Introduction
Hepatocellular carcinoma (HCC) can be divided into
two major categories: primary and secondary liver cancer.
Especially, primary HCC is a highly harmful malignant tumor
worldwide (1). The etiology and
exact molecular mechanism of primary HCC is not fully understood.
It is reported that its pathogenesis is a complex multistep
process, which is controlled by environmental and genetic factors
(2). However, the 5-year survival
rate of HCC remains low at 10% because of poor diagnosis and
prognosis (3). A beneficial
treatment for patients suffering from HCC has not been achieved
although hepatic resection and liver transplantation therapies have
improved in recent years (4).
Therefore, it is still necessary to investigate regulatory pathways
of HCC progression.
MicroRNA (miRNA) can reversely control gene
expression by degrading mRNA and repressing translation although it
cannot encode proteins (5). Research
has identified that miRNA dysregulation influenced the initiation
and development of various cancer types, including HCC. For
instance, miR-448 was downregulated and promoted invasion by
inhibiting Rho-associated coiled-coil containing protein kinase 2
(ROCK2) in HCC (6). Chen et
al demonstrated that miR-490-5p inhibited HCC proliferation and
metastasis by affecting ROBO1 (7).
In addition, miR-96, miR-758-3p and miR-1260b were found to
regulate HCC formation (8–10). Among them, microRNA-185 (miR-185) was
connected with various human cancers such as breast cancer
(11), non-small cell lung cancer
(12), glioma (13) and colorectal cancer (14). However, the role of miR-185 in
tumorigenesis of HCC remains to be elucidated.
ROCK2 belonging to the family of serine/threonine
kinases has been identified to affect tumor development. ROCK2 was
reported to play a carcinogenic role in lung and breast cancers
(15,16). Moreover, RhoE/ROCK2 affects
chemoresistance through NF-κB/IL-6/STAT3 signaling in HCC (17). ROCK2 promoted HCC proliferation by
suppressing CEBPD through phospho-GSK3β/β-catenin signaling
(18). However, the function of
miR-185-5p/ROCK2 in HCC remains to be determined.
In the current study, we paid attention to
miR-185-5p and its association with ROCK2 in HCC. Importantly,
these findings could supplement the regulatory pathway of
miR-185-5p in HCC.
Materials and methods
Clinical tissue samples
Fifty-four surgical tumor specimens and adjacent
tissue samples were obtained from the Linyi Central Hospital
(Linyi, China). None of the patients received treatment before
surgery, and all the participants signed informed consent. Human
tissue was frozen in liquid nitrogen and then stored at −80°C
refrigerator for further use. All the tissue samples of the
experiment were approved by the Linyi Central Hospital
Institutional Ethics Committee.
Cell culture and transfection
The human HCC cell lines Hep-3B and SNU-387, a
normal liver cell line (LO-2) were used in this study. All the cell
lines were obtained from the American Type Culture Collection
(ATCC, Manassas, VA, USA), and were cultured in DMEM containing 10%
fetal bovine serum (FBS). These cells were grown in an incubator at
37°C, with 5% CO2 atmosphere. The medium was replaced
every other day according to the culture state.
The miR-185-5p mimic and inhibitor, ROCK2 siRNA were
purchased from Guangzhou RiboBio Co., Ltd. (Guangzhou, China) and
were transferred into SNU-387 cells with Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc., Carlsbad, CA, USA)
according to the manufacturer's protocols.
RNA extraction and
reverse-transcription quantitative PCR (RT-qPCR)
TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) was used to extract total RNA containing miRNA to
quantify miR-185-5p expression in HCC tissues and cell lines.
RT-qPCR was carried out using the QuantiTect SYBR-Green PCR mixture
on an ABI7900 LightCycler (Roche Diagnostics, Basel, Switzerland).
U6 and GAPDH served as the control of miR-185-5p and ROCK2. The
miR-185-5p and ROCK2 levels were analyzed using the
2−ΔΔCq method (19).
Bioinformatics
We searched the miRNA databases: Target scan
(http://www.targetscan.org/) for miR-185
that target ROCK2, their sequences and their chromosome
localization.
Luciferase assays
The WT-3′-UTR of ROCK2 or MUT-3′-UTR of ROCK2 was
inserted into the pGL3 promoter vector (GenScript Co., Ltd.,
Nanjing, China) for luciferase reporter experiments. Then, the
vector and miR-185-5p mimic were transfected into SNU-387 cells by
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.).
The cells were cultured in a 24-well plate. About 48 h after
transfection, the Dual-Luciferase® Reporter Assay System
(Promega Corp., Madison, WI, USA) was applied to perform luciferase
assays.
Transwell migration and invasion
assay
A total of 5×104 HCC cells without serum
were put in the upper chamber on the non-coated membrane, and the
lower chamber was filled with 20% FBS to induce HCC cells to
migrate or invade through the membrane. The cells were put in the
upper chamber with the coated membrane for invasion assay. Then the
cells were incubated for 48 h to detect cell migration and
invasion. Cells on the lower surface of the membrane were fixed
with 4% paraformaldehyde, stained with 1% crystal violet for 5 min
and subsequently counted in 3 randomly selected fields under an
inverted microscope (Leica Microsystems GmbH, Wetzlar, Germany) at
magnification ×200.
