Introduction
Malignant cells are characterized by uncontrolled
growth and distant metastasis. An abundant energy supply is the
foundation for cancer cell growth and metastasis, and as a result,
metabolism is enhanced in cancer cells. As the primary regulator of
cellular energy, AMP-activated protein kinase (AMPK) not only
serves an important role in energy metabolism, but is also involved
in cancer biology. AMPK is involved in tumorigenesis via the
promotion of epithelial mesenchymal transition (1), regulation of cell growth and
maintenance of tumor cell survival in numerous types of cancer.
AMPK also has the potential to be a therapeutic target for cancer
treatment (2,3). However, whether AMPK acts as an
oncogene to promote cancer initiation and progression or a tumor
suppressor is a matter of debate.
AMPK is a trimeric protein, and each subunit or
isoform may have particular functions in different cancer types,
which may account for the paradoxical effects of AMPK during
tumorigenesis. Thus, it is necessary to demonstrate the role and
mechanism of the AMPK isoforms separately. AMPK α1 (also known as
protein kinase AMP-activated catalytic subunit α1 and encoded by
the PRKAA1 gene), is the most catalytic isoform of AMPK, and
is predominantly expressed in the cytoplasm (4). Reportedly, the expression level of AMPK
α1 was markedly increased in cancerous tissues compared with paired
adjacent normal tissues, and the protein was preferentially
expressed in the less differentiated areas of tumors (5). Furthermore, the proliferative ability
of tumors was suppressed when AMPK α1 was inhibited (6). These findings indicated an oncogenic
role for AMPK α1.
The present study was undertaken to investigate the
role of AMPK α1 in non-small cell lung cancer (NSCLC). Tissue
microarrays were used to determine the expression level of AMPK α1
in tumor tissues and adjacent normal tissues. Furthermore,
lentiviruses were used to modulate the expression level of AMPK α1
in vitro. This aimed to determine whether AMPK α1 acts in a
malignant manner in NSCLC, and ultimately the target of AMPK α1
Materials and methods
Patients and tissue samples
A total of 99 clinical NSCLC specimens were
assessed. The tumor tissues and matched adjacent non-tumor tissues
were collected from patients who had undergone surgical treatment
at Subei People's Hospital (Yangzhou, China) between May 2004 and
October 2012. The mean age of patients was 36–84 years (62.46±9.59)
and patient information is presented in Table I. The present study was approved by
the ethics board of Subei People's Hospital, and informed consent
was obtained from all participants. Fresh samples were
paraffin-embedded and verified by pathological examination
following hematoxylin and eosin staining. Tissues were fixed with
10% formalin for 24 h at room temperature and paraffin embedded.
Samples were sliced into 4-µm sections, which were stained with
hematoxylin and eosin at room temperature for 10 and 2 min,
respectively. Samples were examined by two pathologists who had no
prior knowledge of the patients' clinicopathological information,
using a light microscope in three fields and with a ×400
magnification.
 | Table I.Clinicopathological characteristics of
patients with non-small cell lung cancer. |
Table I.
Clinicopathological characteristics of
patients with non-small cell lung cancer.
| Variables | Cases, n | Cases, % |
|---|
| Age (years) |
|
|
|
<60 | 37 | 34.3 |
| ≥60 | 62 | 65.7 |
| Sex |
|
|
| Male | 73 | 73.7 |
|
Female | 26 | 26.3 |
| Histotype |
|
|
|
Adenocarcinoma | 51 | 51.5 |
| Squamous
cell | 48 | 48.5 |
| Primary tumor |
|
|
| T1 | 21 | 21.2 |
| T2 | 54 | 54.5 |
| T3 | 17 | 17.2 |
| T4 | 7 | 7.1 |
| Regional lymph
node |
|
|
| N0 | 67 | 67.7 |
| N1 | 19 | 19.2 |
| N2 | 11 | 11.1 |
| N3 | 2 | 2.0 |
| Tumor-node-metastasis
stage |
|
|
| I–II | 58 | 58.6 |
|
III–IV | 41 | 41.4 |
Cell culture
The human lung adenocarcinoma cell line A549
(Yingrun Biotechnologies Inc., Changsha, China) was used for
experiments in vitro. Parental A549 cells and
lentivirus-transfected cells were cultured in RPMI-1640 medium
supplemented with 10% fetal bovine serum (Biological Industries,
Kibbutz Beit Haemek, Israel). Cells were maintained at 37°C in a
humidified atmosphere (5% CO2).
