Introduction
Ovarian cancer is one of the leading causes of death
in women. In 2018 alone, 14,070 women have died from this disease
in United States (1). Although the
standard treatment is cytoreductive surgery followed by
chemotherapy, the mortality rate of ovarian cancer remains
substantial and has not significantly improved. Thus, new
treatments and methods of prognostic prediction need to be
developed for women with ovarian cancer.
Recent studies have evaluated the importance of
tumor-infiltrating lymphocytes (TILs) in various types of cancer,
revealing that increased TIL levels are associated with better
prognosis (2–5). Moreover, several studies have
demonstrated that TILs are associated with the survival and
prognosis of individuals with epithelial ovarian cancers (EOCs).
Zhang et al found that a high counts of intratumoral
CD3+ T cells were associated with higher
progression-free and overall survival rates (6). Sato et al reported that high
counts of intraepithelial CD8+ T cells, but not
CD3+ TILs, correlated with improved survival and
prognosis (7). Webb et al
revealed that intraepithelial CD103+ TILs were strongly
associated with patient survival, and affected the prognosis of
high-grade ovarian serous carcinoma (HGSC) (8). Even though all of these studies used
biomarkers to identify the subtypes of inflammatory cells that
affect survival and prognosis, the biomarkers that were used and
the results that were reported have not been consistent.
The International TILs Working Group 2014 on breast
cancer provided recommendations for the evaluation of TILs on
hematoxylin and eosin (H&E)-stained slides. TILs assessed using
these breast cancer recommendations have been applied as predictors
of response to adjuvant or neoadjuvant chemotherapy and prognosis
(9,10). For ovarian cancers, TILs can also be
assessed on H&E-stained slides according to the recommendations
of the International TILs Working Group 2014. However, the clinical
significance of these TIL assessments remains to be determined. To
date, most studies of ovarian cancers have focused only on TILs of
specific subtypes located in the intraepithelial area, as evaluated
using immunohistochemistry. Only one study has evaluated general
TILs on H&E-stained slides.
In the present study, we measured stromal TILs on
H&E-stained slides using the recommendations of the
International TILs Working Group 2014 on breast cancer. Moreover,
we evaluated the associations of stromal TILs with the survival and
prognosis of individuals with EOCs.
Materials and methods
Patients
In total, 270 patients with primary EOCs who
underwent explorative laparotomy at the Department of Gynecology of
Pusan National University Hospital (PNUH) from 1998 to 2013 were
included in the study. All patients provided written informed
consent and underwent surgical procedures. We excluded patients who
were not diagnosed with serous, mucinous, endometriod, and clear
cell carcinoma, and analyzed cases with available tissue slides
from the cohort of all patients. As a result, 256 patients were
included in the study. The biospecimens for this study were
provided by the Biobank of PNUH, a member of the National Biobank
of Korea, which is supported by the Ministry of Health, Welfare and
Family Affairs. All samples derived from the National Biobank of
Korea were obtained with institutional review board approval.
All cases were examined by direct microscopic
observation of H&E-stained slides of formalin-fixed and
paraffin-embedded surgical specimens. Tumor histology was
identified according to the World Health Organization
classification, and tumor stage was diagnosed based on the criteria
of the International Federation of Gynecology and Obstetrics
(FIGO). Other clinical data were obtained from the electronic
medical records of PNUH. The mean age of patients was 53.5 years
(range, 15–78 years). Clinicopathologic data, including tumor
grade, mitosis, nuclear grade, tumor stage, histologic type,
residual tumor, and chemotherapy response, are shown in detail in
Table I. Tumor stage was
reclassified as early stage for stage I and advanced stage for
stages II, III, and IV. Overall survival was measured from
diagnosis to death, and no patients were lost to follow-up.
 | Table I.Clinicopathologic characteristics of
the patients. |
Table I.
Clinicopathologic characteristics of
the patients.
| Cliniopathologic
characteristics | Numbers | Percentage, % |
|---|
| Histologic types |
|
|
|
Serous | 145 | 56 |
|
Mucinous | 48 | 19 |
|
Endometrioid | 20 | 8 |
|
Clear | 43 | 17 |
| Tumor grade |
|
|
| 1 | 57 | 22 |
| 2 | 127 | 50 |
| 3 | 72 | 28 |
| Nuclear grade |
|
|
| Mild | 17 | 7 |
|
Moderate | 125 | 49 |
|
Marked | 114 | 44 |
| Mitosis |
|
|
|
0-9/10HPFs | 95 | 37 |
|
10-24/10HPFs | 97 | 38 |
|
≥25/10HPFs | 64 | 25 |
| Tumor stage |
|
|
| Early
stage | 72 | 38 |
|
Advanced stage | 118 | 62 |
| Chemoresponse |
|
|
|
Regressive disease | 77 | 44 |
|
Stable/progressive
disease | 98 | 56 |
| Residual tumor |
|
|
|
Optimal | 173 | 91 |
|
Suboptimal | 18 | 9 |
Digital slides of high-grade ovarian
serous carcinoma from The Cancer Genome Atlas (TCGA) dataset
The digital slide images of HGSCs were downloaded
from the TCGA data portal (http://tcga-data.nci.nih.gov/). All digital slides
were prepared from frozen specimens, and the digital slides of 475
patients were evaluated.
Evaluation method for stromal
TILs
The stromal TILs were evaluated according to the
recommendations of International TILs Working Group 2014 (11). The authors recommended that TILs
should be reported for the stromal compartment, with percentages,
and evaluated within the tumor border. TILs in the tumor area with
crush artifact, necrosis, or hyalinization should be excluded.
