Introduction
Breast cancer is the most frequently occurring type
of cancer with 1.7 million cases worldwide. In 2012 it was the
leading cause of cancer-associated mortality, accounting for
521,900 cases in females (1). The
identification of biomarkers for breast cancer, including estrogen
receptor (ER), progesterone receptor (PgR) and the human epidermal
receptor 2 (HER2), has enabled the prediction of patient prognosis
and the establishment of novel therapeutic agents (2). Notably, clinicopathological surrogate
definitions for molecular subtypes of breast cancer, including
luminal A-like, luminal B-like with HER2 negative, luminal B-like
with HER2 positive, HER2 positive with ER and PgR negative, and
triple-negative have been used for treatment recommendations
(3). However, other studies have
reported controversial results for the use of molecular subtyping
in the prediction of recurrence (4,5).
One of the hallmarks of cancer is angiogenesis in
conjunction with systemic and local inflammation (6). Crosstalk between angiogenesis and
inflammatory signaling pathways may also contribute to cancer
progression. Interleukin (IL)-6 and C-reactive protein (CRP) are
principal mediators and indicators of the inflammatory response.
CRP, which was named for its capacity to precipitate
C-polysaccharide of Streptococcus pneumoniae, is a sensitive and
widely used inflammatory marker produced primarily in the liver, in
response to IL-1, IL-6, tumor necrosis factor-α and IL-17 (7,8). CRP was
reported to be associated with poor prognosis in nasopharyngeal
(9), hepatocellular (10), pancreatic (11), colorectal (12), renal (13), urothelial (14) and prostate cancer (15), in addition to breast cancer (16,17).
However, the association between CRP and the prognosis of patients
with breast cancer remains controversial (18–20). The
association between serum IL-6 expression levels and breast cancer
has been reported in treatment prognosis (21–23) and
resistance to chemotherapy (24),
and other studies have reported an association between serum IL-6,
CRP and breast cancer (25,26). Although previous studies (21–23) have
investigated the association between IL-6 and prognosis in patients
with metastasis, the prognostic impact of preoperative IL-6 levels
remains to be elucidated.
Galectin-3, a β-galactoside binding lectin is one of
the most highly investigated key factors promoting angiogenesis and
inflammation in breast cancer (27–29).
Galectin-3 has been reported to induce the secretion of angiogenic
factors from blood vascular endothelial cells in vitro, including
as IL-6, vascular endothelial growth factor (VEGF), granulocyte
colony-stimulating factor (G-CSF), granulocyte macrophage
colony-stimulating factor and soluble intercellular adhesion
molecule (sICAM)-1 (30). However,
the association between circulating galectin-3 levels and the
prognosis of breast cancer has yet to be clarified. The present
study aimed to evaluate the prognostic impact of preoperative CRP
and IL-6 expression levels in patients with breast cancer, in
conjunction with angiogenic, inflammatory, immunological and
nutritional parameters.