Western blotting
The protein samples were obtained using RIPA buffer.
Protein concentration was calculated using bicinchoninic acid
(BCA). Equal amounts of protein (30 µg) were separated through 10%
SDS-PAGE and then incubated with 5% skim milk blocked membranes at
room temperature. Next we incubated the membranes overnight at 4°C
with anti-ROCK2 (rabbit polyclonal; dilution, 1:1,000; cat. no.
ab71598; Abcam, Shanghai, China), anti-GAPDH antibodies (rabbit
monoclonal; dilution, 1:1,000; cat. no. ab181602; Abcam) and
subsequently incubated with matched goat anti-rabbit G-horseradish
peroxidase secondary antibody (dilution, 1:2,000; cat. no. sc-2054;
Santa Cruz Biotechnology Inc., Dallas, TX, USA). Then, protein
expression levels were measured by ECL.
Statistical analysis
Statistical and diagram analyses used GraphPad Prism
6.0 and SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA). Data are
presented as the mean ± SD. The difference was analyzed by
Chi-square test or one-way ANOVA using the Tukeys post hoc test.
Differences were considered significant at P<0.05.
Results
miR-185-5p levels are decreased in
HCC
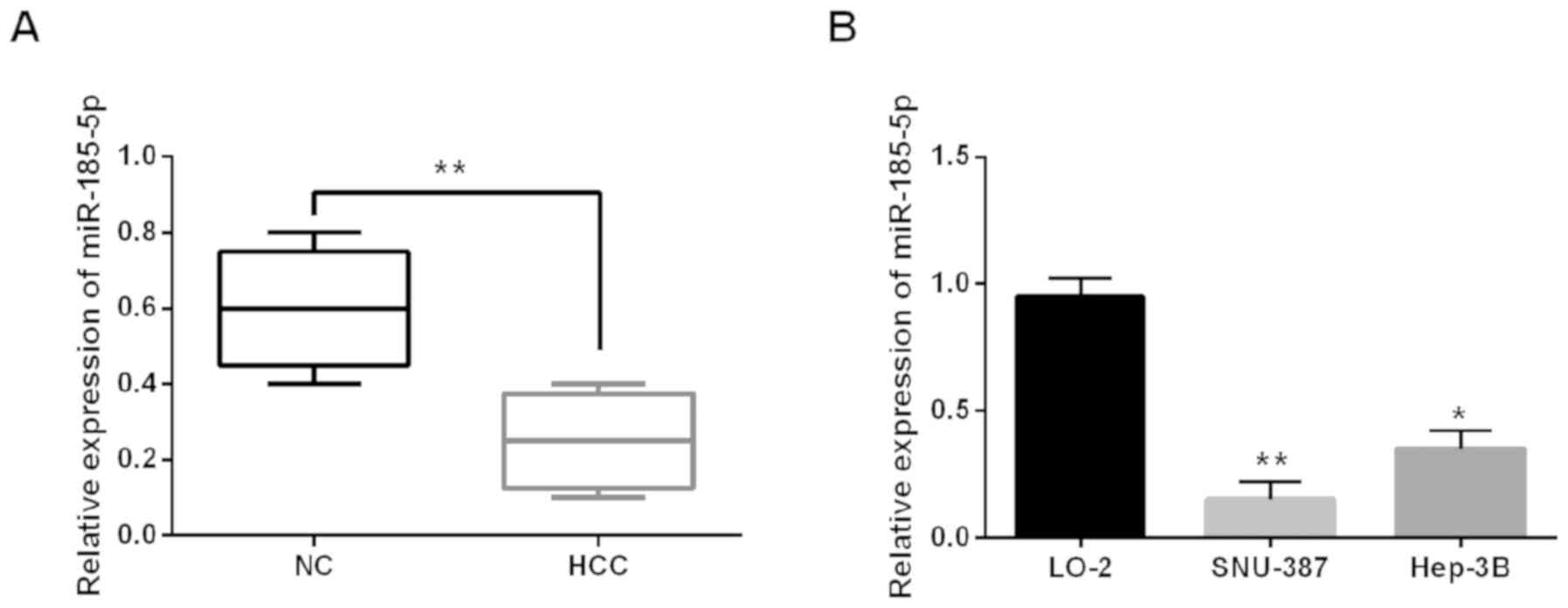
To explore the change of miR-185-5p in HCC, we
conducted RT-qPCR in HCC tissues and cell lines (Hep-3B, SNU-387
and LO-2). Expression of miR-185-5p was obviously downregulated in
HCC tissues (Fig. 1A). miR-185-5p
levels were lower in Hep-3B and SNU-387 than the normal LO-2 cell
line (Fig. 1B). Furthermore, the
correlation between clinical characteristics and miR-185-5p level
was calculated in 54 HCC tissues. We found that a low expression of
miR-185-5p was associated with tumor grade and lymph node
metastasis (P<0.05). There was no association among miR-185-5p
expression and other clinical characteristics (Table I, P>0.05).
 | Table I.Relationship between miR-185-5p
expression and their clinicopathological characteristics in 54
patients with HCC. |
Table I.
