Introduction
Lung cancer is a common type of malignant tumour and
a leading cause of cancer-associated mortality worldwide. Every
year, lung cancer occurs in ~1.8 million individuals and results in
1.6 million mortalities. Despite advances in early diagnosis and
multimodal treatment, over one-half of the patients who are
diagnosed with lung cancer do not survive beyond a year, and the
5-year survival rate is only ~18% (1). The invasive and metastatic mechanisms
of lung cancer remain unclear. Chemokines and their receptors have
emerged as pivotal regulators of tumour growth, progression and
metastasis (2). Therefore,
elucidating the mechanistic roles of chemokines in the recurrence
and metastasis of lung cancer may improve the diagnosis and
treatment of lung cancer.
Chemokine C-X3-C motif ligand 1 (CX3CL1), a
protein-coding gene of fractalkine, serves as a ligand for
chemokine C-X3-C motif receptor 1 (CX3CR1) and integrins. When
CX3CL1 binds to CX3CR1 and integrins, CX3CR1-dependent and
CX3CR1-independent signal pathways may be activated. In contrast to
other chemokines, CX3CL1 has two different forms, a soluble form
and a membrane-bound form, and each form mediates distinct
biological actions. The soluble form is a strong activator of
chemotaxis and causes migration of natural killer cells, cytotoxic
T lymphocytes and macrophages (3).
The membrane-bound form promotes leukocyte-endothelial cell
adhesion, and may serve a role in the processes of leukocyte
adhesion and migration at the endothelium (4,5). CX3CL1
and its receptor are involved in a number of inflammatory
processes, including allergic asthma, rheumatoid arthritis, Crohn's
disease and atherosclerosis (3,6,7). These previous studies demonstrated that
CX3CL1 may be expressed in different tissues and may contribute to
a number of inflammatory diseases by promoting the accumulation of
CX3CR1-positive immune cells at inflammation sites (8,9).
However, under pathogenic conditions with abnormal local and
systemic immune responses, CX3CL1 may additionally induce potent
antitumour and tissue-protective effects (10,11).
It was hypothesised that CX3CL1/CX3CR1 are involved
in cancer pathogenesis and therapeutic approaches targeting this
ligand-receptor pair have additionally demonstrated promising
results in experimental settings. In epithelial ovarian cancer
cells, CX3CL1 promoted cancer cell proliferation by binding to
CX3CR1 and subsequently activating protein kinase B (12). Furthermore, ovarian carcinoma cells
migrated towards CX3CL1, and silencing of CX3CR1 reduced their
migration (13). Li et al
(14), demonstrated that CX3CL1
silencing in the HepG2 cell line inhibited angiogenesis in
vitro and in vivo. However, CX3CL1 has a range of
effects in breast cancer. It is involved in breast cancer
metastasis (15,16); however, increased CX3CL1 expression
is positively correlated with prognosis and tumour-infiltrating
lymphocytes (TIL) levels (16).
The association between CX3CL1 and
clinicopathological parameters in lung cancer remains unclear, and
correlations between its expression levels and its prognostic value
in different lung cancer subtypes require further study. In the
present study, datasets from various public databases were analysed
using statistical models. The prognostic effects identified in the
included studies were pooled to determine the significance of
CX3CL1 in different lung cancer subtypes.
Materials and methods
Search strategy and data
extraction
The Cancer Genome Atlas (TCGA, cancergenome.nih.gov) and the Gene Expression Omnibus
(GEO, www.ncbi.nlm.nih.gov/gds) were
searched using the key words ‘Lung cancer’ and ‘Homo sapiens’.
Subsequently, a preliminary screening based on the title content
was conducted. A total of two independent researchers were asked to
read the contents of each dataset. Datasets with a small sample
size (<50), incomplete clinicopathological parameters or no
clinical data were excluded. In addition, the staging information
of included datasets was based on the 7th Tumor-Node-Metastasis
(TNM) staging system (17). For the
screening results on which the researchers disagreed, a third
researcher was responsible for the final decisions. In total, six
datasets downloaded from the GEO database [GSE30219 (18), GSE37745 (19), GSE42127 (20), GSE50081 (21), GSE68465 (22) and GSE14814 (23)] and two TCGA datasets: lung
adenocarcinoma [LUAD, https://xenabrowser.net/datapages/?cohort=GDC%20TCGA%20
Lung%20Adenocarcinoma%20(LUAD)&removeHub=https%3A%2F%2Fxena.treehouse.gi.ucsc.edu%3A443]
and lung squamous cell carcinoma [LUSC, http://xenabrowser.net/datapages/?cohort=GDC%20TCGA%20Lung%20Squamous%20Cell%20Carcinoma%20(LUSC)&removeHub=https%3A%2F%2Fxena.treehouse.gi.ucsc.edu%3A443]
were included in the present study. The sub-datasets, including
CX3CL1 mRNA expression and associated clinical data, were extracted
for further statistical analysis.
Statistical analysis
All statistical analyses were performed using R
v3.5.0 (R Foundation for Statistical Computing, Vienna, Austria),
RStudio 1.1.456 (RStudio, Boston, MA, USA) and GraphPad Prism 5.0
software package (GraphPad Software, Inc., La Jolla, CA, USA). The
CX3CL1 expression levels were divided into two groups; high
expression and low expression. A web-based function called Cutoff
Finder (http://molpath.charite.de/cutoff) was used to
determine a cutoff point (24).
Independent samples t-test was used to compare means between two
groups, whereas one-way analysis of variance and the Tukey honest
significant difference post hoc test were used for multiple
comparisons. Kaplan-Meier analysis and a log-rank test were used
for comparing survival curves. Cox models were used to calculate
the hazard ratios (HRs) and the 95% confidence intervals (CIs)
based on the CX3CL1 expression levels and their corresponding
clinical parameters. HRs and their 95% CIs from all datasets were
pooled, and the heterogeneity of this pooled analysis based on
CX3CL1 mRNA expression level was appraised by the Cochran Q test
and the I2 test. A random-effects model (the DerSimonian-Laird
method) was applied when significance was P<0.1 or I2>50%;
otherwise, a fixed-effects model (the Mantel-Haenszel method) was
used. P<0.05 was considered to indicate a statistically
significant difference.
Publication bias was assessed by Begg's rank
correlation method. The significance levels of statistical tests
were determined according to two-tailed P-values and all pooled
analyses were performed using the STATA software package (v12.0;
StataCorp LP, College Station, TX, USA).
Functional enrichment analysis
For each dataset, the Pearson correlation
coefficients between the expression level of CX3CL1 and those of
other genes were calculated, and the coefficients were merged by
gene name across all datasets. The genes that had highly ranked
positive or negative correlation coefficients with CX3CL1 were
selected, and functional enrichment analysis was performed using
the R package ‘clusterProfiler’ (www.bioconductor.org/packages/release/bioc/html/clusterProfiler.html).
Dot plots of biological processes and Kyoto Encyclopedia of Genes
and Genomes (KEGG, www.genome.jp/kegg/pathway.html) pathways were drawn
using the R functions ‘enrichGO’ and ‘enrichKEGG’ in
‘clusterProfiler’. Enrichment maps for the enrichment results of
over-representation tests or gene set enrichment analysis were
generated using the R function ‘emapplot’.
Results
Study characteristics
In the present study, six GEO datasets and two TCGA
datasets were included. In the primary screening, 1,092 relevant
datasets were selected using the key word ‘Lung cancer’. The search
filters were set as follows: The sample type was set as ‘tissue’,
selecting 326 datasets, and the sample size was set as >50,
selecting 126 datasets. Subsequent to reading the titles, abstracts
and clinical outcomes of these datasets, a total of six datasets
from GEO met the inclusion criteria and two from TCGA, for a total
of eight datasets in the present study.
The baseline characteristics of all the included
studies is presented in Table I.
Datasets covering 2,443 patients from France, Sweden, Canada and
the USA were included in the present analysis. All of those
datasets included overall survival (OS), and the majority of them
contained sex, age, pathological type, clinical stage and treatment
information; the mRNA expression levels of CX3CL1 and associated
genes were integrated into the information mentioned above.
 | Table I.Baseline characteristics of the eight
included datasets for pooled-analysis. |
Table I.