Polymorphonuclear leukocytes should also bel excluded. Although one
section per patient is sufficient for evaluation according to the
recommendations, we assessed all available slides. The
recommendations provided the table with detailed guidelines for
assessing TILs, representative H&E photographs, and
illustrations for some TIL percentages, which were used to evaluate
the stromal TILs (11). Two
pathologists evaluated stromal TILs together. If their evaluations
had different results, the final stromal TIL value was determined
through consensus. Stromal TIL percentages were evaluated under a
microscope via eye measurement. After evaluating the percentages of
stromal TILs on all of the available H&E-stained slides from
the primary EOCs, the average stromal TIL percentage was calculated
for each patient. This average stromal TIL percentage was used in
the statistical analyses.
Statistical analysis
The χ2 test was used to assess
relationships between stromal TILs and clinicopathologic factors.
The overall survival rates of the low and high stromal TIL groups
were analyzed using Kaplan-Meier survival analysis, and the
statistical significance of between-group differences was evaluated
using the log-rank test. Univariate and multivariate Cox regression
analyses were performed to identify the prognostic significance of
stromal TILs.
Results
Results of stromal TIL evaluations in
the PNUH cohort
Among the patients who were evaluated, the number of
H&E-stained slides ranged from 1 to 15, and the average number
of slides per patient was 4.25. The patients were classified into
the high stromal TIL group if their stromal TIL percentages was
>10%. They were classified into the low stromal TIL group if
their stromal TIL percentage was similar to or <10%.
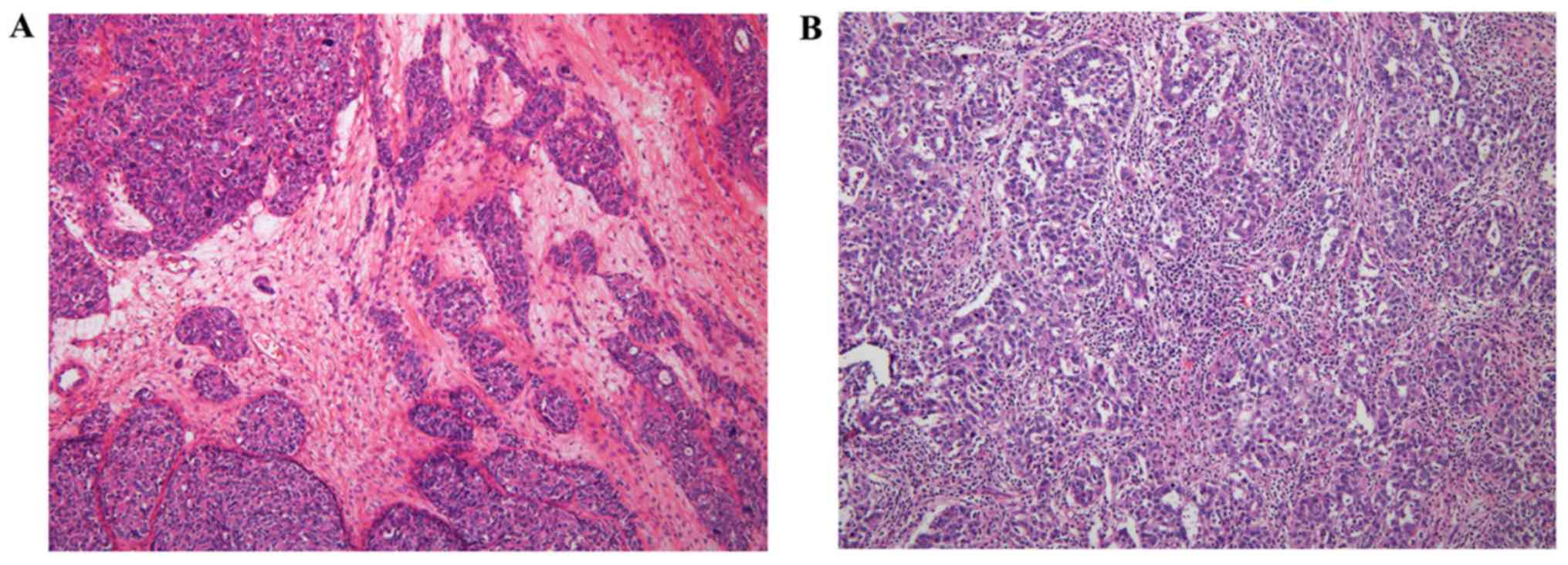
Representative cases of the low and high stromal TIL groups are
shown in Fig. 1.
The overall mean stromal TIL percentage was 6.94%.
As evaluated according to histologic type, the mean stromal TIL
percentages for serous, mucinous, endometrioid, and clear cell
carcinoma were 8.06, 2.98, 8.66, and 6.75%, respectively. Ovarian
endometrioid carcinomas had the highest stromal TIL values, whereas
ovarian mucinous carcinomas had the lowest values.
In total, 56 (22%) of the 256 patients were included
in the high stromal TIL group. Thirty-three (23%) of the 145
patients with ovarian serous carcinomas were included in the high
stromal TIL group. For the other histologic types of ovarian
cancer, the percentages and counts of patients classified into the
high stromal TIL group are presented in Table II.
 | Table II.Association between the stromal TIL
group and clinicopathologic factors in all histologic types of
epithelial ovarian cancer. |
Table II.