Relationship between miR-185-5p
expression and their clinicopathological characteristics in 54
patients with HCC.
|
| miR-185-5p |
|
|---|
|
|
|
|
|---|
| Characteristics | Low | High | P-value |
|---|
| Age (years) |
|
| 0.424 |
| ≥60 | 16 | 13 |
|
|
<60 | 13 | 12 |
|
| Sex |
|
| 0.221 |
| Male | 16 | 14 |
|
|
Female | 13 | 11 |
|
| AFP (ng/ml) |
|
| 0.077 |
| ≤20 | 12 | 10 |
|
|
>20 | 17 | 15 |
|
| Tumor size (cm) |
|
| 0.151 |
| ≥5 | 17 | 11 |
|
|
<5 | 12 | 14 |
|
| Liver cirrhosis |
|
| 0.150 |
| None | 11 | 12 |
|
| Yes | 18 | 13 |
|
| TNM stage |
|
| 0.095 |
| I+II | 15 | 16 |
|
|
III+IV | 14 | 9 |
|
| Tumor stage |
|
| 0.002a |
| I+II | 20 | 14 |
|
| III | 9 | 11 |
|
| Lymph node
metastasis |
|
| 0.016a |
| None | 10 | 10 |
|
| Yes | 19 | 15 |
|
miR-185-5p overexpression inhibits
cell migration and invasion in HCC
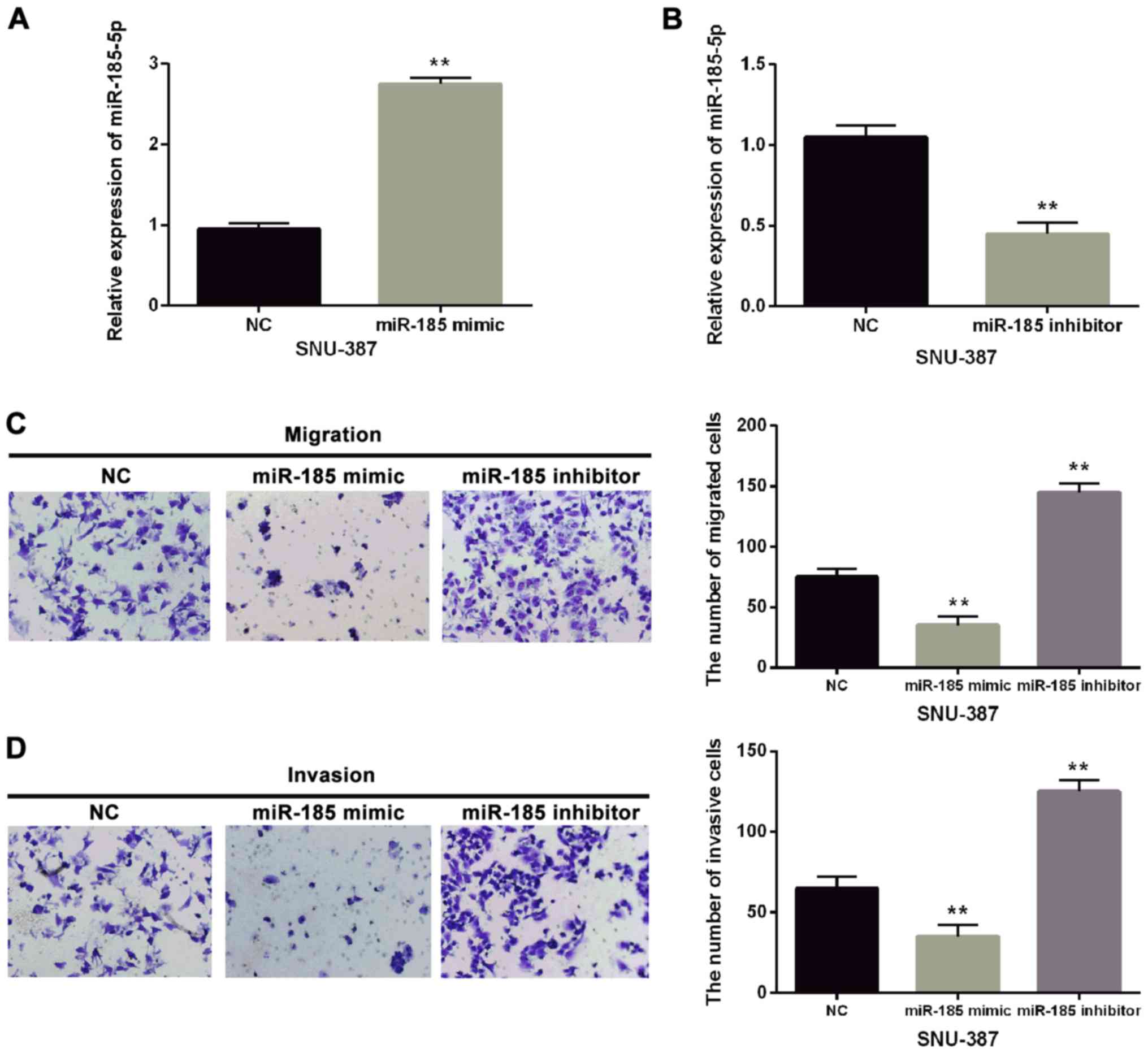
To confirm the effect of miR-185-5p on the
metastasis of HCC, miR-185-5p mimic or inhibitor was transfected
into SNU-387 cells (Fig. 2A and B).