Tissue microarray (TMA) and
immunohistochemistry (IHC)
A total of 99 pairs of NSCLC tumor tissue samples
and adjacent non-tumor tissue samples were fixed with 10% formalin
for 24 h at room temperature and paraffin embedded to obtain the
TMAs. Using biopsy needles, tissue cores (diameter, 3 mm) were
obtained and placed on a recipient block. IHC was carried out using
streptavidin-biotin-peroxidase complex method. TMA slides for IHC
were deparaffinized by bathing in xylene solution for 10 min, and
rehydrated through decreasing ethanol gradient (100, 90, 75 and
50%) for 5 min. Antigen retrieval was performed by incubating
slides with citrate buffer (pH 6.0) at 95°C for 15 min. Endogenous
peroxidase activity was blocked by incubating slides in 3%
H2O2 at room temperature for 15 min. The
slides were then incubated with primary antibodies against AMPK α1
(1:100; cat. no. D63G4; Cell Signaling Technology, Inc., Danvers,
MA, USA) and VEGFA (1:100; cat. no. WL00009b; Wanleibio Co., Ltd.,
Shanghai, China) overnight at 4°C. The slides were incubated with
biotin-labeled anti-rabbit secondary antibodies (1:100; cat. no.
A0279; Beyotime Institute of Biotechnology, Haimen, China) at room
temperature for 30 min, and detected with 3,3-diaminobenzidine
(1:300; cat. no. P0203; Beyotime Institute of Biotechnology).
Staining intensity and extent were examined using a light
microscope at ×400 magnification. Staining intensity was scored as
0, 1, 2 or 3 for negative, weak, moderate or strong staining,
respectively. The extent of staining was scored based on the
percentage of AMPK α1- or VEGF-positive tumor cells. The final
score was obtained by summing the scores for staining intensity and
extent of staining.
Lentivirus and cell infection
To modulate AMPK α1 expression in vitro,
lentiviruses, including LV-PRKAA1-RNAi (expressing a short
interfering RNA targeting the PRKAA1 gene;
5′-CTTTGCTTCTCTTATAAGTTATTGTGA-3′), LV-PRKAA1 (a lentivirus
overexpressing the PRKAA1 gene), and a negative LV-control
were purchased from Shanghai GeneChem Co., Ltd. (Shanghai, China).
A549 cells were infected with lentivirus according to the
manufacturer's protocol. Briefly, 5×104 cells
supplemented with lentivirus (multiplicity of infection, 10) and 1
µl Polybrene (Shanghai GeneChem Co., Ltd.) were grown on 24-well
plates. The supernatant was removed after 24 h and fresh culture
medium was added to the cells. Fluorescence microscopy was used to
observe the infection rate 72 h postinfection, and stable clones
were selected after 2 weeks using puromycin (2 µg/ml) (GE
Healthcare Life Sciences, Little Chalfont, UK). Western blotting
was conducted to confirm the final infection efficiency.
Western blotting
Cells were harvested and lysed with
radioimmunoprecipitation assay buffer (cat. no. P0013B; Beyotime
Institute of Biotechnology) and the protein concentration was
measured using a bicinchoninic acid assay. Sample concentrations
were equalized, and proteins (29 µg, Fig. 4A; 15 µg, Fig. 4B) were separated by SDS-PAGE (10%
gels). The proteins were subsequently transferred to polyvinylidene
fluoride membranes and blocked with 5% skimmed milk in
Tris-buffered saline for 2 h at room temperature. The membranes
were incubated with primary antibodies against AMPK α1 (1:1,000;
cat. no. D63G4; Cell Signaling Technology, Inc.) and vascular
endothelial growth factor A (VEGFA; 1:500; cat. no. WL00009b;
Wanleibio Co., Ltd) overnight at 4°C, prior to incubation with a
secondary horseradish peroxidase-labeled goat anti-rabbit antibody
(1:1,000; cat. no. KGAA35; Nanjing KeyGen Biotech Co., Ltd.,
Nanjing, China) for 1 h at room temperature. Bands were visualized
using BeyoECL Plus (Beyotime Institute of Biotechnology). The
primary antibody against GAPDH, (1:1,000, cat. no. 14C10; Cell
Signaling Technology, Inc.) was used as an internal control.
Densitometric analysis was performed using Image J v1.50i Software
(National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Statistical analysis was performed using SPSS 22.0
(IBM Corp., Armonk, NY, USA). Data were expressed as the mean ±
standard deviation, and analyzed using the Student's t-test. A
Chi-square test was used to evaluate the association between AMPK
α1 and VEGF. Kaplan-Meier curves were generated to assess overall
survival (OS). Patients were classified based on the AMPK α1 or
VEGF expression level in their tumor tissue into high expression (≥
median; n=49) or low expression (< median; n=50) groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Patients characteristics
The characteristics of the 99 surgically resected
NSCLC tumors (51 adenocarcinoma and 48 squamous cell carcinoma
samples) are displayed in Table I.
