Introduction
Cellular components of bone marrow have important
roles in pre-metastatic niche (PMN) formation (1). In lung cancer, distant metastases are
common and this type of cancer usually spreads to the bone (39%),
liver (35%) and central nervous system (47%) (2). Patients with lung cancer and metastasis
have a poor prognosis with a shortened median survival time
following diagnosis (3). In order to
metastasize, tumor cells need an organ with a suitable environment
for their growth and proliferation, which is defined as the
metastatic niche (4).
Cancer cells initiate and establish the
environmental surroundings required for future metastasis through
various mechanisms, including cancer cell intravasation, immune
evasion and arrival at designated site, extravasation, colonization
and tumor growth (5). The bone
marrow microenvironment has been described as fertile ground for
dormant and proliferating tumor cells. For example, bone marrow and
tumor cells can modify the activity of osteoclasts (6), and pro-tumorigenic cells, including
mesenchymal stem cells, have been reported to serve a crucial role
in promoting osteolytic bone metastasis and tumor cell
proliferation in the tumor microenvironment (7). Additional tumor-derived factors have
been reported to promote tumor progression. These factors can
stimulate the differentiation of immature myeloid cells into strong
immune response suppressors and therefore inhibit the activation of
antitumor T cells (8).
Numerous factors, including tumor-derived secreted
factors and extracellular vesicles, are involved in PMN
establishment (5). In addition,
other cell types, including bone marrow-derived cells (BMDCs) such
as mesenchymal stem cells and regulatory T cells, are directed to
the secondary organs. Once these cells have reached the PMN, they
modify its local microenvironment through inflammatory cytokines,
growth factors and proangiogenic molecules to facilitate tumor cell
colonization and proliferation, and therefore promote tumor
metastasis (4,5,9).
Notably, a PMN is established through the
combination of various tumor-derived factors, tumor-mobilized BMDCs
and the local environment (5,9).
However, the role of BMDCs in PMN formation is not yet fully
understood. In the present study, it was hypothesized that lung
cancer cells can remotely modify BMDCs, which could therefore
become actively involved in PMN establishment in target organs. The
present study aimed to investigate the role of BMDCs in lung cancer
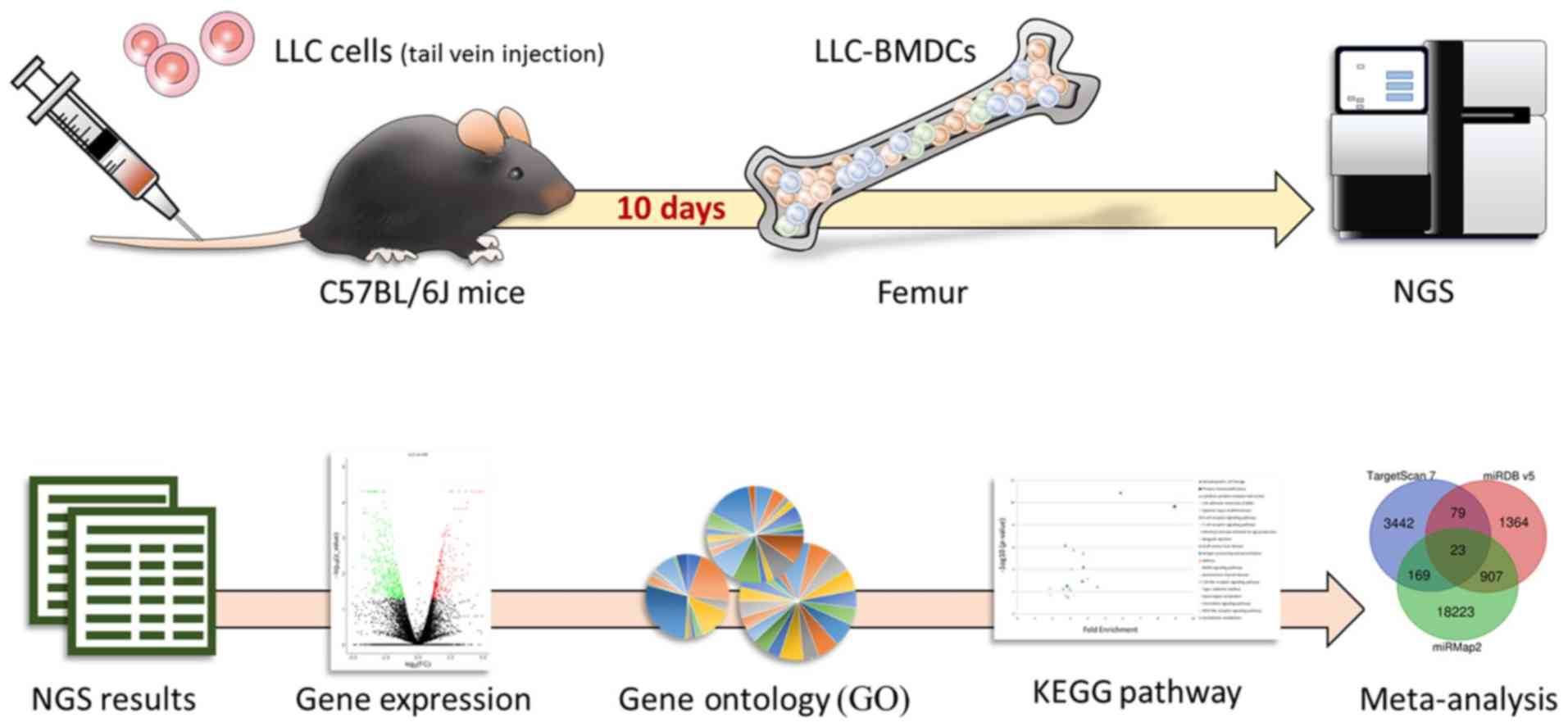
metastasis from a macroscopic perspective (Fig. 1). To do so, bone marrow tissue
samples were examined by next generation sequencing (NGS).
Materials and methods
Cell culture
The LL/2 mouse Lewis lung carcinoma (LLC) cell line
[LLC1; American Type Culture Collection (ATCC)®
CRL-1642™] was purchased from the ATCC (Manassas, VA, USA) and
cultured in Dulbecco's modified Eagle's medium (DMEM) containing
10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) in an incubator containing 5% CO2 at
37°C.
BMDCs isolation
Male C57BL/6J mice (age, 8 weeks; weight, 20–25 g)
were obtained from the National Laboratory Animal Center (Taipei,
Taiwan). These mice were housed at a constant temperature (21±1°C)
and humidity (55–65%) under a 12-h light/dark cycle. Mice had free
access to food and water. Animal experiments were performed
according to the Institutional Animal Care and Use Committee, with
the approval of the Animal Care and Use Committee of the School of
Kaohsiung Medical University (Kaohsiung, Taiwan). The study group
contained six mice that were injected with 1×106 LLC
cells, whereas the control group comprised six mice that received
sham injection with DMEM. All injections were administered into the
tail vein. Mice were sacrificed after 10 days. The LLC-BMDCs and
normal-BMDCs were obtained after femoral bone removal, followed by
flushing the bone marrow out from the femurs bilaterally with PBS.
The collected BMDCs were suspended in DMEM (10). The BMDCs from mice contained in each
group were pooled prior to further experiments.
