Introduction
Metastatic non-small cell lung cancer (NSCLC) is
still characterized by poor prognosis in the majority of patients.
In recent years, development in molecular subtyping and discovery
of predictive biomarkers has led to novel strategies using targeted
therapies that have markedly improved survival rates (1–5).
Additionally, checkpoint inhibition based on programmed
death-ligand 1 expression as a predictive biomarker has been
introduced (6). However, for
adjuvant and palliative treatment, platinum-doublet chemotherapy
remains an important treatment method. The lack of biomarkers that
can predict a favorable response to platinum-doublet chemotherapy
results in exposure to severe toxicity in many patients without
measurable benefit.
Research in recent years increasingly supports the
importance of epithelial-to-mesenchymal transition (EMT) in cancer.
This process was first described in embryonic development and is
characterized by various cellular changes, including the loss of
adhesion properties and the development of a mesenchymal phenotype,
which promote invasion and metastasis of tumor cells (7,8).
Analysis has revealed that EMT markers are associated with survival
in patients with NSCLC (9–14). Various agents that inhibit EMT
pathways are currently being developed, including inhibitors of
transforming growth factor-β, histone deacetylase, focal adhesion
kinase and others (15). Recent
research has outlined the role of EMT in antitumor immunity,
proposing mechanisms of how cancer cells undergoing EMT may
contribute to immune escape, resulting in resistance to
immunotherapy (16). Additionally,
it has been reported that specific anti-cancer therapeutics are
able to increase the immunogenicity of dying cancer cells via a
mechanism described as immunogenic cell death (ICD) (17).
With respect to EMT-associated markers, an
epithelial phenotype is characterized by high expression of
E-cadherin and the mesenchymal phenotype by high expression of
vimentin, among other markers (18).
The function of E-cadherin is associated with tissue integrity and
adhesive properties. Changes in tissue integrity and adhesion lead
to invasiveness in various cancer types; thus, EMT has an important
role in the malignant properties of NSCLC and patient prognosis
(13). Vimentin was originally
identified in mesenchymal tissue, and has been reported to be
associated with cancer progression, patient prognosis and drug
resistance (19). Furthermore,
vimentin expression was demonstrated to predict the occurrence of
metastasis in NSCLC (20); however,
studies that have investigated the role vimentin in NSCLC in
vivo are rare and the findings are controversial.
In unselected patients, EMT characteristics have
been demonstrated to be associated with chemotherapy resistance
(21). For epidermal growth factor
receptor (EGFR) wild-type patients, the EMT phenotype is
potentially be associated with poor response to tyrosine kinase
inhibitor (TKI) treatment (22,23). As
the current study included two treatment regimens, the influence of
EMT in an unselected patient population (with/without chemotherapy
and multiple EGFR genotypes) was assessed.
Tumor specimens obtained from the INNOVATIONS trial
were used to elucidate the potential impact of EMT on treatment
efficacy with and without chemotherapy in conjunction with
anti-angiogenic treatment. This trial included patients with
inoperable stage IIIB/IV non-squamous NSCLC. At the time of this
study, standard treatment for this population was platin-based
combination chemotherapy. One of the longest survival times was
achieved using the regime of carboplatin-paclitaxel plus
bevacizumab, with a median progression-free survival (PFS) of 6
months and a median overall survival (OS) of 12 months (24). The INNOVATIONS study was designed to
assess erlotinib/bevacizumab (EB) compared with cisplatin,
gemcitabine and bevacizumab (PGB) as a first-line treatment in
unselected cisplatin-eligible patients (25).
Patients and methods
Patients
All patients involved in the current study
participated in the INNOVATIONS trial. Written informed consent was
obtained from all patients prior to the collection and use of all
samples. Inclusion criteria and the results have been reported
previously (25). The study was
approved by the ethics committee of each participating institution
and the German regulatory body, the Paul Ehrlich Institute. The
study was conducted in accordance with the Declaration of Helsinki
(version 1996) and the applicable International Conference on
Harmonisation of Technical Requirements for Registration of
Pharmaceuticals for Human Use, Good Clinical Practice guidelines.
The trial was registered on clinicaltrials.gov as NCT00536640 (trial no. EUDRA-CT
2006-004865-32).
Immunostaining for E-cadherin and
vimentin
For immunohistochemical analyses of E-cadherin and
vimentin expression in tumor tissues, tissue microarray blocks were
cut into 4 µm-thick sections and mounted onto poly-L-lysine glass
slides. Tissue sections were deparaffinized with xylene, followed
by rehydration with decreasing concentrations of ethanol and
immersion in 3% H2O2 for 10 min to reduce
endogenous peroxidase activity. Following antigen retrieval in 0.01
M sodium citrate buffer (pH=6.0), the tissue sections were
incubated with mouse anti-E-cadherin (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA; 1:200) or mouse anti-vimentin
(Zymed; Thermo Fisher Scientific, Inc.; 1:400) in PBS overnight in
a cold room. The sections were then washed three times with PBS and
incubated with the corresponding secondary antibodies for 30 min at
37°C; subsequently, the sections were washed with PBS and incubated
for with 3,3′-diaminobenzidine 1 min. The sections were then
counterstained with hematoxylin, dehydrated, cleared and
permanently mounted with mounting medium. All the procedures were
performed at room temperature. Additionally, tissues stained
positive for E-cadherin and vimentin were used as positive
controls. Sections that were processed by replacing the primary
antibody with PBS were used as negative controls.
Microscopic analysis
The degree of immunoreactivity for the proteins was
evaluated semi-quantitatively on the basis of staining intensity
and the proportion of positive tumor cells. Under a microscope at
×400 magnification, five fields of vision were randomly selected
(with no fewer than 200 cells per field). The staining intensity
was graded as follows: 0, no staining; 1, light yellow; 2,
yellowish brown; and 3, brown. The positive cells were graded
according to the percentage of positive cells as follows: 0, no
positive tumor cells; 1, ≤10% positive tumor cells; 2, 11–50%
positive tumor cells; 3, 51–80% positive tumor cells; and 4,
>80% positive tumor cells. The percentage of positive cells and
the staining intensity were then multiplied to generate the
immunoreactivity score (Remmele and Stegner). For e-cadherin
previous studies have used various different cut-offs (26). As there is no standard procedure, we
estimated for our study that E-cadherin <8 is already a
substantial and not incidental loss. For vimentin the respective
literature states that any positive reaction for this marker is a
feature of an ongoing EMT since vimentin is not expressed in the
normal pulmonary epithelium (27).