Association between the stromal TIL
group and clinicopathologic factors in all histologic types of
epithelial ovarian cancer.
|
| Stromal TILs |
|---|
|
|
|
|---|
| Variable | Low stromal TILs
(%) | High stromal TILs
(%) | P-value |
|---|
| Residual tumor |
|
| 0.162 |
|
Optimal | 139 (80) | 34 (20) |
|
|
Suboptimal | 13 (72) | 5 (23) |
|
| Tumor grade |
|
| 0.006 |
| 1 | 51 (89) | 6 (11) |
|
| 2 | 105 (83) | 22 (17) |
|
| 3 | 49 (68) | 23 (32) |
|
| Histologic
type |
|
| 0.002 |
|
Serous | 112 (77) | 33 (23) |
|
|
Mucinous | 47 (98) | 1 (2) |
|
|
Endometrioid | 12 (60) | 8 (40) |
|
|
Clear | 34 (79) | 9 (21) |
|
| Tumor stage |
|
| 1.000 |
| Early
stage | 57 (79) | 15 (21) |
|
|
Advanced stage | 94 (80) | 23 (20) |
|
| Nuclear grade |
|
| 0.032 |
| Mild
and moderate | 121 (85) | 21 (15) |
|
|
Marked | 84 (74) | 30 (26) |
|
| Mitosis |
|
| 0.005 |
|
0-9/10HPFs | 85 (89) | 10 (11) |
|
|
10-24/10HPFs | 76 (78) | 21 (22) |
|
|
≥25/10HPFs | 44 (69) | 20 (31) |
|
| Chemoresponse |
|
| 0.532 |
|
Regressive disease | 59 (77) | 18 (23) |
|
|
Stable/progressive
disease | 80 (82) | 18 (18) |
|
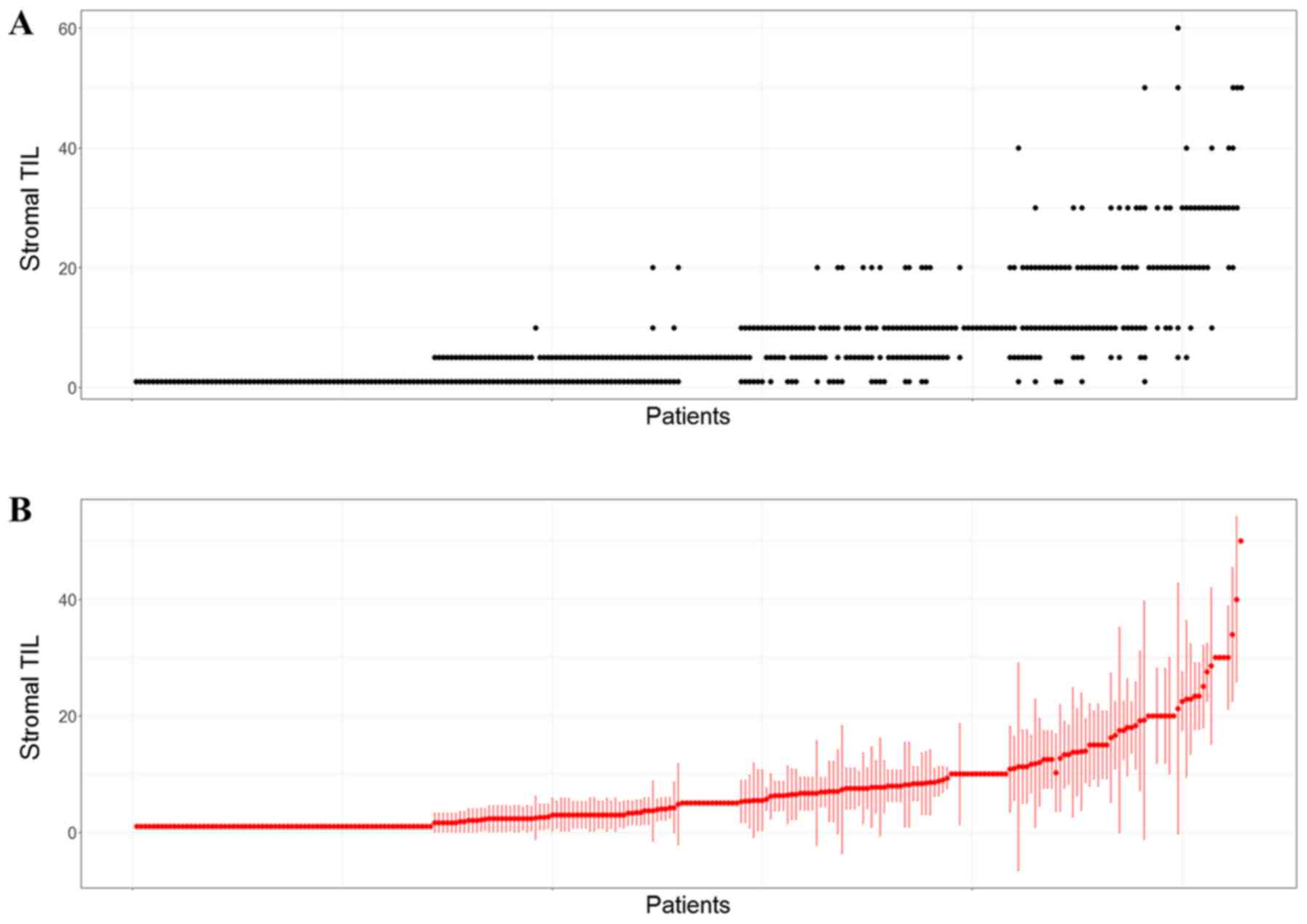
To assess differences in stromal TILs across parts
of the tumors, the standard deviations were calculated for each
case and listed in the order of increasing means (Fig. 2). The result showed that the standard
deviation and mean of the stromal TIL percentages increased
together.
