Introduction
Perihilar cholangiocarcinoma (PCCA), also called
Klatskin tumor, is a rare disease that has a poor prognosis
(1). PCCA presents in the left and
right bile duct bifurcation, accounting for 40–60% of bile duct
carcinomas and 58–75% of extrahepatic bile duct carcinomas
(2). The prognosis of PCCA is
associated with the histopathological results of the surgical tumor
edge, and the tumor histological grading and staging are all
associated with post-operative morbidity and tumor lymph node
invasion (3). Complete tumor
resection remains the only effective treatment method for PCCA and
additional adjuvant treatment is currently absent. However, only
30% of patients with PCCA are able to achieve complete surgical
resection (4). In previous years,
molecular targeting therapy has achieved satisfactory results in
certain cases (5). Therefore, the
investigation of the pathogenesis of PCCA is imperative for the
identification of novel genes that may be targeted
therapeutically.
The metadherin (MTDH) gene is located on chromosome
8 long arm zone 22 (8 q22) and encodes a protein ~64 kDa in size
(6). MTDH was originally cloned in
human embryonic astrocytes that were infected with human
immunodeficiency virus type 1 and was initially named astrocyte
increase gene 1 (7). MTDH has been
demonstrated to participate in breast cancer metastasis to the lung
in a mouse model (7). Consequently,
it was identified as a transfer adhesion gene/protein (8). Previously, a number of studies have
demonstrated high MTDH expression in liver and breast cancer,
osteosarcoma and other malignant tumor types (9–12). In
addition, high MTDH expression is associated with a poor prognosis
in patients with cancer (13).
However, the expression levels and the clinical
significance of MTDH in PCCA have not yet been investigated. In the
present study, an immunohistochemical method was adopted to detect
MTDH expression in 66 cases of PCCA and the potential application
of MTDH as a prognostic factor of PCCA was examined.
Materials and methods
Study population
The present study was ethically approved by the
Medical Ethics Society of Taihe Hospital Affiliated with Hubei
University of Medicine (Hubei, China). A total of 66 patients with
PCCA received surgical treatment at Taihe Hospital Affiliated with
Hubei University of Medicine. The patients provided written
informed consent for the use of their tumor specimens in the
present study. All surgically resected cholangiocellular carcinoma
specimens and non-neoplastic bile ducts exhibited clear
pathological diagnosis with haemotoxylin and eosin-stained slides.
In brief, the 4 µm-thick sections were deparaffinized and hydrated
in 100% alcohol for 5 min and 80% alcohol for 5 min, stained with
hematoxylin for 10 min and stained with eosin for 30 sec at room
temperature. The slides were observed using a light microscope
(magnification, ×200). No postoperative complications were observed
and therefore it was not further discussed in all included
patients. The specimens were fixed with 4% paraformaldehyde for 24
h at room temperature and were paraffin embedded at room
temperature. The clinical data including age, sex, tumor size,
lymph node metastasis, tumor infiltration depth, histological grade
and tumor stage (14) were obtained
from each medical record. The recurrence and distant metastasis
were assessed by clinical and/or imaging diagnostic methods, which
included computer tomography (CT) and magnetic resonance
imaging.
Immunohistochemistry
An immunohistochemical staining method was applied
in order to detect MTDH expression in PCCA paraffin embedded 4
µm-thick slides. In brief, the slides were deparaffinized and
hydrated in a 100% alcohol for 5 min and 80% alcohol for 5 min.
Antigen retrieval was performed with citrate buffer at 98°C for 10
min and endogenous peroxidase activity was blocked with 0.3%
hydrogen peroxide in methanol for 15 min at room temperature. An
anti-human MTDH rabbit monoclonal antibody (rabbit monoclonal, cat.
no. EP4445; 1:100; Abcam, Cambridge, MA, USA) was used to detect
MTDH expression. Vimentin (rabbit monoclonal, cat. no. 5741; 1:100;
Cell Signaling Technology, Inc., Danvers, MA, USA) and E-cadherin
(mouse monoclonal, cat. no. 14472; 1:100; Cell Signaling
Technology, Inc.) were used to detect the expression of
epithelial-to-mesenchymal (EMT) markers. Samples were incubated
with primary antibodies overnight at 4°C. Subsequently, the samples
were incubated with horseradish peroxidase universal IgG secondary
antibody (cat. no. sc69786; 1:1,000. Santa Cruz Biotechnology,
Inc.) for 30 min at 37°C. A histostaining kit (cat. no. SP-9001;
OriGene Technologies, Inc., Beijing, China) was used to visualize
the antibody binding on the slides, according to the manufacturer's
protocol. The slides were then counterstained with hematoxylin for
5 min at 37°C. The slides that contained no primary antibody were
used as negative controls and the breast cancer tissues slides that
had been already confirmed to overexpress the MTDH protein were
used as positive controls. The slides were observed using a light
microscope (magnification, ×200).
Immunohistochemical evaluation
A semi-quantitative assessment of MTDH expression
was performed by measuring the percentage of positive cells, as
previously described (8). The
staining intensity was scored as 0, negative; 1, weak; 2, moderate;
and 3, strong. The percentage of positive cells was scored as 0,
negative or <5%; 1, 6–25%; 2, 26–50%; 3, 51–75%; and 4, >76%.
The final staining score was calculated by the score of the
staining intensity multiplied by the proportion of positively
stained cells. A total score of <2 was considered to indicate a
low MTDH expression, while a score ≥2 indicated a high MTDH
expression. All the sections were assessed by two experienced
pathologists, and 3 cases of inconsistent immunohistochemical
results were reviewed again by the two pathologists in order to
obtain the final pathological diagnosis.