Based on this, the immunoreactivity was divided into two groups:
Low immunoreactivity (a total score of <8) and high
immunoreactivity (a total score of ≥8) for E-cadherin; negative
immunoreactivity (a total score of 0) and positive immunoreactivity
(a total score of >0) for vimentin. Two independent
investigators who were blinded to the cases evaluated the
immunohistochemical staining. When a discrepancy occurred between
the two investigators, a consensus was reached via simultaneous
examinations using a double-headed microscope.
Statistical analysis
All statistical analyses were performed using SAS
software (version 9.4; SAS Institute, Inc., Cary, NC, USA). Tests
were two-sided, P<0.05 was considered to indicate a
statistically significant difference. Time-to-event data were
analyzed using the Kaplan-Meier method. Distributions between
groups were compared by the unstratified log-rank test and also,
for the whole groups, by the stratified log-rank test to adjust for
the E-cadherin and vimentin expression status, respectively. Cox
proportional hazards models were used to identify prognostic
ability for PFS by testing each covariate in the model with a Wald
χ2 test and estimating hazard ratios (HR) with 95%
confidence intervals (CI) based on the Wald tests. Analyses
performed in subgroups were not adjusted, whereas variable
selection methods were applied to include baseline characteristics
(Table I) relevant for the
prediction performance of the model of the whole study populations
(n=95 patients with E-cadherin expression, 7 observations deleted
due to missing values; n=94 patients with vimentin expression, 7
observations deleted due to missing values). Furthermore, an
interaction between treatment and expression status (E-cadherin and
vimentin, respectively) was considered in the variable selection.
Different variable selection methods (forward, backward, stepwise
with a critical P-value of 0.15 for entry in and removal from the
model) identified the same optimal model (minimal value of the
Akaike information criterion of all models in the selection
process) containing treatment, age, and Union for International
Cancer Control stage for patients with E-cadherin expression. This
model was also preferred by forward and stepwise variable selection
for patients with vimentin expression. The interaction variable
proved not statistically significant and did not meet the critical
P-value for model entry in either patient population (with
E-cadherin or vimentin expression).
 | Table I.Patient characteristics with respect
to treatment. |
Table I.
Patient characteristics with respect
to treatment.
|
| Treatment arm |
|---|
|
|
|
|---|
| Characteristic | Arm A (EB) | Arm B (PGB) |
|---|
| Total patients,
n | 46 | 58 |
| Age, years
(range) | 61.2 (40–85) | 59.8 (41–83) |
| Sex |
|
|
|
