Introduction
Oesophageal cancer is considered to be among the
most harmful known tumor types. Previous studies reported that
~480,000 cases of oesophageal cancer are diagnosed worldwide each
year, making it the 8th most commonly occurring cancer. It has also
been reported that >400,000 mortalities occur due to oesophageal
cancer each year wordwide, rendering Oesophageal cancer was the 6th
most deadly tumor worldwide as of 2017 (Cancer Statistics, 2017)
(1). China is a nation with an
exceptionally high prevalence of oesophageal cancer, and
oesophageal cancer is the 4th most common cause of mortality in
China. Contrary to the western nations, the majority of patients
with oesophageal cancer in China have oesophageal squamous cell
carcinoma (ESCC), which represents >90% of cases (2). Tumor invasion and metastasis is one of
the primary explanations for the failure of the oesophageal cancer
treatment and patient mortality (2).
The identification of oesophageal growth associated factors,
together with elucidating the underlying molecular mechanisms, has
been hypothesized to aid in developing more efficient therapeutics
for oesophageal cancer. The aim of the present study was to
identify oesophageal cancer biomarkers and investigate focused
treatment.
Grainyhead like transcription factor 1 (GRHL1) is a
70 kDa protein and belongs in the homologous group of GRH families,
which function as transcription factors in warm-blooded animals
(3). The GRH family was initially
identified in Drosophila, and Drosophila GRH are located on the
surface of the ectoderm, therefore contributing to epidermal
structure, and serve a crucial role in embryonic advancement,
neurodevelopment, epidermal formation and wound-healing (4). The mammalian GRHL family comprises of
three individuals, including GRHL1, GRHL2 and GRHL3 (5). A previous study demonstrated that the
GRHL family serves a role in skin squamous cell carcinoma, oral
squamous cell carcinoma, breast cancer, stomach cancer, liver
cancer, colorectal cancer, renal clear cell carcinoma,
neuroblastoma tumor and cervical cancer (3).
There is a limited amount of research investigating
the role of GRHL1 in tumors. In a neuroblastoma investigation,
Fabian et al (6) demonstrated
that patients with a high expression of GRHL1 had an improved
clinical prognosis and longer disease-free survival. GRHL1, as a
tumor silencer, serves an important role in the repression of tumor
cell clone development, proliferation and tumorigenic capacity in
mice. Mlacki et al (7)
examined the role of GRHL1 knockout in mice with cutaneous squamous
cell carcinoma and identified that the GRHL1 deletion promoted the
advancement of benign papilloma to malignant squamous cell
carcinoma. The aim of the present study was to investigate whether
the low expression of GRHL1 is associated with a poor prognosis in
patients with ESCC.
Materials and methods
Cell lines and cell culture
Immortalized normal esophageal epithelial cell line
NE1 was obtained from Professor George Tsao's laboratory
(Department of Anatomy, The University of Hong Kong) in 2006.
Chinese ESCC cell lines [HKESC1(HK), EC109 and EC9706] and six
Japanese ESCC cell lines [KYSE30(K30), KYSE140(K140),
KYSE180(K180), KYSE410(K410), KYSE510(K510), and KYSE520(K520)]
were kindly provided by Professor Srivastava (Department of
Pathology, The University of Hong Kong). The human ESCC cell lines
HK, EC18, EC109, EC9706, K30, K140, K180, K410, K510 and K520 were
cultured in DMEM (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) supplemented with 10% fetal bovine serum
(Invitrogen; Thermo Fisher Scientific, Inc.). The oesophageal
epithelial cell line (NE1) was cultured in Keratinocyte-SFM &
EpiLife (1:1; Invitrogen; Thermo Fisher Scientific, Inc.),
supplemented with bovine pituitary extract (BPE) (35 ug/ml;
Invitrogen; Thermo Fisher Scientific, Inc.). All cell lines were
cultured at 37°C in a 5% CO2 incubator.
Patients and tissue specimens
A total of 60 matched fresh ESCC samples and normal
oesophageal epithelium specimens were obtained via surgical
resection at Linzhou People's Hospital (Henan, China) between
January 2015 and June 2015 for RNA extraction. Additionally, an
aggregate of 266 formalin-fixed paraffin-embedded (fixed in 4%
paraformaldehyde at room temperature for 10 h, in 4-mm thick
sections) ESCC tissues and the comparing normal oesophageal
epithelia were obtained from Linzhou Cancer Hospital (Henan, China)
between January 2002 and February 2005 for the tumor tissue
microarray (TMA). All patients enrolled in this investigation did
not receive preoperative treatment. The clinical attributes of all
patients are presented in Table I.
The accompanying end focuses (time to the date of death were
assessed). The present study was approved by the Institutional
Ethics Review Board of the First Associated Hospital (Zhengzhou
University), and written informed consent form was obtained from
each patient.
 | Table I.Clinicopathological correlation of
GRHL1 expression in oesophageal squamous cell carcinoma. |
Table I.