Follow-up
The follow-up was examined by carbohydrate antigen
19–9, ultrasonography or abdominal CT and chest radiography every 3
months for the first 2 years following surgery. Overall survival
(OS) rate was calculated from the date of resection to the date of
mortality or last follow-up. Recurrence free survival (RFS) rate
was calculated between the date of resection to the date of tumor
recurrence or the day of mortality or last follow-up.
Statistical analysis
SPSS (version 17.0; SPSS, Inc., Chicago, IL, USA)
was used in the present study. The expression of MTDH and the
clinical and pathological factors including age, sex, tumor size,
capsular invasion, lymph node metastasis, tumor classification
stage and distant metastases during diagnosis were analyzed using
the χ2 test or the exact probability analysis
(χ2 test or Fisher's exact test). The OS time was
defined as the time from the cancer diagnosis until the patient
mortality prior to and during follow-up. The recurrence free
survival (RFS) time was defined as the time from the initial PCCA
diagnosis to the time point of cancer recurrence prior to and
during follow-up. Survival analysis was performed using the Kaplan
Meier method and the log-rank test in order to compare the survival
differences according to the MTDH expression status. Cox's
regression model was used for the survival analysis of multiple
pathological factors. P<0.05 was considered to indicate a
statistically significant difference.
Results
Patient data
The clinical data that were obtained from each
medical record are presented in Table
I, including age at diagnosis, sex, tumor size, the depth of
invasion, histological grade, nodal metastasis and tumor stage
according to the American Joint Committee on Cancer (15). The mean age at diagnosis of the
disease was 57.4 years (range, 32–78 years). In total, 21 (31.82%)
cases were female and 45 (68.18%) were male. Clinical follow-up was
available for all patients.
 | Table I.Association between MTDH protein
expression in perihilar cholangiocarcinoma and clinicopathological
characteristics. |
Table I.
Association between MTDH protein
expression in perihilar cholangiocarcinoma and clinicopathological
characteristics.
|
|
| MTDH expression |