Functionally, miR-185-5p overexpression obviously suppressed
migration and invasion of SNU-387 cells (Fig. 2C). Downregulation of miR-185-5p
significantly promoted cell metastasis of SNU-387 cells (Fig. 2D). Thus, we speculated that the
effect of miR-185-5p on cell metastasis in HCC was dependent on its
altered expression.
miR-185-5p directly targeted
ROCK2
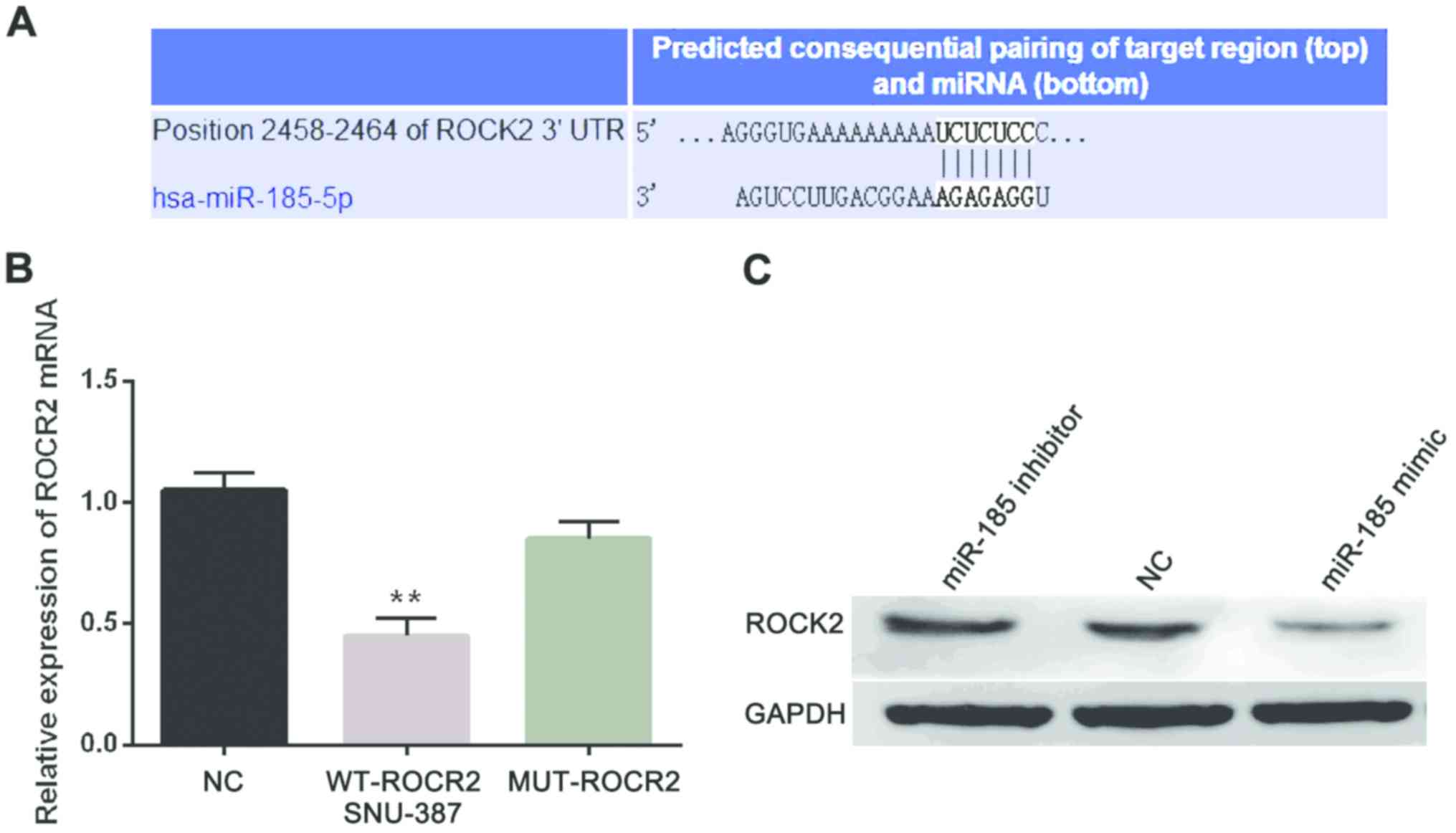
Furthermore, we searched the target genes of
miR-185-5p from TargetScan database (http://www.targetscan.org/vert_71/) to investigate its
downstream regulation mechanism in HCC and it predicted the binding
site of ROCK2 for miR-185-5p (Fig.