Clinicopathological correlation of
GRHL1 expression in oesophageal squamous cell carcinoma.
|
|
| Expression of
GRHL1 |
|
|---|
|
|
|
|
|
|---|
| Characteristic | No. of patients | Downregulated, n
(%) | Normal, n (%) | P-value |
|---|
| Age (years old) |
|
|
| 0.668 |
|
<60 | 139 | 84 (60.4) | 55 (39.6) |
|
|
≥60 | 127 | 80 (63.0) | 47 (37.0) |
|
| Sex |
|
|
| 0.251 |
|
Female | 116 | 67 (57.8) | 49 (42.2) |
|
|
Male | 150 | 97 (64.7) | 63 (35.3) |
|
| Differentiation |
|
|
| 0.574 |
|
Poor | 31 | 20 (64.5) | 11 (35.5) |
|
|
Moderate | 175 | 104 (59.4) | 71 (40.6) |
|
|
Well | 60 | 40 (66.7) | 20 (33.3) |
|
| Tumor invasion |
|
|
| 0.008 |
|
T1 | 21 | 8 (38.1) | 13 (61.9) |
|
|
T2 | 76 | 41 (53.9) | 35 (46.1) |
|
|
T3 | 169 | 115 (68.0) | 54 (32.0) |
|
| Lymph node
metastasis |
|
|
| 0.269 |
|
N0 | 153 | 90 (58.8) | 63 (41.2) |
|
|
N1 | 113 | 74 (65.5) | 39 (34.5) |
|
| Clinical stage |
|
|
| 0.004 |
|
Stage I–II | 178 | 99 (55.6) | 79 (44.4) |
|
|
Stage III–IV | 88 | 65 (73.9) | 23 (26.1) |
|
| Mortality |
|
|
| <0.001 |
|
Yes | 95 | 44 (46.3) | 51 (53.7) |
|
|
No | 171 | 120 (70.2) | 51 (29.8) |
|
Immunohistochemical staining
Briefly, a TMA segment was de-paraffinized
(dimethylbenzene I, II, IIII, each for 5 min) and rehydrated
(alcohol gradient: 100, 95, 75 and 50%, each for 5 min), and the
endogenous peroxidase activity was blocked using 3% hydrogen
peroxide for a period of 15 min. For antigen retrieval, the TMA
slide was microwave-treated (100°C) in 10 mM citrate buffer (pH
6.0,
Na3C6H5O7·2H2O:
1.2054 g, C6H8O7·H2O:
0.189 g, dissolving in 1 liter of ddH2O) for 10 min.
Then Bovine serum albumin (5%, Invitrogen; Thermo Fisher
Scientific, Inc.) was applied to block non-specific binding at
temperature for 1 h. The primary antibody used was rabbit
anti-GRHL1 (cat. no. NBP1-81321, Novus Biologicals, LLC, Littleton,
CO, USA), which was diluted to a ratio of 2 ug/ml and incubated
with the slides for overnight at 4°C. The nucleus was
counterstained with the use of Meyer's haematoxylin (at room
temperature for 20 sec). Representative photomicrographs were
captured with a Vectra (PerkinElmer, Shanghai, China). Slides were
observed under light microscopy at ×40 magnification. GRHL1
immunoreactivity was calculated via the scores for the level of
GRHL1-positive cells 1: ≥5%, <25%; 2: ≥25, <50; 3: ≥50,
<75%; and 4: ≥75% and the force of GRHL1-positive staining
(negative, 0; weak, 1; moderate, 2; or strong, 3). Enlightening
outcomes were observed in 266 sets of ESCC cases. However, invalid
cases included lost cases as well as Non-specific staining.
RNA extraction, reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) and
agarose gel electrophoresis
Total RNA was separated from the cell lines with the
use of TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). The extricated RNA (2 µg) was reverse
transcribed with the use of the Takara PrimeScript™RT reagent kit
with gDNA Eraser FastQuant RT kit (Takara Bio, Inc., Otsu, Japan),
and the cDNA productions were stored at −20°C until subsequent use.
RT-qPCR was conducted on the Roche LightCycler 480 sequence
detection system (Roche Diagnostics, Basel, Switzerland) in 10 µl
responses containing 0.5 µl reverse transcription product, 5 µl
SYBR® Green SuperMix (Roche Diagnostics), 0.25 µl of
each PCR forward primer and reverse primer, and 4 µl
ddH2O. The reactions were pre-incubated at 95°C for 10
min, followed by 45 cycles of denaturation at 95°C for 15 sec and
expansion at 60°C for 1 min, at that point the temperature was
inclined from 60°C to 95°C (via automatic program processing) for
the purpose of obtaining a melting curve. The accompanying PCR
primers were: GRHL1, forward, 5′-CAAACGGCCAGTGTTGGTTC-3′, and
reverse, 5′-TGCTCATCATCGCTTTGGTCG-3′; 18s, forward,
5′-GTAACCCGTTGAACCCCATT-3′, and reverse,
5′-CCATCCAATCGGTAGTAGCG-3′. The gene expression level was expressed
as 2-∆∆Cq, where ∆Cq=Cq (gene)-Cq (18s) (8). The expression level in control cells
was considered as 1. The experiments were conducted in triplicate.
18s was used as the standardization control. Agarose gel
electrophoresis was conducted for the analysis of the mRNA
expression level of GRHL1. The accompanying PCR primers were the
same as RT-qPCR. PCR amplifications were conducted with the use of
the GoTaq Green Master mix kit (Promega Corporation, Madison, WI,
USA) and PCR cycling conditions adhered to the following: 95°C for
5 min, followed by 35 cycles of 95°C for 30 sec, 60°C for 30 sec
and 72°C for 30 sec, with extension at 72°C for 7 min (S1000
Thermal Cycler; Bio-Rad Laboratories, Inc., Hercules, CA, USA;
http://www.bio-rad.com). The PCR items were
examined by 1.5% agarose gel electrophoresis.