Male | 26 (56.5) | 34 (58.6) |
|
Female | 20 (43.5) | 24 (41.4) |
| ECOG performance
status |
|
|
| 0 | 25 (54.3) | 30 (51.7) |
| 1 | 19 (41.3) | 27 (46.6) |
| 2 | 2 (4.3) | 1 (1.7) |
| UICC stage |
|
|
|
IIIB | 3 (6.5) | 10 (17.2) |
| IV | 43 (93.5) | 48 (82.8) |
| Histology |
|
|
|
Adenocarcinoma | 40 (87.0) | 54 (93.1) |
| Large
cell | 2 (4.3) | 2 (3.4) |
| Not
specified | 4 (8.7) | 2 (3.4) |
| Smoking status | 1 (2.2) | 2 (3.4) |
|
Missing |
|
|
| Current
smoker | 14 (30.4) | 18 (31.0) |
| Former
light smoker | 1 (2.2) | 4 (6.9) |
| Former
smoker | 19 (41.3) | 22 (37.9) |
| Never
smoked | 11 (23.9) | 12 (20.7) |
| EGFR mutation
status | 3 (6.5) | 2 (3.4) |
|
Missing |
|
|
|
Mutation | 8 (17.4) | 7 (12.1) |
| Wild
type | 35 (76.1) | 49 (84.5) |
| E-cadherin
immunoreactivity | 1 (2.2) | 1 (1.7) |
|
Missing |
|
|
|
<8 | 24 (52.2) | 30 (51.7) |
| ≥8 | 21 (45.7) | 27 (46.6) |
| Vimentin
immunoreactivity | 3 (6.5) | 0 (0) |
|
Missing |
|
|
| 0 | 32 (69.9) | 35 (60.3) |
|
>0 | 11 (23.9) | 23 (39.7) |
Results
Patient characteristics
Of the 224 patients randomly assigned to one of the
two treatment arms (EB or PGB) in the trial, 102 were
immunohistochemically evaluated for E-cadherin expression and 101
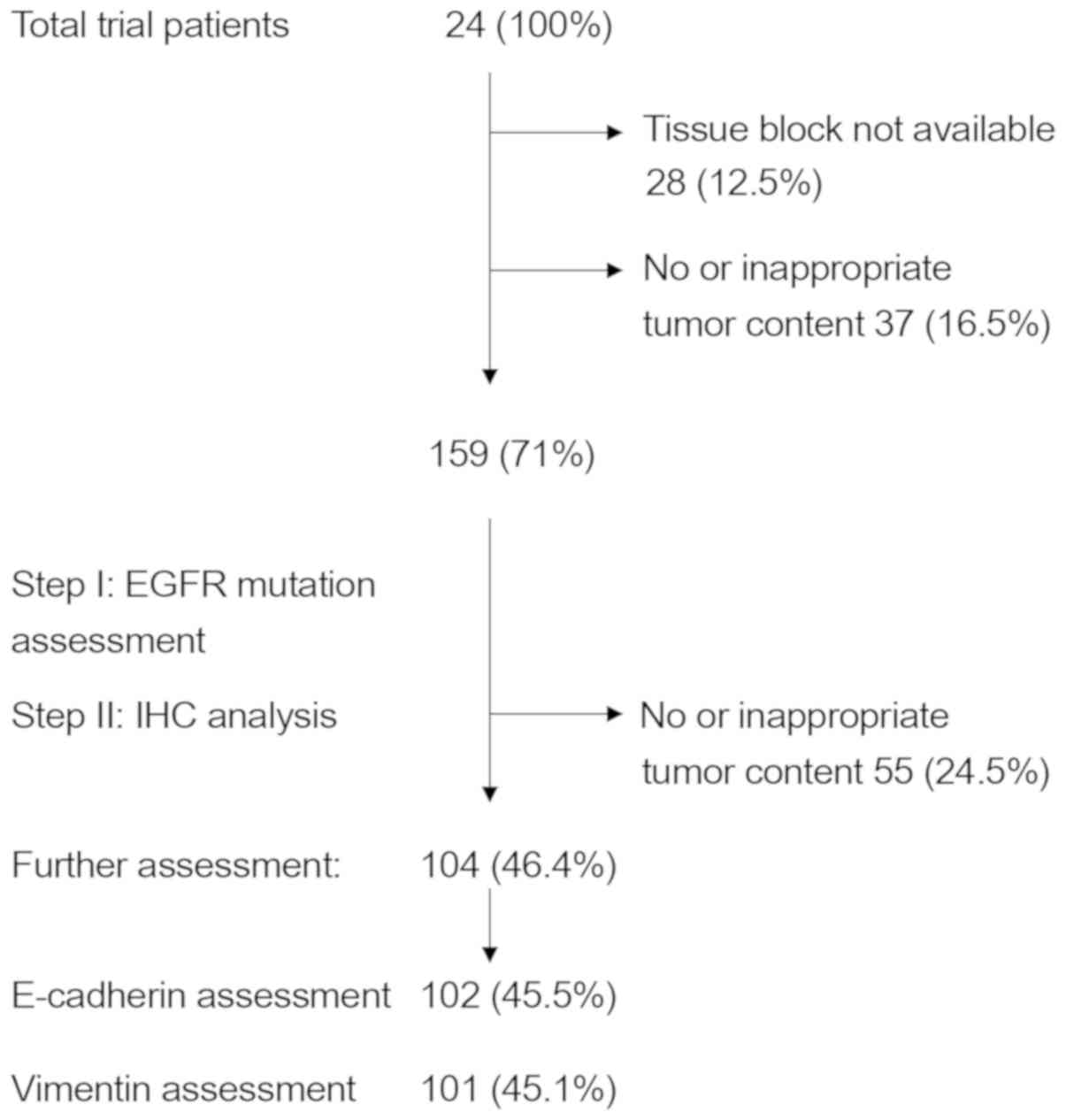
patients for vimentin expression. In total, 104 patient samples
were assessed (99 providing enough material for E-cadherin and
vimentin staining, 3 for E-cadherin only and 2 for vimentin only;
Table I). This drop in sample number
was due to the decision to initially analyze all specimens for EGFR
mutations, as reported earlier (25), and then to proceed to EMT assessment,
leaving less material for further testing, due to unavailable
tissue samples or no tumor tissue left in the paraffin blocks
(Fig. 1).
Survival analysis
In the 102 patients analyzed for E-cadherin
expression (high or low) the comparison of treatment arms revealed
a significant difference in PFS [HR=0.449 (95% CI, 0.292–0.691),
log-rank P=0.0002; PGB vs. EB treatment], and with no significant
difference in OS [HR=0.686 (95% CI, 0.429–1.095), log-rank
P=0.1114; data not shown]. Very similar results were obtained in
the corresponding analyses for the 101 patients analyzed for
vimentin expression (positive or negative): PFS [HR=0.494 (95% CI,
0.319–0.764) PGB vs. EB treatment, log-rank P=0.0012]; OS [HR=0.684
(95% CI, 0.426–1.098), log-rank P=0.1131].
Survival analysis and E-cadherin
expression
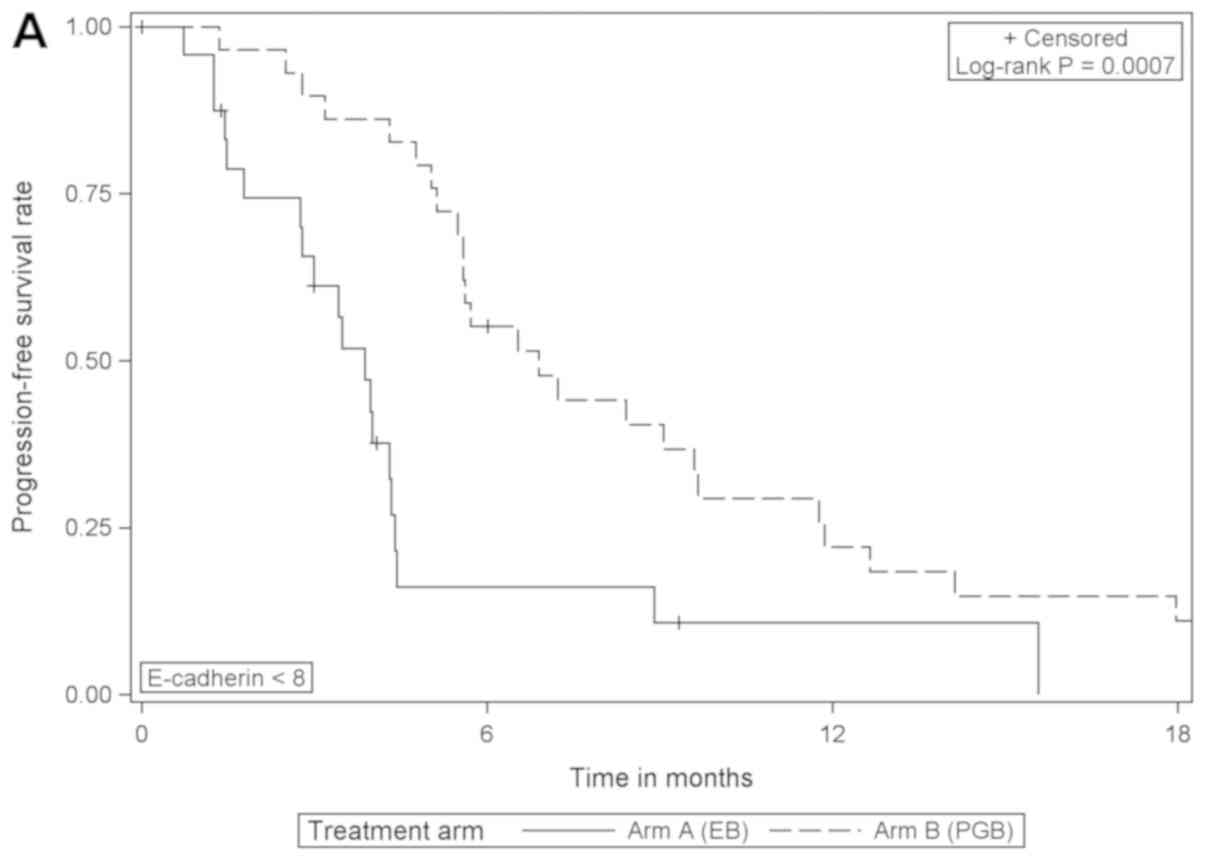
Comparing the treatment arms according to E-cadherin
expression, the analysis revealed that in patients with low
expression of E-cadherin, PFS was significantly increased when
treated with PGB compared with EB [HR=0.353 (95% CI, 0.189–0.658),
log-rank P=0.0007]; but the same effect was not detected in those
with high expression of E-cadherin (Table II; Fig.