Baseline characteristics of the eight
included datasets for pooled-analysis.
| Clinicopathological
parameters | GSE30219 | GSE37745 | GSE42127 | GSE50081 | GSE68465 | GSE14814 | TCGA lung
adenocarcinoma | TCGA lung squamous
cell carcinoma |
|---|
| Public date | 24-May-13 | 12-Oct-12 | 30-Jan-13 | 22-Dec-13 | 2-May-15 | 9-Sep-10 | 24-Feb-15 | 24-Feb-15 |
| Country | France | Sweden | USA | Canada | USA | Canada | USA | USA |
| Case number | 307 | 196 | 173 | 181 | 443 | 132 | 509 | 502 |
| Age (Range),
years | 62.50 | 65.00 | 66.30 | 69.77 | 65.00 | 61.80 | 66.00 | 68.00 |
|
| (15.00–84.00) | (39.00–84.00) | (42.30–86.20) | (40.16–87.93) | (33.00–87.00) | (35.40–81.30) | (38.00–88.00) | (39.00–90.00) |
| Sex |
|
|
|
|
|
|
|
|
|
Male | 250 | 107 | 91 | 98 | 223 | 90 | 235 | 364 |
|
Female | 43 | 89 | 82 | 83 | 220 | 42 | 274 | 127 |
| Pathology |
|
|
|
|
|
|
|
|
|
Adenocarcinoma | 85 | 106 | 131 | 127 | 443 | 70 | 509 | NR |
|
Squamous cell carcinoma | 61 | 66 | 42 | 42 | NR | 52 | NR | 502 |
| Large
cell carcinoma | 3 | 24 | NR | 7 | NR | NR | NR | NR |
| Small
cell carcinoma | 21 | NR | NR | NR | NR | NR | NR | NR |
| Large
cell neuroendocrine | 56 | NR | NR | NR | NR | NR | NR | NR |
| Basal
squamous cell carcinoma | 39 | NR | NR | NR | NR | NR | NR | NR |
|
Other | 28 | NR | NR | 5 | NR | 10 | NR | NR |
| Tumour stage |
|
|
|
|
|
|
|
|
| T1 | 166 | NR | NR | 57 | NR | NR | 167 | 113 |
| T2 | 69 | NR | NR | 122 | NR | NR | 274 | 287 |
| T3 | 31 | NR | NR | 2 | NR | NR | 47 | 069 |
| T4 | 21 | NR | NR | NR | NR | NR | 19 | 022 |
| TX | 6 | NR | NR | NR | NR | NR | 2 | NR |
| Node stage |
|
|
|
|
|
|
|
|
| N0 | 198 | NR | NR | 129 | NR | NR | 326 | 313 |
| N1 | 53 | NR | NR | 52 | NR | NR | 96 | 128 |
| N2 | 50 | NR | NR | NR | NR | NR | 74 | 039 |
| N3 | 10 | NR | NR | NR | NR | NR | 2 | 005 |
| NX | 2 | NR | NR | NR | NR | NR | 10 | 006 |
| Metastasis
stage |
|
|
|
|
|
|
|
|
| M0 | 282 | NR | NR | 181 | NR | NR | 343 | 403 |
| M1 | 8 | NR | NR | 0 | NR | NR | 25 | 7 |
| MX | 3 | NR | NR | NR | NR | NR | 137 | 75 |
| Tumour Node
Metastasis stage |
|
|
|
|
|
|
|
|
| I | NR | 130 | 109 | 127 | 276 | 72 | 276 | 241 |