Establishment of GRHL1 expression and
deletion clone in ESCC cell lines
A lentiviral construct containing GRHL1
(GeneCopoeia, Inc., Rockville, MD, USA) was packaged with the use
of a Lenti-Pac™ HIV Expression Packaging kit (GeneCopoeia, Inc.) in
293 cells. GRHL1-treatment lentivirus was employed to steadily
transfect ESCC cells (EC109 and HKESC1), in order to construct the
GRHL1-overexpressing cells. Empty vector-transfected cells were
established as the controls. Short hairpin (sh)RNA against GRHL1
(GeneCopoeia, Inc.) was transfected into KYSE510 cells with the use
of a Lenti-Pac HIV Expression Packaging kit in 293 cells, according
to the manufacturer's protocol. The cells transfected with the
mixed inhibitor (NC; Shanghai GenePharma Co., Ltd., Shanghai,
China) were employed as the negative controls.
Antibodies and western blotting
Protein was extracted from ESCC cells by ice-cold
lysis buffer supplemented with protease inhibitor cocktail (Pierce;
Thermo Fisher Scientific, Inc.) and quantified using the BCA
Protein Assay kit (Beyotime Institute of Biotechnology, Haimen,
China). Protein samples (30 µg) were separated on a 15% SDS-PAGE
gel and then transferred onto PVDF membrane (Millipore, Billerica,
MA, USA). Following blocking with 5% skimmed milk in Tris-buffered
saline with 0.1% Tween-20 (TBST) at room temperature for 2 h, the
membranes were incubated overnight at 4°C with rabbit anti-human
GRHL1 monoclonal primary antibody (1:250, cat. no. NBP1-81321,
Novus Biologicals, LLC, Littleton, CO, USA). Subsequently, The
membrane was rinsed with TBST and incubated with anti-rabbit IgG
(1:2,000; cat. no. CST-14708, Cell Signaling Technology, Inc.,
Danvers, MA, USA) antibody conjugated to horseradish peroxidase at
room temperature for 2 h. Blots were visualized with enhanced
chemiluminescence (GE Healthcare, Chicago, IL, USA). β-tubulin
primary antibody (1:1,000; cat. no. CST-2146, Cell Signaling
Technology, Inc.) was used as a loading control.
Cell growth assay and foci formation
assay
In order to investigate cell growth, 1×103 cells
(GRHL1, Vec-EC109; GRHL1, Vec-HK; shGRHL1, shCtr-K510) were plated
in 96-well plates and the cell development rate was examined using
a Cell Counting Kit-8 kit (Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan), according to the manufacturer's protocol. For the