2). Furthermore, the comparison of OS between treatment arms
for patients with low E-cadherin expression revealed borderline
statistical significance [HR=0.530 (95% CI, 0.275–1.023), log-rank
P=0.0546; EB arm median, 9.1 months; PGB arm median, 12.0 months;
data not shown].
 | Table II.Progression-free survival with
respect to treatment and E-cadherin immunoreactivity. |
Table II.
Progression-free survival with
respect to treatment and E-cadherin immunoreactivity.
| Comparison | E-cadherin
immunoreactivity <8 | E-cadherin
immunoreactivity ≥8 | Log-rank
P-value | HRa (95% CI): E-cadherin
immunoreactivity ≥8 vs. <8 | Wald chi-square
P-valuea |
|---|
| EB arm: Median PFS
in months | 3.9 | 3.0 | 0.7415 | 1.113
(0.588–2.109) | 0.7423 |
| PGB arm: Median PFS
in months | 6.9 | 5.6 | 0.3924 | 1.275
(0.729–2.227) | 0.3942 |
| Log-rank
P-value | 0.0007 | 0.0626 | – | – | – |
| HRa (95% CI): PGB vs. EB | 0.353
(0.189–0.658) | 0.567
(0.309–1.040) | – | – | – |
| Wald chi-square
P-valuea | 0.0011 | 0.0667 | – | – | – |
Survival analysis and vimentin
expression
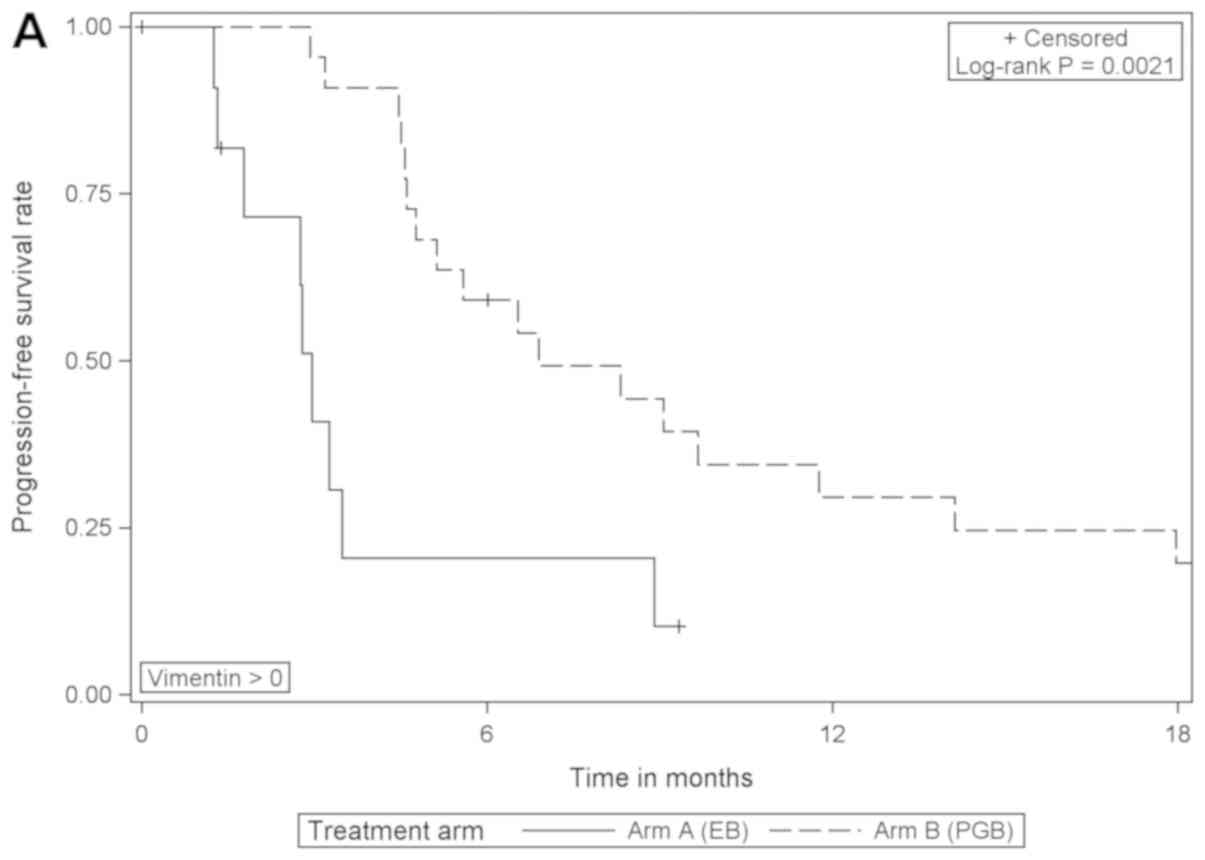
When comparing the treatment arms according to
vimentin expression, the analysis revealed that PFS was
significantly improved in vimentin-positive patients that received
PGB treatment compared with EB [HR=0.276 (95% CI, 0.115–0.659),
log-rank P=0.0021]; however, there was no difference in those that
were negative for vimentin (Table
III; Fig. 3). In patients with
negative or with positive vimentin expression there were no
statistically significant differences in OS when comparing the
different treatment arms (data not shown).
 | Table III.Progression-free survival with
respect to treatment and vimentin immunoreactivity. |
Table III.
Progression-free survival with
respect to treatment and vimentin immunoreactivity.
| Comparison | Vimentin
immunoreactivity=0 | Vimentin
immunoreactivity >0 | Log-rank
P-value | HRa (95% CI): Vimentin
immunoreactivity >0 vs. 0 | Wald chi-square:
P-valuea |
|---|
| EB arm: Median PFS
in months | 4.0 | 3.0 | 0.4594 | 1.336
(0.616–2.899) | 0.4630 |
| PGB arm: Median PFS
in months | 5.7 | 6.9 | 0.0811 | 0.598
(0.334–1.072) | 0.0845 |
| Log-rank
P-value | 0.1809 | 0.0021 | – | – | – |
| HRa (95% CI): PGB vs. EB | 0.703
(0.417–1.183) | 0.276
(0.115–0.659) | – | – | – |
| Wald chi-square
P-valuea | 0.1841 | 0.0038 | – | – | – |
The impact of treatment on PFS all patients with
E-cadherin and vimentin expression was assessed via log-rank tests
comparing PFS in both treatment groups with and without adjustment
for the expression status (unstratified log-rank tests, P=0.0002
E-cadherin group, P=0.0012 vimentin group; stratified log-rank
tests, P=0.0003 adjusted for E-cadherin expression, P=0.0117
adjusted for vimentin expression), and in multivariable analyses of
PFS (Tables IV and V). The results of interaction analyses
between treatment and expression status (E-cadherin and vimentin),
which were performed in the variable selection process, were not
statistically significant (Tables
IV and V).
 | Table IV.Multivariable analysis of PFS with
respect to treatment adjusted for UICC stage, age, E-cadherin
immunoreactivity and interactions between treatment and E-cadherin
immunoreactivity. |
Table IV.