Elderly patients (≥60 years old) accounted for 65.7%, of which 73
were male (73.7%) and 26 female (26.3%). The proportion of patients
with early and late stage NSCLC was 58.6 and 41.4%,
respectively.
High expression of AMPK α1 is
associated with poor prognosis in NSCLC
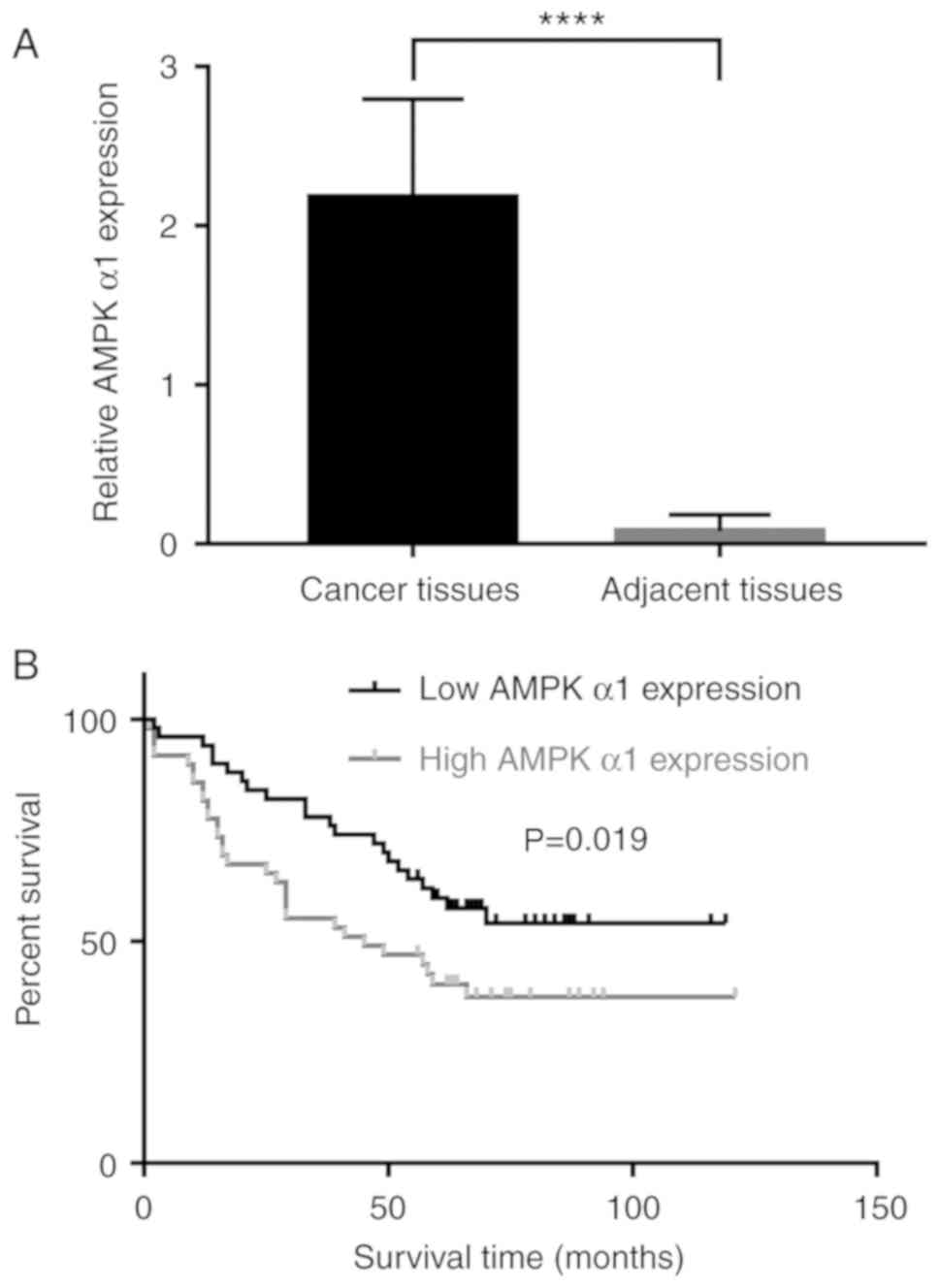
The expression levels of AMPK α1 in NSCLC tumor
tissues were compared with those in adjacent non-tumor lung
tissues; AMPK α1 expression levels were significantly higher in
tumor tissues compared with those in the adjacent tissues (Fig. 1A). The Kaplan-Meier method was used
to determine the association between AMPK α1 expression level and
prognosis. Patients with high AMPK α1 expression levels had a
shorter OS time compared with patients with low AMPK α1 levels
(Fig. 1B). According to these
results, AMPK α1 may serve an aggressive role in NSCLC, and high
levels of AMPK α1 may act as an indicator of poor prognosis in
patients with NSCLC.