3A). Next, we confirmed the above prediction through luciferase
reporter assay. The miR-185-5p mimic and WT-ROCK2 or MUT-ROCK2
plasmid were co-transfected into SNU-387 cells. Moreover, the
luciferase activities were obviously decreased in group with
miR-185-5p mimic and WT-ROCK2 while there was almost no change in
group with miR-185-5p mimic and MUT-ROCK2 (Fig. 3B). In addition, miR-185-5p effect on
ROCK2 expression at protein level was detected in SNU-387 cells
with miR-185-5p mimic or inhibitor. ROCK2 protein levels were
enhanced by miR-185-5p inhibitor but decreased by miR-185-5p mimic
(Fig. 3C). Therefore, miR-185-5p
directly targeted ROCK2 and was negatively associated with ROCK2
expression.
ROCK2 silence inhibits migration and
invasion in HCC
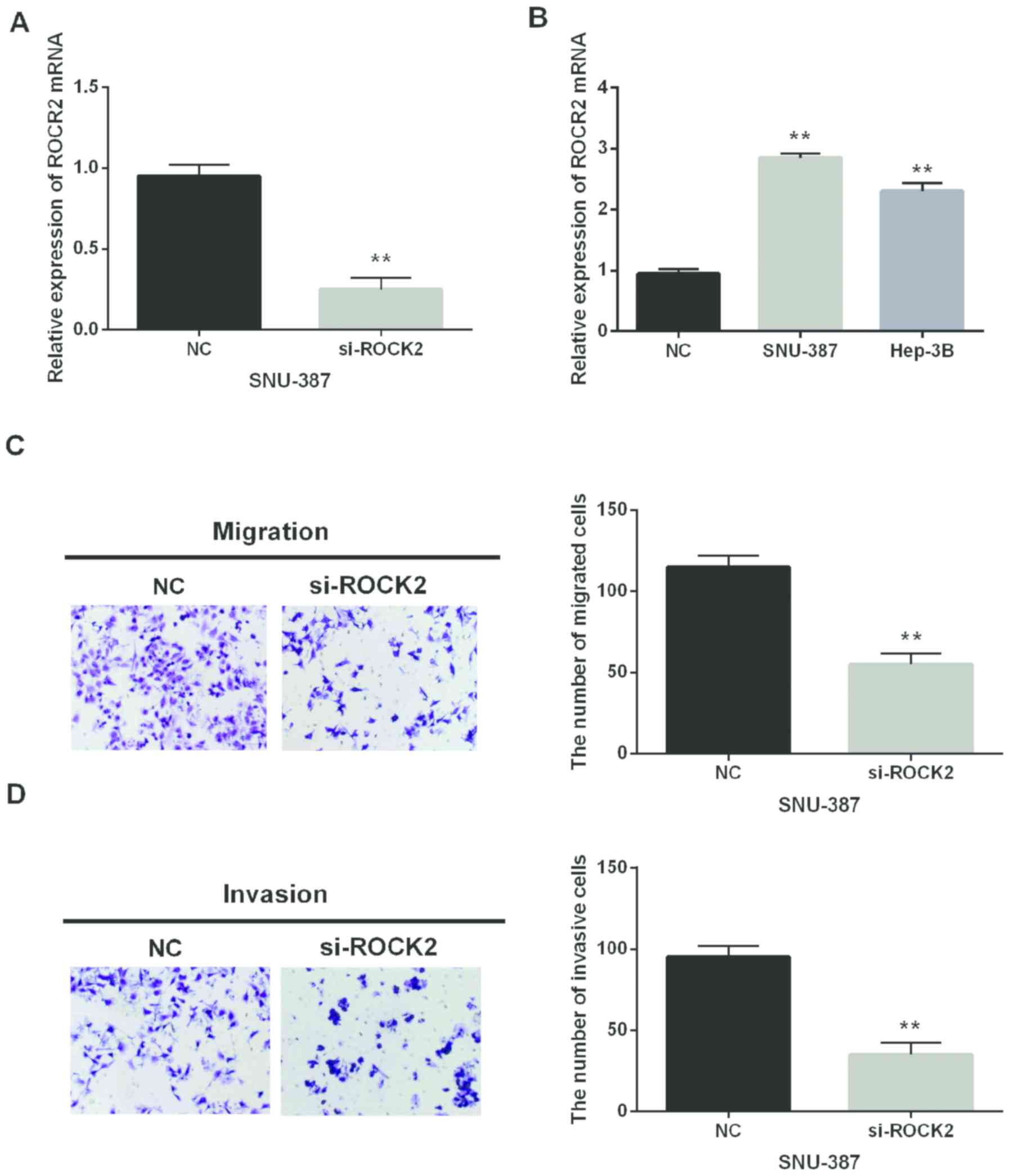
In addition, si-ROCK2 was synthesized and
transfected into SNU-387 cells to investigate ROCK2 in HCC. The
transfection efficiency was assessed by RT-qPCR (Fig. 4A). ROCK2 mRNA expression in HCC cell
lines was also detected. The upregulation of ROCK2 was identified
in Hep-3B and SNU-387 in comparison with the normal LO-2 cell line
(Fig. 4B). Moreover, the Transwell
assay was applied to analyze cell metastasis of HCC. ROCK2 silence
was observed to inhibit cell migration and invasion in HCC
(Fig. 4C and D). Thus, we inferred
that ROCK2 would play an oncogenic role in HCC development.