Multivariable analysis of PFS with
respect to treatment adjusted for UICC stage, age, E-cadherin
immunoreactivity and interactions between treatment and E-cadherin
immunoreactivity.
| A, PFS of treatment
adjusted for UICC stage, and age (AIC=605.819) |
|---|
|
|---|
| Variable | HR (95% CI) | Wald chi-square
P-value |
|---|
| PGB vs. EB | 0.413
(0.263–0.651) | 0.0001 |
| UICC stage IV vs.
IIIB | 0.522
(0.269–1.014) | 0.0551 |
| Age (per year) | 0.980
(0.956–1.005) | 0.1114 |
|
| B, PFS of
treatment adjusted for E-cadherin immunoreactivity and interaction
between treatment and E-cadherin immunoreactivity
(AIC=610.410) |
|
|
Variable | HR (95%
CI) | Wald chi-square
P-value |
|
| PGB vs. EB | E-cadherin
immunoreactivity <8: PGB vs. EB: 0.385 (0.205–0.722) | 0.0029 |
|
| E-cadherin
immunoreactivity ≥8: PGB vs. EB: 0.524 (0.283–0.969) |
|
| E-cadherin
immunoreactivity: ≥8 vs. <8 | EB arm: E-cadherin
immunoreactivity ≥8 vs. <8: 0.904 (0.474–1.722) | 0.7583 |
|
| PGB arm: E-cadherin
immunoreactivity ≥8 vs. <8: 1.230 (0.688–2.198) |
|
| E-cadherin
immunoreactivity × treatment | – | 0.4870 |
 | Table V.Multivariable analysis of
progression-free survival with respect to treatment adjusted for
UICC stage, age, Vimentin immunoreactivity, and interactions
between treatment and Vimentin immunoreactivity. |
Table V.
Multivariable analysis of
progression-free survival with respect to treatment adjusted for
UICC stage, age, Vimentin immunoreactivity, and interactions
between treatment and Vimentin immunoreactivity.
| A, PFS of treatment
adjusted for UICC stage and age (AIC=592.325) |
|---|
|
|---|
| Variable | HR (95% CI) | Wald chi-square
P-value |
|---|
| PGB vs. EB | 0.458
(0.289–0.725) | 0.0009 |
| UICC stage IV vs.
IIIB | 0.532
(0.274–1.034) | 0.0626 |
| Age (per year) | 0.979
(0.954–1.004) | 0.0951 |
|
| B, PFS of
treatment adjusted for Vimentin immunoreactivity, and interactions
between treatment and Vimentin immunoreactivity
(AIC=594.103) |
|
|
Variable | HR (95%
CI) | Wald chi-square
P-value |
|
| PGB vs. EB | Vimentin
immunoreactivity=0: PGB vs. EB: 0.649 (0.382–1.105) | 0.1115 |
|
| Vimentin
immunoreactivity >0: PGB vs. EB: 0.287 (0.123–0.668) |
|
| Vimentin
immunoreactivity ≥8 vs. <8 | EB arm: Vimentin
immunoreactivity >0 vs. 0: 1.337 (0.618–2.895) | 0.4610 |
|
| PGB arm: Vimentin
immunoreactivity >0 vs. 0: 0.591 (0.321–1.087) |
|
| Vimentin
immunoreactivity × treatment | – | 0.1072 |
Although PGB treatment increased PFS compared with
EB treatment in analysis of the whole study population [102
patients with E-cadherin expression (high or low) and 101 patients
with vimentin expression (high or low)] and in subgroups of
patients with low E-cadherin expression or high vimentin
expression, no prognostic or predictive value of either biomarker
expression was identified. In multivariable Cox regression analyses
of PFS with different covariates, only the different treatment
regimens had a statistically significant effect on PFS. The effect
of either biomarker expression, their interaction with treatment or
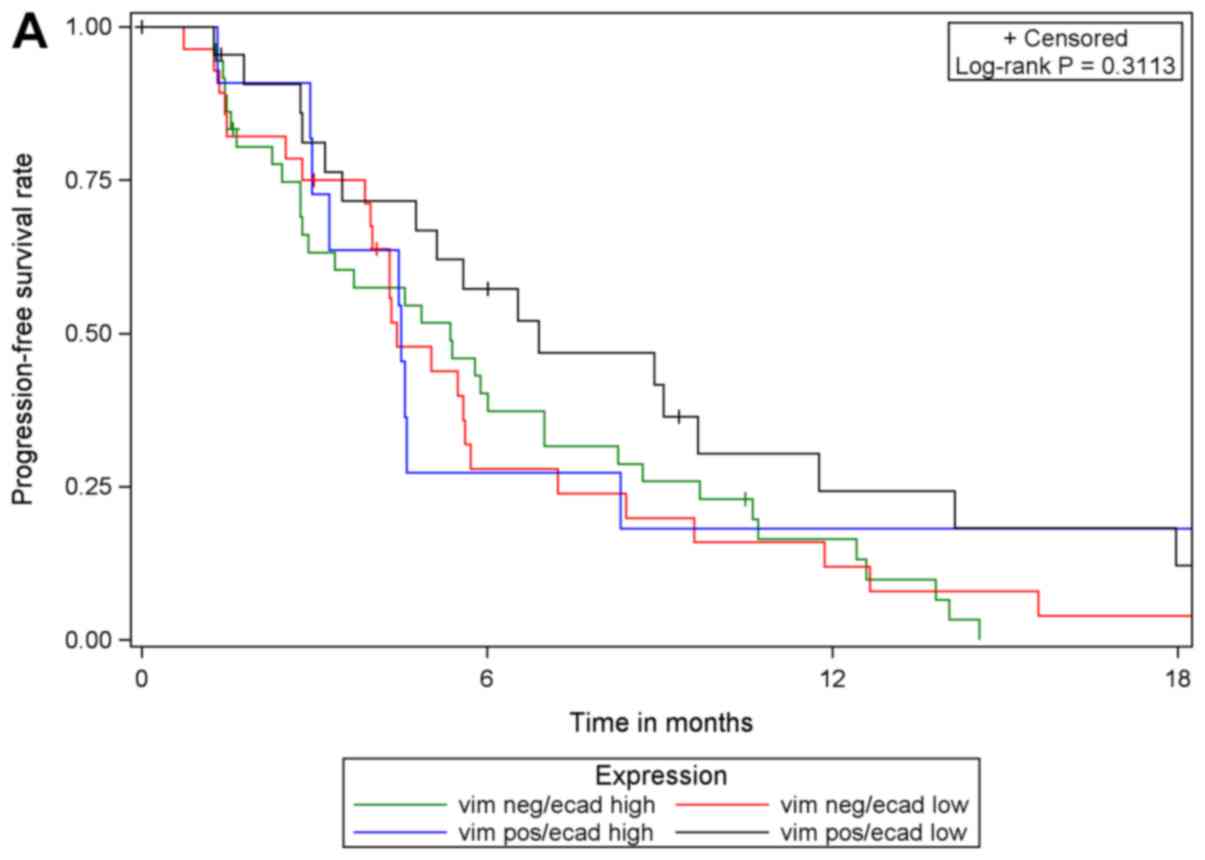
one another was statistically significant. These results were
supported by comparing PFS in the four subgroups with respect to
biomarker expression: E-cadherin low/vimentin negative, E-cadherin
low/vimentin positive, E-cadherin high/vimentin negative,
E-cadherin high/vimentin positive. The results of log-rank tests
were not statistically significant when comparing patients with
valid measurements of E-cadherin and vimentin (n=99) among the four
sub-groups, irrespective of treatment and within each treatment arm
(EB, n=42; PGB, n=57; Fig. 4).