RNA sequencing
Total RNA was isolated from BMDCs using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. RNA purity was
measured by assessing optical density
(OD)260nm/OD280nm absorbance ratio (1.96 for
normal-BMDCs and 2.03 for LLC-BMDCs) with an ND-1000
spectrophotometer (NanoDrop Technologies; Thermo Fisher Scientific,
Inc., Wilmington, DE, USA). RNA concentration was determined
according to the RNA integrity number (RIN; RIN, 10 for
normal-BMDCs and LLC-BMDCs) with an Agilent Bioanalyzer (Agilent
Technologies, Inc., Santa Clara, CA, USA).
Library preparation and deep sequencing were
performed using Illumina Solexa system (Illumina, Inc., San Diego,
CA, USA) according to the manufacturer's protocol, as previously
described (11,12). For small RNA sequencing, total RNA
was reverse transcribed using TruSeq Small RNA Sample Prep kit
(Illumina, Inc.) following the manufacturer's instructions, and
cDNA (18–40-nucleotide RNA fragments; 140–155 nucleotides in length
with both adapters) was sequenced on the Illumina system (75
single-end cycles). After trimming or removing low-quality data
using Trimmomatic software version 0.36 (13), the qualified reads were analyzed
using miRDeep2 software (miRBase 21) (14) and the human genome from the
University of California Santa Cruz database (https://genome.ucsc.edu/). Micro (mi)RNAs with low
levels [<1 normalized read per million (RPM)] in normal- and
LLC-BMDCs were excluded. For transcriptome sequencing, the library
constructed with SureSelect Strand Specific RNA Library Preparation
kit (Agilent Technologies, Inc.) was sequenced using a TruSeq SBS
kit on the Solexa platform (Illumina NextSeq; 75 cycles, single-end
or paired-end). After trimming or removing low-quality data using
Trimmomatic software (13), the
qualified reads were analyzed using TopHat/Cufflinks (15) and Ensembl (https://www.ensembl.org/index.html) databases. Genes
with low expression levels (<0.3 fragment per kilobase of
transcript per million mapped reads) in normal- and LLC-BMDCs were
excluded. The criteria of differentially expressed genes (DEGs)
were -log10 (P-value) >1.3 and >2 fold change
(FC), and -log10 (q-value) >0.6 and q<0.25 for
stringent criteria.
miRNA database analysis
Putative genes targeted by candidate miRNAs were
predicted using the miRmap database (http://cegg.unige.ch/mirmap) (16). The search criteria of the putative
targeted genes by miRNAs were ‘mice species’ and a miRmap score of
>99.0. Potential miRNA interactions were also searched using the
miRmap TargetScan (http://www.targetscan.org/vert_71/) and miRDB
(http://www.mirdb.org/) databases.
Gene Ontology (GO) and Kyoto
Encyclopedia of Genes and Genomes (KEGG) pathway database
analysis
The functions of DEGs were studied by analyzing the
genes of interest according to previous studies (11,12). The
Database for Annotation, Visualization and Integrated Discovery
(https://david.ncifcrf.gov/) was used to
conduct GO and KEGG database analyses. Briefly, a panel of
potential genes was classified into clusters of associated
biological functions, signaling pathways and diseases by
calculating the similarity of global annotation profiles using the
agglomeration algorithm method.
Results
Gene expression profiling and miRNA
alterations in LLC-BMDCs versus normal BMDCs
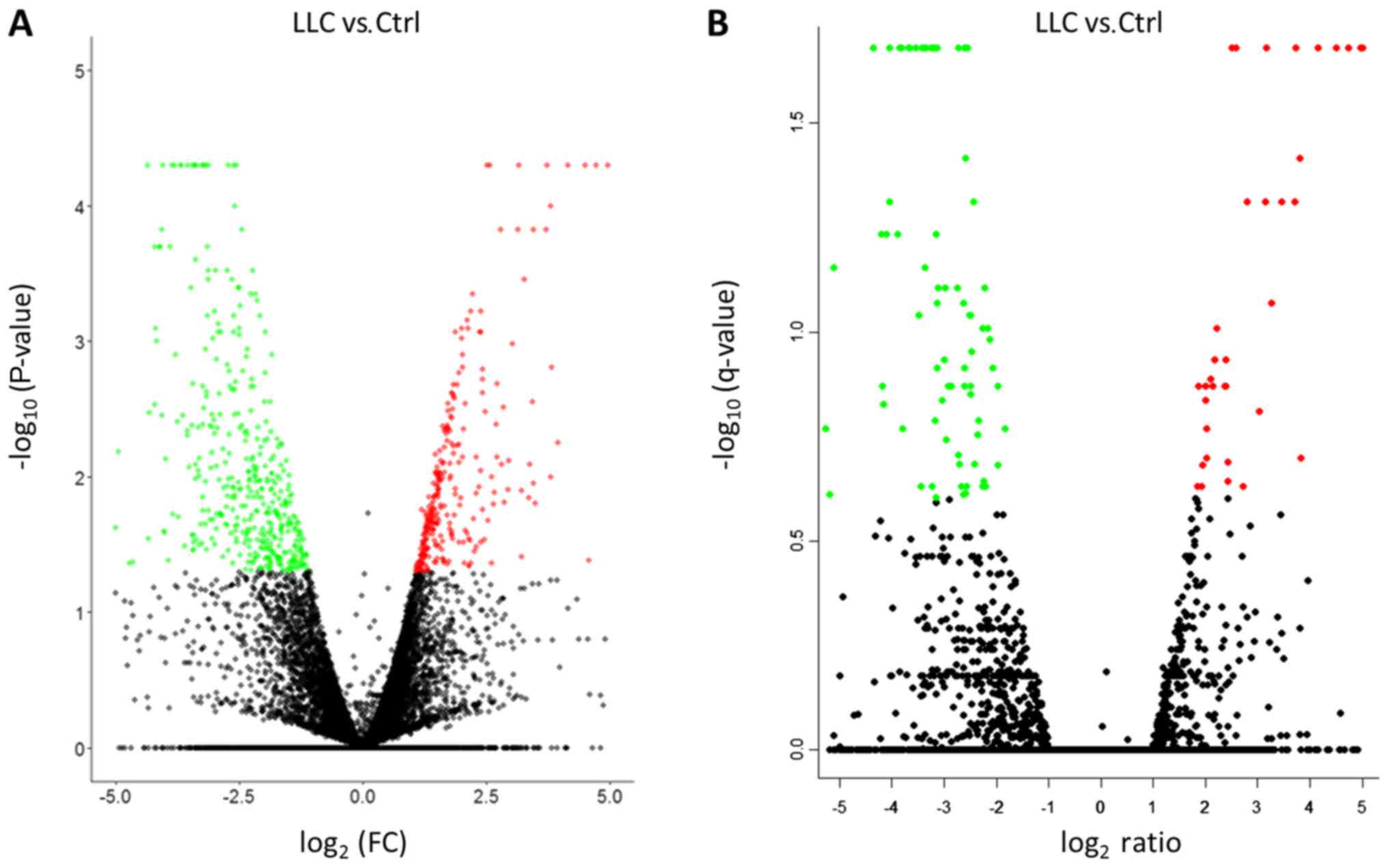
The differentially downregulated (green spots, left
panel) and upregulated (red spots, right panel) genes in the
LLC-BMDCs versus normal-BMDCs were presented as a volcano plot
(Fig. 2). A total of 820 genes with
-log10 (P-value) >1.3 and >2 FC were chosen for
further analyses (Fig. 2A). However,
when using the following more stringent criteria: -log10
[q-value] >0.6 and FDR<0.25, only 139 genes were detected
(Fig. 2B). Using the stringent
criteria, too few genes were obtained to be analyzed.