| II | NR | 35 | 32 | 54 | 95 | 60 | 122 | 154 |
|
III | NR | 27 | 30 | NR | 69 | NR | 84 | 086 |
| IV | NR | 4 | 1 | NR | NR | NR | 26 | 007 |
| Treatment |
|
|
|
|
|
|
|
|
|
Yes | NR | 29 | 49 | NR | 89 | 132 | 126 | 115 |
| No | NR | 71 | 124 | NR | 340 | NR | 258 | 264 |
|
Unknown | NR | 96 | NR | NR | 13 | NR | 127 | 123 |
|
Differentiation |
|
|
|
|
|
|
|
|
|
Well | NR | NR | NR | NR | 60 | NR | NR | NR |
|
Moderate | NR | NR | NR | NR | 209 | NR | NR | NR |
|
Poorly | NR | NR | NR | NR | 166 | NR | NR | NR |
|
Unknown | NR | NR | NR | NR | 7 | NR | NR | NR |
CX3CL1 mRNA expression levels in
different tissue and TNM stages
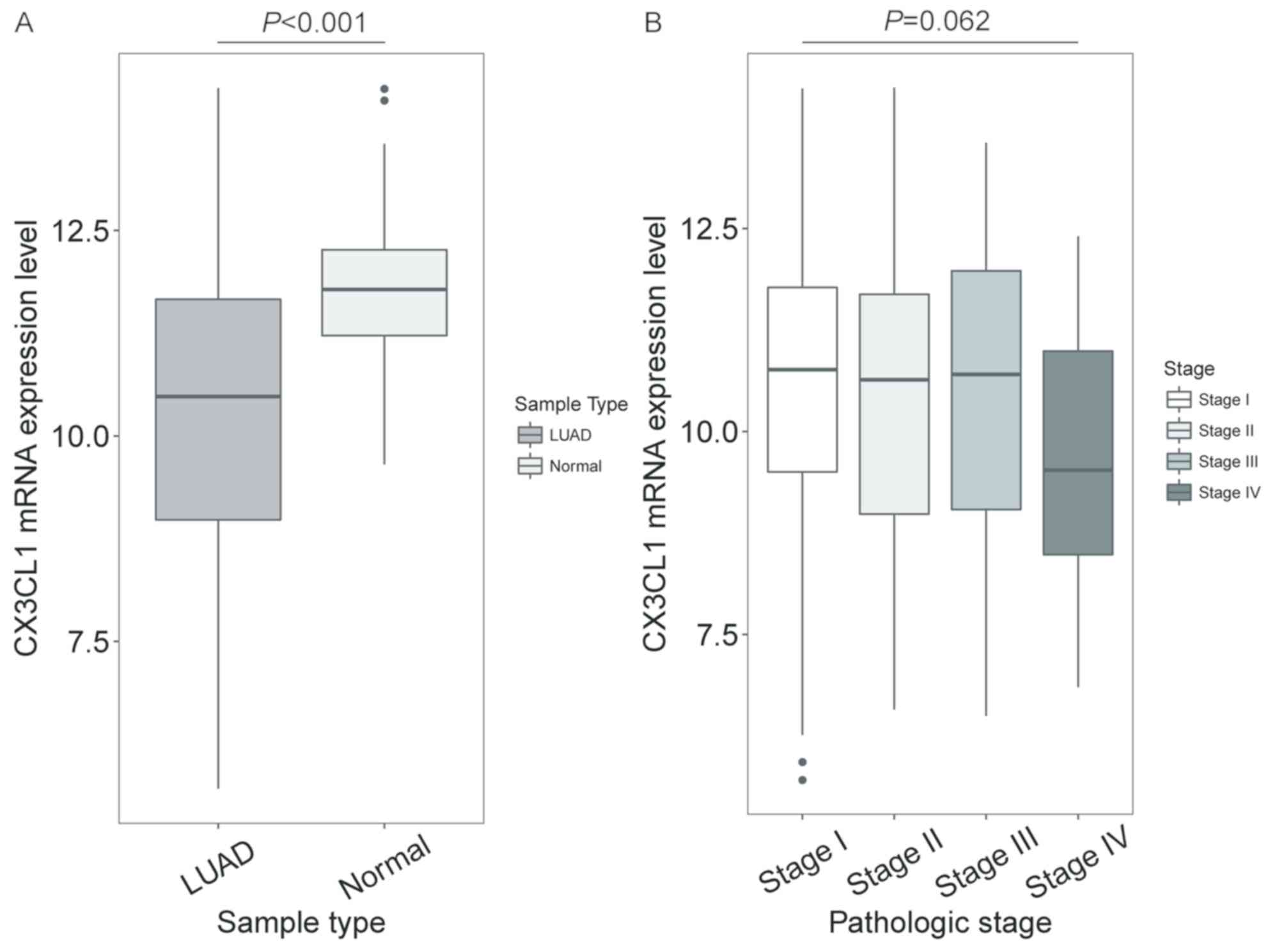
In patients with LUAD, the CX3CL1 mRNA expression
levels in tumour tissue were significantly decreased compared with
normal tissue (t=10.259, P<0.001; Fig. 1A), but no significant difference was
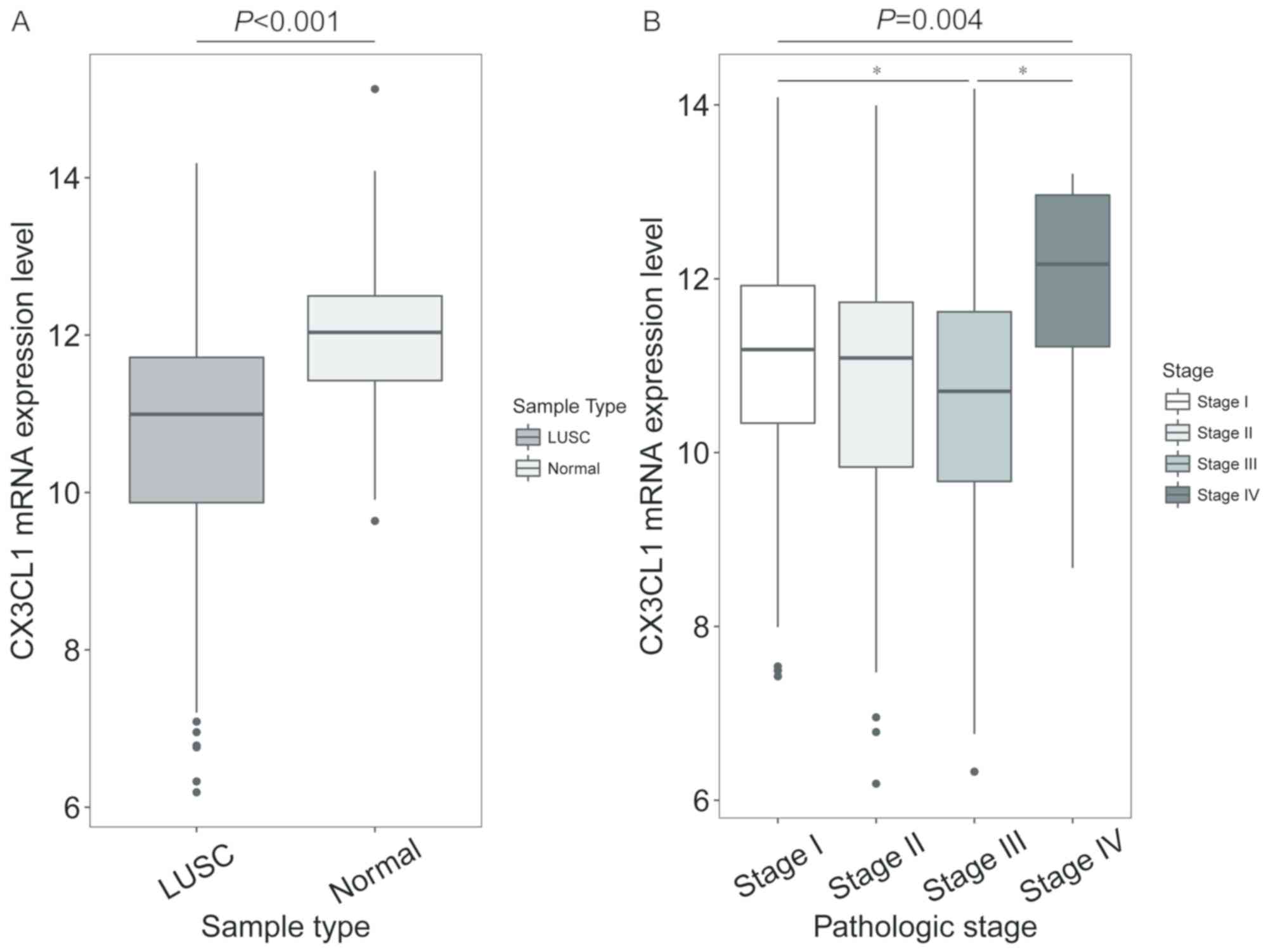
shown in different clinical stages (F=3.512, P=0.062; Fig. 1B). In patients with LUSC, the CX3CL1
mRNA expression levels were significantly decreased compared with
normal tissue (t=7.762, P<0.001; Fig.
2A). In addition, the CX3CL1 mRNA expression levels in stage
III samples were significantly different from those in stage I and
IV samples (F=4.432, P=0.004; stage III vs. stage I, P=0.021; stage
III vs. stage IV, P=0.036; Fig.
2B).
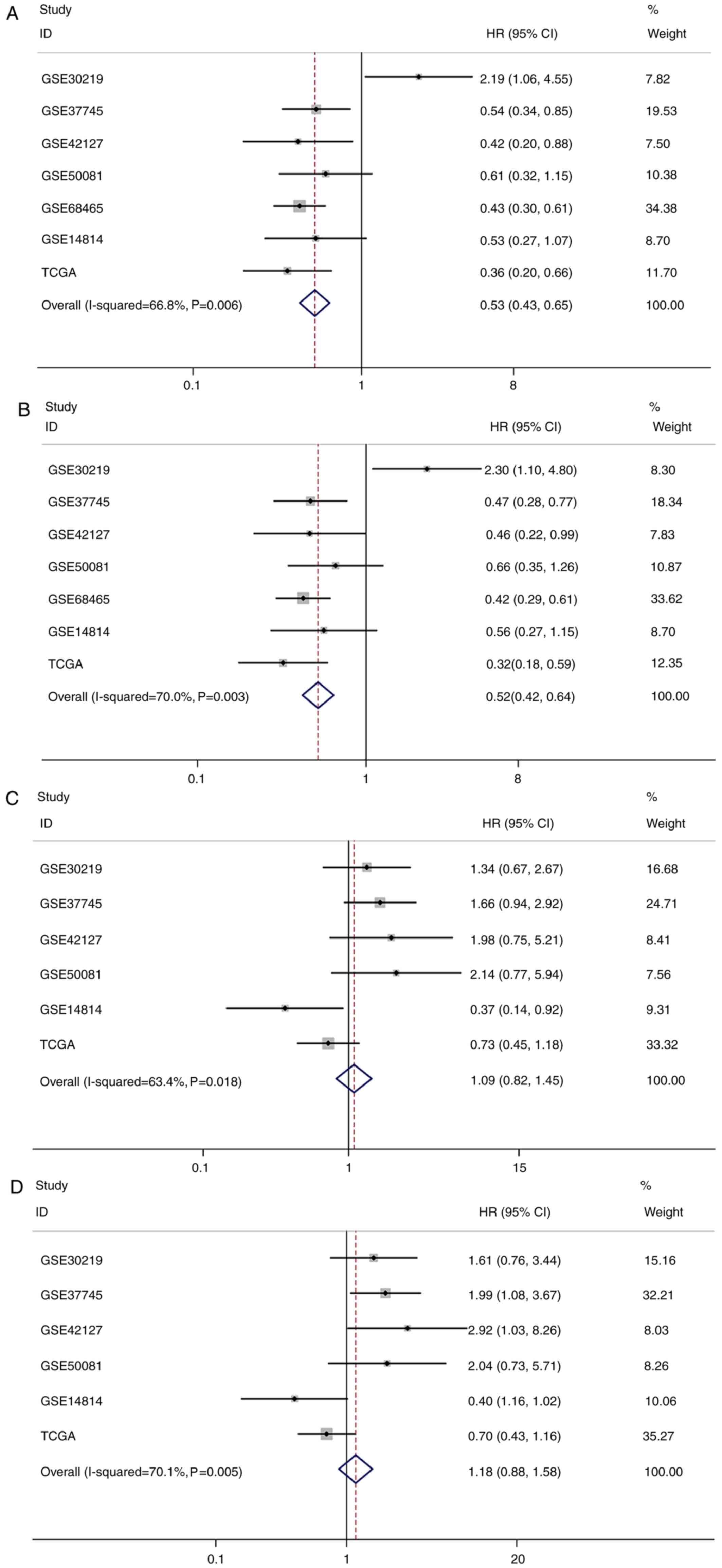
Survival analysis based on CX3CL1
expression level
A total of eight studies, including 2,443 patients,
were used to establish univariate and multivariate Cox models.
P-values, HRs and 95% CIs for each study based on LUAD and LUSC are
presented in Tables II and III. In patients with LUAD, univariate and
multivariate Cox model analyses of the GSE37745, GSE42127, GSE68465
and TCGA datasets revealed that higher CX3CL1 mRNA expression was
significantly associated with improved survival (HR<1,
P<0.05); however, results of the analyses in the GSE30219
dataset revealed an opposite trend (HR>1, P<0.05). In
patients with LUSC, univariate Cox model analysis of the GSE14814
dataset revealed that CX3CL1 mRNA expression exhibited a
significant protective effect on prognosis (HR<1, P<0.05).