miR-185-5p regulates cell metastasis
of HCC through suppressing ROCK2
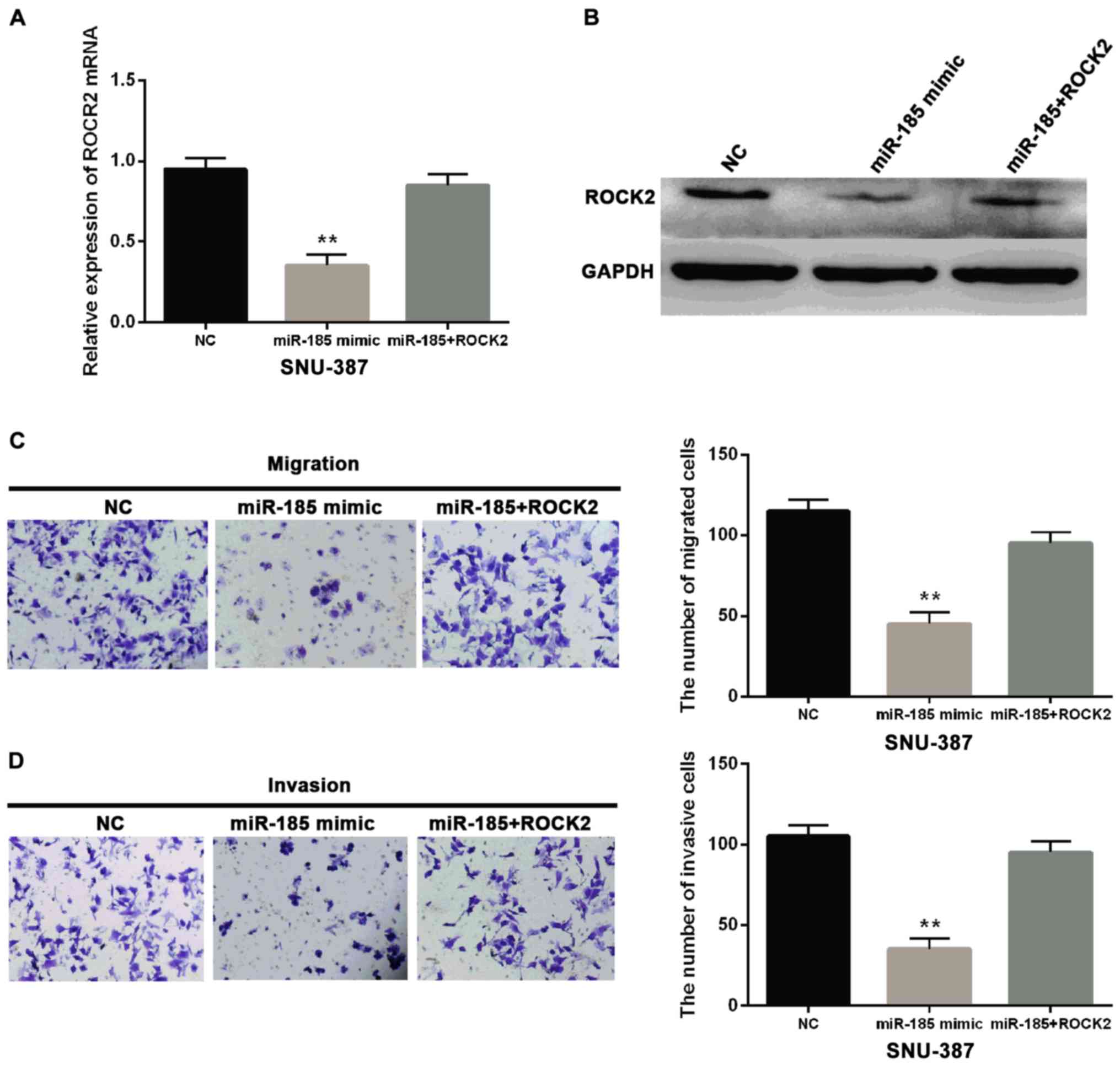
Based on the above results, miR-185-5p was
considered to repress the effect of ROCK2. For confirmation
miR-185-5p mimic was transfected into SNU-387 cells with negative
control or ROCK2 expression vector. The miR-185-5p reduced the
ROCK2 levels at the mRNA and protein expression (Fig. 5A and B). Additionally, miR-185-5p
impaired the effect of ROCK2 on promoting cell metastasis in
SNU-387 cell lines (Fig. 5C and D).
A series of evidence verified that miR-185-5p suppressed cell
migration and invasion in HCC through suppression of ROCK2. These
results may have the potential to affect tumorigenesis of HCC.