Results of stromal TIL evaluations
using the TCGA dataset
The number of digital slides per patient ranged from
1 to 4, and the average number of digital slides per patient was
1.96. The patients were also classified into the high and low
stromal TIL groups based on the same criteria that were applied for
the PNUH cohort. In the TCGA dataset, the average percentage of
stromal TILs was 11.49%, and 164 (35%) of 475 patients were
included in the high stromal TIL group.
Associations between stromal TILs and
clinicopathologic characteristics in the PNUH cohort and TCGA
dataset
The chi-square test revealed that tumor grade,
histologic type, nuclear grade, and mitosis were associated with
stromal TILs (Table II). Higher
tumor grade, nuclear grade, and mitosis were each associated with
higher frequencies of stromal TIL cases.
Ovarian serous carcinoma is the most common type of
EOC. Thus, it has been the focus of the present study. In serous
carcinoma, stromal TILs were associated with tumor grade and
mitosis. Higher tumor grade and mitosis were each associated with
higher frequencies of stromal TIL cases (Table III).
 | Table III.Association between the stromal TIL
group and clinicopathologic factors in ovarian serous
carcinomas. |
Table III.
Association between the stromal TIL
group and clinicopathologic factors in ovarian serous
carcinomas.
|
| Stromal TILs |
|
|---|
|
|
|
|
|---|
| Variable | Low stromal TILs
(%) | High stromal TILs
(%) | P-value |
|---|
| Residual tumor |
|
| 0.924 |
|
Optimal | 72 (77) | 21 (23) |
|
|
Suboptimal | 10 (83) | 2 (17) |
|
| Tumor grade |
|
| 0.002 |
| 1 and
2 | 80 (86) | 13 (14) |
|
| 3 | 32 (62) | 20 (38) |
|
| Simple stage |
|
| 0.596 |
| Early
stage | 15 (71) | 6 (29) |
|
|
Advanced stage | 67 (80) | 17 (20) |
|
| Nuclear grade |
|
| 0.124 |
| Mild
and moderate | 60 (83) | 12 (17) |
|
|
Marked | 52 (71) | 21 (29) |
|
| Mitosis |
|
| 0.006 |
| 0-24/10
HPFs | 79 (85) | 14 (15) |
|
| ≥25/10
HPFs | 33 (63) | 19 (37) |
|
| Chemoresponse |
|
| 0.539 |
|
Regressive disease | 34 (76) | 11 (24) |
|
|
Stable/progressive
disease | 43 (83) | 9 (17) |
|
For high-grade serous ovarian cancer cases from the
TCGA dataset, none of the investigated clinicopathologic factors
(size of residual tumor, tumor stage, tumor grade primary therapy
outcome, or platinum status) were associated with stromal TILs.
Survival analysis of the PNUH
cohort
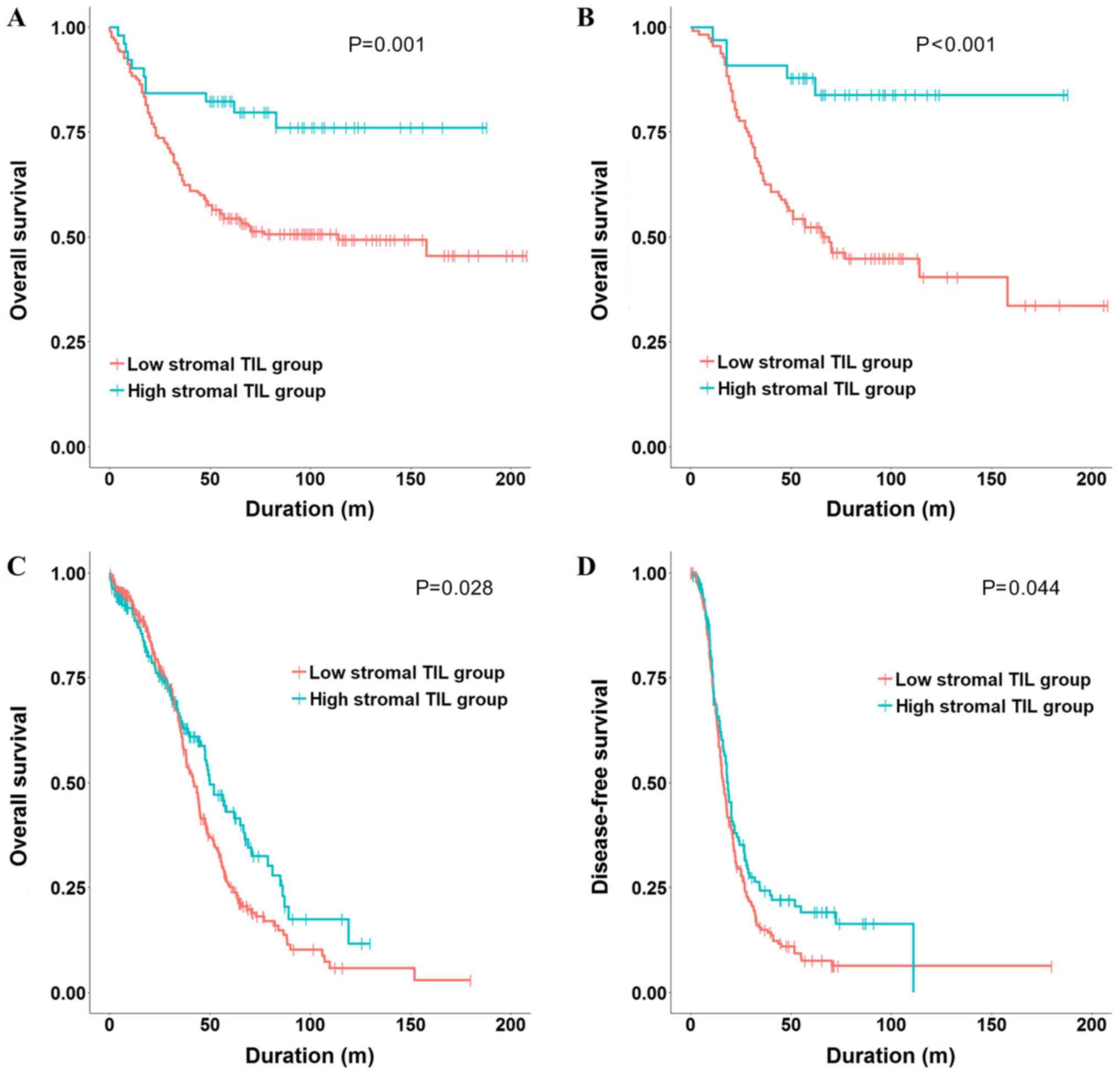
Kaplan-Meier survival analysis and the log-rank test
revealed that the high stromal TIL group had a higher overall
survival rate than the low stromal TIL group (P=0.001) (Fig. 3A). We performed univariate and
multivariate analyses of the pathologic prognostic factors,
including tumor grade, tumor stage, residual tumor after surgery,
and stromal TILs. Results showed that stromal TIL, tumor grade 3,