foci formation assay, 1×103 cells were plated in 6-well plates, and
following two weeks of culture (37°C, 5% CO2), colonies
comprised of >50 cells stained with 1% crystal violet were
counted.
Statistical analysis
Data are presented as the mean ± standard error of
the mean and SPSS standard V.22.0 (IBM Corp., Armonk, NY, USA) was
employed for statistical analysis. The unpaired student's t test or
two-way ANOVA (post-hoc test, mean ± SEM) were used to investigate
differences among two groups or a multi-group comparison. Survival
analysis was carried out with the use of the Kaplan-Meier and
log-rank tests and the associations between GRHL1 expression and
clinicopathological factors were calculated by Pearson's χ2 test or
Fisher's exact test. Univariate and multivariate Cox proportional
hazard regression model were used to calculate the survival hazard
risk. P<0.05 was considered to indicate a statistically
significant difference.
Results
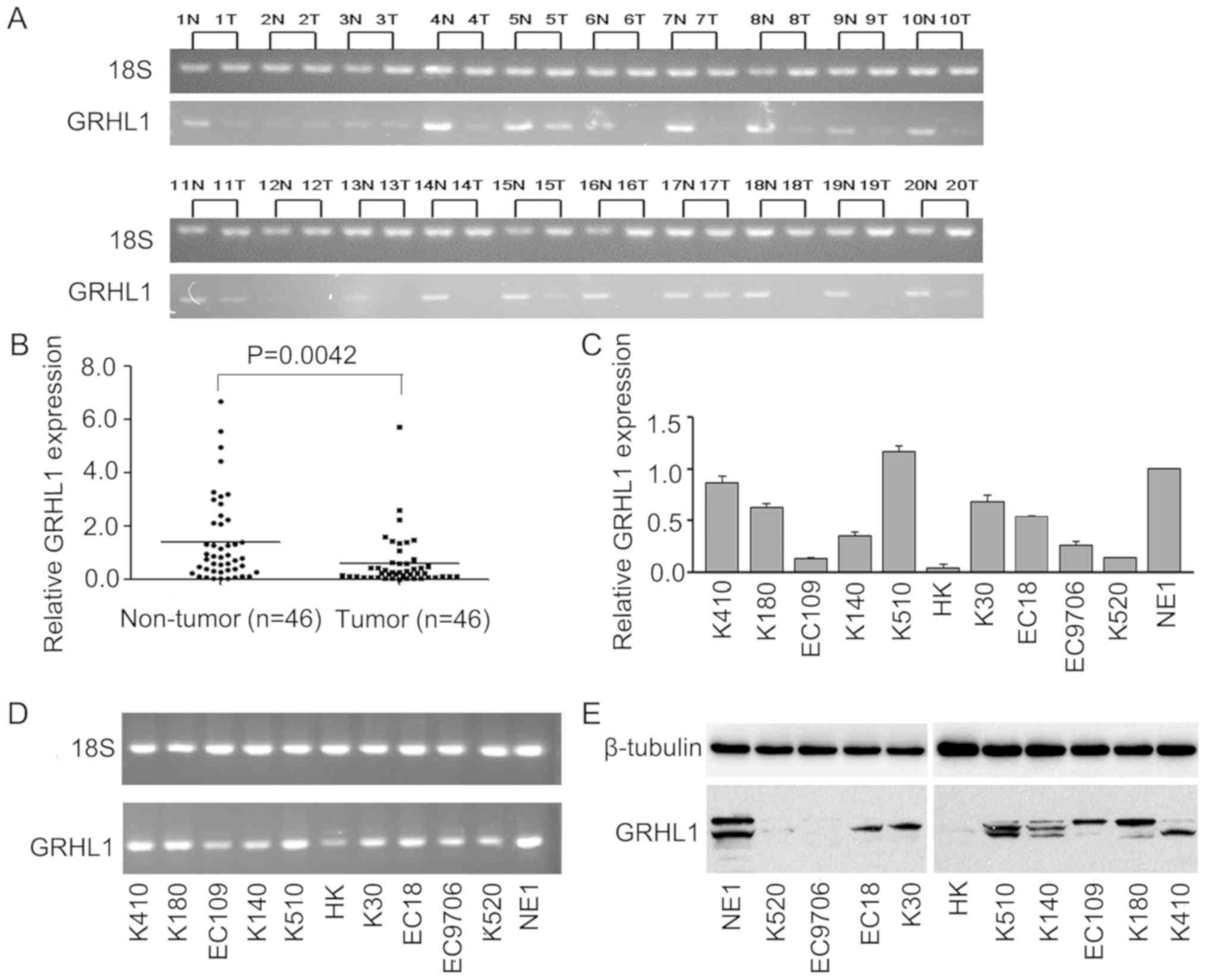
GRHL1 is downregulated in ESCC cells and tissues.
The mRNA levels of GRHL1 in 20 pairs of fresh ESCC tissues and
ordinary oesophageal epithelial tissues, and in 46 combinations of
fresh ESCC tissues and normal oesophageal epithelial tissues
revealed that GRHL1 mRNA was expressed at extensively low levels in
the ESCC tissues (Fig. 1A and B).
The mRNA and protein expression levels of GRHL1 in the normal
oesophageal epithelial cell line NE1 and ESCC cell lines were
evaluated by RT-qPCR, agarose gel electrophoresis and western blot
analysis. In contrast to the NE1 cells, GRHL1 was downregulated in
the majority of the examined ESCC cell lines at the mRNA and
protein levels; however, this downregulation was not exhibited in
KYSE510 cells (Fig. 1C-E).
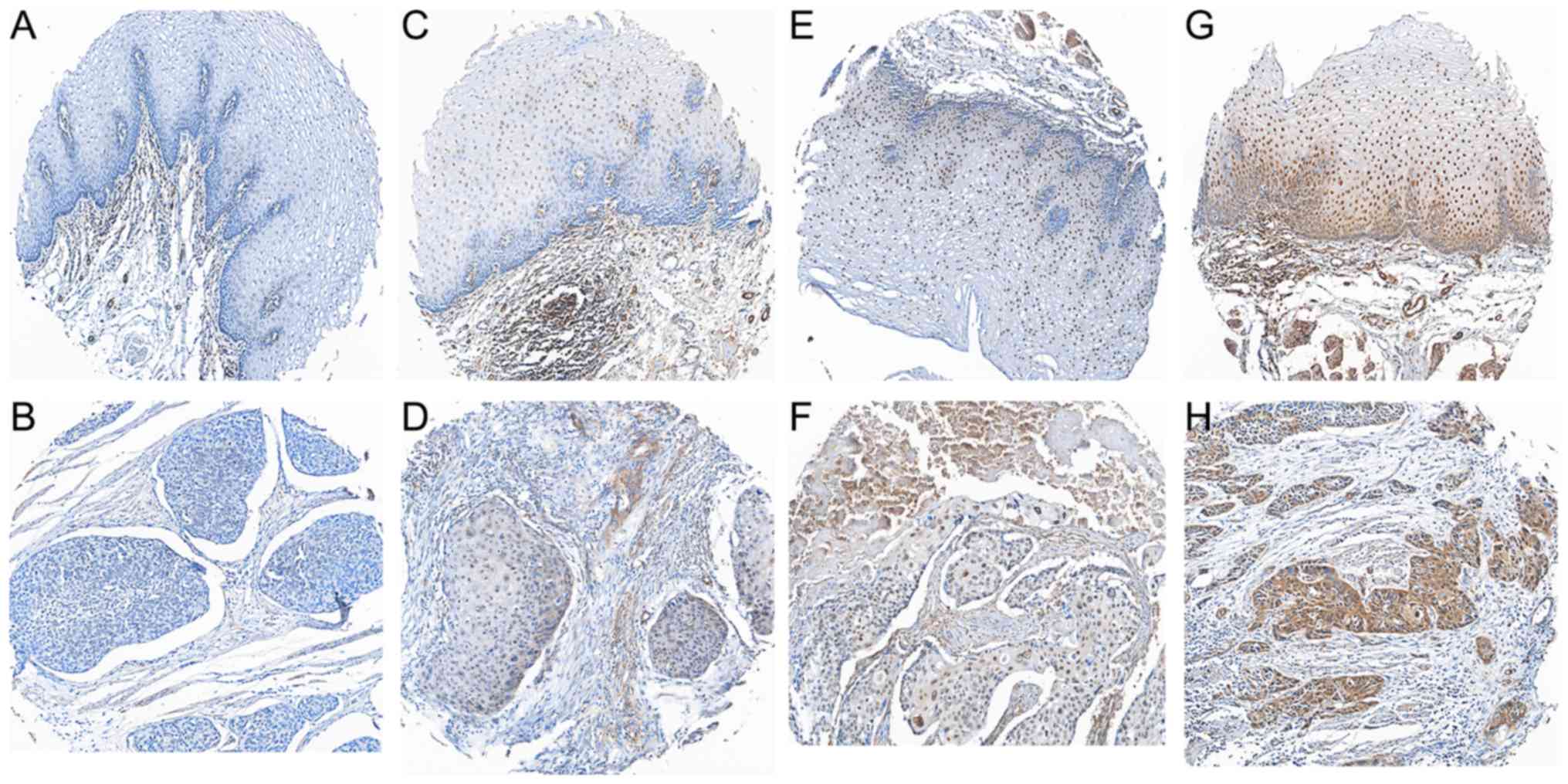
Associations between GRHL1 expression
and the clinicopathological features of ESCC
In an attempt to identify the associations between
the GRHL1 protein expression and the clinical factors of patients
with ESCC, the present study analysed the protein expression of
GRHL1 in an arrangement of 266 paraffin-embedded ESCC TMA using
immunohistochemistry. Delegate images of GRHL1 immunohistochemical
staining in clinical ordinary oesophageal epithelial tissues and
ESCC tissues are presented in Fig.
