Introduction
Epidermal growth factor receptor (EGFR) mutations
are important factors in non-small lung cancer (NSCLC), as EGFR
tyrosine kinase inhibitor (EGFR-TKI) treatment has exhibited high
efficacy in NSCLC patients with EGFR mutations (1–6).
Polymerase chain reaction (PCR) methods are used clinically for the
detection of EGFR mutations. Two widely used PCR methods are
approved as in vitro diagnostic (IVD) methods, namely, the
Scorpion Amplification Refractory Mutation System (ARMS; QIAGEN
therascreen® EGFR; Qiagen, Inc., Valencia, CA, USA) and
the cobas® EGFR Mutation Test v2 (Roche Diagnostics,
Indianapolis, IN, USA) (7,8). These 2 methods are a real-time PCR test
for the qualitative detection of defined mutations of the EGFR gene
in DNA derived from formalin-fixed paraffin-embedded (FFPE) tumor
tissue from NSCLC patients. The test is intended to aid in
identifying patients with NSCLC whose tumors have defined EGFR
mutations and for whom safety and efficacy of EGFR-TKI have been
established. The first EGFR-TKI is gefitinib, which was approved
from July 2002 in Japan. Erlotinib, afatinib, dacomitinib and
osimertinib are subsequently approved as EGFR-TKIs. Dacomitinib is
a second-generation, irreversible EGFR-TKI. In NSCLC patients with
EGFR mutations detected by Scorpion-ARMS method, dacomitinib
significantly improved progression-free survival over gefitinib in
first-line treatment (5).
Osimertinib is a third-generation, irreversible EGFR-TKI. In the
first-line treatment of EGFR mutation-positive advanced NSCLC
identified by cobas v2, osimertinib showed efficacy superior to
that of gefitinib or erlotinib with a similar safety profile and
lower rates of serious adverse events (6). In addition, cobas v2 can be used with
plasma samples, as a companion diagnostic for NSCLC therapy. The
Scorpion-ARMS and the cobas v2 are useful, rapid and cost-effective
methods as a companion diagnostic. However, they can only identify
a small proportion of the different types of mutation, including
common exon 19 deletions and exon 21 L858R. The present study
therefore analyzed the frequency of detectable EGFR mutations and
the clinical significance of mutations that are not detected by
these 2 methods.
Materials and methods
Patients
The present study included a cohort of 73 Japanese
patients with NSCLC, from whom written informed consent was
obtained for the use of their samples in this research. These
patients presented with recurrent disease following surgery between
1992 and 2004. The response of patients with EGFR mutations to
EGFR-TKI treatment, which was a daily dose of gefitinib (250 mg)
administered between April 2002 and October 2005, was evaluated.
During this period, only gefitinib was approved as an EGFR-TKI
therapy for NSCLC patients in Japan (Table I). The present study received ethics
approval from the Institutional Review Board of Tokyo Medical
University (Tokyo, Japan).
 | Table I.Background information of the 73
patients with NSCLC. |
Table I.
Background information of the 73
patients with NSCLC.
| Variable | Total n, (%) |
|---|
| Age, years, range
(median) | 30–92 (65.6) |
| Sex: Male/Female | 45 (61.6)/28
(38.4) |
| Histology:
ADC/SCC/La/Pleomorphic | 59 (80.8)/9 (12.3)/4
(5.5)/1 (1.4) |
| Smoking: Never/Former
and Current | 29 (39.7)/44
(60.3) |
| EGFR mutation | 35
(47.9) |
DNA extraction and direct sequencing
analysis
All 73 surgical samples were FFPE; analysis of EGFR
mutations was performed. Tumor cells were microdissected under
stereoscopic microscopy, and DNA was extracted from tumor cells
with a DNeasy Tissue kit (Qiagen, Inc.). PCR was performed using a
primer set flanking exons 18 to 21. PCR products were run on a gel,
and the appropriate bands were cut, and then DNA was extracted from
the gel with the QIAquick Gel Extraction kit (Qiagen, Inc.).
Sequencing analysis was performed using an ABI Prism 3100-Avant
genetic analyzer (Applied Biosystems; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) according to the manufacturer's
protocol.
Determination of the efficacy of
EGFR-TKI therapy
Definition of the efficacy of EGFR-TKI therapy was
applied according to the Response Evaluation Criteria in Solid
Tumors (9). In the case of a partial
response (PR) or complete response (CR), changes in tumor
measurements were confirmed by repeated assessments that were
performed no less than 4 weeks following the fulfillment of the
response criteria. Stable disease (SD) was defined as fulfilling
the SD criteria for at least 8 weeks following the start of
EGFR-TKI therapy. Patients receiving >4 weeks of treatment were
considered assessable, and patients who had received EGFR-TKI
therapy for <4 weeks were considered not assessable. Response
rate (RR) was defined as the percentage of PR and CR divided by the
total of the assessable patients, and disease control rate (DCR)
was the percentage of response and SD divided by the total of the
assessable patients (Table II).
 | Table II.Information on 35 NSCLC patients with
EGFR mutations receiving EGFR- tyrosine kinase inhibitor
therapy. |
Table II.