|
|---|
|
|
|
|
|
|---|
| Characteristics | Number | Low (%) | High (%) | P-value |
|---|
| Age |
|
|
| 0.455 |
| ≤55
years | 32 | 18 (56.3) | 14 (43.8) |
|
| >55
years | 34 | 16 (47.1) | 18 (52.9) |
|
| Sex |
|
|
| 0.249 |
|
Female | 21 | 13 (61.9) | 8 (38.1) |
|
|
Male | 45 | 21 (46.7) | 24 (53.3) |
|
| Tumor diameter |
|
|
| 0.177 |
| <3
cm | 24 | 15 (62.5) | 9 (37.5) |
|
| ≥3
cm | 42 | 19 (45.2) | 23 (54.8) |
|
|
Differentiation |
|
|
| 0.007 |
|
Well/moderately | 36 | 24 (66.7) | 12 (33.3) |
|
|
Poorly | 30 | 10 (33.3) | 20 (66.7) |
|
| Lymph node
metastasis |
|
|
| 0.023 |
| No | 28 | 19 (67.9 | 9 (32.1) |
|
|
Yes | 38 | 15 (39.5) | 23 (60.5) |
|
| pT status |
|
|
| 0.068 |
|
T1/T2 | 17 | 12 (70.6) | 5 (29.4) |
|
|
T3/T4 | 49 | 22 (44.9) | 27 (55.1) |
|
| Nerve invasion |
|
|
| 0.088 |
|
Negative | 38 | 23 (60.5) | 15 (39.5) |
|
|
Positive | 28 | 11 (39.3) | 17 (60.7) |
|
| Disease stage |
|
|
| 0.339 |
|
I/II/III | 18 | 117 (61.1) | 7 (38.9) |
|
| IV | 48 | 237 (47.9) | 25 (52.1) |
|
Expression of MTDH in non-neoplastic
bile ducts and cholangiocellular carcinoma
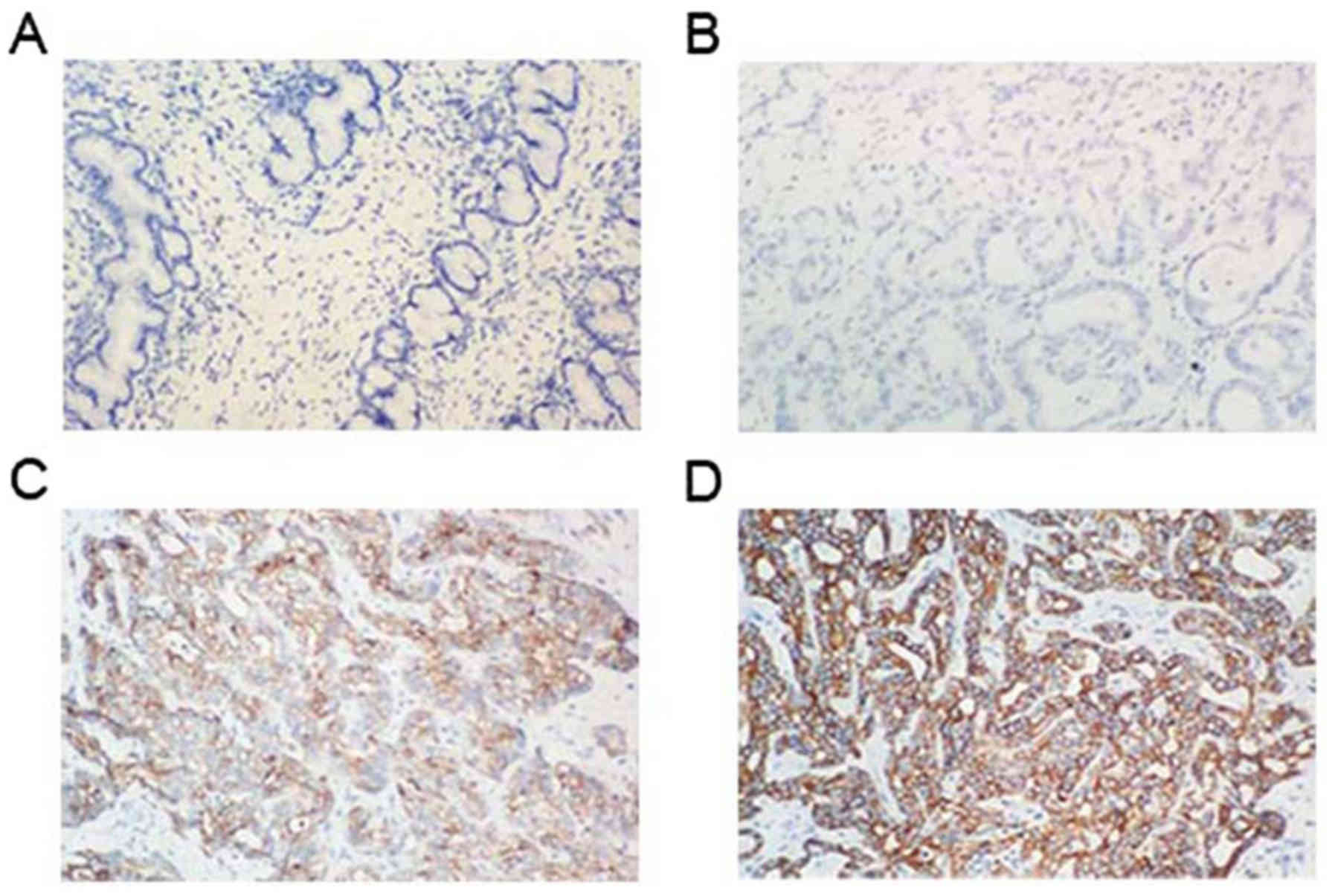
MTDH expression was detected by immunohistochemical
methods in patients with PCCA. Fig.
1 represents the MTDH expression in tumor specimens and in
matched normal tissues. MTDH was negatively and/or weakly expressed
in the cytoplasm and in the cell membrane of cholangiocytes derived
from normal bile ducts. However, high MTDH expression was noted in
the PCCA tumor tissues compared with the normal tissues. The MTDH
positive expression rate was 48.5% (32/66) in PCCA tumor
tissues.
Association of MTDH overexpression
with clinicopathological data
The association between MTDH expression levels and
PCCA clinicopathological parameters is summarized in Table I. High MTDH expression in PCCA was
revealed to be positively associated with tumor differentiation and
lymph node metastasis. Overexpression of MTDH occurred
significantly more frequently in patients with cancer with regional
lymph nodes metastasis (60.5%) compared with patients with cancer
with N0-stage tumors (32.1%; P=0.023). It is important to note that
MTDH expression was significantly higher in poorly differentiated
