur J
Radiol. 81:547–552. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Landis JR and Koch GG: The measurement of
observer agreement for categorical data. Biometrics. 33:159–174.
1977. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Farghadani M, Momeni M, Hekmatnia A,
Momeni F and Baradaran Mahdavi MM: Anatomical variation of celiac
axis, superior mesenteric artery, and hepatic artery: Evaluation
with multidetector computed tomography angiography. J Res Med Sci.
21:1292016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schernthaner R, Stadler A, Lomoschitz F,
Weber M, Fleischmann D, Lammer J and Loewe Ch: Multidetector CT
angiography in the assessment of peripheral arterial occlusive
disease: Accuracy in detecting the severity, number, and length of
stenoses. Eur Radiol. 18:665–671. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Delgado Almandoz JE, Romero JM, Pomerantz
SR and Lev MH: Computed tomography angiography of the carotid and
cerebral circulation. Radiol Clin North Am. 48:265–281. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fang JF, Shih LY, Wong YC, Lin BC and Hsu
YP: Repeat transcatheter arterial embolization for the management
of pelvic arterial hemorrhage. J Trauma. 66:429–435. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Salehi M, Jalilian N, Salehi A and Ayazi
M: Clinical efficacy and complications of uterine artery
embolization in symptomatic uterine fibroids. Glob J Health Sci.
8:245–250. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang E, Liu L and Owen R: Pelvic artery
embolization in the management of obstetrical hemorrhage:
Predictive factors for clinical outcomes. Cardiovasc Intervent
Radiol. 38:1477–1486. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
de Assis AM, Moreira AM, de Paula
Rodrigues VC, Yoshinaga EM, Antunes AA, Harward SH, Srougi M and
Carnevale FC: Prostatic artery embolization for treatment of benign
prostatic hyperplasia in patients with prostates >90 g: A
prospective single-center study. J Vasc Interv Radiol. 26:87–93.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Al-Thunyan A, Al-Meshal O, Al-Hussainan H,
Al-Qahtani MH, El-Sayed AA and Al-Qattan MM: Buttock necrosis and
paraplegia after bilateral internal iliac artery embolization for
postpartum hemorrhage. Obstet Gynecol. 120:468–470. 2012.
View Article : Google Scholar : PubMed/NCBI
|