Information on 35 NSCLC patients with
EGFR mutations receiving EGFR- tyrosine kinase inhibitor
therapy.
| Variable | Total n |
|---|
| Assessable
patients/patients with EGFR mutations | 30/35 |
| Mutations of exon
18/19/21 | 1/23/11 |
| Mutation types in 30
assessable patients |
|
| Mutations
of exon 18/19/21 | 1/19/10 |
| EGFR
mutation RR | 36.7% (11/30) |
| EGFR
mutation DCR | 97% (29/30) |
| Exon
mutations |
|
|
Exon 18 mutation
RR | 100% (1/1) |
|
Exon 19 mutations
RR | 42.1% (8/19) |
|
Exon 21 mutations
RR | 20% (2/10) |
Frequency of detectable mutations by
the 2 IVD PCR methods
Two widely used IVD PCR methods (Scorpion ARMS and
cobas v2) were used in the present study. Scorpion-ARMS can detect
29 types of EGFR nucleotide mutations, and cobas v2 can detect 42
types of EGFR nucleotide mutations (Table III) (7,8). The two
methods of detection have previously identified common mutations
such as specific exon 19 deletions and specific exon 21 mutations
including L858R. Using the 2 methods, patients who harbored
mutations that could not be identified were confirmed, and the
frequency of detectable EGFR mutations was calculated. The results
from the 2 methods were compared with the predicted frequency of
detectable mutations from the catalog of somatic mutations in
cancer (COSMIC) version 84 database. COSMIC is the world's largest
and most comprehensive resource for analyzing the effects of
somatic mutations in human cancers. EGFR consists of 564
nucleotides from exon 18 to exon 21 (codons 688–875), and EGFR
mutations include substitutions, deletions, insertions, and
combinations of these (9–12). The COSMIC v84 database was used in
the present study to count the types of EGFR mutations (released on
February 13, 2018).
 | Table III.Types of EGFR mutations identified by
the 2 IVD PCR methods. |
Table III.
Types of EGFR mutations identified by
the 2 IVD PCR methods.
| PCR method | Exon 18 | Exon 19 | Exon 20 | Exon 21 | Total n |
|---|
| Scorpion-ARMS | 3 | 19 | 5 | 2 | 29 |
| Cobas v2 | 3 | 29 | 7 | 3 | 42 |
Detection of EGFR mutations by the 2
IVD PCR methods
Owing to the large number of clinical samples, DNA
was extracted from FFPE tumor specimens without microdissection,
for analysis by the 2 IVD PCR methods. The DNA was subjected to
Scorpion-ARMS at SRL Inc. (Tokyo, Japan) and cobas v2 at BML Inc.
(Tokyo, Japan), along with sizing capillary electrophoresis using