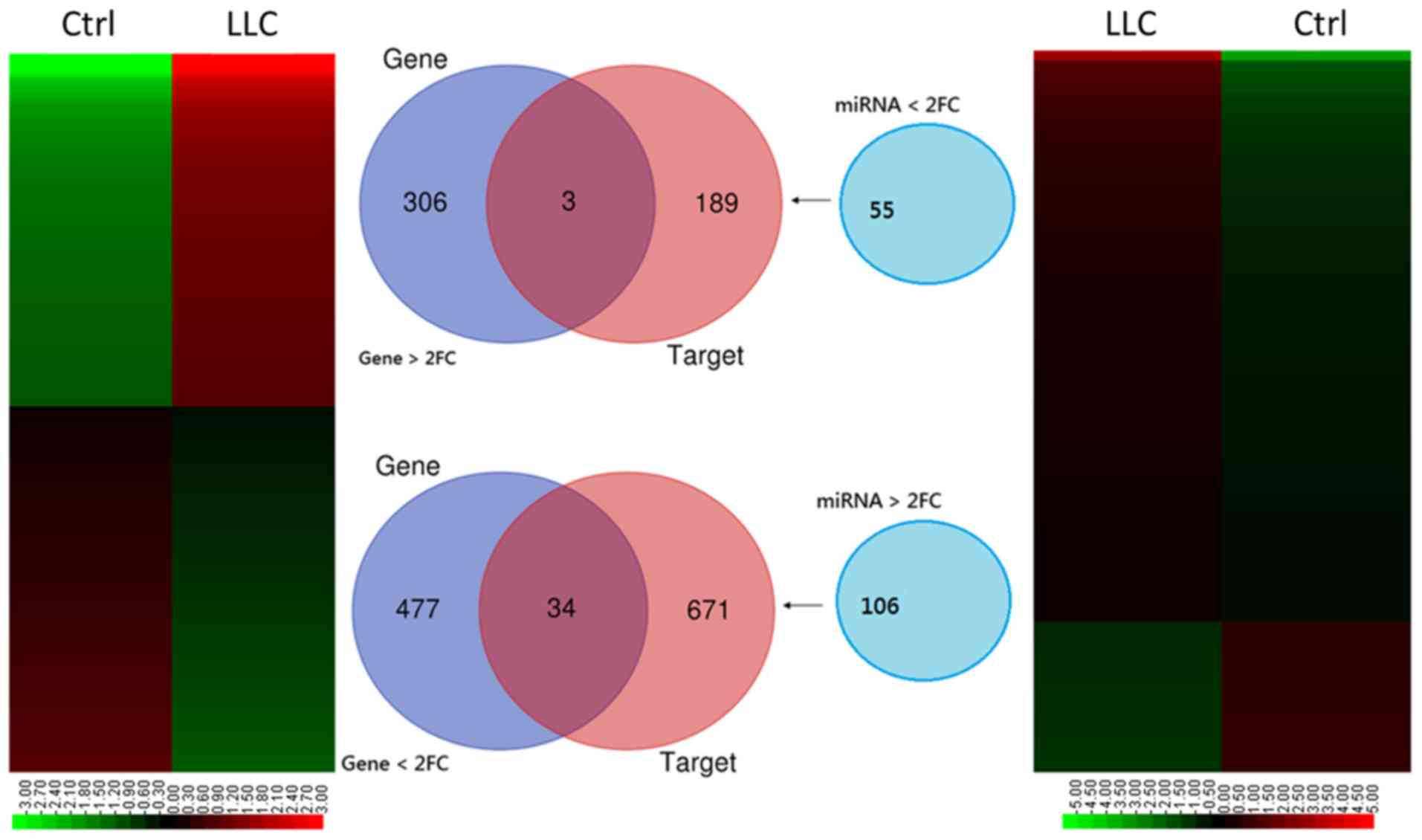
The 820 genes expressed on the RNA sequencing
heatmap (Fig. 3; left panel)
revealed DEGs with FC>2 (either increase or decrease). The
‘gene’ Venn diagram demonstrated that 309 genes were upregulated
and 511 were downregulated in the LLC-BMDCs compared with
normal-BMDCs (Fig. 3). NGS miRNA
heatmap (Fig. 3; right panel)
analysis highlighted 161 differentially expressed miRNAs with
FC>2 and P<0.05. Results from the ‘miRNA’ Venn diagram
revealed the miRNAs with a threshold of RPM>1, which included 55
precursor miRNAs that were downregulated and 106 precursor miRNAs
that were upregulated in BDMCs. The ‘targets’ Venn diagram
exhibited the predicted genes of the miRNAs from the ‘miRNA’ Venn
diagram using the miRmap online database. The selection threshold
was a miRmap score ≥99.0. The intersection in the Venn diagram
between ‘genes’ and ‘targets’ revealed 37 potential miRNA-mRNA
interactions (Fig. 3), of which 34
included downregulated and three included upregulated genes
(Table I).
 | Table I.Potential miRNA-mRNA interactions in
LLC-BMDCs. |
Table I.
Potential miRNA-mRNA interactions in
LLC-BMDCs.