E-cadherin and vimentin association
with EGFR or smoking status
Fisher's exact test was performed to determine
whether the proportion of patients with different
E-cadherin/vimentin expression varies with different EGFR status
and smoking histories. There was no association between the
biomarker expression and specific EGFR or smoking status
(E-cadherin low/vimentin positive; data not shown).
Discussion
In this study, the expression of the EMT-associated
markers E-cadherin and vimentin in NSCLC was semi-quantitated using
immunostaining, and their value in predicting clinical outcomes in
patients from the INNOVATIONS trial was evaluated. There is
evidence indicating that the loss of E-cadherin predicts a poor
response to treatment with EGFR inhibitors, including TKIs
(erlotinib and gefitinib) and monoclonal antibodies (cetuximab)
(28,29). In the study published by Ren et
al (28) the epithelial
phenotype was associated with a significantly higher objective
response rate, longer PFS and longer OS in the wild-type EGFR
subgroup. Contradictory to these findings, in the current study,
there was no difference in the outcome of patients treated with EB
when categorized according to epithelial phenotype (high E-cadherin
or negative vimentin).
Previous studies have reported that including
bevacizumab with erlotinib treatment is beneficial for patients
harboring activating EGFR mutations (30,31),
with relevance at least in molecularly defined subgroups (32). In the EGFR wild-type population there
is currently no defined biomarker identified to predict whether
EGFR TKI treatment will be beneficial. Evaluation of EMT markers in
the present study addressed this question, as one of the treatment
arms included the TKI erlotinib in combination with bevacizumab,
without cytostatic chemotherapy. However, limitations of the
present study are the non-predefined exclusion of EGFR-mutated
patients and the restriction of analysis to patients with samples
available for EMT assessment, resulting in reduced patient numbers.
Furthermore, reflecting on histological subtype and expression of
EMT markers, variations have been described, with lower E-cadherin
expression in squamous cell carcinoma compared with adenocarcinoma
(33).
Regarding the potential prognostic impact of EMT
markers, negative or low E-cadherin expression has been associated
with poor prognosis in patients with NSCLC undergoing resection
(11,14); however, results are variable,
potentially due to heterogeneous study conditions (use of different
antibodies, immunohistochemical staining techniques and scoring
systems). Thus, the clinical significance of E-cadherin expression
in NSCLC remains controversial. In the present study, no prognostic
impact of E-cadherin or vimentin was identified in EB-treated
patients.
In an EGFR wild-type population, Garassino et
al (34) reported that
cytostatic treatment with docetaxel significantly improves PFS
compared with EGFR TKI treatment in a second-line setting. From
further trials (35,36), it has been established that the
addition of vascular endothelial growth factor receptor (VEGFR)
inhibition to docetaxel treatment (ramucirumab or nintedanib,
respectively) improves survival in previously treated patients with
advanced NSCLC.
In the current analysis, treatment with chemotherapy
and bevacizumab (PGB) was superior to EB treatment in patients
displaying a mesenchymal phenotype (low E-cadherin or high
vimentin), but not in those with an epithelial phenotype (high
E-cadherin or low vimentin). These results are contradictory to
previously published data, which stated that EMT facilitates
drug-resistance (21). According to
the findings of the present study, it may be hypothesized that
patients with a mesenchymal phenotype in particular would benefit
from VEGFR inhibition (bevacizumab) in addition to combination
chemotherapy (cisplatin/gemcitabine). Unfortunately, the trial did
not include a comparison of cytostatic treatment with and without
anti-VEGFR treatment, or even an arm containing anti-VEGFR
treatment alone. This impedes more precise conclusions and should
be performed in further research.
As EMT can be reversible, the role of bevacizumab as
implemented in the present trial setting, or anti-angiogenesis in
general, should be considered more thoroughly. In vitro data
have demonstrated that VEGFR activation mediates EMT (37,38). Li
et al (39) reported that the
inhibitory effect of vandetanib (inhibitor of VEGFR and EGFR
tyrosine kinase activities) were associated with EMT, leading to a
loss of E-cadherin and gain of vimentin expression. Pretreatment
with vandetanib increased cisplatin sensitivity of the tumor cells
suggesting that blocking the effects of the growth factors
associated with EMT may reverse chemotherapy resistance. In
addition to this pathway, the concept of ICD has been introduced.
Certain anticancer therapeutics may have the potential to induce
ICD, maximizing antitumor immunity through this mechanism to
increase the effect of a treatment (17). As none of the agents used in the
INNOVATIONS trial have specifically been described as ICD-inducers
previously, further research is required to explore possible
associations with the findings of the current study.
To better understand the effect of bevacizumab, it
may be worthwhile to design trials with anti-angiogenic treatment
in conjunction with chemotherapy to assess the predictive impact of
EMT markers, or even to consider stratification according to those.
Furthermore, the increasing evidence for EMT-mediated tumor immune
escape supports the rationale for the development of EMT-blockers
to increase the response to checkpoint inhibitor immunotherapy
(16).
In conclusion, even though with the inherent
limitations outlined, the present study raises interest regarding
the therapeutic relevance of EMT markers, the mesenchymal phenotype
and the impact of anti-angiogenic treatment. Targeting EMT may
offer important perspectives for the therapeutic approaches in
advanced lung cancer.
Acknowledgements
Not applicable.