Materials and methods
Patients
Sera from 64 female preoperative patients with
invasive breast cancer were collected between March 2011 and May
2013 at the Department of Breast Surgery, Fukushima Medical
University Hospital (Fukushima, Japan), prior to starting
treatment. Among these patients, 55 underwent curative-intent
surgery (either modified radical mastectomy or partial mastectomy
followed by irradiation); the remaining 9 patients had distant
metastasis, and were therefore excluded. Thus, 55 preoperative
patients with breast cancer (median age, 52; range, 37–83 years)
were ultimately enrolled in the present study. Of the 55 patients,
17 were preoperatively diagnosed as having T4 tumor (n=6) and/or
axillary lymph node metastasis (n=22), and subsequently received
neoadjuvant chemotherapy using fluorouracil, epirubicin and
cyclophosphamide. Following surgery, cancer stage was determined
pathologically according to the Tumor-Node-Metastasis (TNM)
classification system of malignant tumors, published in the Union
for International Cancer Control, 8th edition (31). A total of 29 patients received
adjuvant chemotherapy, including docetaxel, paclitaxel or
capecitabine, and subsequent treatment with neoadjuvant
chemotherapy (n=17) due to a postoperative diagnosis of metastasis
to the axillary lymph nodes (n=20), and/or having a triple-negative
(n=8) or HER2 (n=4) subtype. Patient characteristics are summarized
in Table I. The study protocol was
approved by the Ethics Committee of Fukushima Medical University
(approval no. 1095) and written informed consent was obtained from
the enrolled patients.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Category | Patients, n (total
55) | % |
|---|
| Pathological T
factor |
| 1a | 0 | 0.0 |
| 1b | 1 | 1.8 |
| 1c | 21 | 38.2 |
| 2 | 25 | 45.5 |
| 3 | 2 | 3.6 |
| 4a | 0 | 0.0 |
| 4b | 6 | 10.9 |
| Pathological N
factor |
| 0 | 35 | 63.6 |
| 1a | 13 | 23.6 |
| 1b | 0 | 0.0 |
| 1c | 0 | 0.0 |
| 2a | 6 | 11.0 |
| 2b | 0 | 0.0 |
| 3a | 0 | 0.0 |
| 3b | 1 | 1.8 |
| 3c | 0 | 0.0 |
|
Tumor-Node-Metastasis stage |
| IA | 17 | 31.0 |
| IB | 0 | 0.0 |
|
IIA | 18 | 32.7 |
|
IIB | 7 | 12.7 |
|
IIIA | 7 | 12.7 |
|
IIIB | 5 | 9.1 |
|
IIIC | 1 | 1.8 |
| Pathological
subtype |
|
Papillo-tubular | 18 | 32.7 |
|
Solid-tubular | 9 | 16.4 |
|
Scirrhous | 25 | 45.4 |
|
Other | 3 | 5.5 |
| Histological
grade |
| 1 | 24 | 43.6 |
| 2 | 17 | 30.9 |
| 3 | 11 | 20.0 |
| Not
graded | 3 | 5.5 |
| Molecular
subtype |
| Luminal
A-like | 19 | 34.5 |
| Luminal
B/HER- | 9 | 16.4 |
| Luminal
B/HER+ | 15 | 27.3 |
|
HER2 | 4 | 7.3 |
|
Triple-negative | 8 | 14.5 |
| Surgical
procedure |
|
Bp+SN | 19 | 34.5 |
|
Bp+AX | 8 | 14.5 |
|
Bt+SN | 11 | 20.0 |
|
Bt+AX | 17 | 31.0 |
Measurement of parameters
Using an ELISA (R&D Systems, Inc., Minneapolis,
MN, USA) according to the manufacturer's protocol, patient sera
were evaluated to determine the concentrations of galectin-3 (cat.
no. DGAL30), IL-6 (cat. no. D6050), VEGF (cat. no. DVE00), sICAM-1
(cat. no. DCD540) and G-CSF (cat. no. DCS50). The nutritional
status of each patient was using a combination of the body mass
index (BMI) and serum concentrations of total protein, albumin,
retinol binding protein (RBP), transthyretin (TTR) and transferrin
(TF). These parameters were assessed at the Central Clinical
Laboratory of Fukushima Medical University Hospital. Indicators of
inflammation, including CRP, white blood cell count, neutrophil,
lymphocyte and monocyte counts, in addition to the
neutrophil-to-lymphocyte ratio (NLR) and lymphocyte-to-monocyte
ratio (LMR), were also verified.