Discussion
The downregulation and inhibitory effect of
miR-185-5p in HCC were identified by us and upregulation and
promoted effect of ROCK2 were detected in HCC. Moreover, miR-185-5p
was confirmed to directly target ROCK2 in HCC. These results might
help us better understand the regulatory mechanism of HCC
progression.
Previous studies demonstrated that miR-185 function
varied from cancer to cancer. For instance, miR-185 as an oncogenic
miRNA was found upregulated in gastric and bladder cancers
according to miRNA profiling (20,21). On
the other hand, miR-185 expression was low and suppressed
colorectal cancer cell proliferation and invasion (22). Similarly, Zhu et al reported
that miR-185 level was decreased in HCC (23). Not surprisingly, we also detected
downregulation of miR-185-5p in HCC which could be used as a
biomarker for diagnosing HCC. Zhi et al also reported that
miR-185 was a potential prognostic biomarker for HCC in early stage
(24). Functionally, miR-185-5p has
been confirmed to inhibit cell proliferation, tumor growth and EMT
by regulating Six1, Six2 and DNMT1/PTEN/Akt Pathway in HCC
(11,23,25).
However, scarce research has been performed to explore the effect
of miR-185 on cell metastasis in HCC. Thus, we detected the exact
function of miR-185-5p for cell migration and invasion and proved
that miR-185-5p had suppressive effect on cell metastasis in
HCC.
To the best of our knowledge, the relationship
between miR-185-5p and ROCK2 has not been investigated in human
cancers. However, we proved that miR-185-5p directly targeted ROCK2
in the current investigation. ROCK2 was reported to be
overexpressed in HCC and to promote cell invasion through
depredating MMP2 (26).
Consistently, ROCK2 in HCC was also upregulated in our study.
Moreover, ROCK2 was identified to influence cell metastasis and
aggressiveness which was regulated by miR-124 and miR-139 in HCC
(27,28). In this study, ROCK2 was negatively
associated with miR-185-5p and promoted HCC cell migration and
invasion. Thus, we considered that miR-185-5p would impede cell
migration and invasion by directly repressing ROCK2. However, we
still need to explore whether miR-185-5p/ROCK2 regulates HCC
progression through specific signaling pathways.
Collectively, miR-185-5p function as anti-metastatic
miRNA was identified to be downregulated in HCC, and miR-185-5p
upregulation repressed migration and invasion of HCC cells.
Moreover, miR-185-5p directly targeted ROCK2 and had negative
correlation with its expression. ROCK2 promoted cell metastasis in
HCC. Therefore, miR-185-5p inhibited cell metastasis of HCC by
suppressing ROCK2.
Acknowledgements
Not applicable.
Funding
Not applicable.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YN contributed to the conception of the study and
wrote the manuscript. GT performed the data analyses. Both authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Linyi Central Hospital (Linyi, China). Signed informed consents
were obtained from the patients or guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Llovet JM, Zucman-Rossi J, Pikarsky E,
Sangro B, Schwartz M, Sherman M and Gores G: Hepatocellular
carcinoma. Nat Rev Dis Primers. 2:160182016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Farazi PA and DePinho RA: Hepatocellular
carcinoma pathogenesis: From genes to environment. Nat Rev Cancer.
6:674–687. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kinoshita A, Onoda H, Fushiya N, Koike K,
Nishino H and Tajiri H: Staging systems for hepatocellular
carcinoma: Current status and future perspectives. World J Hepatol.
7:406–424. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Allen MD, Luong P, Hudson C, Leyton J,
Delage B, Ghazaly E, Cutts R, Yuan M, Syed N, Lo Nigro C, et al:
Prognostic and therapeutic impact of argininosuccinate synthetase 1
control in bladder cancer as monitored longitudinally by PET
imaging. Cancer Res. 74:896–907. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhu H, Zhou X, Ma C, Chang H, Li H, Liu F
and Lu J: Low expression of miR-448 induces EMT and promotes
invasion by regulating ROCK2 in hepatocellular carcinoma. Cell
Physiol Biochem. 36:487–498. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen W, Ye L, Wen D and Chen F: MiR-490-5p
inhibits hepatocellular carcinoma cell proliferation, migration and
invasion by directly regulating ROBO1. Pathol Oncol Res. 2:1–9.
2017.
|
|
8
|
Li Z and Wang Y: MiR-96 targets SOX6 and
promotes proliferation, migration and invasion of hepatocellular
carcinoma. Biochem Cell Biol bcb-2017-0183. 2017.
|
|
9
|
Jiang D, Cho W, Li Z, Xu X, Qu Y, Jiang Z,
Guo L and Xu G: MiR-758-3p suppresses proliferation, migration and
invasion of hepatocellular carcinoma cells via targeting MDM2 and
mTOR. Biomed Pharmacother. 96:535–544. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li X, Song H, Liu Z and Bi Y: miR-1260b
promotes cell migration and invasion of hepatocellular carcinoma by
targeting the regulator of G-protein signaling 22. Biotechnol Lett.