Funding
The INNOVATIONS trial was supported by Roche
Diagnostics GmbH (Mannheim, Germany), who also provided erlotinib
and bevacizumab, with an unrestricted grant (grant no. ML20262) to
our sponsor ‘ABC Aktion Bronchialkarzinom e.V.’ group (Study ID,
ABC-2006-NSCLC-01) as well as a grant for conducting the study and
the analyses of the associated biomarker.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MV, PC, PAS and MT designed the study. MV, PC and
PAS analyzed the probes (immunohistochemical examination). MV, PC
and MT interpreted the results. PN and AR performed the statistical
analysis. NR, JRF, SA, CK, MS, MW and MT enrolled the patients,
performed the diagnostic procedures and treatments, and assisted
with the implementation and execution of the trial. All authors
read, reviewed and approved the manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the study are appropriately
investigated and resolved.
Ethics approval and consent to
participate
The present study was approved by the ethics
committee of each participating institution and the German body,
The Paul Ehrlich Institute. The study was conducted in accordance
with the Declaration of Helsinki (version 1996) and the applicable
International Conference on Harmonisation of Technical Requirements
for Registration of Pharmaceuticals for Human Use, Good Clinical
Practice guidelines. It was registered on clinicaltrials.gov as NCT00536640 (EUDRA-CT
2006-004865-32). Written informed consent was obtained from all
patients prior to the collection and use of all samples.
Patient consent for publication
Not applicable.
Competing interests
MT received an honorarium (grant) from Roche
Diagnostics GmbH. All other authors declare no competing
interests.
References
|
1
|
Duruisseaux M, Besse B, Cadranel J, Pérol
M, Mennecier B, Bigay-Game L, Descourt R, Dansin E,
Audigier-Valette C, Moreau L, et al: Overall survival with
crizotinib and next generation ALK-inhibitors in ALK-positive
non-small-cell lung cancer (IFCT-1302 CLINAKL): A French nationwide
cohort retrospective study. Oncotarget. 8:21903–21917. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Planchard D, Smit EF, Groen HJM, Mazieres
J, Besse B, Helland Å, Giannone V, D'Amelio AM Jr, Zhang P,
Mookerjee B and Johnson BE: Dabrafenib plus trametinib in patients
with previously untreated BRAF V600E-mutant metastatic
non-small-cell lung cancer: An open-label, phase 2 trial. Lancet
Oncol. 18:1307–1316. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wu YL, Cheng Y, Zhou X, Lee KH, Nakagawa
K, Niho S, Tsuji F, Linke R, Rosell R, Corral J, et al: Dacomitinib
versus gefitinib as first-line treatment for patients with
EGFR-mutation positive non-small-cell lung cancer (ARCHER 1050): A
randomised, open-label, phase 3 trial. Lancet Oncol. 18:1454–1466.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shaw AT, Ou SH, Bang YJ, Camidge DR,
Solomon BJ, Salgia R, Riely GJ, Varella-Garcia M, Shapiro GI, Costa
DB, et al: Crizotinib in ROS1-rearranged non-small-cell lung
cancer. N Engl J Med. 371:1963–1971. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shea M, Costa DB and Rangachari D:
Management of advanced non-small cell lung cancers with known
mutations or rearrangements: Latest evidence and treatment
approaches. Ther Adv Respir Dis. 10:113–129. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Reck M, Rodriguez-Abreu D, Robinson AG,
Hui R, Csoszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe
S, et al: Pembrolizumab versus Chemotherapy for PD-L1-positive
non-small-cell lung cancer. N Engl J Med. 375:1823–1833. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kupferman ME, Jiffar T, El-Naggar A,
Yilmaz T, Zhou G, Xie T, Feng L, Wang J, Holsinger FC, Yu D and
Myers JN: TrkB induces EMT and has a key role in invasion of head
and neck squamous cell carcinoma. Oncogene. 29:2047–2059. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Burdsal CA, Damsky CH and Pedersen RA: The
role of E-cadherin and integrins in mesoderm differentiation and
migration at the mammalian primitive streak. Development.
118:829–844. 1993.PubMed/NCBI
|
|
9
|
Lee YC, Wu CT, Chen CS and Chang YL:
E-cadherin expression in surgically-resected non-small cell lung
cancers-a clinicopathological study. Thorac Cardiovasc Surg.
48:294–299. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kase S, Sugio K, Yamazaki K, Okamoto T,
Yano T and Sugimachi K: Expression of E-cadherin and ß-catenin in
human non-small cell lung cancer and the clinical significance.
Clin Cancer Res. 6:4789–4796. 2000.PubMed/NCBI
|
|
11
|
Choi YS, Shim YM, Kim SH, Son DS, Lee HS,
Kim GY, Han J and Kim J: Prognostic significance of E-cadherin and
ß-catenin in resected stage I non-small cell lung cancer. Eur J
Cardiothorac Surg. 24:441–449. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Richardon F, Young GD, Sennello R, Wolf J,
Argast GM, Mercado P, Davies A, Epstein PM and Wacker B: The
evaluation of E-cadherin and vimentin as biomarkers of clinical
outcomes among patients with non-small cell lung cancer treated
with erlotinib as second- and third-line therapy. Anticancer Res.
32:537–552. 2012.PubMed/NCBI
|
|
13
|
Soltermann A, Tischler V, Arbogast S,
Braun J, Probst-Hensch N, Weder W, Moch H and Kristiansen G:
Prognostic significance of epithelial-mesenchymal and
mesenchymal-epithelial transition protein expression in non-small
cell lung cancer. Clin Cancer Res. 14:7430–7437. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang H, Liu J, Yue D, Gao L, Wang D,
Zhang H and Wang C: Clinical significance or E-cadherin, ß-catenin,
vimentin and S100A4 expression in completely resected squamous cell
lung carcinoma. J Clin Pathol. 66:937–945. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Voon DC, Huang RY, Jackson RA and Thiery
JP: The EMT spectrum and therapeutic opportunities. Mol Oncol.
11:878–891. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Terry S, Savagner P, Ortiz-Cuaran S,
Mahjoubi L, Saintigny P, Thierry JP and Chouaib S: New insights
into the role of EMT in tumor immune escape. Mol Oncol. 11:824–846.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gark AD, Dudek-Peric A, Romanio E and
Agostinis P: Immunogenic cell death. Int J Dev Biol. 59:131–140.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sato M, Shames DS and Hasegawa Y: Emerging
evidence of epithelial-to-mesenchymal transition in lung
carcinogenesis. Respirology. 17:1048–1059. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhihua Y, Zhang X, Luo Y, Li S, Huang L,
Li Z, Li P and Chen G: Prognostic values of vimentin expression and
its clinicopathological significance in non-small cell lung cancer:
A meta-analysis of observational studies with 4118 cases. PLoS One.