| Gene symbol | Associated
miRNA | Gene | log2
ratio (LLC/Ctrl) | Expression |
|---|
| BMPR1A |
mmu-miR-135a-5p | Bone morphogenetic
protein receptor, type 1A | 2.80 | Up |
| LRRC75B | mmu-miR-150-5p | Leucine rich repeat
containing 75B | 5.41 | Up |
| FAM217B |
mmu-miR-195a-5p | Family with
sequence similarity 217, member B | 2.93 | Up |
| AXL |
mmu-miR-1249-5p | AXL receptor
tyrosine kinase | −2.89 | Down |
| PHYHIP |
mmu-miR-1249-5p | Phytanoyl-CoA
hydroxylase interacting protein | −1.73 | Down |
| FOSB |
mmu-miR-1249-5p | FosB
proto-oncogene, AP-1 transcription factor subunit | −1.75 | Down |
| CACNA1E |
mmu-miR-1249-5p | Calcium channel,
voltage-dependent, R type, α1E subunit | −1.92 | Down |
| MPP2 |
mmu-miR-1249-5p | Membrane protein,
palmitoylated 2 (MAGUK p55 subfamily member 2) | −1.79 | Down |
| ZBTB4 |
mmu-miR-1249-5p | Zinc finger and BTB
domain containing 4 | −2.02 | Down |
| ADAM11 |
mmu-miR-1249-5p | A disintegrin and
metallopeptidase domain 11 | −1.69 | Down |
| OTUB2 |
mmu-miR-3085-3p | OTU domain,
ubiquitin aldehyde binding 2 | −1.87 | Down |
| NR1D2 |
mmu-miR-148a-5p | Nuclear receptor
subfamily 1, group D, member 2 | −1.93 | Down |
| LYNX1 |
mmu-miR-1249-5p | Ly6/neurotoxin
1 | −2.04 | Down |
| Fhl1 |
mmu-miR-1249-5p | Four and a half LIM
domains 1 | −2.91 | Down |
| Cd4 |
mmu-miR-1249-5p | CD4 antigen | −2.09 | Down |
| EHD3 |
mmu-miR-1249-5p | EH-domain
containing 3 | −1.68 | Down |
|
DNASE1L3 |
mmu-miR-1249-5p | Deoxyribonuclease
1-like 3 | −3.31 | Down |
| SDC3 |
mmu-miR-1249-5p | Syndecan 3 | −2.92 | Down |
| NFATC2 |
mmu-miR-1249-5p | Nuclear factor of
activated T cells 2 | −1.33 | Down |
| TNIK |
mmu-miR-1198-3p | TRAF2 and NCK
interacting kinase | −1.71 | Down |
| PDE4B |
mmu-miR-1249-5p | Phosphodiesterase
4B | −1.55 | Down |
| BCL7A |
mmu-miR-1249-5p | BAF chromatin
remodeling complex subunit BCL7A | −1.50 | Dpwn |
| DUSP16 |
mmu-miR-1249-5p | Dual specificity
phosphatase 16 | −1.64 | Down |
| SORBS2 | mmu-miR-21a-3p | Sorbin and SH3
domain containing 2 | −4.21 | Down |
| THY1 |
mmu-miR-1249-5p | Thy-1 cell surface
antigen | −1.48 | Down |
| CAPN5 |
mmu-miR-1249-5p | Calpain 5 | −1.53 | Down |
| TRIM58 |
mmu-miR-1249-5p | Tripartite
motif-containing 58 | −1.95 | Down |
| CAMK1D |
mmu-miR-1249-5p |
Calcium/calmodulin-dependent protein
kinase ID | −1.15 | Down |
| FHDC1 |
mmu-miR-193b-3p | FH2 domain
containing 1 | −2.42 | Down |
| SBK1 |
mmu-miR-1249-5p | SH3-binding kinase
1 | −2.25 | Down |
| DUSP18 |
mmu-miR-1249-5p | Dual specificity
phosphatase 18 | −1.65 | Down |
|
2900026A02RIK |
mmu-miR-1249-5p | RIKEN cDNA
2900026A02 gene | −1.90 | Down |
| MAPK11 |
mmu-miR-1249-5p | Mitogen-activated
protein kinase 11 | −2.78 | Down |
|
SH3PXD2A |
mmu-miR-1249-5p | SH3 and PX domains
2A | −2.08 | Down |
| CECR2 |
mmu-miR-1249-5p | Cat eye syndrome
chromosome region, candidate 2 | −2.43 | Down |
| SOX4 | mmu-miR-129-5p | SRY-box 4 | −1.36 | Down |
| ACSF2 |
mmu-miR-1249-5p | Acyl-CoA synthetase
family member 2 | −1.39 | Down |
KEGG analysis of DEGs in
LLC-BMDCs
The three upregulated and 34 downregulated genes in
the ‘genes’ and ‘targets’ diagram (Fig.
3) were mapped to KEGG pathways. The results identified eight
potential pathways that may involve the LLC-BMDCs, including the
‘T-cell receptor signaling pathway’, ‘osteoclast differentiation’,
‘MAPK signaling pathway’ ‘VEGF signaling pathway’, ‘leukocyte
transendothelial migration’, ‘signaling pathways regulating
pluripotency of stem cells’, ‘oxytocin signaling pathway’ and ‘cell
adhesion molecules (CAMs)’ (Table
II).
 | Table II.KEGG analysis of differentially
expressed genes predicted by miRmap in LLC-BMDCs. |
Table II.
KEGG analysis of differentially
expressed genes predicted by miRmap in LLC-BMDCs.
| KEGG pathway | Count | P-value | Upregulated
genes | Downregulated
genes | Fold
enrichment |
|---|
| T-cell receptor
signaling pathway | 3 | 0.01 |
| Cd4, Mapk11,
Nfatc2 | 18.56 |
| Osteoclast
differentiation | 3 | 0.01 |
| Fosb, Mapk11,
Nfatc2 | 15.32 |
| MAPK signaling
pathway | 3 | 0.05 |
| Dusp16, Mapk11,
Cacna1e |
7.63 |
| VEGF signaling
pathway | 2 | 0.08 |
| Mapk11,
Nfatc2 | 21.44 |
| Leukocyte
transendothelial migration | 2 | 0.16 |
| Mapk11,
Thy1 | 10.63 |
| Signaling pathways
regulating pluripotency of stem cells | 2 | 0.18 | BMPR1A | Mapk11 |
9.32 |
| Oxytocin signaling
pathway | 2 | 0.20 |
| Camk1d,
Nfatc2 |
8.14 |
| Cell adhesion
molecules (CAMs) | 2 | 0.21 |
| Cd4,
Sdc3 |
7.94 |
Analysis of DEGs in LLC-BMDCs versus
normal-BMDCs
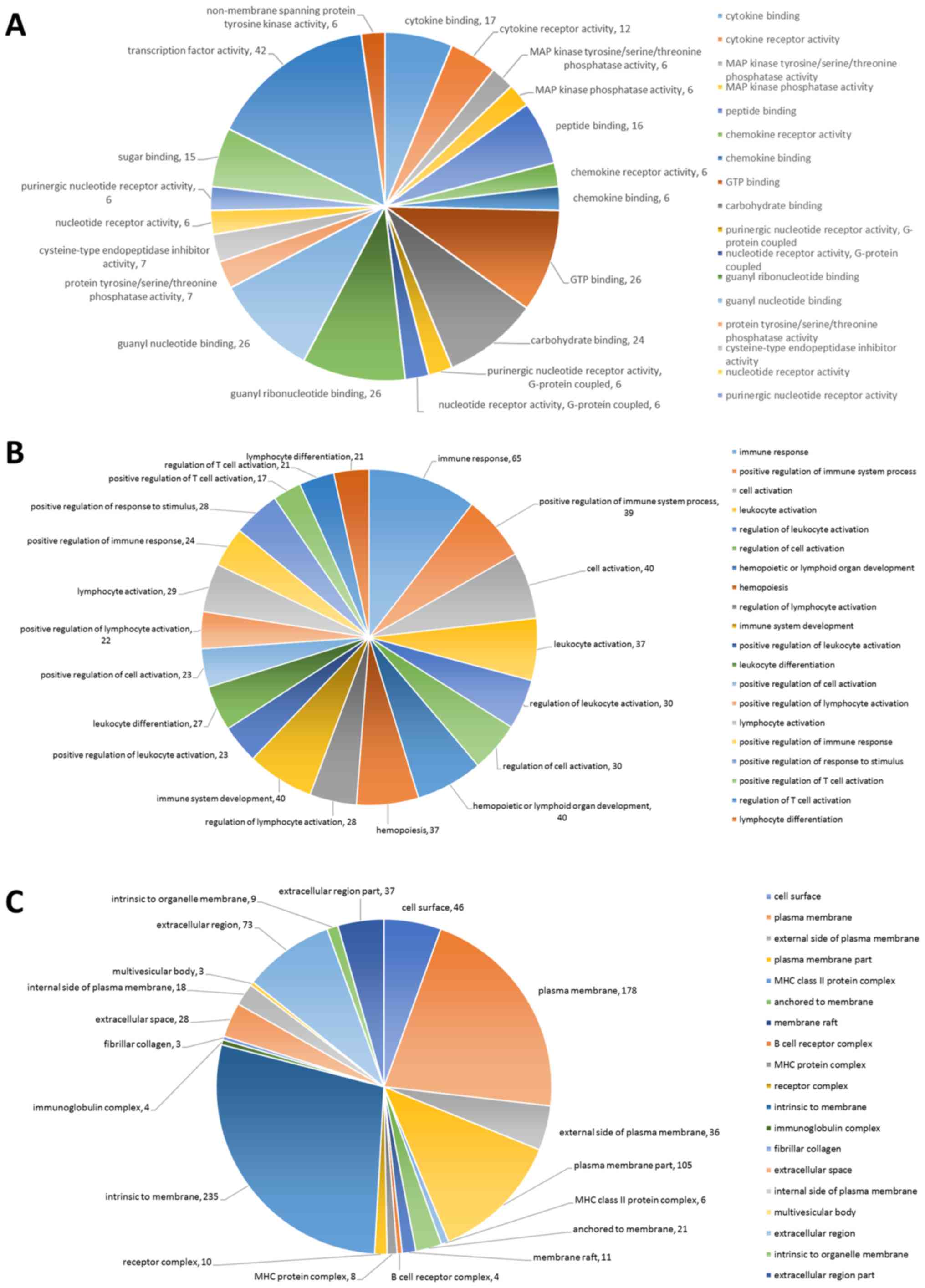
DEGs were analyzed using GO in order to investigate
the enriched functions of the 820 potential miRNA-mRNA
interactions. The top 20 cellular components, top 20 biological
processes and top 20 molecular functions of the DEGs in the
LLC-BMDCs are presented in Fig.