Conversely, multivariate Cox model analysis of the GSE37745 and
GSE42127 datasets indicated that higher CX3CL1 mRNA expression was
a risk factor for LUSC prognosis (HR>1, P<0.05). Survival
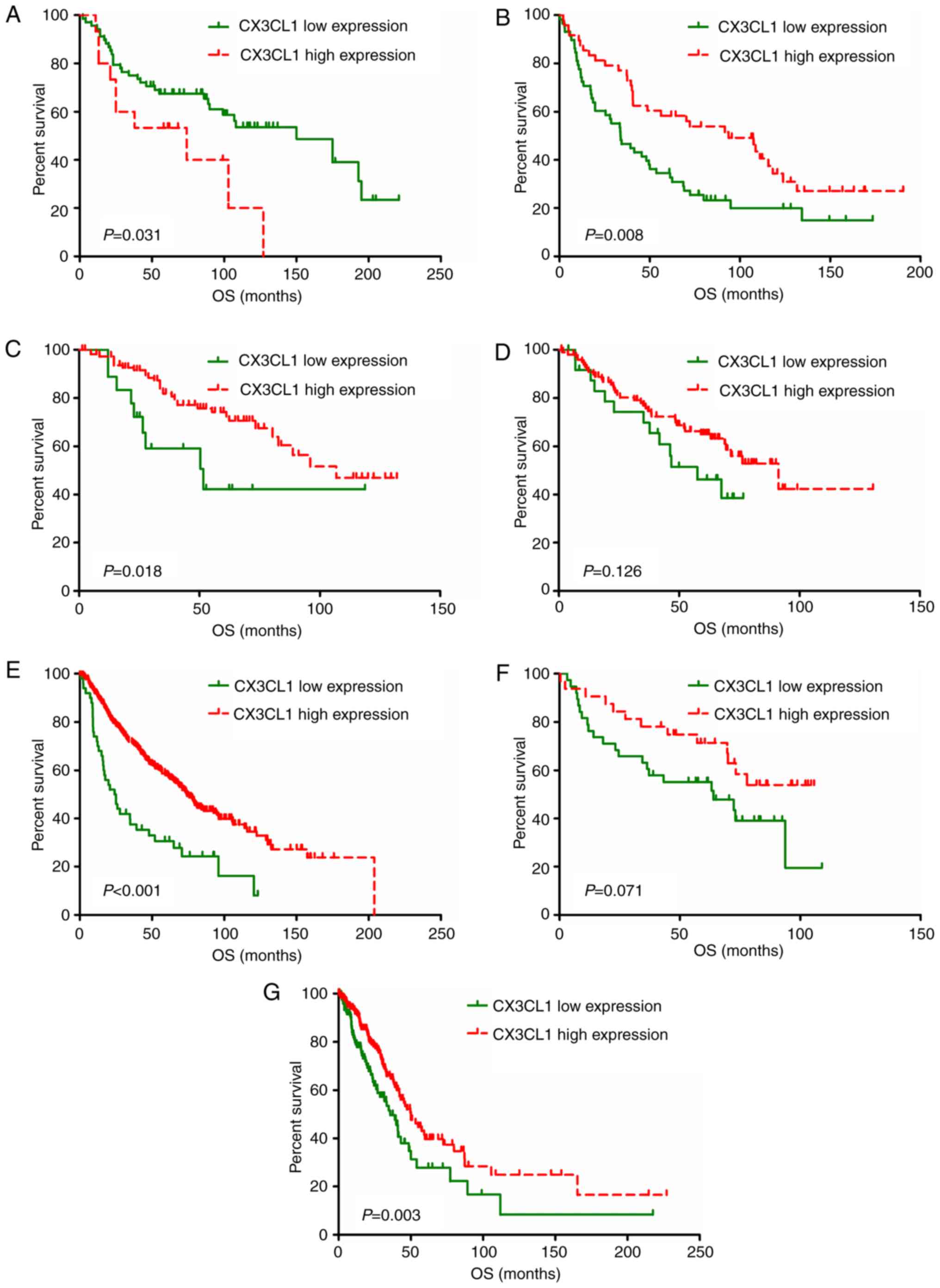
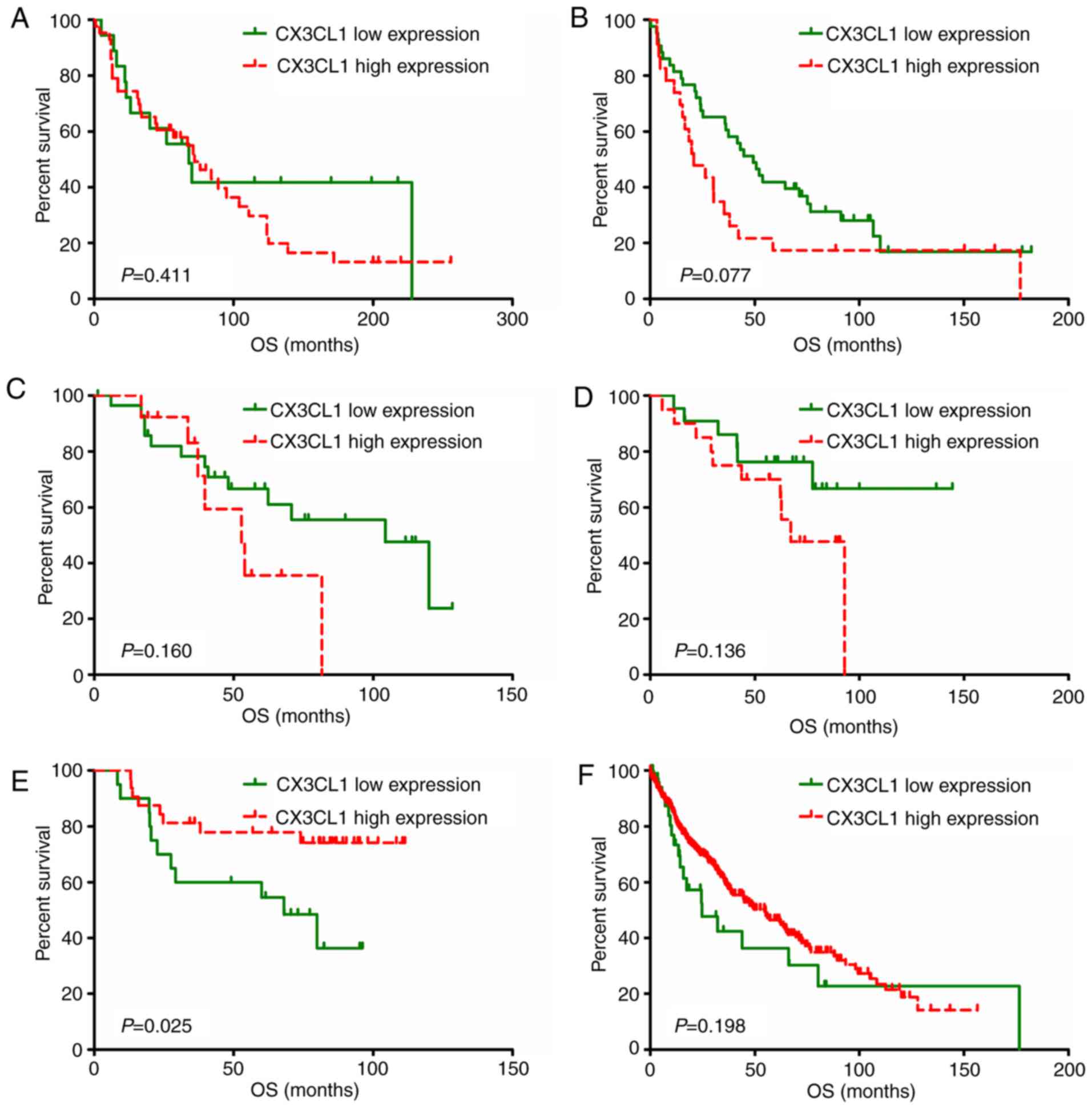
plots of each dataset are presented in Figs. 3 and 4. In six out of seven datasets, high
expression of CX3CL1 was associated with a decreased risk of
mortality in patients with LUAD (Fig.
3). Amongst patients with LUSC, two datasets demonstrated that
CX3CL1 was associated with a decreased risk of mortality, whereas,
in four datasets, CX3CL1 was associated with an increased risk of
mortality (Fig. 4). These findings
indicated that CX3CL1 may be a candidate prognostic indicator for
patients with LUAD but not for patients with LUSC.
 | Table II.Univariate and multivariate Cox model
analysis of the prognostic effect of CX3CL1 based on the lung
adenocarcinoma datasets. |
Table II.