MCE-202 MultiNA (Shimazu Corporation, Kyoto, Japan) at BML Inc. for
the comprehensive detection of exon 19 deletions. Sizing capillary
electrophoresis measures the length of DNA to distinguish between
wild-type exons and exons with deletions, and covers all exon 19
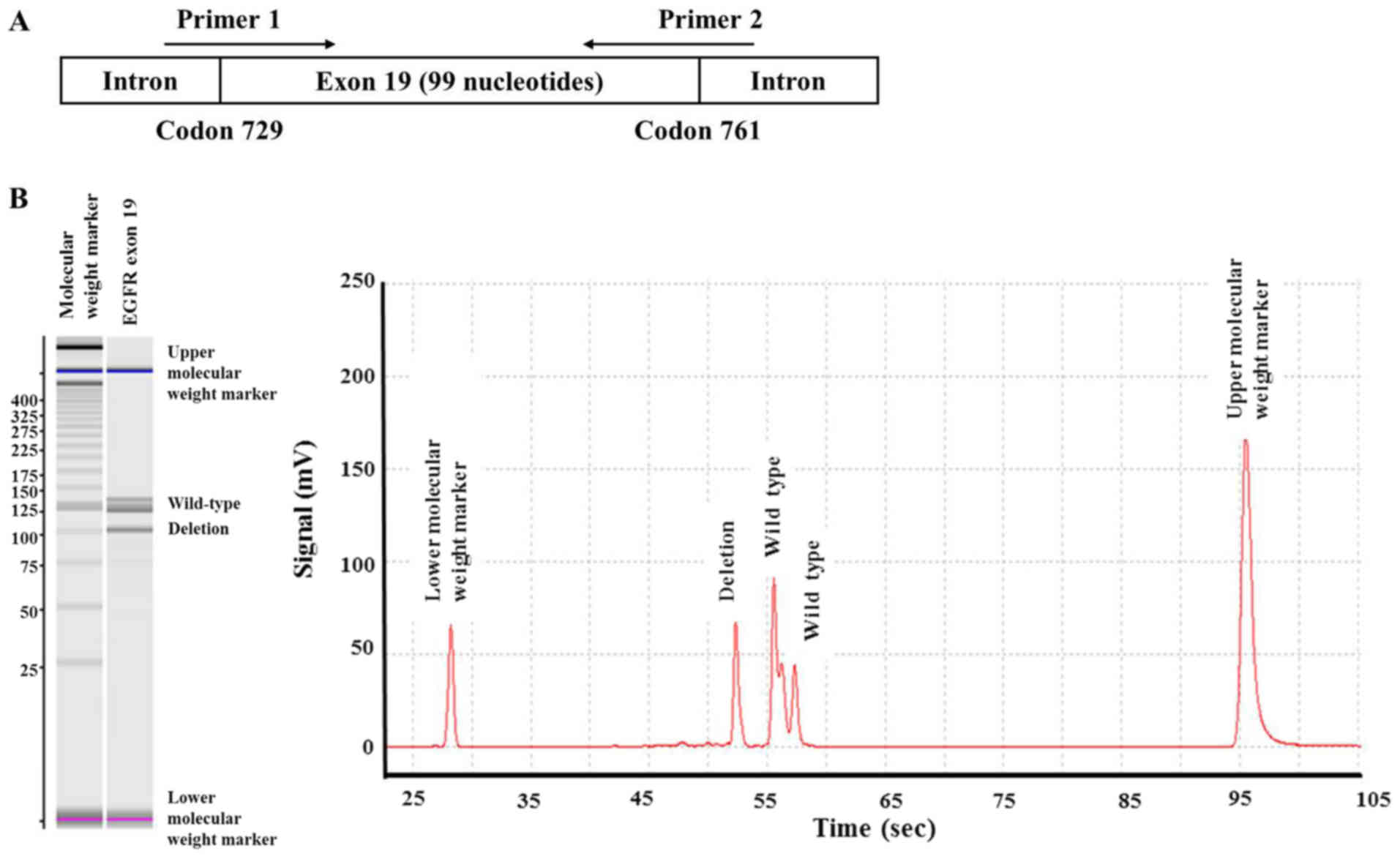
deletions along the 99 nucleotides from codon 729 to 761 (Fig. 1A). This was performed to detect exon
19 deletions that were not identified by the 2 IVD PCR methods.
Results
EGFR mutation status and response to
EGFR-TKI therapy
EGFR mutations were detected by direct sequencing in
35 patients (47.9% of clinical samples), including, 1 exon 18
mutation, 23 exon 19 mutations and 11 exon 21 mutations. Among the
35 patients with EGFR mutations who received EGFR-TKI therapy, 30
patients were assessable, which included 1 patient with an exon 18
mutation, 19 patients with exon 19 mutations and 10 patients with
exon 21 mutations. The RR of the EGFR mutations was 36.7% and the
DCR was 97%. In exons 19 and 21, including common mutations, the RR
of exon 19 mutations was 42.1% and the RR of exon 21 mutations was
20% (Table II).
Frequency of mutations identified by
the 2 IVD PCR methods
In all 73 cases, there were 11 types of EGFR
mutations among the 35 patients with an EGFR mutation, including 1
type of exon 18 mutation, 9 types of exon 19 mutations and 1 type
of exon 21 mutation, as detected by direct sequencing. The exon 18
mutation was G719S, the exon 19 mutations were all deletions (9
types), including deletion and insertion compound mutations, and
the exon 21 mutation was L858R. All exon 18 and 21 mutations were
identified by Scorpion ARMS and cobas v2. However, among the 9
types of exon 19 deletions, 3 types were not identified by the 2
methods. These unidentified deletions were the E746_S752 deletion,
the T751_I759 deletion with insertion S and the K757_L760 deletion
with insertion N. Two patients with unidentified deletions had
achieved disease control (CR and SD) via EGFR-TKI therapy (Table IV). Of the 35 patients with EGFR
mutations, the frequency of detectable EGFR mutations by the 2
methods was 91.4% (32/35); that of exon 19 mutations was 87.0%
(20/23) and that of exon 21 mutations was 100% (11/11).
 | Table IV.Information on 35 EGFR mutations
detected by direct sequencing and the EGFR-TKI response of the
patients. |
Table IV.