VEGF expression levels are associated
with prognosis in patients with NSCLC
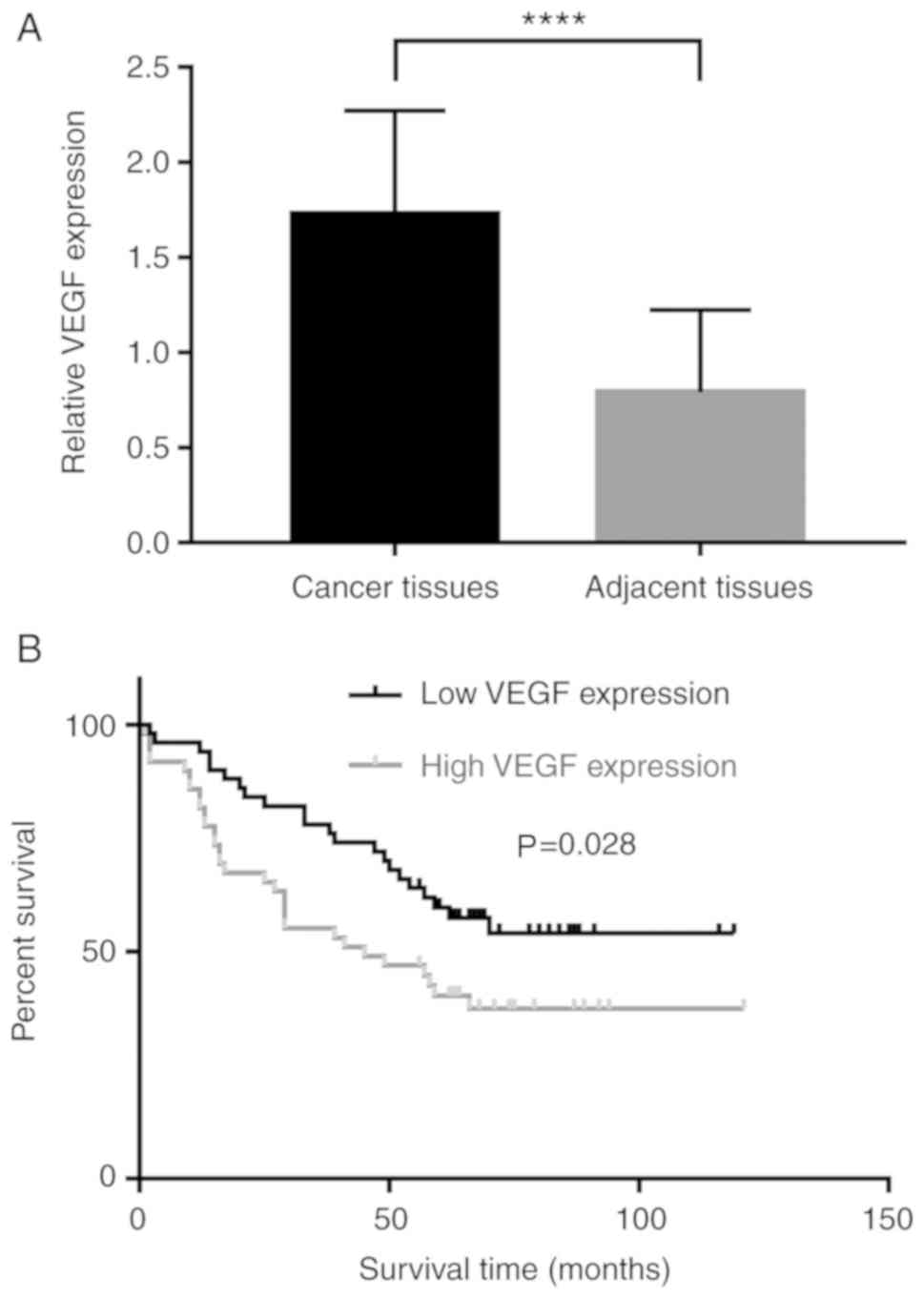
Angiogenesis has a prominent role during cancer
occurrence, development, and metastasis. VEGF, an
angiogenesis-driving factor, influences malignancy and is
associated with poor prognosis in various types of cancer (7,8).