Univariate and multivariate Cox model
analysis of the prognostic effect of CX3CL1 based on the lung
adenocarcinoma datasets.
|
|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|
|---|
| Gene expression
Omnibus datasets | Cases | Cutoff value | HR | LCI | UCI | P-value | HR | LCI | UCI | P-value |
|---|
| GSE30219 | 83 | 7.091 | 2.192 | 1.056 | 4.55 | 0.035a | 2.296 | 1.098 | 4.802 | 0.027a |
| GSE37745 | 106 | 7.241 | 0.538 | 0.339 | 0.854 | 0.009b | 0.468 | 0.285 | 0.769 | 0.003b |
| GSE42127 | 131 | 6.680 | 0.42 | 0.199 | 0.884 | 0.022a | 0.463 | 0.217 | 0.991 | 0.047a |
| GSE50081 | 127 | 5.686 | 0.612 | 0.325 | 1.154 | 0.129 | 0.659 | 0.346 | 1.256 | 0.205 |
| GSE68465 | 442 | 68.560 | 0.429 | 0.303 | 0.608 |
<0.001c | 0.424 | 0.294 | 0.612 |
<0.001c |
| GSE14814 | 70 | 6.825 | 0.534 | 0.267 | 1.066 | 0.075 | 0.561 | 0.273 | 1.154 | 0.116 |
| The cancer genome
Atlas | 483 | 7.530 | 0.363 | 0.2 | 0.66 |
<0.001c | 0.322 | 0.176 | 0.59 |
<0.001c |
 | Table III.Univariate and multivariate Cox model
analysis of the prognostic effect of CX3CL1 based on the lung
squamous cell carcinoma datasets. |
Table III.
Univariate and multivariate Cox model
analysis of the prognostic effect of CX3CL1 based on the lung
squamous cell carcinoma datasets.
|
|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|
|---|
| Gene expression
Omnibus datasets | Cases | Cutoff value | HR | LCI | UCI | P-value | HR | LCI | UCI | P-value |
|---|
| GSE30219 | 61 | 6.172 | 1.342 | 0.674 | 2.67 | 0.403 | 1.613 | 0.756 | 3.442 | 0.216 |
| GSE37745 | 66 | 7.529 | 1.657 | 0.941 | 2.916 | 0.080 | 1.989 | 1.078 | 3.67 | 0.028a |
| GSE42127 | 42 | 9.755 | 1.977 | 0.75 | 5.211 | 0.168 | 2.916 | 1.029 | 8.263 | 0.044a |
| GSE50081 | 42 | 7.033 | 2.138 | 0.769 | 5.941 | 0.145 | 2.044 | 0.732 | 5.706 | 0.172 |
| GSE14814 | 52 | 7.055 | 0.365 | 0.145 | 0.916 | 0.032a | 0.401 | 0.158 | 1.016 | 0.054 |
| The cancer genome
Atlas | 474 | 8.728 | 0.727 | 0.447 | 1.184 | 0.200 | 0.705 | 0.429 | 1.159 | 0.168 |
Pooled analysis based on the results
of survival analysis
Pooled analysis results for LUAD and LUSC are
presented in Tables IV and V. Given the statistical heterogeneity in
univariate and multivariate analysis of LUAD and LUSC data (I2
value >50% and P<0.1), random-effect models were used to pool
the HRs and 95% CIs. The results of this pooled analysis
demonstrated that increased expression of CX3CL1 mRNA was
significantly associated with an improved OS in patients with LUAD
(univariate Cox model: Pooled HR=0.53; 95% CI=0.43–0.65;
P<0.001; multivariate Cox model: Pooled HR=0.52; 95%
CI=0.42–0.64; P<0.001; Table
IV); however, there was no significant association between the
CX3CL1 mRNA expression level and OS in patients with LUSC
(univariate Cox model: Pooled HR=1.09; 95% CI=0.82–1.45; P=0.536;
multivariate Cox model: Pooled HR=1.18; 95% CI=0.88–1.58; P=0.282;
Table V). The forest plots of this
pooled analysis are presented in Fig.
5.
 | Table IV.Pooled hazard ratio of chemokine
C-X3-C motif ligand 1 high expression in patients based on lung
adenocarcinoma datasets. |
Table IV.
Pooled hazard ratio of chemokine
C-X3-C motif ligand 1 high expression in patients based on lung
adenocarcinoma datasets.
|
| Random effect
model | Heterogeneity |
|---|
|
|
|
|
|---|
| Analysis | Hazard ratio (95%
confidence interval) | P-value | I2,
% | P-value |
|---|
| Univariate | 0.53
(0.43–0.65) | <0.001 | 66.8 | 0.006 |
| Multivariate | 0.52
(0.42–0.64) | <0.001 | 70.0 | 0.003 |
 | Table V.Pooled hazard ratio of chemokine
C-X3-C motif ligand 1 high expression in patients based on lung
squamous cell carcinoma datasets. |
Table V.
Pooled hazard ratio of chemokine
C-X3-C motif ligand 1 high expression in patients based on lung
squamous cell carcinoma datasets.
|
| Random effect
model | Heterogeneity |
|---|
|
|
|
|
|---|
| Analysis | Hazard ratio (95%
confidence interval) | P-value | I2,
% | P-value |
|---|
| Univariate | 1.09
(0.82–1.45) | 0.536 | 63.4 | 0.018 |
| Multivariate | 1.18
(0.88–1.58) | 0.282 | 70.1 | 0.005 |
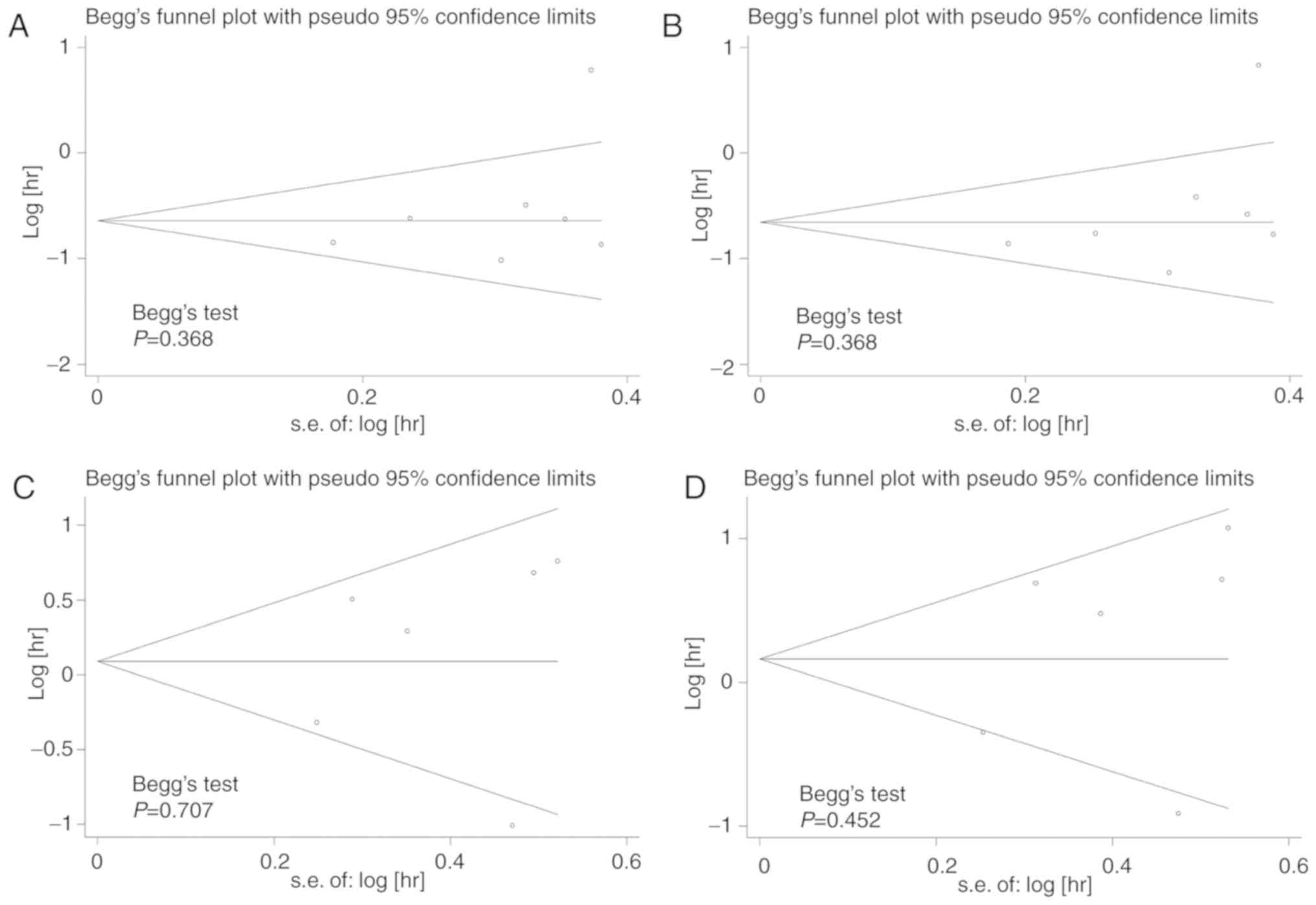
Begg's test was used to test for publication bias
among the included studies, and funnel plots were used to
demonstrate any publication bias graphically. The results indicated
that there was no significant publication bias amongst the included
studies; Begg's test demonstrated all P>0.1 for the studies
(Fig. 6).