40:57–62. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Imam JS, Buddavarapu K, Lee-Chang JS,
Ganapathy S, Camosy C, Chen Y and Rao MK: MicroRNA-185 suppresses
tumor growth and progression by targeting the Six1 oncogene in
human cancers. Oncogene. 29:4971–4979. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Takahashi Y, Forrest ARR, Maeno E,
Hashimoto T, Daub CO and Yasuda J: MiR-107 and MiR-185 can induce
cell cycle arrest in human non small cell lung cancer cell lines.
PLoS One. 4:e66772009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tang H, Wang Z, Liu X, Liu Q, Xu G, Li G
and Wu M: LRRC4 inhibits glioma cell growth and invasion through a
miR-185-dependent pathway. Curr Cancer Drug Targets. 12:1032–1042.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Akçakaya P, Ekelund S, Kolosenko I,
Caramuta S, Ozata DM, Xie H, Lindforss U, Olivecrona H and Lui WO:
miR-185 and miR-133b deregulation is associated with overall
survival and metastasis in colorectal cancer. Int J Oncol.
39:311–318. 2011.PubMed/NCBI
|
|
15
|
Kalender ME, Demiryürek S, Oztuzcu S,
Kizilyer A, Demiryürek AT, Sevinc A, Dikilitas M, Yildiz R and
Camci C: Association between the Thr431Asn polymorphism of the
ROCK2 gene and risk of developing metastases of breast cancer.
Oncol Res. 18:583–591. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vigil D, Kim TY, Plachco A, Garton AJ,
Castaldo L, Pachter JA, Dong H, Chen X, Tokar B, Campbell SL, et
al: ROCK1 and ROCK2 are required for non-small cell lung cancer
anchorage-independent growth and invasion. Cancer Res.
72:5338–5347. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ma W, Sze KM, Chan LK, Lee JM, Wei LL,
Wong CM, Lee TK, Wong CC and Ng IO: RhoE/ROCK2 regulates
chemoresistance through NF-κB/IL-6/STAT3 signaling in
hepatocellular carcinoma. Oncotarget. 7:41445–41459.
2016.PubMed/NCBI
|
|
18
|
Li M, Zhou W, Yuan R, Chen L, Liu T, Huang
D, Hao L, Xie Y and Shao J: ROCK2 promotes HCC proliferation by
CEBPD inhibition through phospho-GSK3β/β-catenin signaling. FEBS
Lett. 589:1018–1025. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yao Y, Suo AL, Li ZF, Liu LY, Tian T, Ni
L, Zhang WG, Nan KJ, Song TS and Huang C: MicroRNA profiling of
human gastric cancer. Mol Med Rep. 2:963–970. 2009.PubMed/NCBI
|
|
21
|
Gottardo F, Liu CG, Ferracin M, Calin GA,
Fassan M, Bassi P, Sevignani C, Byrne D, Negrini M, Pagano F, et
al: MicroRNA profiling in kidney and bladder cancers. Urol Oncol.
25:387–392. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu M, Lang N, Chen X, Tang Q, Liu S,
Huang J, Zheng Y and Bi F: miR-185 targets RhoA and Cdc42
expression and inhibits the proliferation potential of human
colorectal cells. Cancer Lett. 301:151–160. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhu SM, Chen CM, Jiang ZY, Yuan B, Ji M,
Wu FH and Jin J: MicroRNA-185 inhibits cell proliferation and
epithelial-mesenchymal transition in hepatocellular carcinoma by
targeting Six2. Eur Rev Med Pharmacol Sci. 20:1712–1719.
2016.PubMed/NCBI
|
|
24
|
Zhi Q, Zhu J, Guo X, He S, Xue X, Zhou J,
Hu B, Li H, Chen S, Zhao H, et al: Metastasis-related miR-185 is a
potential prognostic biomarker for hepatocellular carcinoma in
early stage. Biomed Pharmacother. 67:393–398. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Qadir XV, Han C, Lu D, Zhang J and Wu T:
miR-185 inhibits hepatocellular carcinoma growth by targeting the
DNMT1/PTEN/Akt pathway. Am J Pathol. 184:2355–2364. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang D, Du X, Yuan R, Chen L, Liu T, Wen
C, Huang M, Li M, Hao L and Shao J: Rock2 promotes the invasion and
metastasis of hepatocellular carcinoma by modifying MMP2
ubiquitination and degradation. Biochem Biophys Res Commun.
453:49–56. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zheng F, Liao YJ, Cai MY, Liu YH, Liu TH,
Chen SP, Bian XW, Guan XY, Lin MC, Zeng YX, et al: The putative
tumour suppressor microRNA-124 modulates hepatocellular carcinoma
cell aggressiveness by repressing ROCK2 and EZH2. Gut. 61:278–289.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wong CC, Wong CM, Tung EK, Au SL, Lee JM,
Poon RT, Man K and Ng IO: The microRNA miR-139 suppresses
metastasis and progression of hepatocellular carcinoma by
down-regulating Rho-kinase 2. Gastroenterology. 140:322–331. 2011.
View Article : Google Scholar : PubMed/NCBI
|