2A-H. Positive staining for GRHL1 was primarily observed in the
nucleus of typical oesophageal epithelial cells and ESCC cells.
Using a staining index of 5 as the cut-off point in the 266
patients with ESCC inspection, 164 (61.7%) patients were
categorised as exhibiting low GRHL1 expression, and 102 (38.3%)
patients exhibited a high expression of GRHL1. As presented in
Table I, low GRHL1 expression was
significantly associated with the tumor invasion (P=0.008),
clinical stage (P=0.004) and patient mortality (P<0.001). No
significant associations were observed between GRHL1 expression and
other clinical factors, including age, sex, differentiation or
lymph node (LN) metastasis (P>0.05).
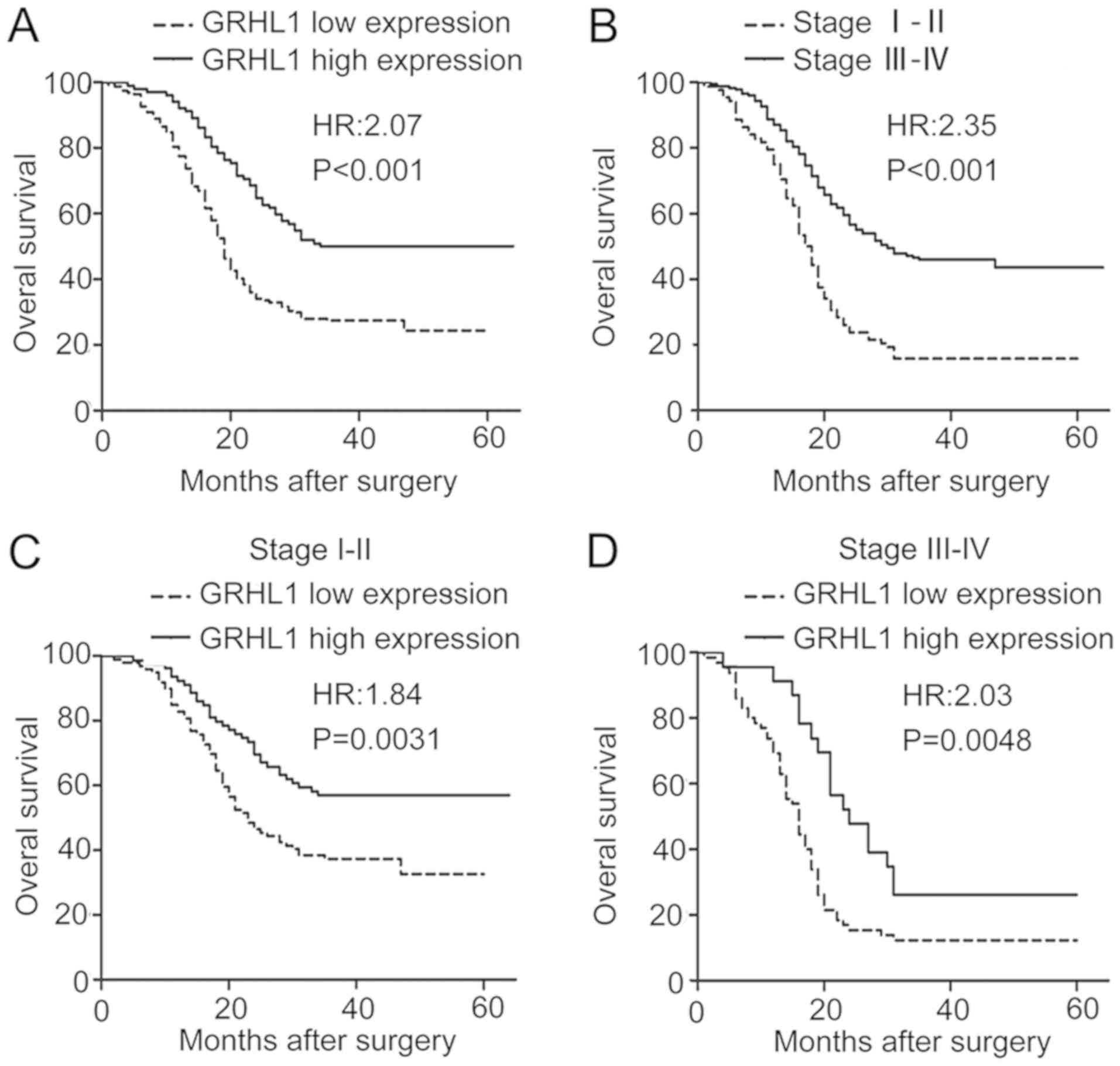
Downregulation of GRHL1 is associated
with a poor prognosis and is an independent prognostic factor in
ESCC
The association between GRHL1 expression and OS in
ESCC was examined using Kaplan-Meier analysis as well as the
log-rank test. As evident from Fig.