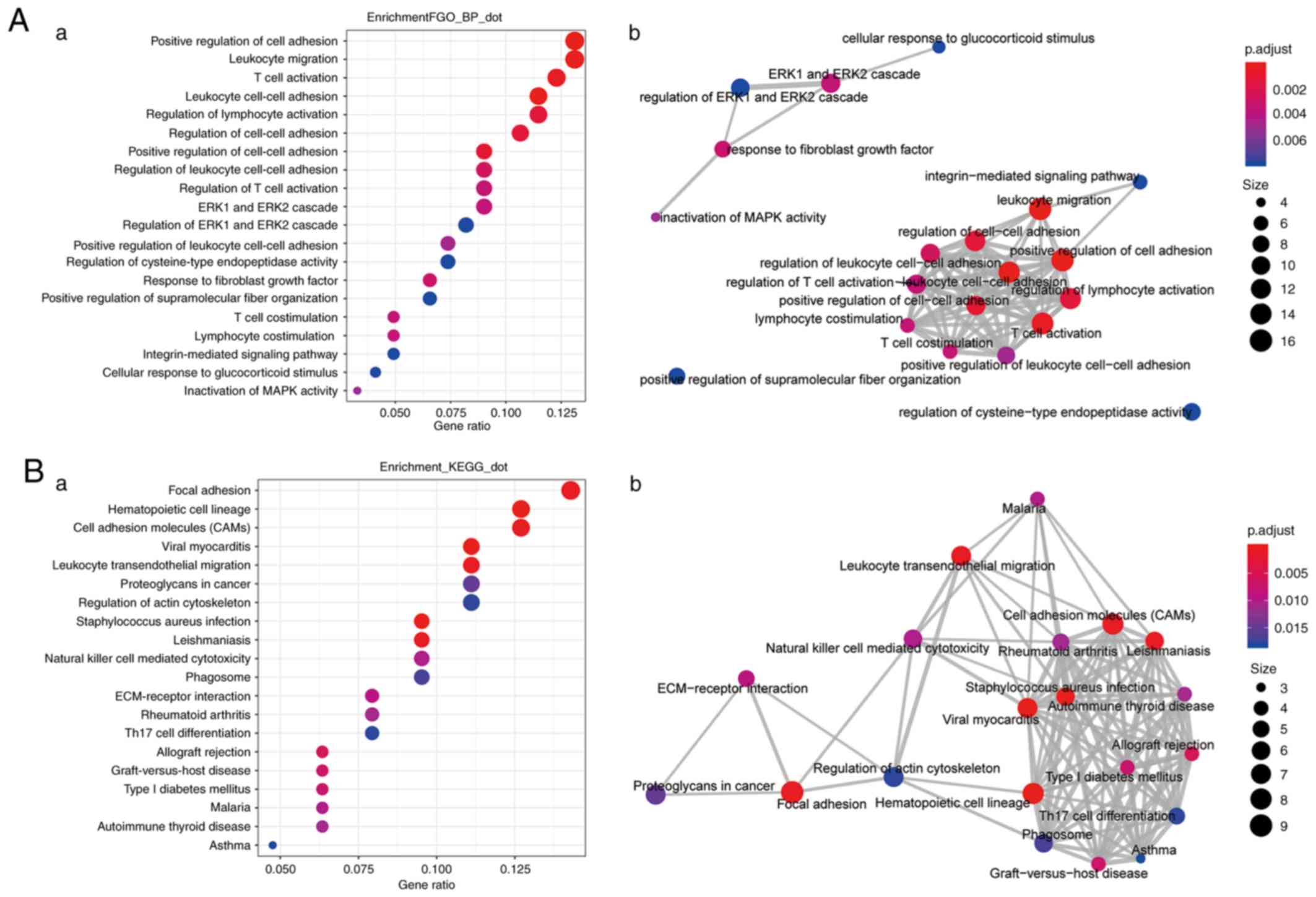
Enrichment analysis of genes whose
expression is highly correlated with CX3CL1 expression
In patients with LUAD, genes whose expression
correlation coefficients (r)>0.4 with CX3CL1 expression
(Table SI) were included in the
enrichment analysis. The top 20 enriched biological processes and
pathways are presented in Fig. 7.
The most significant biological processes included ‘positive
regulation of cell adhesion’ (GO:0045785), ‘leukocyte cell-cell
adhesion’ (GO:0007159), ‘leukocyte migration’ (GO:0050900) and
‘T-cell activation’ (GO:0042110), and the top 20 important pathways
associated with tumour immunity included ‘cell adhesion molecules
(CAMS)’ (KEGG: hsa04514), ‘leukocyte transendothelial migration’
(KEGG: hsa04670) and ‘natural killer cell mediated cytotoxicity’
(KEGG: hsa04650). However, in the patients with LUSC, the number of
genes that were highly correlated with CX3CL1 (r>0.4) was too
small and these genes were not enriched in any biological processes
or signalling pathways (Table
SII).
Discussion
Chemokines are a superfamily of proteins that
regulate the transmission and involvement of leukocytes in
vivo and are involved in inflammatory responses, including the
migration processes of lymphocytes, dendritic cells, macrophages
and stem cells (25,26). CX3CL1 (additionally termed
fractalkine) is a large cytokine protein of 373 amino acids; it
contains multiple domains and is the only known ligand of the CX3C
chemokine family. It is synthesised as a membrane-bound form that
may be released by proteolytic cleavage (5,27).
Membrane-bound CX3CL1 serves as a molecule that promotes adhesion
of leukocytes to endothelial cells, whereas, soluble CX3CL1 serves
as a potent chemoattractant of T-cells and monocytes, causing them
to move towards sites of inflammation (28). In contrast to other chemokines,
CX3CL1 has a cell-adhesion function in addition to its chemotactic
function. CX3CL1 is able to attract immune effector cells to the
tumour location site and exert an antitumour immune effect
(29). CX3CR1, the receptor of
CX3CL1, is expressed on human natural killer (NK) cells, monocytes,
T-lymphocytes and mast cells (5).
CX3CL1 may additionally promote the adhesion of CX3CR1-positive
tumour cells to target organs, causing the migration of tumour
cells, thus promoting tumourigenesis (13). This may, in theory, help to elucidate
how CX3CL1 serves seemingly opposing roles in a number of tumour
types.
A previous study demonstrated that CX3CR1 expression
was upregulated in solid tumours, including breast cancer and
prostate cancer (15); its
overexpression may promote the migration of tumour cells to the
brain and bones due to the high expression of soluble CX3CL1 in
these tissues. CX3CL1 expression in human bone marrow endothelial
cells and osteoblasts is involved in the process of prostate cancer
metastasis to bone marrow (30).
Blocking CX3CL1 with a specific antibody significantly reduced the
migration of prostate cancer cells to the bone marrow epithelium
(31). A previous study on
pancreatic adenocarcinoma models additionally demonstrated that
CX3CR1 was involved in the process of metastatic spread of tumour
cells to specific tissues that had increased expression of CX3CL1
(21). Therefore, high expression of
CX3CR1 in pancreatic ductal adenocarcinoma (PDAC) may be one of the
mechanisms that promote the spread of pancreatic cancer cells along
the peripheral nerves, which results in a risk of early recurrence
in patients with PDAC (32). Gaudin
et al (12) demonstrated that
epithelial cells from the surface and fallopian tubes of healthy
ovaries, and from benign, borderline and malignant tumours, all
stained positive for CX3CL1. Additionally, an increased expression
of CX3CL1 was closely associated with rapid tumour growth (12). Zhou et al (33) demonstrated that the expression of
cytokine SCM-1 β and CX3CL1 was significantly increased in lung
cancer compared with adjacent matched normal tissues, and was
correlated with pathological stage. Interactions between CX3CL1 and
CX3CR1 mediated the process of cell migration and adhesion between
EOC cells and peritoneal mesothelial cells, thereby promoting EOC
cell proliferation (13). Similarly,
previous studies on the mechanism of CX3CL1 in glioma and
neuroblastoma demonstrated its negative regulatory function in
these tumours (34–36).