PCCA (66.7%) compared with that observed in well differentiated
PCCA (33.3%; P=0.007). However, no significant associations were
identified with patient age, sex, tumor diameter, tumor grade and
tumor classification stage.
MTDH overexpression in patients with
PCCA is associated with the expression of EMT markers
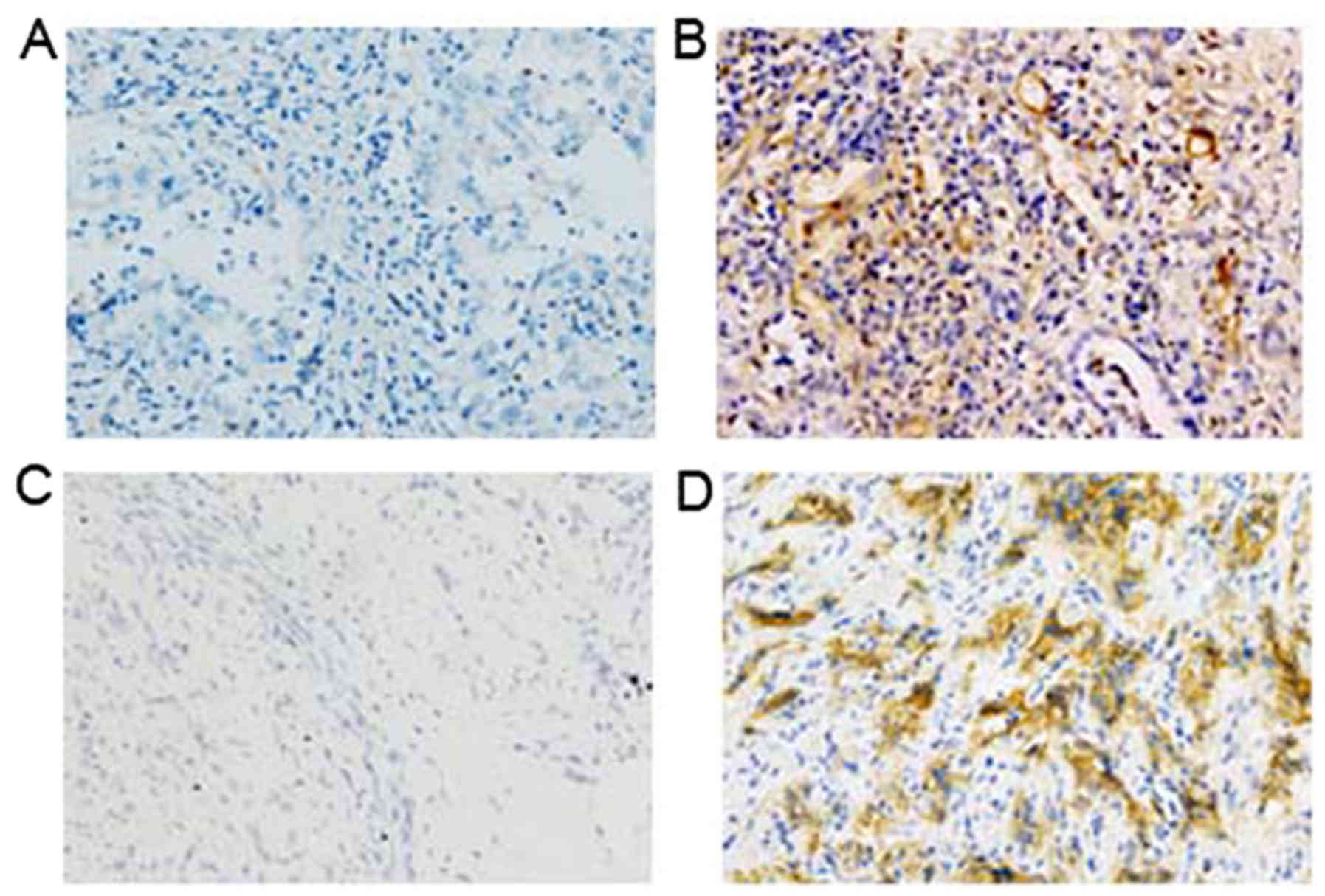
The association between MTDH expression and the PCCA
EMT was analyzed. An immunohistochemical method was employed in
order to detect E-cadherin and vimentin expression (Fig. 2). The results indicated that high
MTDH expression was significantly positively associated with
vimentin expression levels in PCCA tissues compared with negative
vimentin expression levels (P=0.037). However, a significant
inverse association was noted between MTDH expression and positive
E-cadherin compared with negative E-cadherin expression (P=0.030;
Table II).
 | Table II.Association between MTDH protein
expression in perihilar cholangiocarcinoma and EMT markers. |
Table II.
Association between MTDH protein
expression in perihilar cholangiocarcinoma and EMT markers.
|
|
| MTDH
expression |
|
|---|
|
|
|
|
|
|---|
| EMT | Number | Low (%) | High (%) | P-value |
|---|
| E-cadherin |
|
|
| 0.030 |
|
Positive | 23 | 15 (65.2) | 8
(34.8) |
|
|
Negative | 43 | 16 (37.2) | 27 (62.8) |
|
| Vimentin |
|
|
| 0.037 |
|
Positive | 39 | 13 (33.3) | 26 (66.7) |
|
|
Negative | 27 | 16 (59.3) | 11 (40.7) |
|
MTDH expression is associated with a
poor prognosis in patients with PCCA
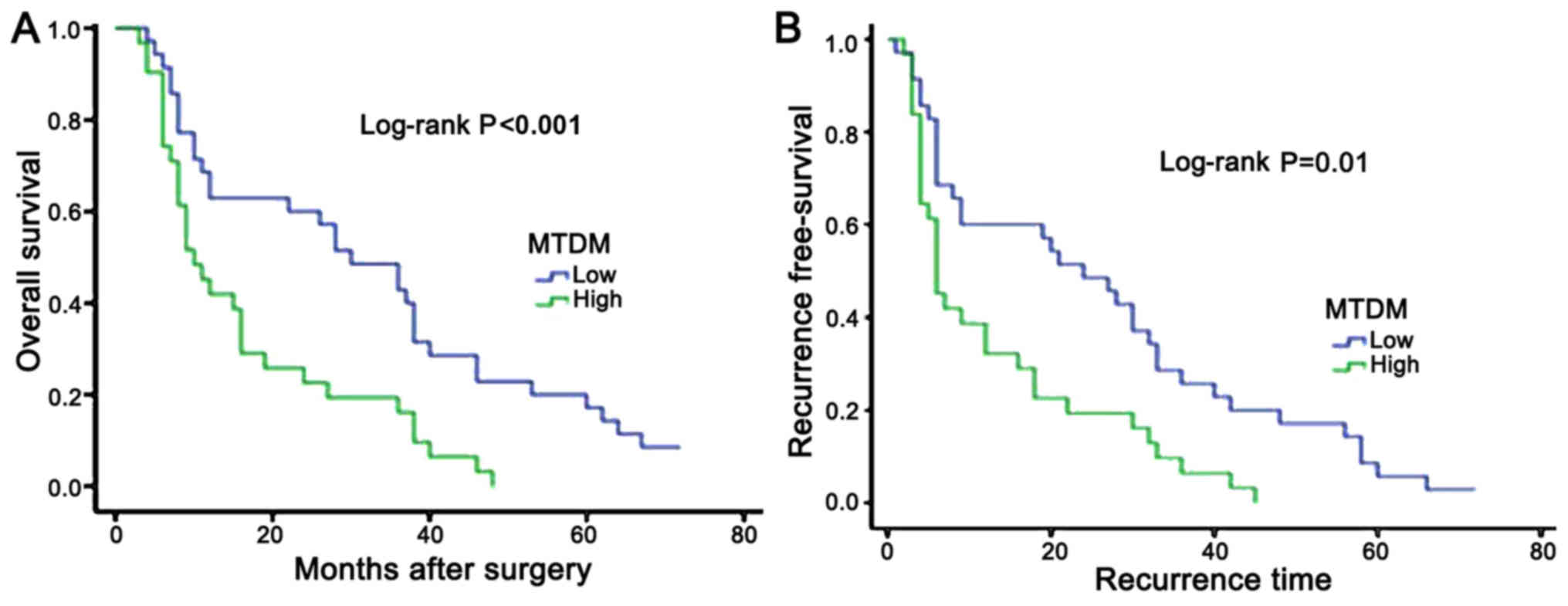
The 1-, 3- and 5-year OS rates were revealed to be
64.7, 44.1 and 17.6%, respectively, in the low MTDH expression
group. However, in the high MTDH expression group, the 1-, 3- and
5-year OS rates were 40.6, 15.6 and 0.0%, respectively (Fig. 3A). The 1-, 3- and 5-year RFS rates
were 55.9, 26.5 and 8.8%, respectively, in the low MTDH expression
group. However, in the high MTDH expression group, the 1-, 3- and
5-year RFS rates were 31.3, 9.4 and 0.0%, respectively (Fig. 3B). Kaplan Meier analysis and a
log-rank test suggested that the high MTDH expression group
exhibited significantly worse OS and RFS rates compared with the
low MTDH expression group (P<0.001 and P=0.01,
respectively).