The expression levels of the immunological cytokines
IL-10, −12 and −17 were obtained. Peripheral blood mononuclear
cells (PBMCs) were isolated using Ficoll-Hypaque columns
(Pharmacia-Biotech, Uppsala, Sweden), and washed twice with
RPMI-1640 medium (Wako Pure Chemical Industries, Ltd., Osaka,
Japan). Isolated PBMCs were incubated at a concentration of
1×106 cells/ml in 1 ml RPMI-1640 (10% heat-inactivated
fetal calf serum; Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) for 24 h at 37°C and 5% CO2, with the following
stimuli: i) 20 µg/ml phytohemagglutinin for the IL-10 and IL-17
production assays; and ii) 0.01% Staphylococcus aureus Cowan strain
1 for the IL-12 assays. The supernatant was aliquoted and stored at
−80°C until use. Supernatant samples were subsequently thawed and
used to determine the concentrations of IL-10, IL-12, and IL-17
using an ELISA. Following thawing, samples were used only once, and
not all blood samples were of sufficient volume for all
measurements.
Statistical analysis
All statistical calculations were performed using
SPSS® v.24 (IBM Corp., Armonk, NY, USA). Data are
presented as frequencies or percentages for categorical variables,
and means ± standard deviation for continuous variables, unless
otherwise indicated. For categorical clinical variables, the
differences between two groups were evaluated using the
χ2 or the Fisher's exact test. The differences between
continuous data were analyzed using the Mann-Whitney U test.
With regard to survival analysis, the mean
observation period was 69.6 months (range, 55.3–81.4). The final
assessment of disease status was made on December 12, 2017.
Receiver operating characteristic (ROC) analysis was used to
evaluate the prognostic value of the selected parameters. Overall
survival (OS) and recurrence-free survival (RFS) rates were
determined using the Kaplan-Meier method, and the differences
between the groups were assessed using the log-rank test.
Prognostic factor candidates were subjected to univariate and
multivariate analysis using a Cox proportional hazard model to