4A-C. All top 20 results, including molecular functions,
biological processes and cellular components, were statistically
significant (P<0.05).
KEGG pathway enrichment analysis of
820 differentially expressed mRNAs in LLC-BMDCs versus
normal-BMDCs
The top 20 pathways with the most significant
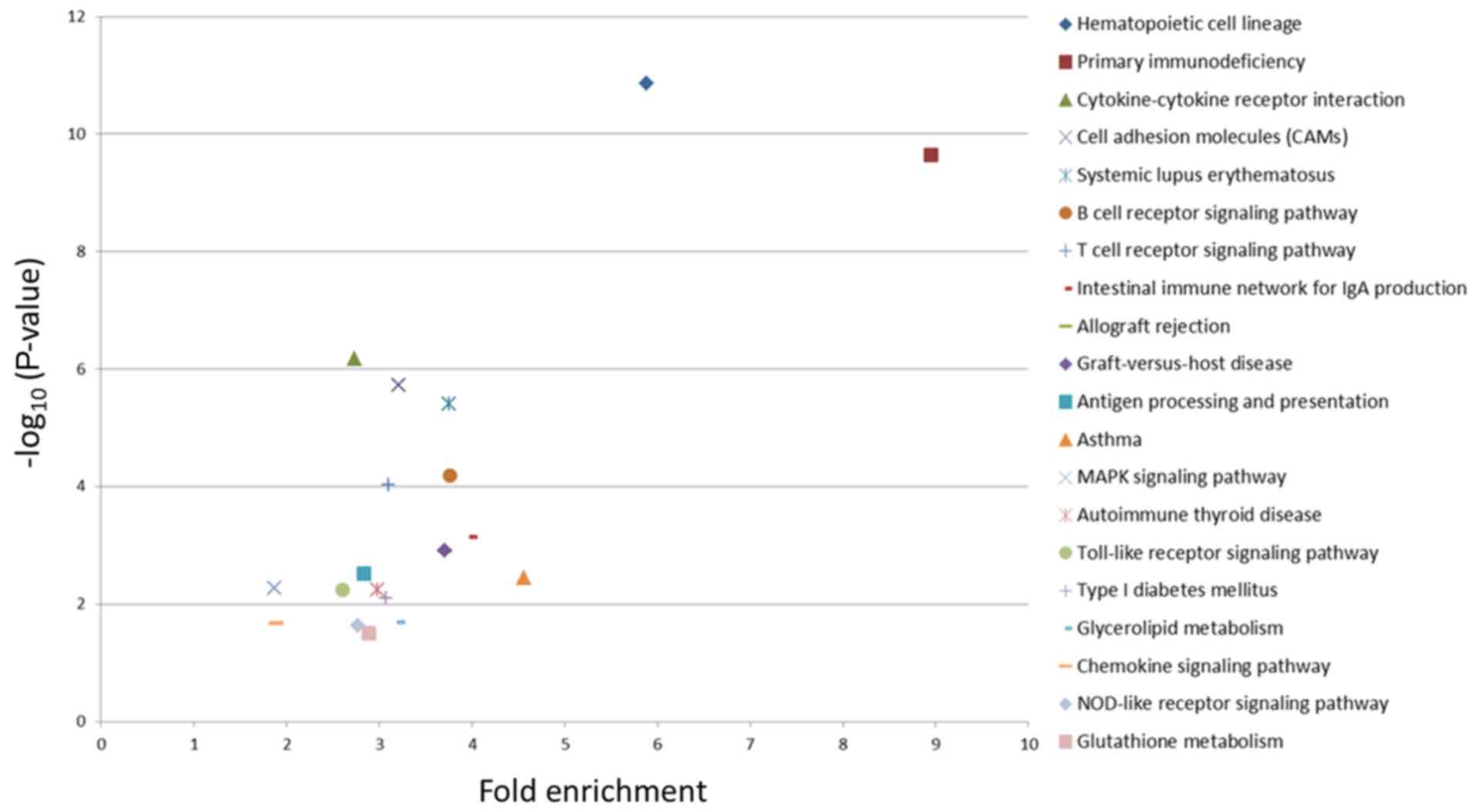
enrichment are presented in Fig. 5,
where each marker represents a KEGG pathway. The fold enrichment
represents the ratio of the proportion of DEGs annotated to the
pathway to all genes. The larger the fold enrichment score, the
more significant the enrichment level of the DEGs in the pathway.
In addition, the greater -log10(P-value), the more
reliable the enrichment significance of the DEGs in the pathway.
Two pathways (‘Hematopoietic cell lineage’ and ‘Primary
immunodeficiency’) were most significantly enriched in LLC-BMDCs
(Fig. 5).