Using univariate factor analysis, it was
demonstrated that tumor differentiation, tumor degree, American
Joint Committee on Cancer stage (15,16),
MTDH expression and surgery margin were associated with the OS and
RFS. Lymph node metastasis was also associated with RFS in PCCA. In
contrast to univariate analysis, Cox's analysis indicated that
patients with stage IV TNM (P=0.023), high MTDH expression
(P=0.030) and a surgery margin (P=0.042) were significant
independent prognostic factors of the OS in multivariate factors
analysis (Table III). Furthermore,
tumor patients with stage IV TNM (P=0.018), high MTDH expression
(P=0.041) and a surgery margin (P=0.043) were also significant
independent prognostic factors of RFS in patients with PCCA
(Table IV).
 | Table III.Univariate and multivariate analysis
of different parameters in perihilar cholangiocarcinoma overall
survival rates by Cox's proportional hazard model. |
Table III.
Univariate and multivariate analysis
of different parameters in perihilar cholangiocarcinoma overall
survival rates by Cox's proportional hazard model.
|
| Univariate survival
analysis | Multivariate
survival analysis |
|---|
|
|
|
|
|---|
| Parameters | HR | (95% CI) | P-value | HR | (95% CI) | P-value |
|---|
| Age
(>55/≤55) | 1.307 | 0.791–2.159 | 0.296 |
|
|
|
| Sex
(male/female) | 0.686 | 0.402–1.170 | 0.166 |
|
|
|
|
Differentiation | 1.820 | 1.061–3.123 | 0.030 | 1.580 | 0.906–2.757 | 0.107 |
| Tumor diameter
(≥3/<3) | 1.212 | 0.720–2.040 | 0.118 |
|
|
|
| Tumor degree
(T3-4/T1-2) | 1.605 | 0.887–2.906 | 0.019 | 0.918 | 0.483–1.748 | 0.795 |
| Lymph node
metastasis (yes/no) | 1.667 | 0.972–2.858 | 0.064 |
|
|
|
|
Tumor-Node-Metastasis [IV/(I/II/III)] | 2.307 | 1.124–3.694 | 0.019 | 2.030 | 1.105–3.729 | 0.023 |
| Nerve invasion | 1.453 | 0.871–2.427 | 0.153 |
|
|
|
| Tumor recurrence
(yes/no) | 1.584 | 1.039–2.414 | 0.249 |
|
|
|
| Metadherin
expression (high/low) | 2.438 | 1.433–4.147 | 0.001 | 1.736 | 1.065–3.388 | 0.030 |
| Surgery margin
(yes/no) | 2.292 | 1.330–3.950 | 0.003 | 1.308 | 1.023–3.326 | 0.042 |
 | Table IV.Univariate and multivariate analysis
of different parameters in perihilar cholangiocarcinoma
recurrence-free survival rates by Cox proportional hazard
model. |
Table IV.
Univariate and multivariate analysis
of different parameters in perihilar cholangiocarcinoma
recurrence-free survival rates by Cox proportional hazard
model.
|
| Univariate survival
analysis | Multivariate
survival analysis |
|---|
|
|
|
|
|---|
| Parameters | HR | (95% CI) | P-value | HR | (95% CI) | P-value |
|---|
| Age
(>55/≤55) | 1.290 | 0.784–2.122 | 0.316 |
|
|
|
| Sex
(male/female) | 0.674 | 0.395–1.148 | 0.146 |
|
|
|
|
Differentiation | 1.759 | 1.032–2.999 | 0.038 | 1.527 | 0.887–2.626 | 0.126 |
| Tumor diameter
(≥3/<3) | 1.246 | 0.749–2.070 | 0.397 |
|
|
|
| Tumor degree
(T3-4/T1-2) | 1.499 | 0.847–2.652 | 0.164 |
|
|
|
| Lymph node
metastasis (yes/no) | 1.844 | 1.067–3.187 | 0.028 | 1.142 | 0.639–2.040 | 0.654 |
|
Tumor-Node-Metastasis [IV/(I/II/III)] | 2.037 | 1.146–3.620 | 0.015 | 2.033 | 1.128–3.662 | 0.018 |
| Nerve invasion | 1.569 | 0.937–2.627 | 0.087 |
|
|
|
| Metadherin
expression (high/low) | 2.466 | 1.440–4.223 | 0.001 | 1.853 | 1.025–3.352 | 0.041 |
| Surgery margin
(yes/no) | 2.296 | 1.326–3.974 | 0.003 | 1.809 | 1.018–3.214 | 0.043 |
Discussion
PCCA is a relatively rare bile duct tumor, which
accounts for 2% of all cancer types identified in humans (17). The incidence of PCCA in Asian
countries appears to be associated with liver infection caused by
parasites from the Opistorchiidae family, while in Western
countries PCCA is caused by chronic bile duct inflammation, notably
primary sclerosing cholangitis (18). Surgical removal of the tumor is still
considered the most effective treatment method for PCCA.