and tumor stage were independent prognostic factors (Table IV).
 | Table IV.Univariate and multivariate Cox
regression analysis in all histologic types of epithelial ovarian
cancer. |
Table IV.
Univariate and multivariate Cox
regression analysis in all histologic types of epithelial ovarian
cancer.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Variable | HR (95% CI for
HR) | P-value | HR (95% CI for
HR) | P-value |
|---|
| Stromal TILs |
|
|
|
|
| Low
group | 1 [Reference] |
| 1 [Reference] |
|
| High
group | 0.379
(0.203–0.707) | 0.002 | 0.343
(0.17–0.691) | 0.003 |
| Residual tumor |
|
|
|
|
|
Optimal | 1 [Reference] |
| 1 [Reference] |
|
|
Suboptimal | 2.551
(1.377–4.723) | 0.003 | 1.687
(0.903–3.15) | 0.101 |
| Tumor grade |
|
|
|
|
| Grade
1 | 1 [Reference] |
| 1 [Reference] |
|
| Grade
2 | 3.685
(1.895–7.168) |
<0.001 | 2.119
(0.929–4.836) | 0.074 |
| Grade
3 | 3.517
(1.738–7.119) |
<0.001 | 2.753
(1.158–6.546) | 0.022 |
| Simple stage |
|
|
|
|
| Early
stage | 1 [Reference] |
| 1 [Reference] |
|
|
Advanced stage | 5.598
(2.956–10.6) |
<0.001 | 4.303
(2.207–8.389) |
<0.001 |
For patients with ovarian serous carcinoma, stromal
TILs were significantly associated with the overall survival rate
(P<0.001) (Fig. 3B). Univariate
and multivariate analyses showed that stromal TILs and tumor stage
were independent prognostic factors (Table V).
 | Table V.Univariate and multivariate Cox
regression analysis in ovarian serous carcinomas. |
Table V.
Univariate and multivariate Cox
regression analysis in ovarian serous carcinomas.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Variable | HR (95% CI for
HR) | P-value | HR (95% CI for
HR) | P-value |
|---|
| Stromal TILs |
|
|
|
|
| Low
group | 1 [Reference] |
| 1 [Reference] |
|
| High
group | 0.218
(0.087–0.543) | 0.001 | 0.255
(0.09–0.72) | 0.010 |
| Residual tumor |
|
|
|
|
|
Optimal | 1 [Reference] |
| 1 [Reference] |
|
|
Suboptimal | 2.458
(1.143–5.283) | 0.021 | 1.638
(0.759–3.533) | 0.208 |
| Tumor grade |
|
|
|
|
| Grade
1 | 1 [Reference] |
| 1 [Reference] |
|
| Grade
2 | 8.337
(1.148–60.52) | 0.036 | 3.35
(0.451–24.881) | 0.237 |
| Grade
3 | 6.077
(0.817–45.21) | 0.078 | 3.424
(0.446–26.29) | 0.237 |
| Tumor stage |
|
|
|
|
| Early
stage | 1 [Reference] |
| 1 [Reference] |
|
|
Advanced stage | 17.19
(2.365–124.9) | 0.005 | 13.21
(1.976–97.1) | 0.011 |
Stromal TIL was significantly associated with
overall survival in individuals with ovarian mucinous carcinoma,
according to Kaplan-Meier survival analysis and the log-rank test
(P=0.029). However, this result was not reliable because only one
case was included in the high stromal TIL group. The associations
between stromal TILs and the overall survival rates of individuals
with endometrioid and clear cell carcinoma were not statistically
significant (P=0.241 and 0.317, respectively).