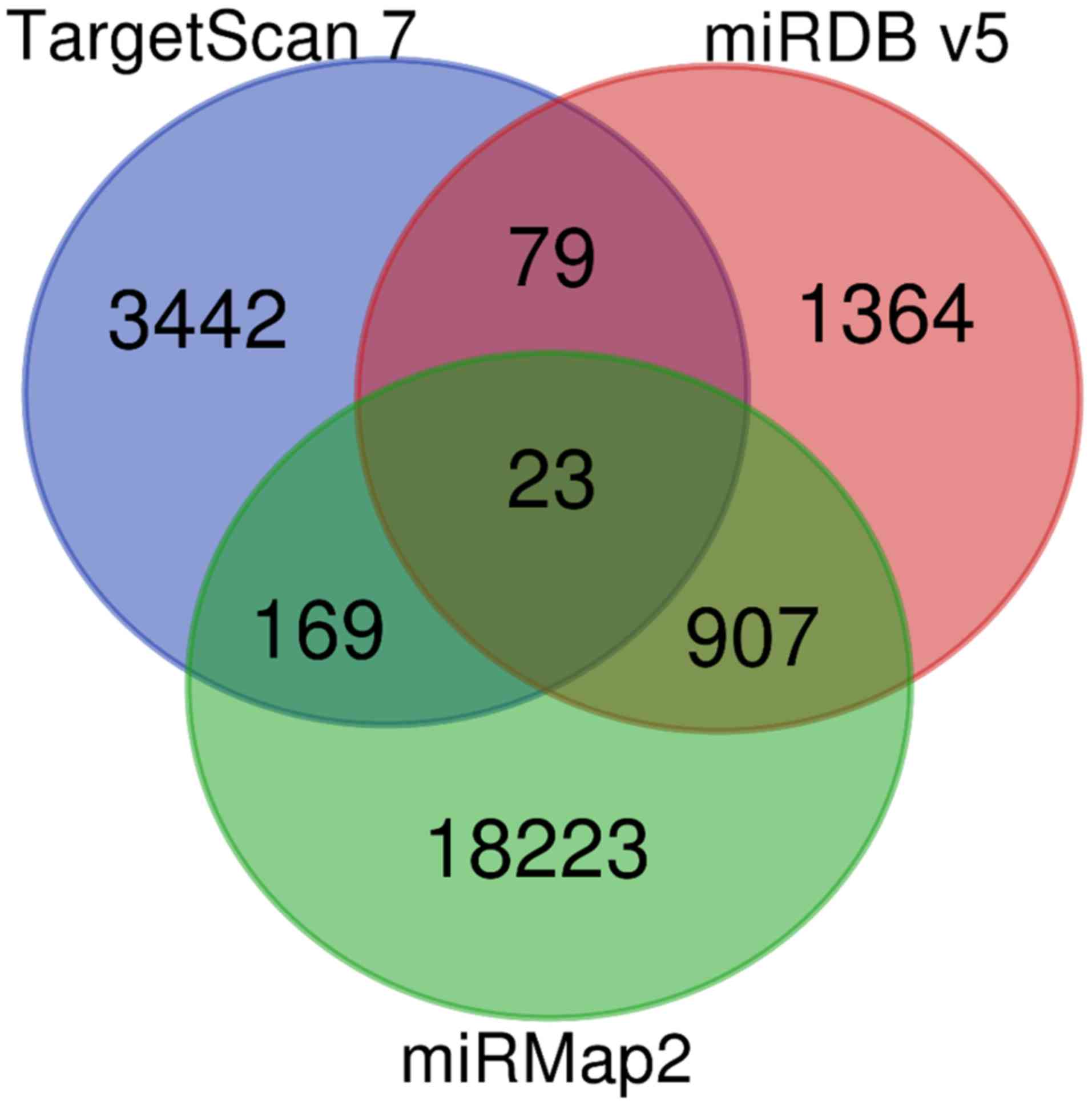
Potential miRNA-mRNA interactions from
miRmap, TargetScan and miRDB databases
The miRmap (selection criteria: miRmap score
>97.0), TargetScan (selection criteria: TargetScan score
>99.0) and miRDB (selection criteria: miRDB score >95.0)
databases were used to explore the potential miRNA-mRNA
interactions of the miRNAs with >2 FC (Fig. 6). By combining the results from the
three databases, 23 miRNA-mRNA interactions were identified
(Table III). Due to the
insufficiency of gene interactions, the miRmap and miRDB scores
were modified to >99 and >95.0, respectively.
 | Table III.Potential miRNA-mRNA interactions of
miRNAs with 2-fold change validated in miRmap, TargetScan and miRDB
databases. |
Table III.
Potential miRNA-mRNA interactions of
miRNAs with 2-fold change validated in miRmap, TargetScan and miRDB
databases.
| miRNAs | Gene symbol |
|---|
| mmu-miR-33-5p | Abca1 |
| mmu-miR-205-5p | Lrrk2 |
| mmu-miR-129-5p |
Cdc42ep3 |
| mmu-miR-151-3p | Ago2 |
| mmu-miR-205-5p | Cdh11 |
| mmu-miR-342-3p | Fam208a |
| mmu-miR-150-5p | Prkar1a |
| mmu-miR-342-3p | Fam53c |
| mmu-miR-136-5p | Mtmr4 |
| mmu-miR-411-5p | Pxdc1 |
| mmu-miR-342-3p | Rictor |
| mmu-miR-150-5p |
Prickle2 |
| mmu-miR-129-5p | Glcci1 |
| mmu-miR-129-5p | Chmp2b |
| mmu-miR-455-5p | Yipf6 |
| mmu-miR-150-5p | Myb |
| mmu-miR-129-5p | ZFP36L1 |
| mmu-miR-129-5p | BACH2 |
| mmu-miR-455-5p | ADD3 |
| mmu-miR-147-3p | NDUFA4 |
| mmu-miR-33-5p | CNTN4 |
| mmu-miR-409-3p | ZDHHC20 |
| mmu-miR-33-5p | SLC12A5 |
Discussion
Patients with lung cancer and metastasis have a poor
prognosis. Notably, PMN establishment is crucial for the
development of distant metastases. The predicted mRNAs of specific
miRNAs imply potential regulatory mechanisms of miRNA targets in
the formation of a PMN. In the present study, ‘gene’ and
miRNA-predicted ‘target’ genes were matched to strengthen the
significance of the selected genes that mediate PMN. Eight
potential pathways that may be involved in the bone marrow
modification observed during lung cancer progression were revealed.
These eight pathways were derived from the common genes of the
‘genes’ and ‘targets’, as follows: ‘T-cell receptor signaling
pathway’, ‘MAPK signaling pathway’, ‘osteoclast differentiation’,
‘VEGF signaling pathway’, ‘leukocyte transendothelial migration’,
‘signaling pathways regulating pluripotency of stem cells’,
‘oxytocin signaling pathway’ and ‘cellular adhesion molecules
(CAMs)’. In addition, the present study analyzed the KEGG pathways
derived from mRNAs with >2 FC in the LLC-BMDCs compared with the
control-BDMCs. Results demonstrated that three pathways were
identical, including the ‘T-cell receptor signaling pathway’, ‘MAPK
signaling pathway’ and ‘cellular adhesion molecules (CAMs)’.
The T-cell receptor (TCR) signaling pathway
represents a branching network, which is initiated by cognate
peptide-major histocompatibility complex molecules, and is
associated with the mitogen-activated protein kinase (MAPK) and
nuclear factor-κB (NF-κB) signaling pathways (17). It has also been reported that the TCR
signaling pathway involves the mobilization of transcription
factors. Subsequently, it serves a crucial role in T-cell gene
expression and is essential for T-cell growth and differentiation
(17). Furthermore, it induces
expression of the inducible costimulatory molecule (ICOS) and
programmed cell death 1 (PD1), which are members of the CD28
family. ICOS and PD1 can control the sustained phase of T-cell
signaling (18), whereas PD1
inhibitors can target the binding between T cell PD1 receptors and
PD-ligands 1 and 2. PD1 inhibitors have also been reported to block
inhibitory signaling, which results in the activation of T-cell
effector function, which provides an anticancer potential for
activated T cells (19).
The Ras/Raf/mitogen-activated protein kinase
kinase/extracellular signal-regulated kinase (ERK) signaling
pathway, which is one of the major intracellular axes that regulate
intracellular signaling trafficking, is associated with cell
proliferation, growth, invasion, metastasis, resistance to
apoptosis and angiogenesis in lung cancer (20). It has been reported that the MAPK
pathway interferes in non-small cell lung cancer tumorigenesis
through terminal differentiation-induced noncoding RNA (21), although the role of the MAPK/ERK
pathway in lung cancer treatment remains unclear. The therapeutic
inhibition of elements from this pathway has been reported to be
beneficial in cancer treatment, including colorectal and ovarian
cancer (22). However, treatment
with small inhibitors that specifically target proteins from the
MAPK/ERK pathway can lead to the development of secondary
malignancies. For example, Raf inhibitors have been reported to
potentially induce abnormal skin cell proliferation and cause
secondary squamous cell cancers (22).