Approximately 60% of patients with PCCA have a considerably wide
margin resection (19). Adjuvant
therapy methods including chemical drug treatment and radiotherapy
may be applied in PCCA treatment and have exhibited satisfactory
curative effects (20). However, the
prognosis of PCCA remains very poor and even radical surgery (R0
resection) cannot increase the 5-year survival rate considerably.
The 5-year survival rate of PCCA is ~40%, the recurrence rate is as
high as 50–70%, and in R1/2 resection the 5-year survival rate is
almost zero (21). Consequently, it
is urgent to investigate the molecular mechanism underlying PCCA
progression and provide novel therapies for its treatment.
In previous years, MTDH has been proposed to possess
oncogenic functions by various studies (8,22–24).
High MTDH expression was associated with poor clinical pathological
characteristics in the patients in the present study, including
tumor stage, lymphatic metastasis, tumor recurrence and disease
prognosis. High MTDH expression may result in tumorigenesis via the
increased expression of phosphorylated (p-) protein kinase B,
p-MDM2 proto-oncogene and p-glycogen synthase kinase-3β (GSK-3β)
and via the inhibition of P53 and P21 expression (25). In liver cancer, exogenous MTDH
expression in HepG3 cells indicated strong activity of
mitogen-activated protein kinases, including activated
extracellular signal-regulated kinase and p38. These enzymes
inactivated GSK-3β through phosphorylation, and increased β-catenin
phosphorylation and nuclear translocation. In this manner MTDH is
able to activate the Wnt/β-catenin signaling pathway and promote
tumor gene expression (26–29). The downregulation of the expression
of the tumor suppressor gene phosphatase and tensin homolog, and
the promotion of B-cell lymphoma 2 expression by MTDH were also
reported as important anticancer mechanisms required for the
treatment of human breast cancer (30). These data suggested that MTDH serves
an important function in tumor development and may be considered a
potential therapeutic target.
In the present study, the MTDH expression in 66
tissues of PCCA were examined using immunohistochemical methods,
and it was revealed that MTDH was positive in 48.5% of PCCA
tissues. Further analysis revealed that positive MTDH expression
was associated with lymph node metastasis and poor differentiation
in patients with PCCA. Survival prognostic analysis suggested that
a high MTDH expression in PCCA resulted in a worse RFS and OS
rates. Although the number of clinical samples was limited, the
included patients were only 66 cases in the present study, and the
results suggested that high MTDH expression in PCCA may provide a
meaningful tumor marker that is able to predict patient
prognosis.
Previous studies have clearly demonstrated that MTDH
participates in breast cancer metastasis to the lung (8,31,32). In
addition, MTDH was reported to have an effect in promoting tumor
metastasis in a number of human cancer types (33,34). EMT
is an important characteristic for the initiation of tumor cell
migration and invasion. One previous study demonstrated that MTDH
participates in EMT by upregulating N-cadherin, Snail family
transcriptional repressor 1 and Snail family transcriptional
repressor 2 expression and by inhibiting E-cadherin expression
(35). It has been further reported
that certain microRNAs (miRNAs) may regulate EMT by targeting MTDH
(18). These results are consistent
with the analysis in the present study and indicate that high MTDH
expression in PCCA is highly associated with lymph node
metastasis.
The oncogenic function of MTDH may be associated
with with tumor metastasis for various reasons. Firstly, MTDH may
promote angiogenesis through enhancing the expression of multiple
angiogenesis molecular markers (36). Secondly, MTDH may inhibit the
expression of cell cycle protein inhibitors, including p53, p21 and
p27 and induce the expression of cell cycle promoting proteins,
including cyclin D1 and cyclin E (37). In prostate cancer, the inhibition of
MTDH promoted apoptosis, reduced cell viability and increased cell
sensitivity to cisplatin (38). MTDH
may also promote human cancer growth by regulating the expression
of specific miRNAs (39). In
contrast to the present study, miRNA-630 may inhibit breast cancer
cell growth by targeting MTDH (40).
In the present study, it was demonstrated that high MTDH expression
was associated with poor differentiation in PCCA. However, no
significant difference was noted with regard to high MTDH
expression and patients with PCCA with a large tumor diameter
compared with small tumor diameter. These results strongly suggest
that MTDH may promote PCCA growth and malignant transformation
through multiple mechanisms of action. Further experiments
including analyzing the association of MTDH and Ki67 in tumor
tissues and using in vivo experiments to confirm the
importance of MTDH expression are required.
The present study demonstrated that high MTDH
expression was noted in PCCA cases, and that high MTDH expression
in patients with PCCA was associated with poor tumor
differentiation, lymph node metastasis and a worse disease
prognosis. MTDH may serve a vital function in promoting malignant
transformation and is a potential therapeutic target in the
treatment of PCCA. To the best of our knowledge, this is the first
study to report MTDH expression in PCCA and its association with
the clinicopathological characteristics of this disease. The
detailed molecular mechanisms of the function of MTDH in PCCA
require further investigation.