3, a low GRHL1 expression was associated with a significantly
reduced OS [hazard ratio (HR), 2.07 95% confidence interval (CI),
1.491–2.881; P<0.001; Fig. 3A].
Additionally, stage III–IV was associated with a reduced OS (HR,
2.345; 95% CI, 1.727–3.186; P <0.001; Fig. 3B). In addition, the early stage
(stage I–II, n=178) and the advanced stage subgroup (stage III–IV,
n=88) of patients with a low GRHL1 expression had a significantly
reduced OS (stage I–II: HR, 1.840; 95% CI, 1.229–2.756; P=0.0031;
and stage III–IV: HR, 2.032; 95% CI, 1.241–3.325; P=0.0048) in
comparison with those who had a high GRHL1 expression (Fig. 3C and D). These outcomes indicated
that the low expression of GRHL1 is associated with a reduced
prognosis in ESCC. Univariate and multivariate Cox regression
analyses were performed in order to investigate the impact of
various clinical factors, including GRHL1 expression, sex, age,
differentiation, tumor invasion, LN metastasis and clinical stage.
As evident from Table II, the
univariate analyses demonstrated that low GRHL1 expression was
associated with notably reduced OS (P<0.001), compared with high
GRHL1 expression. Furthermore, in multivariate analysis, poor
differentiation was also associated with a significantly reduced OS
(P=0.049), compared with patients with moderate and well
differentiation. In univariate analysis, T3 (Fibre layer invasion)
was associated with reduced OS (P<0.001), compared with T1
(mucosae invasion). LN metastasis was associated with significantly
reduced OS (P<0.001), compared with patients without LN
metastasis. The advanced stage of disease was also associated with
significantly reduced OS (P<0.001), compared with early stage.
The results also revealed that sex and age had no significant
effect on OS (P>0.05), with multivariate analysis confirming
that the low expression levels of GRHL1 and differentiation degrees
were independent prognostic factors associated with the OS in ESCC
(P<0.05).
 | Table II.Cox proportional hazard regression
analyses for overall survival. |
Table II.
Cox proportional hazard regression
analyses for overall survival.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Clinicopathological
features | HR (95% Cl) | P-value | HR (95% Cl) | P-value |
|---|
| GRHL1
downregulation | 2.073
(1.491–2.881) |
<0.001a | 1.930
(1.375–2.709) |
<0.001a |
| Sex | 1.181
(0.869–1.606) | 0.280 | – | – |
| Age | 1.313
(0.973–1.773) | 0.069 | – | – |
| Differentiation | 1.388
(1.065–1.810) | 0.044a | 1.298
(1.001–1.682) | 0.049a |
| Tumor invasion | 1.704
(1.303–2.229) |
<0.001a | 1.353
(0.995–1.840) | 0.054 |
| LN metastasis | 2.053
(1.518–2.776) |
<0.001a | 1.714
(0.945–3.109) | 0.076 |
| Clinical stage | 2.345
(1.727–3.186) |
<0.001a | 1.124
(0.584–2.163) | 0.726 |
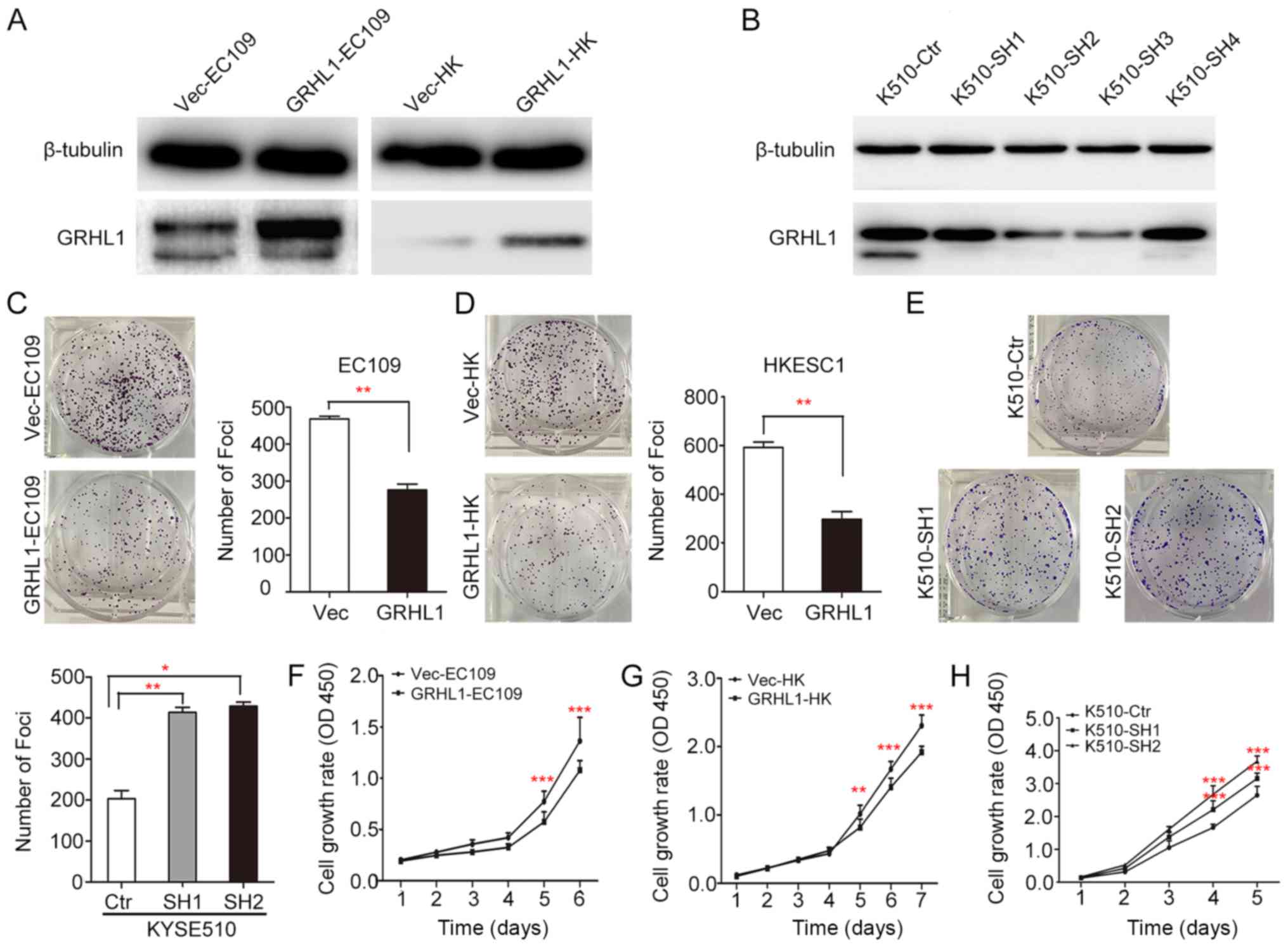
GRHL1 exerts tumor-suppressive
ability
In order to investigate the tumor-suppressive
ability of GRHL1, GRHL1 was transfected into the EC109 and HKESC1
ESCC cell lines (termed GRHL1-EC109 and GRHL1-HK cells,
respectively). EC109 and HKESC1 cells transfected with an empty
vector (Vec-EC109 and Vec-HK, respectively) were used as controls.