However, CX3CL1 additionally has tumour-suppressive
activity. Vitale et al (37)
investigated the antitumour effect of fractalkine in its three
molecular forms on animal models, and compared the extent of tumour
development between C26 colon cancer cells with no fractalkine
expression, and those expressing either native, soluble or
membrane-bound fractalkine; the results demonstrated that native
fractalkine exhibited the strongest antitumour effect, reducing
tumours size by 93 and 99% in skin and orthotopic models,
respectively. The effects depend on a critical balance between the
soluble and membrane-bound forms. The soluble form exerted a marked
effect in reducing liver and lung metastases, whereas, the
membrane-bound form had very little impact in the liver and
promoted tumour growth in the lungs (37). Xin et al (38) transduced CX3CL1 into mouse
mesenchymal stem cells (MSCs) ex vivo using an adenoviral
vector with the Arg-Gly-Asp-4C peptide in the fibre knob. Systemic
administration of CX3CL1-expressing MSCs to the mice bearing lung
metastases of C26 and B16F10 cells significantly inhibited the
development of lung metastases and thus, improved the survival of
these tumour-bearing mice. Transduction of CX3CL1 and CX3CR1 in
colorectal cancer (CRC) cell lines induced cell aggregation that
markedly inhibited in vitro migration in chemotaxis assays,
and overexpression of CX3CL1-CX3CR1 inhibited the spread of cancer
to the liver in a mouse model of spleen-liver metastasis (39). Ohta et al (11) demonstrated a significant correlation
between CX3CL1 expression with TIL recruitment and prognosis in CRC
cases. Hyakudomi et al (10)
suggested that patients with gastric adenocarcinoma with increased
levels of CX3CL1 expression had elevated levels of cluster of
differentiation 8 T-cells and NK cells, which may induce innate and
adaptive immunity, and are correlated with a more favourable
prognosis. In hepatocellular carcinoma, CX3CL1 overexpression was
additionally associated with a favourable prognosis. Intra- and
extrahepatic recurrence rates were significantly reduced in tumours
with high expression of CX3CL1 and CX3CR1 (40).
Lung cancer, with its rapidly growing morbidity and
mortality rates in recent years, is one of the principal threats to
human health and life amongst all the malignant tumours (1). Therefore, the identification of a
biomarker for the treatment and prognosis of lung cancer may be of
considerable value. At present, the roles of CX3CL1 in lung cancer
and the mechanisms of those roles remain unclear. In the present
study, the results demonstrated that the CX3CL1 and CX3CR1 mRNA
expression levels in tumour tissue were significantly decreased
compared with levels in normal tissue in patients with LUAD or
LUSC. The pooled analysis results demonstrated that higher CX3CL1
mRNA expression was significantly associated with improved OS in
patients with LUAD; however, no significant association was
identified between CX3CL1 mRNA expression and OS in patients with
LUSC. In all the LUAD datasets, the genes that were highly
correlated (r>0.4) with CX3CL1 were included for the enrichment
analysis. The most significantly altered biological processes
included ‘positive regulation of cell adhesion’ (GO:0045785),
‘leukocyte cell-cell adhesion’ (GO:0007159), ‘leukocyte migration’
(GO:0050900) and ‘T cell activation’ (GO:0042110), and the top 20
important pathways associated with tumour immunity included ‘cell
adhesion molecules (CAMS)’ (KEGG: hsa04514), ‘leukocyte
transendothelial migration’ (KEGG: hsa04670) and ‘natural killer
cell mediated cytotoxicity’ (KEGG: hsa04650). However, in the
patients with LUSC, the genes that were highly correlated with
CX3CL1 were not enriched for any biological processes or signalling
pathways. These results demonstrated that CX3CL1 may be a positive
prognostic indicator in LUAD. CX3CL1 may promote the adhesion of
CX3CR1-positive tumour cells to target organs, causing migration of
tumour cells (31,41). However, in LUAD, CX3CL1
overexpression may lead to increased chemotactic efficiency and
increased immune effector cell infiltration, which results in an
improved prognosis.
The present study has certain limitations; specific
datasets from the GEO database did not provide complete clinical
information, meaning that further hierarchical analysis was
difficult to achieve. The distribution of clinical features amongst
the patients was uneven. Certain datasets included in this analysis
were not based on the same detection platform; thus, a certain
degree of heterogeneity existed between different datasets. Despite
a number of limitations, the present study may provide insight for
other researchers; the prognostic value of CX3CL1 using seven
similar datasets in patients with LUAD was demonstrated.
In conclusion, the results of the present study
demonstrated that increased CX3CL1 mRNA expression levels in
patients with LUAD may be associated with improved prognosis.
However, this was not observed in patients with LUSC. The
favourable results of increased expression levels of CX3CL1 in
patients with LUAD may occur through biological processes and
signalling pathwats, including ‘T cell activation’ (GO:0042110),
‘leukocyte cell-cell adhesion’ (GO:0007159), ‘leukocyte migration’
(GO:0050900), ‘cell adhesion molecules (CAMS)’ (KEGG: hsa04514),
‘leukocyte transendothelial migration’ (KEGG: hsa04670) and
‘natural killer cell mediated cytotoxicity’ (KEGG: hsa04650).
Further prospective studies are required to confirm the prognostic
value of CX3CL1 in patients with LUAD. In addition, the detailed
mechanisms of the action of CX3CL1 in LUAD require confirmation by
further experimental studies.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Mr. Bin Xu
(Department of Tumor Biological Treatment, The Third Affiliated
Hospital of Soochow University, Changzhou, Jiangsu, China) for his
expertise and useful suggestions in the statistical and
bioinformatics analysis.
Funding
The present study was supported by a grant from The
International Science and Technology Cooperation Project of the
Changzhou Science and Technology Bureau (grant no. CZ20140016;
China).
Availability of data and materials
The datasets used and/or analysed during the present
study are available from The Cancer Genome Atlas and Gene
Expression Omnibus.
Authors' contributions
JL and CL conceived and designed the study. YL, JL
and XQZ contributed to data cleansing, the statistical and
bioinformatics analysis, and manuscript writing. QL, JX, XHL, SH
and WDP contributed to the literature search, data collection,
figure and table generation, and manuscript writing. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zappa C and Mousa SA: Non-small cell lung
cancer: Current treatment and future advances. Transl Lung Cancer
Res. 5:288–300. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Borsig L, Wolf MJ, Roblek M, Lorentzen A
and Heikenwalder M: Inflammatory chemokines and metastasis-Tracing
the accessory. Oncogene. 33:3217–3224. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu W, Jiang L, Bian C, Liang Y, Xing R,
Yishakea M and Dong J: Role of CX3CL1 in diseases. Arch Immunol
Ther Exp. 64:371–383. 2016. View Article : Google Scholar
|
|
4
|
Pan Y, Lloyd C, Zhou H, Dolich S, Deeds J,
Gonzalo JA, Vath J, Gosselin M, Ma J, Dussault B, et al:
Neurotactin, a membrane-anchored chemokine upregulated in brain
inflammation. Nature. 387:611–617. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bazan JF, Bacon KB, Hardiman G, Wang W,
Soo K, Rossi D, Greaves DR, Zlotnik A and Schall TJ: A new class of
membrane-bound chemokine with a CX3C motif. Nature. 385:640–644.
1997. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Julia V, Staumont-Salle D and Dombrowicz
D: Role of fractalkine/CX3CL1 and its receptor CX3CR1 in allergic
diseases. Med Sci (Paris). 32:260–266. 2016.(Article in French).
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Thomas S and Baumgart DC: Targeting
leukocyte migration and adhesion in Crohn's disease and ulcerative
colitis. Inflammopharmacology. 20:1–18. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yoneda O, Imai T, Goda S, Inoue H,
Yamauchi A, Okazaki T, Imai H, Yoshie O, Bloom ET, Domae N and
Umehara H: Fractalkine-mediated endothelial cell injury by NK
cells. J Immunol. 164:4055–4062. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gu X, Tang XD, Gu SY, Yang SQ, Zhou PJ and
Tan JM: Expression of fractalkine and its receptor in acute cardiac
allografts rejection. Zhonghua wai ke za zhi. 41:139–142. 2003.(In
Chinese). PubMed/NCBI
|
|
10
|
Hyakudomi M, Matsubara T, Hyakudomi R,
Yamamoto T, Kinugasa S, Yamanoi A, Maruyama R and Tanaka T:
Increased expression of fractalkine is correlated with a better
prognosis and an increased number of both CD8+ T cells and natural
killer cells in gastric adenocarcinoma. Ann Surg Oncol.
15:1775–1782. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ohta M, Tanaka F, Yamaguchi H, Sadanaga N,
Inoue H and Mori M: The high expression of Fractalkine results in a
better prognosis for colorectal cancer patients. Int J Oncol.