Therefore, the association between VEGF and AMPK α1 levels in NSCLC
were investigated. Consistent with previous reports (9,10), it
was concluded that VEGF was more highly expressed in NSCLC tissues
compared with adjacent non-tumor lung tissues (Fig. 2A), and higher levels of VEGF were
associated with a poorer prognosis (Fig.
2B).
Patients with NSCLC that express high
levels of AMPK α1 and VEGF have a shorter OS
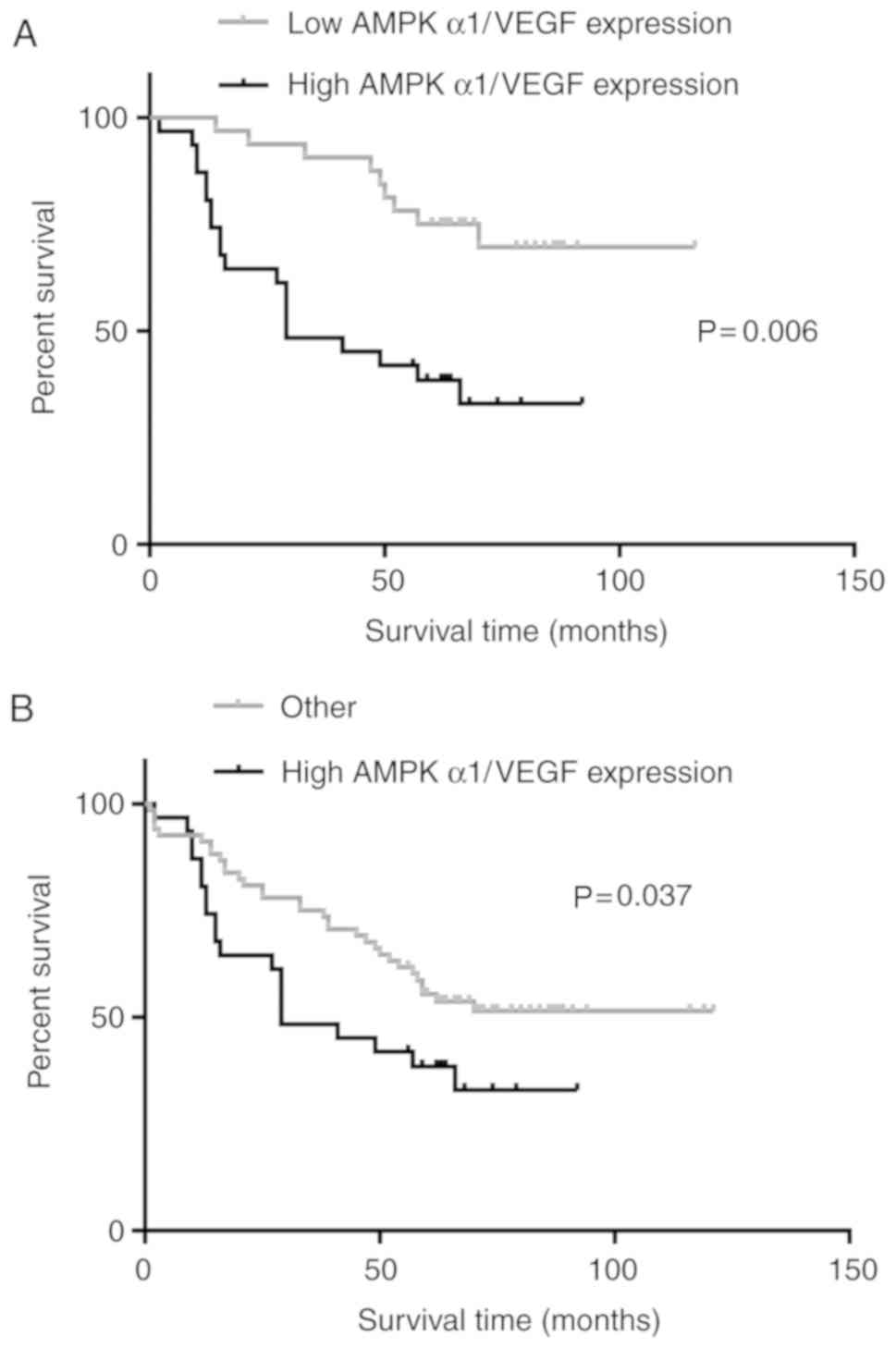
Based on the conclusion that AMPK α1 and VEGF are
associated with NSCLC, patients that expressed high levels of AMPK
α1 and VEGF (n=31; Table II) were
selected and further classified. Patients that expressed high
levels of AMPK α1 and VEGF had a significantly shorter OS compared
with those that expressed low levels of AMPK α1 and VEGF, and those
with all other expression level combinations (AMPK α1 low, VEGF
low; AMPK α1 high, VEGF low; and AMPK α1 low, VEGF high; Fig. 3A and B).
 | Table II.Association between the expression
levels of AMPK α1 and VEGF. |
Table II.