Expression of the GRHL1 gene and protein in these transfectants
were confirmed using western blotting (Fig. 4A). KYSE510 cells were transfected
using shGRHL1 (K510-SH2 and K510-SH3) or shCtr (K510-ctr).
Expression of the depleted GRHL1 gene and protein in these
transfectants were analysed by western blotting (Fig. 4B). In comparison with the control
cells, the in vitro experiments revealed that the high
expression of GRHL1 could successfully suppress tumorigenic
capacity in its transfected cells, which was evidenced by a
significant decrease in foci formation frequency (P<0.01, ANOVA;
Fig. 4C-E), together with the
inhibition of cell development rate (P<0.001, ANOVA; Fig. 4F-H).
Discussion
With the advancement of diagnostic techniques and
therapeutic interventions in recent years, the clinical prognosis
of patients with ESCC has improved significantly. However, patients
in the same clinical phase of ESCC exhibit differing clinical
results when receiving identical treatments (9). Therefore, it is imperative to
distinguish novel biomarkers, which may accurately predict patient
prognosis and aid in developing individualized treatments for
patients with ESCC. As demonstrated by the present study, GRHL1 is
down-regulated in ESCC at the mRNA and protein levels, and a low
expression of GRHL1 is associated with a reduced prognosis in
patients with ESCC. Additionally, GRHL1 was recognized as an
independent prognostic factor in the multivariate investigation,
which demonstrated that GRHL1 has the potential to serve as a novel
prognostic biomarker for the control of the clinical practice and
research on ESCC.
In the present study, depleted GRHL1 was observed in
164/266 (61.7%) ESCC tissues. Additionally, the downregulation of
GRHL1 was revealed to be associated with a reduced patient
prognosis, and may serve as an independent prognostic factor in
ESCC. The study by Fabian et al reanalyzed the microarray
expression data from a cohort of 476 neuroblastoma cases (6,10).
Kaplan-Meier analysis demonstrated that the high level of GRHL1
expression in tumors is closely associated with improve the
patient's overall survival rate (6).
Mlacki et al investigated the role of GRHL1 in knockout mice
with cutaneous squamous cell carcinoma, and observed that the
tumors in knockout mice were also bigger in size and had earlier
onset (7). In the light of these
results we can state that loss of GRHL1 supports the progression of
papillomas to carcinomas in a mouse model. However, the loss of
GRHL1 does not effect the initiation of cancerous transformation in
keratinocytes. Considering Mlacki's observations, the present study
concluded that the loss of GRHL1 promotes the progression of
papillomas to carcinomas in the knockout mice model (7). It was consequently hypothesized that
GRHL1 exhibits tumor heterogeneity; however, further investigations
are required in order to investigate the function of GRHL1 in
various malignancies. Collectively, the results of the present
study indicated that low GRHL1 expression is associated with tumor
progression and patient clinical outcomes in ESCC.