Survival analysis using the TCGA
dataset
Kaplan-Meier survival analysis and the log-rank test
revealed that the high stromal TIL group had significantly better
overall and disease-free survival rates than the low stromal TIL
group (P=0.028 and 0.044, respectively) (Fig. 3C and D, respectively). We performed
Cox regression analyses of the pathologic prognostic factors,
including tumor stage, tumor grade, residual tumor, and stromal
TILs. In the univariate analysis of the overall survival rate,
stromal TILs, tumor stage, and residual tumor were considered as
prognostic factors, and stromal TIL was a favorable prognostic
factor (Table VI). Tumor stage
remained a prognostic factor in the multivariate Cox regression
analysis of the overall survival rate. However, stromal TIL and
residual tumor were not prognostic factors in the multivariate
analysis.
 | Table VI.Univariate and multivariate cox
regression analysis for overall survival in high-grade ovarian
serous carcinoma of The Cancer Genome Atlas dataset. |
Table VI.
Univariate and multivariate cox
regression analysis for overall survival in high-grade ovarian
serous carcinoma of The Cancer Genome Atlas dataset.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Variable | HR (95% CI for
HR) | P-value | HR (95% CI for
HR) | P-value |
|---|
| Stromal TILs |
|
|
|
|
|
Low | 1 [Reference] |
| 1 [Reference] |
|
|
High | 0.744
(0.57–0.971) | 0.029 | 0.764
(0.576–1.014) | 0.063 |
| Tumor stage |
|
|
|
|
| Stage
II | 1 [Reference] |
| 1 [Reference] |
|
| Stage
III | 2.399
(1.128–5.102) | 0.023 | 2.535
(1.023–6.285) | 0.045 |
| Stage
IV | 3.153
(1.425–6.977) | 0.005 | 3.094
(1.202–7.965) | 0.019 |
| Tumor grade |
|
|
|
|
| Grade
2 | 1 [Reference] |
| 1 [Reference] |
|
| Grade
3 | 1.325
(0.917–1.915) | 0.134 | 1.344
(0.904–1.999) | 0.144 |
| Residual tumor |
|
|
|
|
|
Optimal | 1 [Reference] |
| 1 [Reference] |
|
|
Suboptimal | 1.33
(1.014–1.742) | 0.039 | 1.263
(0.955–1.67) | 0.102 |
In the univariate and multivariate Cox regression
analyses of the disease-free survival rate, stromal TIL was a
statistically independent and favorable prognostic factor (Table VII).
 | Table VII.The result of univariate and
multivariate cox regression analysis for disease-free survival in
HGSC of TCGA dataset. |
Table VII.
The result of univariate and
multivariate cox regression analysis for disease-free survival in
HGSC of TCGA dataset.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Variable | HR (95% CI for
HR) | P-value | HR (95% CI for
HR) | P-value |
|---|
| Stromal TIL |
|
|
|
|
|
Low | 1 [Reference] |
| 1 [Reference] |
|
|
High | 0.77
(0.597–0.994) | 0.044 | 0.748
(0.568–0.985) | 0.039 |
| Tumor stage |
|
|
|
|
| Stage
II | 1 [Reference] |
| 1 [Reference] |
|
| Stage
III | 1.895
(1.103–3.257) | 0.021 | 1.812
(0.963–3.411) | 0.066 |
| Stage
IV | 2.475
(1.351–4.534) | 0.003 | 2.451
(1.223–4.914) | 0.012 |
| Tumor grade |
|
|
|
|
| Grade
2 | 1 [Reference] |
| 1 [Reference] |
|
| Grade
3 | 1.329
(0.938–1.882) | 0.109 | 1.393
(0.939–2.064) | 0.099 |
| Residual tumor |
|
|
|
|
|
Optimal | 1 [Reference] |
| 1 [Reference] |
|
|
Suboptimal | 1.164
(0.881–1.537) | 0.285 | 1.13
(0.851–1.5) | 0.398 |
Discussion
Several previous studies have evaluated TILs on
H&E-stained slides using a method suggested by the
International TILs Working Group 2014 on breast, lung, stomach, and
esophageal cancer. The results of these studies showed that stromal
TILs were associated with survival rates and had prognostic effects
(9,12–17). The
results were particular striking for breast cancer, stromal TIL was
not only a prognostic factor, but also a predictor of responses to
both adjuvant and neoadjuvant chemotherapy in certain types of
breast cancer. For example, Loi et al reported that an
increase in stromal TILs was associated with benefits of
anthracycline-only chemotherapy in individuals with HER2-positive
breast cancer (9). Additionally,
Herrero-Vicent et al demonstrated that the rate of
pathologic complete remission of tumor and lymphadenopathy was
higher in individuals with lymphocyte-predominant triple-negative
breast cancer when neoadjuvant chemotherapy was performed before
surgery (10). Furthermore, it is
known that stromal TIL is more stable and reproducible than
intratumoral TIL in breast cancer (18). These results suggested that TILs
assessed on H&E-stained slides can be used as a predictor of
prognosis or therapeutic response. The results also suggested that
it would be worth investigating whether TILs assessed using
H&E-stained slides were clinically significant in ovarian
cancers.
Nevertheless, only one study of EOC has evaluated
TILs with H&E-stained slides using the method suggested by an
International TILs Working Group 2014 on breast cancer (19). To date, most studies of EOC have
instead evaluated TILs using immunostaining for immune-related
biomarkers, such as CD3, CD8, or FoxP3 (7,20,21).