CAMs, which comprise cadherins, integrins, selectins
and members of the immunoglobulin family, are important components
involved in cell-to-cell and cell-to-extracellular matrix
anchoring. Their main roles are to maintain cell and tissue
structure, cell signaling, tissue repair and wound healing
(23). In addition, integrins serve
an important role in platelet aggregation, hematopoietic cell
mobilization, neoangiogenesis and stromal function. They are also
associated with bone metastasis, which suggests that they may
represent potential novel therapeutic targets in the prevention and
treatment of bone metastasis (24).
For example, CAMs, including CD44, N-cadherin, neural cell adhesion
molecule and integrins, are involved in the metastatic cascade
observed in a metastatic neuroblastoma model (25).
Osteoclast differentiation is associated with bone
metastasis in lung cancer. Non-small cell lung cancer can
metastasize to the bone, which results in bone osteolytic lesions
via osteoclast lineage activation. Through the downregulation of
the receptor activator of NF-κB signaling pathway, osteoclast
differentiation attenuates osteoclastogenesis and reduces
osteolysis following bone metastasis (26).
Additional pathways predicted in this study, such as
VEGF, leukocyte transendothelial migration, cancer stem cells,
etc., are associated with lung cancer metastasis. To support
cellular function, tumor cells release VEGF that stimulates
angiogenesis through vascular sprouting, intussusception and
incorporation of bone marrow-derived endothelial precursors
(27). In gastric cancer, celecoxib
has been used as an inhibitor of cysteine and glycine rich protein
1, thrombospondin 1, myosin light chain 9, filamin A, actinin α1,
vinculin, laminin subunit γ2 and claudin 1 expression, and can also
suppress leukocyte transendothelial migration and focal adhesion,
which highlights its anti-gastric cancer effect (28). Furthermore, cancer stem cells possess
self-renewal ability and are involved in the initiation of cancer,
malignant transformation and metastatic progression, and in the
post-treatment recurrence of various types of human cancer
(29). Understanding the underlying
mechanisms of transcription factors associated with pluripotency is
therefore crucial to clarify human carcinogenesis. In addition, the
hypothalamic nonapeptide, oxytocin, serves as a tumor growth
regulator by activating specific G-coupled transmembrane receptor
through oxytocin receptor. Oxytocin attenuates proliferation of
cancer cells that originate from epithelium, nerves and bone
(30).
In conclusion, results from the present study
demonstrated that bone marrow may serve a crucial role in mediating
lung cancer metastasis. Data revealed that numerous pathways were
involved in the LLC-BMDC microenvironment, including the ‘T-cell
receptor signaling pathway’, ‘MAPK signaling pathway’, ‘osteoclast
differentiation’, ‘VEGF signaling pathway’, ‘leukocyte
transendothelial migration’, ‘signaling pathways regulating
pluripotency of stem cells’, ‘oxytocin signaling pathway’ and
‘cellular adhesion molecules (CAMs)’. These eight pathways were all
associated with cancer progression. In addition, the present study
provided macroscopic evidence of the association between lung
cancer cells and BMDCs (Fig. 7).
Present findings suggested that these genetic interactions may be
associated with lung cancer cell progression. Molecular alterations
in gene expression may therefore represent a novel signature in
lung cancer, which may be used to develop diagnostic and
therapeutic strategies for patients with lung cancer and bone
metastasis. Further investigation is required to study the role of
BMDCs in the bone microenvironment in lung cancer metastasis, and
to identify the main cells that mediate bone metastasis in lung
cancer.
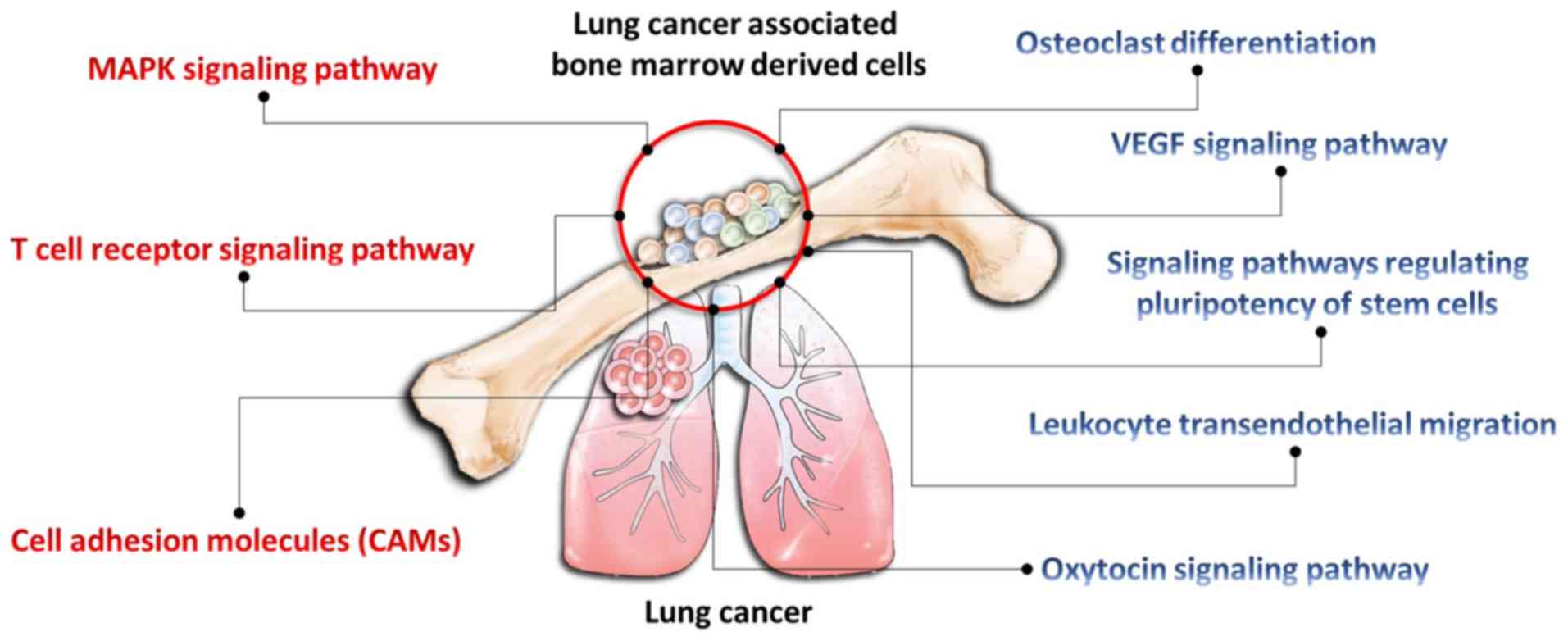 | Figure 7.Potential pathways involved in the
microenvironment of lung cancer and bone marrow-derived cells. The
injection of Lewis lung carcinoma cells into the mice modified bone
marrow-derived cells and initiated a pre-metastatic niche. Through
next generation sequencing, eight potential pathways, including the
‘T-cell receptor signaling pathway’, ‘osteoclast differentiation’,
‘MAPK signaling pathway’ (red font, P<0.05), ‘VEGF signaling
pathway’, ‘leukocyte transendothelial migration’, ‘signaling
pathways regulating pluripotency of stem cells’, ‘oxytocin
signaling pathway’, and ‘cell adhesion molecules (CAMs)’ (blue
font, P>0.05) were identified. These pathways may mediate the
pre-metastatic niche in lung cancer metastasis. |
Acknowledgements
The authors would like to thank Mr. Chi-Hsien Chou
and other staff members of the Center for Research Resources and
Development of Kaohsiung Medical University for their assistance in
bioinformatics analysis.