Association between the expression
levels of AMPK α1 and VEGF.
|
| AMPK α1
expression |
|
|---|
|
|
|
|
|---|
| VEGF expression | High | Low | P-value |
|---|
| High | 31 | 18 | 0.006 |
| Low | 18 | 32 |
|
AMPK α1 positively regulates VEGF
expression in NSCLC in vitro
The potential regulatory association between AMPK α1
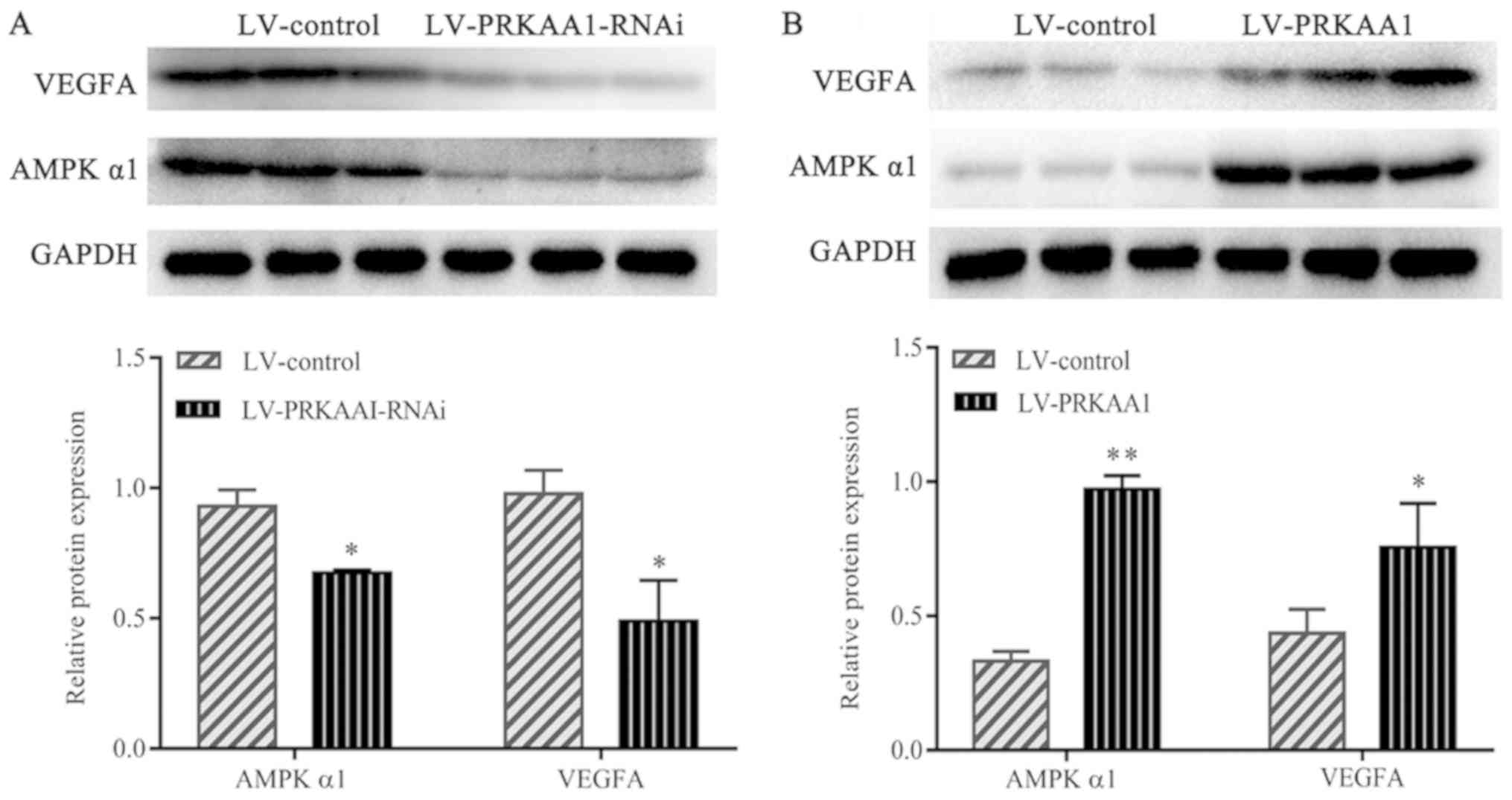
and VEGF in NSCLC was investigated. Western blotting was performed
to detect the effects AMPK α1 expression level on that of VEGF. As
illustrated in Fig. 4A,
downregulation of AMPK α1 resulted in decreased VEGFA protein
expression in A549 cells, and upregulation of AMPK α1 resulted in
increased VEGFA protein expression (Fig.