26:41–47. 2005.PubMed/NCBI
|
|
12
|
Gaudin F, Nasreddine S, Donnadieu AC,
Emilie D, Combadière C, Prévot S, Machelon V and Balabanian K:
Identification of the chemokine CX3CL1 as a new regulator of
malignant cell proliferation in epithelial ovarian cancer. PLoS
One. 6:e215462011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim M, Rooper L, Xie J, Kajdacsy-Balla AA
and Barbolina MV: Fractalkine receptor CX(3)CR1 is expressed in
epithelial ovarian carcinoma cells and required for motility and
adhesion to peritoneal mesothelial cells. Mol Cancer Res. 10:11–24.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li F, Wang Z, Liu Y and Li J:
Down-regulation of fractalkine inhibits the in vitro and in vivo
angiogenesis of the hepatocellular carcinoma HepG2 cells. Oncol
Rep. 24:669–675. 2010.PubMed/NCBI
|
|
15
|
Andre F, Cabioglu N, Assi H, Sabourin JC,
Delaloge S, Sahin A, Broglio K, Spano JP, Combadiere C, Bucana C,
et al: Expression of chemokine receptors predicts the site of
metastatic relapse in patients with axillary node positive primary
breast cancer. Ann Oncol. 17:945–951. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jamieson-Gladney WL, Zhang Y, Fong AM,
Meucci O and Fatatis A: The chemokine receptor CX(3)CR1 is directly
involved in the arrest of breast cancer cells to the skeleton.
Breast Cancer Res. 13:R912011. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Edge SB and Compton CC: The American joint
committee on cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rousseaux S, Debernardi A, Jacquiau B,
Vitte AL, Vesin A, Nagy-Mignotte H, Moro-Sibilot D, Brichon PY,
Lantuejoul S, Hainaut P, et al: Ectopic activation of germline and
placental genes identifies aggressive metastasis-prone lung
cancers. Sci Transl Med. 5:186ra1662013. View Article : Google Scholar
|
|
19
|
Botling J, Edlund K, Lohr M, Hellwig B,
Holmberg L, Lambe M, Berglund A, Ekman S, Bergqvist M, Pontén F, et
al: Biomarker discovery in non-small cell lung cancer: Integrating
gene expression profiling, meta-analysis, and tissue microarray
validation. Clin Cancer Res. 19:194–204. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tang H, Xiao G, Behrens C, Schiller J,
Allen J, Chow CW, Suraokar M, Corvalan A, Mao J, White MA, et al: A
12-gene set predicts survival benefits from adjuvant chemotherapy
in non-small cell lung cancer patients. Clin Cancer Res.
19:1577–1586. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Der SD, Sykes J, Pintilie M, Zhu CQ,
Strumpf D, Liu N, Jurisica I, Shepherd FA and Tsao MS: Validation
of a histology-independent prognostic gene signature for
early-stage, non-small-cell lung cancer including stage IA
patients. J Thorac Oncol. 9:59–64. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shedden K, Taylor JM, Enkemann SA, Tsao
MS, Yeatman TJ, Gerald WL, Eschrich S, Jurisica I, Giordano TJ,
Misek DE, et al: Gene expression-based survival prediction in lung
adenocarcinoma: A multi-site, blinded validation study. Nat Med.
14:822–827. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhu CQ, Ding K, Strumpf D, Weir BA,
Meyerson M, Pennell N, Thomas RK, Naoki K, Ladd-Acosta C, Liu N, et
al: Prognostic and predictive gene signature for adjuvant
chemotherapy in resected non-small-cell lung cancer. J Clin Oncol.
28:4417–4424. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Budczies J, Klauschen F, Sinn BV, Győrffy
B, Schmitt WD, Darb-Esfahani S and Denkert C: Cutoff Finder: A
comprehensive and straightforward web application enabling rapid
biomarker cutoff optimization. PLoS One. 7:e518622012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yao X, Qi L, Chen X, Du J, Zhang Z and Liu
S: Expression of CX3CR1 associates with cellular migration,
metastasis, and prognosis in human clear cell renal cell carcinoma.
Urol Oncol. 32:162–170. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Johnson LA and Jackson DG: The chemokine
CX3CL1 promotes trafficking of dendritic cells through inflamed
lymphatics. J Cell Sci. 126:5259–5270. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Umehara H, Bloom ET, Okazaki T, Nagano Y,
Yoshie O and Imai T: Fractalkine in vascular biology: From basic
research to clinical disease. Arterioscler Thromb Vasc Biol.
24:34–40. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fong AM, Robinson LA, Steeber DA, Tedder
TF, Yoshie O, Imai T and Patel DD: Fractalkine and CX3CR1 mediate a
novel mechanism of leukocyte capture, firm adhesion, and activation
under physiologic flow. J Exp Med. 188:1413–1419. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Park MH, Lee JS and Yoon JH: High
expression of CX3CL1 by tumor cells correlates with a good
prognosis and increased tumor-infiltrating CD8+ T cells, natural
killer cells, and dendritic cells in breast carcinoma. J Surg
Oncol. 106:386–392. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jamieson WL, Shimizu S, D'Ambrosio JA,
Meucci O and Fatatis A: CX3CR1 is expressed by prostate epithelial
cells and androgens regulate the levels of CX3CL1/fractalkine in
the bone marrow: Potential role in prostate cancer bone tropism.
Cancer Res. 68:1715–1722. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shulby SA, Dolloff NG, Stearns ME, Meucci
O and Fatatis A: CX3CR1-fractalkine expression regulates cellular
mechanisms involved in adhesion, migration, and survival of human
prostate cancer cells. Cancer Res. 64:4693–4698. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Marchesi F, Piemonti L, Fedele G, Destro
A, Roncalli M, Albarello L, Doglioni C, Anselmo A, Doni A, Bianchi
P, et al: The chemokine receptor CX3CR1 is involved in the neural
tropism and malignant behavior of pancreatic ductal adenocarcinoma.
Cancer Res. 68:9060–9069. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhou B, Xu H, Ni K, Ni X and Shen J:
Expression of chemokine XCL2 and CX3CL1 in lung cancer. Med Sci
Monit. 22:1560–1565. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Locatelli M, Boiocchi L, Ferrero S,
Martinelli Boneschi F, Zavanone M, Pesce S, Allavena P, Maria Gaini
S, Bello L and Mantovani A: Human glioma tumors express high levels
of the chemokine receptor CX3CR1. Eur Cytokine Netw. 21:27–33.
2010.PubMed/NCBI
|
|
35
|
Erreni M, Solinas G, Brescia P, Osti D,
Zunino F, Colombo P, Destro A, Roncalli M, Mantovani A, Draghi R,
et al: Human glioblastoma tumours and neural cancer stem cells
express the chemokine CX3CL1 and its receptor CX3CR1. Eur J Cancer.
46:3383–3392. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sciume G, Soriani A, Piccoli M, Frati L,
Santoni A and Bernardini G: CX3CR1/CX3CL1 axis negatively controls
glioma cell invasion and is modulated by transforming growth
factor-β1. Neuro Oncol. 12:701–710. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Vitale S, Cambien B, Karimdjee BF, Barthel
R, Staccini P, Luci C, Breittmayer V, Anjuère F, Schmid-Alliana A
and Schmid-Antomarchi H: Tissue-specific differential antitumour
effect of molecular forms of fractalkine in a mouse model of
metastatic colon cancer. Gut. 56:365–372. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xin H, Kanehira M, Mizuguchi H, Hayakawa
T, Kikuchi T, Nukiwa T and Saijo Y: Targeted delivery of CX3CL1 to
multiple lung tumors by mesenchymal stem cells. Stem Cells.
25:1618–1626. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Erreni M, Siddiqui I, Marelli G, Grizzi F,
Bianchi P, Morone D, Marchesi F, Celesti G, Pesce S, Doni A, et al:
The fractalkine-receptor axis improves human colorectal cancer
prognosis by limiting tumor metastatic dissemination. J Immunol.
196:902–914. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Matsubara T, Ono T, Yamanoi A, Tachibana M
and Nagasue N: Fractalkine-CX3CR1 axis regulates tumor cell cycle
and deteriorates prognosis after radical resection for
hepatocellular carcinoma. J Surg Oncol. 95:241–249. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Marchesi F, Locatelli M, Solinas G, Erreni
M, Allavena P and Mantovani A: Role of CX3CR1/CX3CL1 axis in
primary and secondary involvement of the nervous system by cancer.
J Neuroimmunol. 224:39–44. 2010. View Article : Google Scholar : PubMed/NCBI
|