James et al (19) indicated
that the findings of studies that used immunostaining differed in
terms of immune-related biomarkers that affect prognosis. These
results suggested that the evaluation of all types of inflammatory
cells on H&E-stained slides would be more effective in
confirming tumor immunity than in assessing TILs of a particular
subtype. Therefore, we evaluated stromal TILs on H&E-stained
slides using a method suggested by the International TIL Working
Group 2014 on breast cancer and identified their clinical
significance.
Based the standard deviations of stromal TILs that
were observed in the present study, the evaluation of as many tumor
slides as possible may help to improve the accuracy of stromal TIL
measurements in cases of EOC. However, the International TIL
Working Group 2014 recommendations showed that the evaluation of
only one representative slide is sufficient. EOCs generally have
ambiguous boundaries with normal tissue, and they are larger than
breast cancers. In addition, more intratumoral changes, such as
necrosis, are observed. Therefore, in EOCs, evaluating as many
slides as possible may improve the accuracy of stromal TIL
measurements.
Our study showed that stromal TIL was an independent
prognostic factor for EOC overall (all histologic types) and for
ovarian serous carcinoma, specifically. James et al
(19) also evaluated TILs on
H&E-stained slides and showed that stromal TILs were not an
independent prognostic factor for EOCs, which is contrary to our
results. Our study and the study of James et al both
followed the standardized method described by Salgado et al
(11) for breast cancer, and both
studies assessed TILs via eye measurement. The proportion of cases
with stromal TIL values above the cutoff value of 10% was
approximately the same in both studies, which indicates that there
is no difference in stromal TIL readings. In the previous study,
James et al evaluated a large number of cases and performed
survival analysis by dividing the cases into three groups according
to TILs, and this is the only difference from the present
study.
Although the cytotoxic T lymphocyte-mediated direct
killing mechanism is an important part of T cell-mediated cancer
regression, T cells exposed to tumor antigens must first migrate to
the tumor site. To overcome the various mechanisms of immune
invasion by cancer cells, it is necessary that as many T cells as
possible accumulate at the tumor site (22). Schietinger et al (23) showed that T cell-induced destruction
of stromal components, including blood vessels, leads to cancer
necrosis, and this phenomenon also occurs in individuals with
antigen-negative cancer cell variants. These results suggest that
the accumulation of TILs in the stroma without direct interaction
with cancer cells is extremely important for cancer removal and
that stromal TILs can influence the clinical outcomes.
In conclusion, we evaluated stromal TILs on
H&E-stained slides using a method suggested by the
International TILs Working Group 2014 on breast cancer. We found
that this evaluation of stromal TILs could be useful for predicting
the prognosis of patients with EOC, particularly those with serous
ovarian cancers. More studies should be performed to evaluate the
precise clinical significance of stromal TILs assessed on
H&E-stained slides.
Acknowledgements
Not applicable.
Funding
This study was supported by a 2-Year Research Grant
of Pusan National University.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
CH and KUC designed the experiments and wrote the
manuscript. KHK, DSS and BSK contributed to the acquisition of data
and approved the final version of the version to be published. CH,
SJL and JHL analyzed the data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
This study was approved by the ethics committee of
Pusan National University Hospital (Busan, South Korea) and
informed consent was obtained from all individual participants
included in the study.
Patient consent for publication
Consent was obtained from all individual
participants included in the study.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
TIL
|
tumor-infiltrating lymphocyte
|
|
EOC
|
epithelial ovarian cancer
|
|
H&E
|
hematoxylin and eosin
|
|
HGSC
|
high grade ovarian serous
carcinoma
|
|
PNUH
|
Pusan National University Hospital
|
|
FIGO
|
International Federation of Gynecology
and Obstetrics
|
|
TCGA
|
The Cancer Genome Atlas
|
References
|
1
|
Seigel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Clemente CG, Mihm MC Jr, Bufalino R,
Zurrida S, Collini P and Cascinelli N: Prognostic value of tumor
infiltrating lymphocytes in the vertical growth phase of primary
cutaneous melanoma. Cancer. 77:1303–1310. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mahmoud SM, Paish EC, Powe DG, Macmillan
RD, Grainge MJ, Lee AH, Ellis IO and Green AR: Tumor-infiltrating
CD8+ lymphocytes predict clinical outcome in breast
cancer. J Clin Oncol. 29:1949–1955. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ropponen KM, Eskelinen MJ, Lipponen PK,
Alhava E and Kosma VM: Prognostic value of tumour-infiltrating
lymphocytes (TILs) in colorectal cancer. J Pathol. 182:318–324.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fukunaga A, Miyamoto M, Cho Y, Murakami S,
Kawarada Y, Oshikiri T, Kato K, Kurokawa T, Suzuoki M, Nakakubo Y,
et al: CD8+ tumor-infiltrating lymphocytes together with
CD4+ tumor-infiltrating lymphocytes and dendritic cells
improve the prognosis of patients with pancreatic adenocarcinoma.
Pancreas. 28:e26–e31. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang L, Conejo-Garcia JR, Katsaros D,
Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H,
Schlienger K, Liebman MN, et al: Intratumoral T cells, recurrence,
and survival in epithelial ovarian cancer. N Engl J Med.