Information on 35 EGFR mutations
detected by direct sequencing and the EGFR-TKI response of the
patients.
| Amino acid/deletion
type | Nucleotide | No. | Histology
ADC/SCC | Response
CR/PR/SD/PD/NA |
|---|
| Exon 18 |
|
|
|
|
|
G719S | G2155A | 1 | 2/0 | 0/1/0/0/0 |
| Exon 19 |
| 23 | 20/3 | 2/6/10/1/4 |
|
E746-A750 del | 2,235-2,249 | – | – | – |
|
| 2,236-2,250 | 13 | 11/2 | 0/3/7/1/2 |
|
E746-S752 dela | 2,236-2,256 | 1 | 1/0 | 1/0/0/0/0 |
|
L747-E749 del | 2,239-2,247 | 1 | 1/0 | 1/0/0/0/0 |
|
L747-L751 del | 2,239-2,253 | – | – | – |
|
| 2,240-2,254 | 3 | 3/0 | 0/2/0/0/1 |
|
L747-P753 ins S | 2,240-2,257 | 3 | 3/0 | 0/1/2/0/0 |
|
T751-I759 ins Sa | 2,252-2,275
T2276G | 1 | 1/0 | 0/0/1/0/0 |
|
K757-L760 ins Na | 2,271-2,279 | 1 | 0/1 | 0/0/0/0/1 |
| Exon 21 |
|
|
|
|
|
L858R | T2573G | 11 | 11/0 | 0/2/8/0/1 |
| Total EGFR