4B). The results indicated that AMPK α1 positively regulates
VEGF expression in NSCLC.
Discussion
Lung cancer is a commonly occurring malignancy with
high mortality rates worldwide (11). Based on cell morphology, lung cancer
is predominantly divided into two types: NSCLC and small cell lung
cancer (SCLC), which account for 80 and 20% of all lung cancer
cases, respectively. Although the detection rate and treatment have
improved over time, the clinical outcomes of patients with lung
cancer remain unsatisfactory (12,13). In
addition, compared with SCLC, patients with NSCLC are less
sensitive to chemotherapy. To achieve an improved outcome for
patients with NSCLC, further studies to understand the mechanisms
underlying tumorigenesis and metastasis, and the development
effective treatments are required.
In the present study it was demonstrated that AMPK
α1 was highly expressed in tumor tissues compared with non-tumor
lung tissues, and overexpression of AMPK α1 was negatively
associated with prognosis in patients with NSCLC. This observation
of the aggressive role of AMPK α1 in NSCLC was consistent with
previous findings (6,14). However, AMPK α1 has also been
indicated as a tumor suppressor (15), and its deletion reportedly promotes
cell proliferation and angiogenesis (16). This discrepancy in different cancer
types may be the result of different tissue systems, in addition to
different targets and associated pathways. Furthermore, it was
revealed that AMPK α1 positively regulated VEGF expression.
Considering that VEGF is a well-known tumor driver gene, it was
hypothesized that the malignant role of AMPK α1 may be influenced
by the regulation of VEGF expression in NSCLC. Anti-angiogenesis of
cancer cells is a promising method to treat cancer. However to
date, its efficacy has been limited (17). In the present study, a positive
regulatory association was observed between AMPK α1 and VEGF, such
that patients possessing tumors with high levels of these proteins
had poorer clinical outcomes. Therefore, simultaneous targeting of
AMPK α1 and VEGF may enhance the anti-cancer efficacy of anti-VEGF
therapy.
The present study was limited in the following ways:
Firstly, this was a retrospective, single institution study, and
the number of specimens used to analyze the association between
AMPK α1 and prognosis was small. Moreover, the exact mechanism of
AMPK α1 regulation of VEGF in NSCLC was not investigated.
In conclusion, the present study identified an
aggressive pro-cancerous effect of high levels of AMPK α1 in
patients with NSCLC. A regulatory association between AMPK α1 and
VEGF was also demonstrated. The results indicated that AMPK α1 is a
potential biomarker and therapeutic target in NSCLC, which requires
further investigation in vitro and in vivo.
Acknowledgements
Not applicable
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81870033, 81302016
and 81302015), the National Natural Science Foundation of Jiangsu
Province (grant nos. BK20130456 and BK20140101) and the Six Talent
Peaks Project of Jiangsu Province (grant no. WSN-106).
Availability of data and materials
The datasets used during the current study are
available from the corresponding author on reasonable request.
Authors' contributions
XXX, HSW and MLF made contributions to conception
and design of this study. WYX, CY, CBY, ZJ, GJJ and YJJ analyzed
and interpreted the patients' data. GDH contributed to the
conception and the design of this study and wrote the manuscript.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics Board
of Subei People's Hospital (Yangzhou, China), and written informed
consent was obtained from all participants.
Patient consent for publication
All of the patients provided written informed
consent for the publication of any associated data.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AMPK α1
|
AMP-activated protein kinase α1
|
|
VEGF
|
vascular endothelial growth factor
|
|
NSCLC
|
non-small cell lung cancer
|
|
OS
|
overall survival
|
References
|
1
|
Cazarin JM, Coelho RG, Hecht F, Andrade BM
and Carvalho DP: 5′-AMP-activated protein kinase regulates
papillary (TPC-1 and BCPAP) thyroid cancer cell survival,
migration, invasion, and epithelial-to-mesenchymal transition.