Funding
This study was supported by the Ministry of Science
and Technology (grants nos. MOST 107-2314-B-037-107-MY3, MOST
106-2314-B-037-016-MY2 and MOST 106-2314-B-037-064), the Kaohsiung
Medical University Hospital (grants nos. KMUHS10701, KMUHS10712 and
KMUH103-3R09) and the Kaohsiung Medical University (grants nos.
105KMUOR05, KMU-DK108003 and KMU-DK108008).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WAC and JYH designed the study. WAC, YMT, YCT, CYW,
KFC, CTL, JYH, YLH, and PLK analyzed the data and interpreted the
results. WAC, YMT and JYH wrote the manuscript. The final version
of the manuscript has been read and approved by all authors.
Ethical approval and consent to
participate
Animal experiments were performed according to the
Institutional Animal Care and Use Committee, and with the approval
of the Animal Care and Use Committee of the School of Kaohsiung
Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kaplan RN, Riba RD, Zacharoulis S, Bramley
AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, et
al: VEGFR1-positive haematopoietic bone marrow progenitors initiate
the pre-metastatic niche. Nature. 438:820–827. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Riihimäki M, Hemminki A, Fallah M, Thomsen
H, Sundquist K, Sundquist J and Hemminki K: Metastatic sites and
survival in lung cancer. Lung Cancer. 86:78–84. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Coleman RE: Clinical features of
metastatic bone disease and risk of skeletal morbidity. Clin Cancer
Res. 12:6243s–6249s. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Peinado H, Zhang H, Matei IR, Costa-Silva
B, Hoshino A, Rodrigues G, Psaila B, Kaplan RN, Bromberg JF, Kang
Y, et al: Pre-metastatic niches: Organ-specific homes for
metastases. Nat Rev Cancer. 17:302–317. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kaplan RN, Psaila B and Lyden D: Bone
marrow cells in the ‘pre-metastatic niche’: Within bone and beyond.
Cancer Metastasis Rev. 25:521–529. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Roato I: Bone metastases: When and how
lung cancer interacts with bone. World J Clin Oncol. 5:149–155.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bergfeld SA and DeClerck YA: Bone
marrow-derived mesenchymal stem cells and the tumor
microenvironment. Cancer Metastasis Rev. 29:249–261. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rutkowski MR, Svoronos N, Perales-Puchalt
A and Conejo-Garcia JR: The tumor macroenvironment:
Cancer-promoting networks beyond tumor beds. Adv Cancer Res.
128:235–262. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu Y and Cao X: Characteristics and
significance of the pre-metastatic niche. Cancer Cell. 30:668–681.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu X and Quan N: Immune cell isolation
from mouse femur bone marrow. Bio Protoc. 5:e16312015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sheu CC, Tsai MJ, Chen FW, Chang KF, Chang
WA, Chong IW, Kuo PL and Hsu YL: Identification of novel genetic
regulations associated with airway epithelial homeostasis using
next-generation sequencing data and bioinformatics approaches.
Oncotarget. 8:82674–82688. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen SC, Chen FW, Hsu YL and Kuo PL:
Systematic analysis of transcriptomic profile of renal cell
carcinoma under long-term hypoxia using next-generation sequencing
and bioinformatics. Int J Mol Sci. 18:E26572017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bolger AM, Lohse M and Usadel B:
Trimmomatic: A flexible trimmer for illumina sequence data.
Bioinformatics. 30:2114–2120. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Friedlander MR, Mackowiak SD, Li N, Chen W
and Rajewsky N: miRDeep2 accurately identifies known and hundreds
of novel microRNA genes in seven animal clades. Nucleic Acids Res.
40:37–52. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Trapnell C, Roberts A, Goff L, Pertea G,
Kim D, Kelley DR, Pimentel H, Salzberg SL, Rinn JL and Pachter L:
Differential gene and transcript expression analysis of RNA-seq
experiments with TopHat and Cufflinks. Nat Protoc. 7:562–578. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vejnar CE and Zdobnov EM: MiRmap:
Comprehensive prediction of microRNA target repression strength.
Nucleic Acids Res. 40:11673–11683. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Brownlie RJ and Zamoyska R: T cell
receptor signalling networks: Branched, diversified and bounded.
Nat Rev Immunol. 13:257–269. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huse M: The T-cell-receptor signaling
network. J Cell Sci. 122:1269–1273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Niyongere S, Saltos A and Gray JE:
Immunotherapy combination strategies (non-chemotherapy) in
non-small cell lung cancer. J Thorac Dis. 10:S433–S450. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Reungwetwattana T and Dy GK: Targeted
therapies in development for non-small cell lung cancer. J
Carcinog. 12:222013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhu ZJ and He JK: TINCR facilitates
non-small cell lung cancer progression through BRAF-activated MAPK
pathway. Biochem Biophys Res Commun. 497:971–977. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Burotto M, Chiou VL, Lee JM and Kohn EC:
The MAPK pathway across different malignancies: A new perspective.
Cancer. 120:3446–3456. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Farahani E, Patra HK, Jangamreddy JR,
Rashedi I, Kawalec M, Rao Pariti RK, Batakis P and Wiechec E: Cell
adhesion molecules and their relation to (cancer) cell stemness.
Carcinogenesis. 35:747–759. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schneider JG, Amend SR and Weilbaecher KN:
Integrins and bone metastasis: Integrating tumor cell and stromal
cell interactions. Bone. 48:54–65. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schwankhaus N, Gathmann C, Wicklein D,
Riecken K, Schumacher U and Valentiner U: Cell adhesion molecules
in metastatic neuroblastoma models. Clin Exp Metastasis.
31:483–496. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ihn HJ, Kim JA, Bae YC, Shin HI, Baek MC
and Park EK: Afatinib ameliorates osteoclast differentiation and
function through downregulation of RANK signaling pathways. BMB
Rep. 50:150–155. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Niu G and Chen X: Vascular endothelial
growth factor as an anti-angiogenic target for cancer therapy. Curr
Drug Targets. 11:1000–1017. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jin GH, Xu W, Shi Y and Wang LB: Celecoxib
exhibits an anti-gastric cancer effect by targeting focal adhesion
and leukocyte transendothelial migration-associated genes. Oncol
Lett. 12:2345–2350. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kashyap V, Rezende NC, Scotland KB,
Shaffer SM, Persson JL, Gudas LJ and Mongan NP: Regulation of stem
cell pluripotency and differentiation involves a mutual regulatory
circuit of the NANOG, OCT4, and SOX2 pluripotency transcription
factors with polycomb repressive complexes and stem cell microRNAs.
Stem Cells Dev. 18:1093–1108. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cassoni P, Sapino A, Marrocco T, Chini B
and Bussolati G: Oxytocin and oxytocin receptors in cancer cells
and proliferation. J Neuroendocrinol. 16:362–364. 2004. View Article : Google Scholar : PubMed/NCBI
|