In the present study, MTDH expression in patients
with PCCA was investigated and attempted to determine its clinical
and pathological significance. It was demonstrated that high MTDH
expression may serve an important function in PCCA tumor growth and
metastasis, and thus targeting MTDH potentially has important
therapeutic applications for patients with PCCA.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Health Bureau of
Shiyan City (grant no. 2012-1-035).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YZ and WFL performed the experiments and wrote the
manuscript. WFL made substantial contributions to conception and
design of the manuscript. YZ was responsible for the design of the
experiments. KZ and FS analyzed the experimental data. LLR and WFL
assisted with the statistical analysis. GW critically revised the
manuscript and provided final approval of the version to be
published and also made substantial contributions to conception and
design. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Taihe Hospital Affiliated to Hubei University of
Medicine (Hubei, China) and written informed consent was obtained
from each patient prior to enrolment.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that there are no competing
interests.
References
|
1
|
Patel T: Cholangiocarcinoma. Nat Clin
Pract Gastroenterol Hepatol. 3:33–42. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhao W, Zhang B, Guo X, Zhang X, Hu J, Hu
X and Lu Y: Expression of Ki-67, Bax and p73 in patients with hilar
cholangiocarcinoma. Cancer Biomark. 14:197–202. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chauhan A, House MG, Pitt HA, Nakeeb A,
Howard TJ, Zyromski NJ, Schmidt CM, Ball CG and Lillemoe KD:
Post-operative morbidity results in decreased long-term survival
after resection for hilar cholangiocarcinoma. HPB (Oxford).
13:139–147. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cheng Y, Chen Y and Chen H: Application of
portal parenchyma-enterostomy after high hilar resection for
Bismuth type IV hilar cholangiocarcinoma. Am Surg. 76:182–187.
2010.PubMed/NCBI
|
|
5
|
Liu Y, Su ZW, Li G, Yu C, Ren S, Huang D,
Fan S, Tian Y, Zhang X and Qiu Y: Increased expression of
metadherin protein predicts worse disease-free and overall survival
in laryngeal squamous cell carcinoma. Int J Cancer. 133:671–679.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hu G, Wei Y and Kang Y: The multifaceted
role of MTDH/AEG-1 in cancer progression. Clin Cancer Res.
15:5615–5620. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Su ZZ, Kang DC, Chen Y, Pekarskaya O, Chao
W, Volsky DJ and Fisher PB: Identification and cloning of human
astrocyte genes displaying elevated expression after infection with
HIV-1 or exposure to HIV-1 envelope glycoprotein by rapid
subtraction hybridization, RaSH. Oncogene. 21:3592–3602. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Brown DM and Ruoslahti E: Metadherin, a
cell surface protein in breast tumors that mediates lung
metastasis. Cancer Cell. 5:365–374. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bruix J and Sherman M: Practice Guidelines
Committee, American Association for the Study of Liver Diseases:
Management of hepatocellular carcinoma. Hepatology. 42:1208–1236.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sarkar D and Fisher PB: AEG-1/MTDH/LYRIC:
Clinical significance. Adv Cancer Res. 120:39–74. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yu C, Liu Y, Tan H, Li G, Su Z, Ren S, Zhu
G, Tian Y, Qiu Y and Zhang X: Metadherin regulates metastasis of
squamous cell carcinoma of the head and neck via AKT signalling
pathway-mediated epithelial-mesenchvmal transition. Cancer Lett.
343:258–267. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hu G, Chong RA, Yanu Q, Wei Y, Blanco MA,
Li F, Reiss M, Au JL, Haffty BG and Kang Y: MTDH activation by 8q22
genomic gain promotes chemoresistance and metastasis of
poor-prognosis breast cancer. Cancer Cell. 15:9–20. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhu K, Dai Z, Pan Q, Wang Z, Yang GH, Yu
L, Ding ZB, Shi GM, Ke AW, Yang XR, et al: Metadherin promotes
hepatocellular carcinoma metastasis through induction of
epithelial-mesenchymal transition. Clin Cancer Res. 17:7294–7302.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Madhusudhan KS, Gamanagatti S and Gupta
AK: Imaging and interventions in hilar cholangiocarcinoma: A
review. World J Radiol. 7:28–44. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Poruk KE, Pawlik TM and Weiss MJ:
Perioperative management of hilar cholangiocarcinoma. J
Gastrointest Sura. 19:1889–1899. 2015. View Article : Google Scholar
|
|
16
|
Amin MB: AJCC Cancer Staging Manual. 8th.
American Joint Committee on Cancer. Springer; New York, NY:
2016
|
|
17
|
Wang F, Liu Y and Zhang H: Loss of MTSS1
expression is an independent prognostic factor for hilar
cholangiocarcinoma. Pathol Oncol Res. 19:815–820. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shin HR, Oh JK, Masuyer E, Curado MP,
Bouvard V, Fang YY, Wiangnon S, Sripa B and Hong ST: Epidemiology
of cholangiocarcinoma: An update focusing on risk factors. Cancer
Sci. 101:579–585. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Endo I, House MG, Klimstra DS, Gönen M,
D'Angelica M, Dematteo RP, Fong Y, Blumgart LH and Jarnagin WR:
Clinical significance of intraoperative bile duct margin assessment
for hilar cholangiocarcinoma. Ann Surg Oncol. 15:2104–2112. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Welling TH, Feng M, Wan S, Hwang SY, Volk
ML, Lawrence TS, Zalupski MM and Sonnenday CJ: Neoadjuvant
stereotactic body radiation therapy, capecitabine, and liver
transplantation for unresectable hilar cholangiocarcinoma. Liver
Transpl. 20:81–88. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Otani K, Chijiiwa K, Kai M, Ohuchida J,
Nagano M, Tsuchiya K and Kondo K: Outcome of surgical treatment of
hilar cholangiocarcinoma. J Gastrointest Surg. 12:1033–1040. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu G, Huang X, Cui X, Zhang J, Wei L, Ni
R and Lu C: High SKIP expression is correlated with poor prognosis
and cell proliferation of hepatocellular carcinoma. Med Oncol.