mutations | – | 35 | 32/3 | 2/9/18/1/5 |
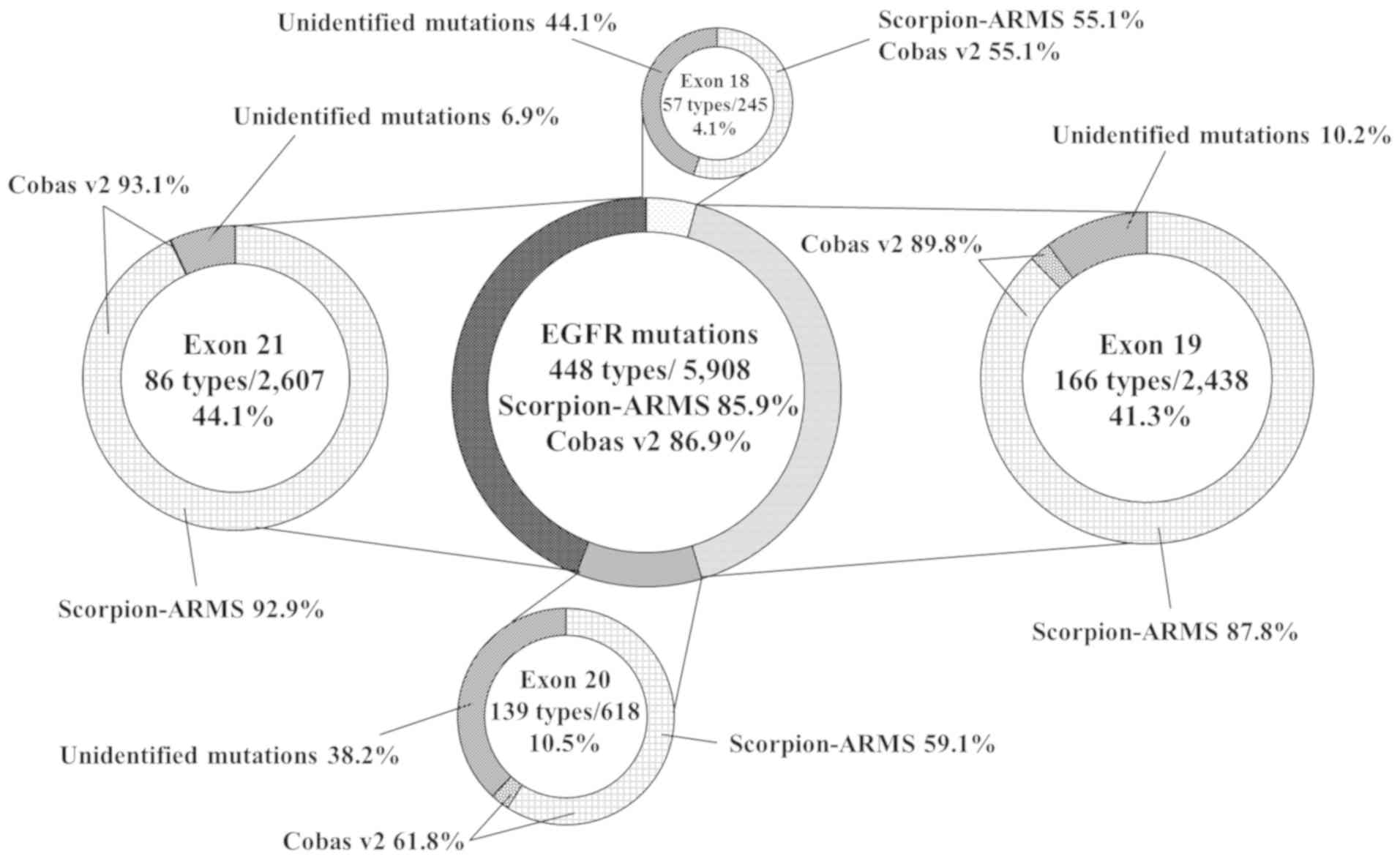
The COSMIC v84 database contains a total of 5,908
EGFR mutations comprising 448 types. The distribution of the
mutations was 4.1% in exon 18, 41.3% in exon 19, 10.5% in exon 20
and 44.1% in exon 21. In the COSMIC v84 database, the predicted
frequencies of detectable EGFR mutations by Scorpion-ARMS and cobas
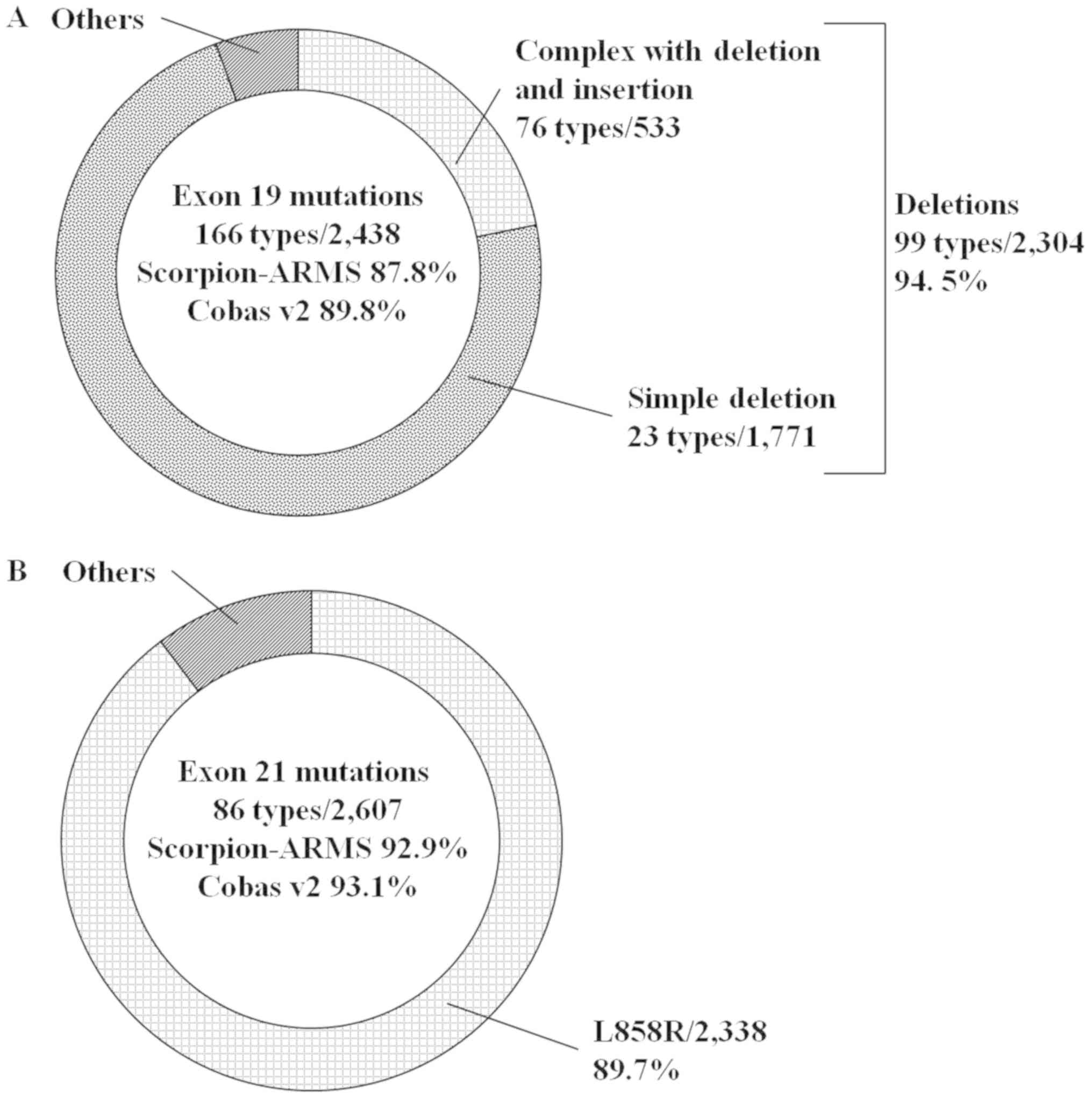
v2 were 85.9 and 86.9%, respectively (Fig. 2). Regarding exon 19 mutations, the
distribution of deletions, including deletion and insertion
compound mutations, was 94.5%, and the predicted frequencies of
these mutations by the two methods were 87.8 and 89.8%,
respectively (Fig. 3A). Regarding
exon 21 mutations, the distribution of L858R was 89.7%, and the
predicted frequencies of the two methods were 92.9 and 93.1%,
respectively (Fig. 3B).