11:e01631622016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dauphin M, Barbe C, Lemaire S,
Nawrocki-Raby B, Lagonotte E, Delepine G, Birembaut P, Gilles C and
Polette M: Vimentin expression predicts the occurrence of
metastases in non small cell lung carcinomas. Lung Cancer.
81:117–122. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sui H, Zhu L, Deng W and Li Q:
Epithelial-mesenchymal transition and drug resistance: Role,
molecular mechanisms and therapeutic strategies. Oncol Res Treat.
37:584–589. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yauch RL, Januario T, Eberhard DA, Cavet
G, Zhu W, Fu L, Pham TQ, Soriano R, Stinson J, Seshagiri S, et al:
Epithelial versus mesenchymal phenotype determines in vitro
sensitivity and predicts clinical activity of erlotinib in lung
cancer patients. Clin Cancer Res. 11:8686–8698. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Witta SE, Gemmill RM, Hirsch FR, Coldren
CD, Hedman K, Ravdel L, Helfrich B, Dziadziuszko R, Chan DC, Sugita
M, et al: Restoring E-cadherin expression increases sensitivity to
epidermal growth factor receptor inhibitors in lung cancer cell
lines. Cancer Res. 66:944–950. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sandler A, Gray R, Perry MC, Brahmer J,
Schiller JH, Dowlati A, Lilenbaum R and Johnson DH:
Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell
lung cancer. N Engl J Med. 355:2542–2550. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Thomas M, Fischer J, Andreas S, Kortsik C,
Grah C, Serke M, von Eiff M, Witt C, Kollmeier J, Müller E, et al:
Erlotinib and bevacizumab versus cisplatin, gemcitabine and
bevacizumab in unselected nonsquamous nonsmall cell lung cancer.
Eur Respir J. 46:219–229. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu Y, Liu H, Ding M, Liu J, Zhan P, Fu X
and Gan L: The impact of E-cadherin expression on non-small cell
lung cancer survival: A meta-analysis. Mol Biol Rep. 39:9621–9628.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Satelli A and Li S: Vimentin in cancer and
its potential as a molecular target for cancer therapy. Cell Mol
Life Sci. 68:3033–3046. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ren S, Su C, Wang Z, Li J, Fan L, Li B, Li
X, Zhao C, Wu C, Hou L, et al: Epithelial phenotype as a predictive
marker for response to EGFR-TKIs in non-small cell lung cancer
patients with wild-type EGFR. Int J Cancer. 135:2962–2971. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nikolova DA, Asangani IA, Nelson LD,
Hughes DP, Siwak DR, Mills GB, Harms A, Buchholz E, Pilz LR,
Manegold C and Allgayer H: Cetuximab attenuates metastasis and
u-PAR expression in non-small cell lung cancer: u-PAR and
E-cadherin are novel biomarkers of cetuximab sensitivity. Cancer
Res. 69:2461–2470. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Herbst RS, Ansari R, Bustin F, Flynn P,
Hart L, Otterson GA, Vlahovic G, Soh CH, O'Connor P and Hainsworth
J: Efficacy of bevacizumab plus erlotinib versus erlotinib alone in
advanced non-small-cell lung cancer after failure of standard
first-line chemotherapy (BeTa): A double-blind, placebo-controlled,
phase 3 trial. Lancet. 377:1846–1854. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Seto T, Kato T, Nishio M, Goto K, Atagi S,
Hosorni Y, Yamamoto N, Hida T, Maemondo M, Nakagawa K, et al:
Erlotinib alone or with bevacizumab as first-line therapy in
patients with advanced non-squamous non-small-cell lung cancer
harbouring EGFR mutations (JO25567): An open-label, randomized,
mutlicentre, phase 2 study. Lancet Oncol. 15:1236–1244. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rosell R, Dafni U, Felip E,
Curioni-Fontecedro A, Gautschi O, Peters S, Massuti B, Palmero R,
Aix SP, Carcereny E, et al: Erlotinib and bevacizumab in patients
with advanced non-small-cell lung cancer and activating EGFR
mutations (BELIEF): An international, multicentre, single-arm,
phase 2 trial. Lancet Respir Med. 5:435–444. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Prudkin L, Liu DD, Ozburn NC, Sun M,
Behrens C, Tang X, Brown KC, Bekele BN, Moran C and Wistuba II:
Epithelial-to-mesenchymal transition in the development and
progression of adenocarcinoma and squamous cell carcinoma of the
lung. Mod Pathol. 22:668–678. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Garassino MC, Martelli O, Broggini M,
Farina G, Veronese S, Rulli E, Bianchi F, Bettini A, Longo F,
Moscetti L, et al: Erlotinib versus docetaxel as second-line
treatment of patients with advanced non-small-cell lung cancer and
wild-type EGFR tumours (TAILOR): A randomized controlled trial.
Lancet Oncol. 14:981–988. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Garon EB, Ciuleanu TE, Arrieta O, Prabhash
K, Syrigos KN, Goksel T, Park K, Gorbunova V, Kowalyszyn RD, Pikiel
J, et al: Ramucirumab plus docetaxel versus placebo plus docetaxel
for second-line treatment of stage IV non-small-cell lung cancer
after disease progression on platinum-based therapy (REVEL): A
multicentre, double-blind, randomized phase 3 trial. Lancet.
384:665–673. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Reck M, Kaiser R, Mellemgaard A, Douillard
JY, Orlov S, Krzakowski M, von Pawel J, Gottfried M, Bondarenko I,
Liao M, et al: Docetaxel plus nintedanib versus docetaxel plus
placebo in patients with previously treated non-small-cell lung
cancer (LUME-Lung 1): A phase 3, double-blind, randomized
controlled trial. Lancet Oncol. 15:143–155. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fantozzi A, Gruber DC, Pisarsky L, Heck C,
Kunita A, Yilmaz M, Meyer-Schaller N, Cornille K, Hopfer U,
Bentires-Alj M and Christofori G: VEGF-mediated angiogenesis links
EMT-induced cancer stemness to tumor initiation. Cancer Res.
74:1566–1575. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yi ZY, Feng LJ, Xiang Z and Yao H:
Vascular endothelial growth factor receptor-1 activation mediates
epithelial to mesenchymal transition in hepatocellular carcinoma
cells. J Invest Surg. 24:67–76. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li Y, Yang X, Su LJ and Flaig TW: VEGFR
and EGFR inhibition increases epithelial cellular characteristics
and chemotherapy sensitivity in mesenchymal bladder cancer cell.
Oncol Rep. 24:1019–1028. 2010.PubMed/NCBI
|