30:5372013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang W, Yang L, Liang S, Liu D, Chen X,
Ma Z, Zhai S, Li P and Wang X: AEG-1 is a target of perifosine and
is over-expressed in gastric dysplasia and cancers. Dig Dis Sci.
58:2873–2880. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yao Y, Gu X, Liu H, Wu G, Yuan D, Yang X
and Song Y: Metadherin regulates proliferation and metastasis via
actin cytoskeletal remodelling in non-small cell lung cancer. Br J
Cancer. 111:355–364. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Emdad L, Darker D, Su ZZ, Randolph A,
Boukerche H, Valerie K and Fisher PB: Activation of the nuclear
factor kappaB pathway by astrocyte elevated gene-1: Implications
for tumor progression and metastasis. Cancer Res. 66:1509–1516.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nikpour M, Emadi-Baygi M, Fischer U,
Niegisch G, Schulz WA and Nikpour P: MTDH/AEG-1 contributes to
central features of the neoplastic phenotype in bladder cancer.
Urol Oncol. 32:670–677. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yu C, Liu Y, Tan H, Li G, Su Z, Ren S, Zhu
G, Tian Y, Qiu Y and Zhang X: Metadherin regulates metastasis of
squamous cell carcinoma of the head and neck via AKT signaling
pathway-mediated epithelial-mesenchymal transition. Cancer Lett.
343:258–267. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hui AB, Bruce JP, Alajez NM, Shi W, Yue S,
Perez-Ordonez B, Xu W, O'Sullivan B, Waldron J, Cummings B, et al:
Significance of dysregulated metadherin and microRNA-375 in head
and neck cancer. Clin Cancer Res. 17:7539–7550. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Singal AG, Conjeevaram HS, Volk ML, Fu S,
Fontana RJ, Askari F, Su GL, Lok AS and Marrero JA: Effectiveness
of hepatocellular carcinoma surveillance in patients with
cirrhosis. Cancer Epidemiol Biomarkers Prev. 21:793–799. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Luo Z, Hu X, Xiong H, Qiu H, Yuan X, Zhu
F, Wang Y and Zou Y: A polysaccharide from Huaier induced apoptosis
in MCF-7 breast cancer cells via down-regulation of MTDH protein.
Carbohydr Polym. 151:1027–1033. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang Y, Klijn JG, Zhang Y, Sieuwerts AM,
Look MP, Yang F, Talantov D, Timmermans M, Meijer-van Gelder ME, Yu
J, et al: Gene-expression profiles to predict distant metastasis of
lymph-node-negative primary breast cancer. Lancet. 365:671–679.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chan SL, Mo F, Johnson PJ, Siu DY, Chan
MH, Lau WY, Lai PB, Lam CW, Yeo W and Yu SC: Performance of serum
α-fetoprotein levels in the diagnosis of hepatocellular carcinoma
in patients with a hepatic mass. HPB (Oxford). 16:366–372. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gopal P, Yopp AC, Waljee AK, Chiang J,
Nehra M, Kandunoori P and Singal AG: Factors that affect accuracy
of α-fetoprotein test in detection of hepatocellular carcinoma in
patients with cirrhosis. Clin Gastroenterol Hepatol. 12:870–877.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Moore RF, Sholl AB, Kidd L, Al-Qurayshi Z,
Tsumagari K, Emejulu OM, Kholmatov R, Friedlander P, Abd Elmageed
ZY and Kandil E: Metadherin expression is associated with
extrathyroidal extension in papillary thyroid cancer patients. Ann
Surg Oncol. 23:2883–2888. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yu J, Wang JG, Zhang L, Yang HP, Wang L,
Ding D, Chen Q, Yang WL, Ren KH, Zhou DM, et al: MicroRNA-320a
inhibits breast cancer metastasis by targeting metadherin.
Oncotarget. 7:38612–38625. 2016.PubMed/NCBI
|
|
36
|
Liu Y, Kong X, Li X, Li B and Yang Q:
Knockdown of metadherin inhibits angiogenesis in breast cancer. Int
J Oncol. 46:2459–2466. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yu C, Chen K, Zheng H, Guo X, Jia W, Li M,
Zeng M, Li J and Song L: Overexpression of astrocyte elevated
gene-1 (AEG-1) is associated with esophageal squamous cell
carcinoma (ESCC) progression and pathogenesis. Carcinogenesis.
30:894–901. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wei YB, Guo Q, Gao YL, Yan B, Wang Z, Yang
JR and Liu W: Repression of metadherin inhibits biological behavior
of prostate cancer cells and enhances their sensitivity to
cisplatin. Mol Med Rep. 12:226–232. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Huang S, Wu B, Li D, Zhou W, Deng G, Zhang
K and Li Y: Knockdown of astrocyte elevated gene-1 inhibits tumor
growth and modifies microRNAs expression profiles in human
colorectal cancer cells. Biochem Biophys Res Commun. 444:338–345.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhou CX, Wang CL, Yu AL, Wang QY, Zhan MN,
Tang J, Gong XF, Yin QQ, He M, He JR, et al: MiR-630 suppresses
breast cancer progression by targeting metadherin. Oncotarget.
7:1288–1299. 2016.PubMed/NCBI
|