Detection of EGFR mutations by the 2
IVD PCR methods
Scorpion-ARMS and cobas v2 analyses were performed
on 25 samples among the 35 patients who had EGFR mutations that
were detected by direct sequencing, which included 1 exon 18
mutation (G719S), 18 exon 19 deletions comprised of 6 different
types (including 1 exon 19 deletion not identified by the 2
methods), and 6 exon 21 mutations (L858R). All exon 18 and 21
mutations were detected by the two methods; however, both methods
failed to detect exon 19 deletions in 4 patients (2 squamous cell
carcinoma and 2 adenocarcinoma patients). The undetected deletions
of exon 19 from 3 samples were actually the common E746-A750
mutation, which was expected to be identified by the two methods.
One undetected complex with a deletion and insertion of exon 19 was
T751_I759 and insertion S (COSMIC ID 1667027), which were not
identified by either of the methods. The deletion was identified in
a patient with adenocarcinoma, with long SD (43.4 months) by
EGFR-TKI therapy. Subsequent sizing capillary electrophoresis was
able to detect the deletion (Fig.
1B).
Discussion
Gefitinib and erlotinib were approved as a
first-generation EGFR-TKI. Subsequently, afatinib and dacomitinib
as a second-generation and osimertinib as a third-generation were
approved. EGFR mutations are the major factors determining the
efficacy of EGFR-TKI therapy in patients with NSCLC (1–6). EGFR
consists of 564 nucleotides from exons 18 to 21, and EGFR mutations
include substitutions, deletions, insertions, and a combination of
any those (10–13). Therefore, different types of
mutations are continuously being identified. In the COSMIC v84
database, exon 19 and 21 mutations account for the majority of EGFR
mutations, and the distribution of these mutations among each exon
is similar to previous studies (14,15)
Scorpion-ARMS and cobas v2 can detect a proportion of the EGFR
mutations, including common exon 19 deletions and the exon 21 L858R
mutation. The present results revealed that the 2 methods did not
identify 3 exon 19 deletions, in which 2 deletions were
adenocarcinomas and 1 deletion was squamous cell carcinoma. In
squamous cell carcinoma with EGFR mutations, a patient with a
common exon 19 deletion (E746-A750) had achieved disease control
via EGFR TKI therapy. Regardless of the histological type of lung
cancer, exon 19 deletions are important for EGFR TKI therapy.
In the present study and COSMIC v84 database, the
frequencies of detectable EGFR mutations by the 2 methods were
similar, despite the different number of mutations identified by
the 2 methods. In particular, in regard to exon 19 mutations,
Scorpion-ARMS and cobas v2 identified 19 and 29 deletions,
respectively. Mutations identified by the two methods include
common mutations (exon 19 E746-A750 deletion and exon 21 L858R
mutation); however, there are many other types of mutations, for
example there are 99 different types of exon 19 deletions listed in
the COSMIC database v84. In a recent clinical study, the efficacy
of EGFR-TKI therapy in NSCLC patients with common exon 19 deletions
and the exon 21 L858R mutation were analyzed (4–6).
Methodological studies tend to use methods with higher sensitivity
for liquid biopsy. Scorpion-ARMS and cobas v2 are highly sensitive
PCR methods for the detection of common mutations. Cobas v2 is a
recently established reliable PCR method, which is the companion
diagnostic for osimertinib (6).
However, the frequency of detectable EGFR mutations and the
clinical significance of unidentified mutations by the 2 methods
are unclear. Previous studies have demonstrated that NSCLC patients
with tumors harboring exon 19 deletions have a longer survival rate
when compared with those harboring the L858R point mutation of exon
21, following treatment with EGFR-TKIs, which was reported between
2006 and 2007 prior to the approval of cobas v2 as an IVD (16–18).
Afatinib treatment resulted in a significant improvement in the
overall survival of NSCLC patients with exon 19 deletions, compared
with cytotoxic chemotherapy (4). In
the present study, the RR of patients with exon 19 deletions was
higher than that of exon 21 L858R. Among patients with exon 19
deletions, that were not identified by Scorpion-ARMS and cobas v2,
there were patients who responded to EGFR-TKI therapy.