Thyroid. 26:933–942. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zou J, Hong L, Luo C, Li Z, Zhu Y, Huang
T, Zhang Y, Yuan H, Hu Y, Wen T, et al: Metformin inhibits
estrogen-dependent endometrial cancer cell growth by activating the
AMPK-FOXO1 signal pathway. Cancer Sci. 107:1806–1817. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zadra G, Photopoulos C, Tyekucheva S,
Heidari P, Weng QP, Fedele G, Liu H, Scaglia N, Priolo C, Sicinska
E, et al: A novel direct activator of AMPK inhibits prostate cancer
growth by blocking lipogenesis. EMBO Mol Med. 6:519–538. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Choi CH, Chung JY, Cho H, Kitano H, Chang
E, Ylaya K, Chung EJ, Kim JH and Hewitt SM: Prognostic significance
of AMP-dependent kinase alpha expression in cervical cancer.
Pathobiology. 82:203–2011. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huang FY, Chiu PM, Tam KF, Kwok YK, Lau
ET, Tang MH, Ng TY, Liu VW, Cheung AN and Ngan HY:
Semi-quantitative fluorescent PCR analysis identifies PRKAA1 on
chromosome 5 as a potential candidate cancer gene of cervical
cancer. Gynecol Oncol. 103:219–225. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Laderoute KR, Calaoagan JM, Chao WR, Dinh
D, Denko N, Duellman S, Kalra J, Liu XH, Papandreou I, Sambucetti L
and Boros LG: Experimental human breast cancer supports the growth
of aggressive 5′-AMP-activated protein kinase (AMPK). J Biol Chem.
289:22850–22864. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lin Y, Liu F, Fan Y, Qian X, Lang R, Gu F,
Gu J and Fu L: Both high expression of pyruvate kinase M2 and
vascular endothelial growth factor-C predicts poorer prognosis in
human breast cancer. Int J Clin Exp Pathol. 8:8028–8037.
2015.PubMed/NCBI
|
|
8
|
Yalniz Z, Tigli H, Tigli H, Sanli O, Dalay
N and Buyru N: Novel mutations and role of the LKB1 gene as a tumor
suppressor in renal cell carcinoma. Tumour Biol. 35:12361–12368.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yuan Y, Min SJ, Xu DQ, Shen Y, Yan HY,
Wang Y, Wang W and Tan YJ: Expressions of VEGF and miR-21 in tumor
tissues of cervical cancer patients with HPV infection and their
relationships with prognosis. Eur Rev Med Pharmacol Sci.
22:6274–6279. 2018.PubMed/NCBI
|
|
10
|
Wang F, Peng L, Wang Y and Liu X: A
Meta-analysis of vascular endothelial growth factor for
nasopharyngeal cancer prognosis. Front Oncol. 8:4862018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bagcchi S: Lung cancer survival only
increases by a small amount despite recent treatment advances.
Lancet Respir Med. 5:1692017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ettinger DS, Wood DE, Akerley W, Bazhenova
LA, Borghaei H, Camidge DR, Cheney RT, Chirieac LR, D'Amico TA,
Demmy TL, et al: Non-small cell lung cancer, version 6.2015. J Natl
Compr Canc Netw. 13:515–524. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu H, Zhou Y, Coughlan KA, Ding Y, Wang S,
Wu Y, Song P and Zou M: AMPKα1 deficiency promotes cellular
proliferation and DNA damage via p21 reduction in mouse
embryonic fibroblasts. Biochim Biophys Acta. 1853:65–73. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Faubert B, Boily G, Izreig S, Griss T,
Samborska B, Dong Z, Dupuy F, Chambers C, Fuerth BJ, Viollet B, et
al: AMPK is a negative regulator of the Warburg effect and
suppresses tumor growth in vivo. Cellmetab. 17:113–1124. 2013.
|
|
16
|
Zhou Y, Xu H, Ding Y, Lu Q, Zou MH and
Song P: AMPKα1 deletion in fibroblasts promotes tumorigenesis in
athymic nude mice by p52-mediated elevation of
erythropoietin and CDK2. Oncotarget. 7:53654–53667. 2016.PubMed/NCBI
|
|
17
|
Singh R, Kim WJ, Kim PH and Hong HJ:
Combined blockade of HER2 and VEGF exerts greater growth inhibition
of HER2-overexpressing gastric cancer xenografts than individual
blockade. Exp Mol Med. 45:e522013. View Article : Google Scholar : PubMed/NCBI
|