GRHL1 has tumor-suppressive capacity, Fabian et
al demonstrated that GRHL1 is epigenetically and
transcriptionally subdued by the chromatin-altering chemical
histone deacetylase 3 (HDAC3) in association with MYCN (6). It has also been reported that the
re-expression of GRHL1 in MYCN-enhanced neuroblastoma cells
inhibits cell proliferation, in addition to impeding the
development in xenografts associated with the tumor at a molecular
level (6). The molecular mechanism
of GRHL1, in Lodrini's exploration, demonstrated that MYCN and
HDAC2 work together in order to inhibit microRNA-183, and has
tumor-suppressive attributes in neuroblastoma cells, via
co-localization to the miR-183 promoter (11). The particular molecular mechanism
through which GRHL1 assumes a tumor silencer role in ESCC has not
yet been completely elucidated, and therefore requires further
investigation.
The present study demonstrated that GRHL1 serves a
crucial role in the progression of ESCC. However, due to certain
limitations in the present study, the results obtained require
further investigation and verification. For example, due to
objective factors, the cell function experiments following the
inhibition of GRHL1 in cell lines were not performed completely. In
addition, the present study lacks sufficient immunohistochemistry
results for Clinical Stage IV ESCC tissues. Furthermore, the
specific molecular mechanism of the GRHL1 gene in tumor suppression
has not been completely elucidated, further research is still
required, and future studies should investigate whether the
promoter region of GRHL1 is methylated, resulting in downregulation
of its expression in ESCC. At the same time, it was detected
whether the downregulation of GRHL1 expression caused abnormal
apoptosis in ESCC, and the antitumor protection mechanism of the
organism was destroyed, resulting in uncontrolled tumor growth and
advanced cancer. We hypothesized that further research into the
function and mechanism of activity of GRHL1 will identify novel
therapeutic targets for ESCC, and future research will investigate
the exact mechanisms by which GRHL1 inhibits the progression of
ESCC.
In conclusion, the present study demonstrated that
GRHL1 is downregulated at the mRNA and protein levels in ESCC and
the low expression of GRHL1 is associated with a reduced OS in
ESCC. GRHL1 has tumor-suppressive capacity in ESCC, and has the
potential to serve as a novel prognostic biomarker and potential
therapeutic target in ESCC.
Acknowledgements
Not applicable.
Funding
The present study received support from the National
Natural Science Foundation of China (grant no. H1602).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ML, ZL, YQ and XG were responsible for study
conception and design. ML and ZL developed the methodology. ML, ZL
and YQ undertook the acquisition and analysis and interpretation of
data. ML, ZL and YQ undertook the writing, review, and/or revision
of the manuscript. Administrative, technical, or material support
was provided by ML, ZL and YQ, and YQ and XG supervised the study.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Ethics Review Board of the First Associated Hospital (Zhengzhou
University). All patients gave written informed consent to
participate in the study and the data were anonymized.
Patient consent for publications
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mlacki M, Kikulska A, Krzywinska E, Pawlak
M and Wilanowski T: Recent discoveries concerning the involvement
of transcription factors from the Grainyhead-like family in cancer.
Exp Biol Med (Maywood). 240:1396–1401. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bray SJ, Burke B, Brown NH and Hirsh J:
Embryonic expression pattern of a family of Drosophila proteins
that interact with a central nervous system regulatory element.
Genes Dev. 3:1130–1145. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wilanowski T, Tuckfield A, Cerruti L,
O'Connell S, Saint R, Parekh V, Tao J, Cunningham JM and Jane SM: A
highly conserved novel family of mammalian developmental
transcription factors related to Drosophila grainyhead. Mech Dev.
114:37–50. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fabian J, Lodrini M, Oehme I, Schier MC,
Thole TM, Hielscher T, Kopp-Schneider A, Opitz L, Capper D, von
Deimling A, et al: GRHL1 acts as tumor suppressor in neuroblastoma
and is negatively regulated by MYCN and HDAC3. Cancer Res.
74:2604–2616. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mlacki M, Darido C, Jane SM and Wilanowski
T: Loss of Grainy head-like 1 is associated with disruption of the
epidermal barrier and squamous cell carcinoma of the skin. PLoS
One. 9:e892472014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dorth JA, Pura JA, Palta M, Willett CG,
Uronis HE, D'Amico TA and Czito BG: Patterns of recurrence after
trimodality therapy for esophageal cancer. Cancer. 120:2099–2105.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Oberthuer A, Juraeva D, Li L, Kahlert Y,
Westermann F, Eils R, Berthold F, Shi L, Wolfinger RD, Fischer M
and Brors B: Comparison of performance of one-color and two-color
gene-expression analyses in predicting clinical endpoints of
neuroblastoma patients. Pharmacogenomics J. 10:258–266. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lodrini M, Oehme I, Schroeder C, Milde T,
Schier MC, Kopp-Schneider A, Schulte JH, Fischer M, De Preter K,
Pattyn F, et al: MYCN and HDAC2 cooperate to repress miR-183
signaling in neuroblastoma. Nucleic Acids Res. 41:6018–6133. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rice TW, Ishwaran H, Blackstone EH,
Hofstetter WL, Kelsen DP and Apperson-Hansen C; Worldwide
Esophageal Cancer Collaboration Investigators, : Recommendations
for clinical staging (cTNM) of cancer of the esophagus and
esophagogastric junction for the 8th edition AJCC/UICC staging
manuals. Dis Esophagus. 29:913–919. 2016. View Article : Google Scholar : PubMed/NCBI
|