Sizing capillary electrophoresis not only detected
the exon 19 deletions identified by Scorpion-ARMS and cobas v2, but
also detected the exon 19 deletion that was not identified by the 2
methods. This was observed in a patient with adenocarcinoma for
whom EGFR-TKI therapy was effective. The samples of 3 patients with
the exon 19 E746–750 deletion (common mutation), which was detected
by sequencing were concluded to be wild-type by the 2 methods. The
reason for this was that the tumors are heterogeneous, comprised of
a mixture of wild-type cells and cells with mutations, and as there
was a time lag between performing sequencing and the 2 methods, the
DNA may have been damaged during this delay.
Sequencing has the ability to analyze the whole
exome. EGFR contains 564 nucleotides in exons 18 to 21 (codons
688–875), and for the majority of NSCLC patients, sequencing of the
whole exome of EGFR is difficult due to the high cost and long time
required (19,20). The frequency of false negatives is an
important factor in screening prior to treatment, as patients may
lose the opportunity to receive EGFR-TKI therapy. Exon 19 mutations
in particular had 10% false negatives in Scorpion-ARMS and cobas
v2, and were expected to achieve improved overall survival via EGFR
TKIs therapy when compared with other exon mutations. In addition,
different types of exon 19 deletions have continuously increased in
the COSMIC database between v84 and v87 over the 10 month time
period between versions, therefore the number of false negatives is
increasing. In conclusion, exon 19 deletions are the most important
EGFR mutations in exons 18 to 21 for EGFR-TKI therapy, and the
frequencies of exon 19 deletions detected by Scorpion-ARMS and
cobas v2 were less than those of exon 21 mutations with L858R.
Therefore, the results of the present study suggest that decreasing
the rate of the false negatives of exon 19 deletions via widely
used PCR methods may be important for the clinical testing of EGFR
mutations.
Acknowledgements
The authors would like to thank the Department of
International Medical Communications of Tokyo Medical University
(Tokyo, Japan) for their assistance with the manuscript.
Funding
No funding was received.
Availability of data and materials
The datasets regarding the clinical samples used and
analyzed during this study are available from the corresponding
author on reasonable request. The datasets analyzed in this study
are available in the COSMIC database repository (cancer.sanger.ac.uk/cosmic/).
Authors' contributions
EN and MS conceived and designed the study. KF
obtained the clinical samples from Tokyo Medical University Ibaraki
Medical Center (Ibaraki, Japan). HT and OU obtained the clinical
samples from Tokyo Medical University Hachioji Medical Center
(Tokyo, Japan). YK, TO and NI obtained the clinical samples from
Tokyo Medical University Hospital (Tokyo, Japan). EN, MS and TO
processed and analyzed the samples. TO, NI and FRH interpreted the
data. JM and WAF analyzed and interpreted the pathological samples.
All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study received ethics approval from the
Institutional Review Board of Tokyo Medical University (Tokyo,
Japan), and written informed consent to participate was obtained
from all of the patients.
Patient consent for publication
The present study obtained consent for publication
from each patient.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
EGFR
|
epidermal growth factor receptor
|
|
NSCLC
|
non-small cell lung cancer
|
|
TKI
|
tyrosine kinase inhibitor
|
|
PCR
|
polymerase chain reaction
|
|
IVD
|
in vitro diagnostics
|
|
Scorpion-ARMS
|
Scorpion Amplification Refractory
Mutation System
|
|
FFPE
|
formalin-fixed and
paraffin-embedded
|
|
COSMIC
|
the catalog of somatic mutations in
cancer
|
|
PR
|
partial response
|
|
CR
|
complete response
|
|
SD
|
stable disease
|
|
RR
|
response rate
|
|
DCR
|
disease control rate
|
References
|
1
|
Mok TS, Wu YL, Thongprasert S, Yang CH,
Chu DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, et
al: Gefitinib or carboplatin-paclitaxel in pulmonary
adenocarcinoma. N Engl J Med. 361:947–957. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mitsudomi T, Morita S, Yatabe Y, Negoro S,
Okamoto I, Tsurutani J, Seto T, Satouchi M, Tada H, Hirashima T, et
al: Gefitinib versus cisplatin plus docetaxel in patients with
non-small-cell lung cancer harbouring mutations of the epidermal
growth factor receptor (WJTOG3405): An open label, randomised phase
3 trial. Lancet Oncol. 11:121–128. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Maemondo M, Inoue A, Kobayashi K, Sugawara
S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I,
et al: Gefitinib or chemotherapy for non-small cell lung cancer
with mutated EGFR. N Engl J Med. 362:2380–2388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang JC, Wu YL, Schuler M, Sebastian M,
Popat S, Yamamoto N, Zhou C, Hu CP, O'Byrne K, Feng J, et al:
Afatinib versus cisplatin-based chemotherapy for EGFR
mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6):
Analysis of overall survival data from two randomised, phase 3
trials. Lancet Oncol. 16:141–151. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu YL, Cheng Y, Zhou X, Lee KH, Nakagawa
K, Niho S, Tsuji F, Linke R, Rosell R, Corral J, et al: Dacomitinib
versus gefitinib as first-line treatment for patients with
EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): A
randomised, open-label, phase 3 trial. Lancet Oncol. 18:1454–1466.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Soria JC, Ohe Y, Vansteenkiste J,
Reungwetwattana T, Chewaskulyong B, Lee KH, Dechaphunkul A, Imamura
F, Nogami N, Kurata T, et al: Osimertinib in untreated EGFR-mutated
advanced non-small-cell lung cancer. N Engl J Med. 378:113–125.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kimura H, Kasahara K, Kawaishi M, Kunitoh
H, Tamura T, Holloway B and Nishio K: Detection of epidermal growth
factor receptor mutations in serum as a predictor of the response
to gefitinib in patients with non-small-cell lung cancer. Clin
Cancer Res. 12:3915–3921. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kimura H, Ohira T, Uchida O, Matsubayashi
J, Shimizu S, Nagao T, Ikeda N and Nishio K: Analytical performance
of the cobas EGFR mutation assay for Japanese non-small lung
cancer. Lung Cancer. 83:329–333. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Therasse P, Arbuck SG, Eisenhauer EA,
Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van
Oosterom AT, Christian MC and Gwyther SG: New guidelines to
evaluate the response to treatment in solid tumors. European
Organization for Research and Treatment of Cancer, National Cancer
Institute of the United States, National Cancer Institute of
Canada. J Natl Cancer Inst. 92:205–216. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kosaka T, Yatabe Y, Endoh H, Kuwano H,
Takahashi T and Mitsudomi T: Mutations of the epidermal growth
factor receptor gene in lung cancer: Biological and clinical
implications. Cancer Res. 64:8919–8923. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tokumo M, Toyooka S, Kiura K, Shigematsu
H, Tomii K, Aoe M, Ichimura K, Tsuda T, Yano M, Tsukuda K, et al:
The relationship between epidermal growth factor receptor mutations
and clinicopathologic features in non-small cell lung cancers. Clin
Cancer Res. 11:1167–1173. 2005.PubMed/NCBI
|
|
12
|
Sonobe M, Manabe T, Wada H and Tanaka F:
Mutations in the epidermal growth factor receptor gene are linked
to smoking-independent, lung adenocarcinoma. Br J Cancer.
93:355–363. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shigematsu H, Lin L, Takahashi T, Nomura
M, Suzuki M, Wistuba II, Fong KM, Lee H, Toyooka S, Shimizu N, et
al: Clinical and biological features associated with epidermal
growth factor receptor gene mutations in lung cancers. J Natl
Cancer Inst. 97:339–346. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mitsudomi T, Kosaka T and Yatabe Y:
Biological and clinical implications of EGFR mutations in lung
cancer. Int J Clin Oncol. 11:190–198. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kobayashi Y and Mitsudomi T: Not all
epidermal growth factor receptor mutations in lung cancer are
created equal: Perspectives for individualized treatment strategy.
Cancer Sci. 107:1179–1186. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Riely GJ, Pao W, Pham D, Li AR, Rizvi N,
Venkatraman ES, Zakowski MF, Kris MG, Ladanyi M and Miller VA:
Clinical course of patients with non-small cell lung cancer and
epidermal growth factor receptor exon 19 and exon 21 mutations
treated with gefitinib or erlotinib. Clin Cancer Res. 12:839–844.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jackman DM, Yeap BY, Sequist LV, Lindeman
N, Holmes AJ, Joshi VA, Bell DW, Huberman MS, Halmos B, Rabin MS,
et al: Exon 19 deletion mutations of epidermal growth factor
receptor are associated with prolonged survival in non-small cell
lung cancer patients treated with gefitinib or erlotinib. Clin
Cancer Res. 12:3908–3914. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hirsch FR, Varella-Garcia M, Cappuzzo F,
McCoy J, Bemis L, Xavier AC, Dziadziuszko R, Gumerlock P, Chansky
K, West H, et al: Combination of EGFR gene copy number and protein
expression predicts outcome for advanced non-small-cell lung cancer
patients treated with gefitinib. Ann Oncol. 18:752–760. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lin MT, Mosier SL, Thiess M, Beierl KF,
Debeljak M, Tseng LH, Chen G, Yegnasubramanian S, Ho H, Cope L, et
al: Clinical validation of KRAS BRAF, and EGFR mutation detection
using next-generation sequencing. Am J Clin Pathol. 141:856–866.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu X, Yang Y, Li H, Chen Z, Jiang G and
Fei K: Assessment of the clinical application of detecting EGFR,
KRAS, PIK3CA and BRAF mutations in patients with non-small cell
lung cancer using next-generation sequencing. Scand J Clin Lab
Invest. 76:386–392. 2016. View Article : Google Scholar : PubMed/NCBI
|