348:203–213. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sato E, Oison SH, Ahn J, Bundy B,
Nishikawa H, Qian F, Jungbluth AA, Frosina D, Gnjatic S, Ambrosone
C, et al: Intraepithelial CD8+ tumor-infiltrating
lymphocytes and a high CD8+/regulatory T cell ratio are
associated with favorable prognosis in ovarian cancer. Proc Natl
Acad Sci USA. 102:18538–18543. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Webb JR, Milne K, Watson P, Deleeuw RJ and
Nelson BH: Tumor-infiltrating lymphocytes expressing the tissue
resident memory marker CD103 are associated with increased survival
in high-grade serous ovarian cancer. Clin Cancer Res. 20:434–444.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Loi S, Sirtaine N, Piette F, Salgado R,
Viale G, Van Eenoo F, Rouas G, Francis P, Crown JP, Hitre E, et al:
Prognostic and predictive value of tumor-infiltrating lymphocytes
in a phase III randomized adjuvant breast cancer trial in
node-positive breast cancer comparing the addition of docetaxel to
doxorubicin with doxorubicin-based chemotherapy: BIG 02–98. J Clin
Oncol. 31:860–867. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Herrero-Vicent C, Guerrero A, Gavila J,
Gozalbo F, Hernandez A, Sandiego S, Algarra MA, Calatrava A,
Guillem-Porta V and Ruiz-Simon A: Predictive and prognostic impact
of tumour-infiltrating lymphocytes in triple-negative breast cancer
treated with neoadjuvant chemotherapy. Ecancermedicalscience.
11:7592017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Salgado R, Denkert C, Demaria S, Sirtaine
N, Klauschen F, Pruneri G, Wienert S, Van den Eynden G, Baehner FL,
Penault-Llorca F, et al: The evaluation of tumor-infiltrating
lymphocytes (TILs) in breast cancer: recommendations by an
International TILs Working Group 2014. Ann Oncol. 26:259–271. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Loi S, Michiels S, Salgado R, Sirtaine N,
Jose V, Fumagali D, Kellokumpu-Lehtinen PL, Bono P, Kataja V,
Desmedt C, et al: Tumor infiltrating lymphocytes are prognostic in
triple negative breast cancer and predictive for trastuzumab
benefit in early breast cancer: Results from the FinHER trial. Ann
Oncol. 25:1544–1550. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Denkert C, von Minckwitz G, Brase JC, Sinn
BV, Gade S, Kronenwett R, Pfitzner BM, Salat C, Loi S, Schmitt WD,
et al: Tumor-infiltrating lymphocytes and response to neoadjuvant
chemotherapy with or without carboplatin in human epidermal growth
factor receptor 2-positive and triple-negative breast cancers. J
Clin Oncol. 33:983–991. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Adams S, Gray RJ, Demaria S, Goldstein L,
Perez EA, Shulman LN, Martino S, Wang M, Jones VE, Saphener TJ, et
al: Prognostic value of tumor-infiltrating lymphocytes in
triple-negative breast cancers from two phase III randomized
adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin
Oncol. 32:2959–2966. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mignon S, Wilard-Gallo K, Van den Eynden
G, Salgado R, Waelput W, Decoster L, Marien K, Vansteenkiste J,
Teugels E and De Greve J: The relationship of TILs and PD-L1
expression in NSCLC adenocarcinoma in little to non-smokers with
driver mutations and outcome parameters. J Thorac Oncol.
12:S13312017. View Article : Google Scholar
|
|
16
|
Kang BW, Seo An, Yoon S, Bae HI, Jeon SW,
Kwon OK, Chung HY, Yi W, Kang H and Kim JG: Prognostic value of
tumor-infiltrating lymphocytes in Epstein-Barr virus-associated
gastric cancer. Ann Oncol. 27:494–501. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sudo T, Nishida R, Kawahara A, Saisho K,
Mimori K, Yamada A, Mizogchi A, Kadoya K, Matono S, Mori N, et al:
Clinical impact of tumor-infiltrating lymphocytes in esophageal
squamous cell carcinoma. Ann Surg Oncol. 24:3763–3770. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ahn SG, Jeong J, Hong S and Jung WH:
Current issues and clinical evidence in tumor-infiltrating
lymphocytes in breast cancer. J Pathol Transl Med. 49:355–363.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
James FR, Jiminez-Linan M, Alsop J, Mack
M, Song H, Brenton JD, Pharoah PDP and Ali HR: Association between
tumor infiltrating lymphocytes, histotype and clinical outcome in
epithelial ovarian cancer. BMC Cancer. 17:6572017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tomsová M, Melichar B, Sedlákovál I and
Steiner I: Prognostic significance of CD3+
tumor-infiltrating lymphocytes in ovarian carcinoma. Gynecol Oncol.
108:415–420. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hermans C, Anz D, Engel J, Kirhner T,
Endres S and Mayr D: Analysis of FoxP3+ T-regulatory
cells and CD8+ T-cells in ovarian carcinoma: Location
and tumor infiltrating patterns are key prognostic markers. PLos
One. 9:e1117572014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Slaney CY, Kershaw MH and Darcy PK:
Trafficking of T cells into tumor. Cancer Res. 74:7168–7174. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schietinger A, Arina A, Liu RB, Wells S,
Huang J, Engels B, Bindokas V, Bartkowiak T, Lee D, Hermann A, et
al: Longitudinal confocal microscopy imaging of solid tumor
destruction following adoptive T cell transfer. Oncoimmunology.
2:e266772013. View Article : Google Scholar : PubMed/NCBI
|