identify independent predictors of prognosis; when P<0.1 for
univariate analysis, the candidate was also analyzed using
multivariate analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
ROC curve analysis
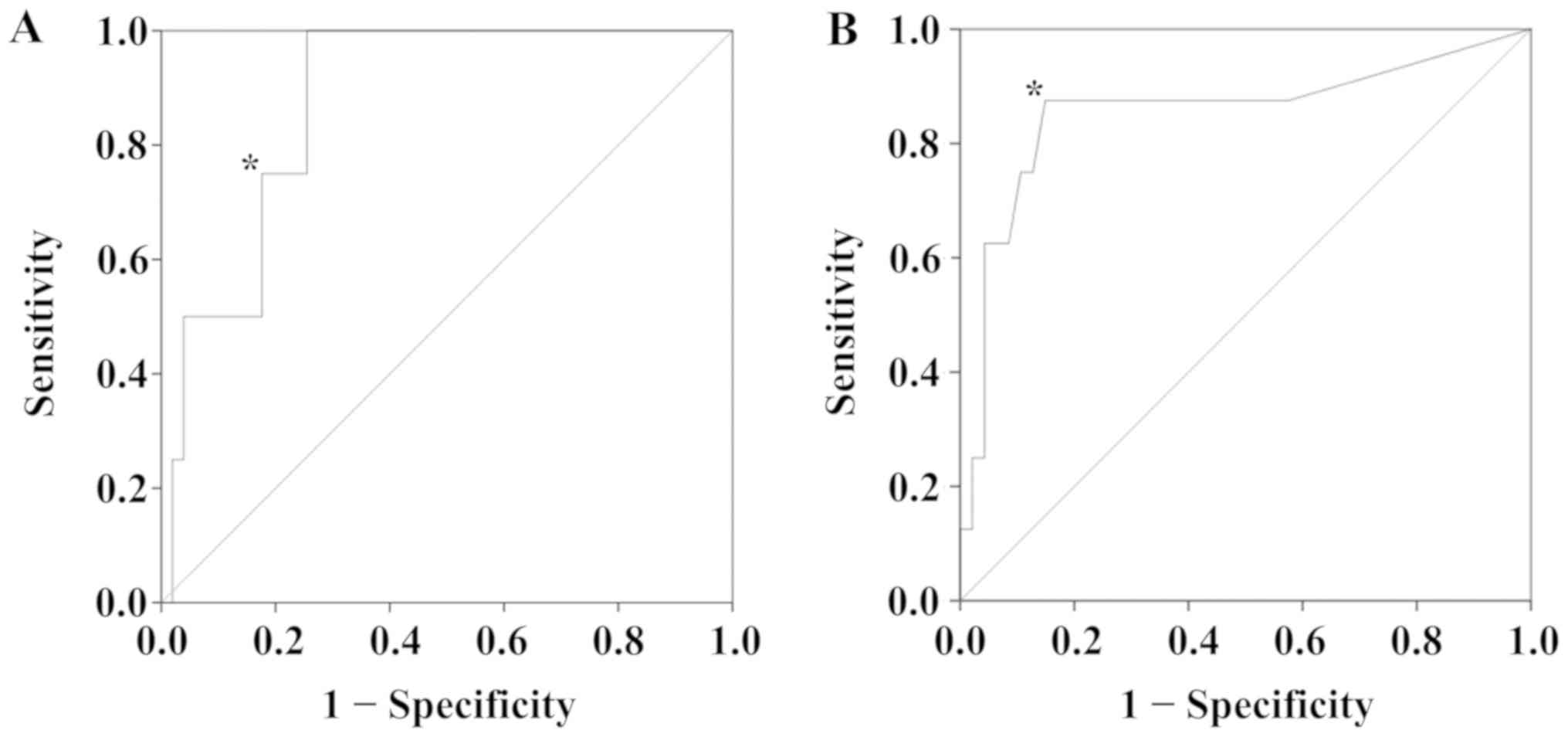
Using ROC curve analysis, the serum expression level
of IL-6 was determined to be a biomarker for the prediction of OS
(P=0.013), with a cutoff value of 10.0 pg/ml (Fig. 1A), a sensitivity of 0.750 and a
specificity of 0.824. Additionally, the serum expression level of
CRP was evaluated as a biomarker to predict RFS (P=0.001), with a
cutoff threshold of 0.12 mg/dl (Fig.
1B). At this cutoff value, the sensitivity was 0.875 and the
specificity was 0.851.
Associations between serum expression
levels of IL-6 and CRP, and patient characteristics
Table II displays
patient characteristics according to serum IL-6 or CRP levels.
There were no statistically significant differences in pathological
T factor, pathological N factor, TNM stage, administration of
neoadjuvant or adjuvant chemotherapy, ER, PgR, or HER2 expression
levels, lymphatic invasion or microscopic vascular invasion
associated with IL-6 and CRP expression levels between groups.
 | Table II.Patient characteristics according to
IL-6 and CRP expression levels. |
Table II.
Patient characteristics according to
IL-6 and CRP expression levels.
| Category | IL-6 <10.0 pg/ml
N=43 | IL-6 ≥10.0 pg/ml
N=12 | P-value | CRP <0.12 pg/dl
N=41 | CRP ≥0.12 pg/dl
N=14 | P-value |
|---|
| pT |
|
| 1.000 |
|
| 0.678 |
|
1+2 | 36 | 10 |
| 35 | 11 |
|
|
3+4 | 7 | 2 |
| 6 | 3 |
|
| pN |
|
| 0.319 |
|
| 0.755 |
| 0 | 29 | 6 |
| 26 | 8 |
|
| ≥1 | 14 | 6 |
| 15 | 6 |
|
| Stage |
|
| 0.477 |
|
| 0.736 |
|
I+II | 33 | 8 |
| 31 | 10 |
|
|
III+IV | 10 | 4 |
| 10 | 4 |
|
| Neoadjuvant
chemotherapy |
|
| 0.735 |
|
| 0.742 |
| − | 29 | 9 |
| 29 | 9 |
|
| + | 14 | 3 |
| 12 | 5 |
|
| Adjuvant
chemotherapy |
|
| 1.000 |
|
| 0.130 |
| − | 20 | 6 |
| 22 | 4 |
|
| + | 23 | 6 |
| 19 | 10 |
|
| Estrogen
receptor |
|
| 0.689 |
|
| 0.477 |
| − | 8 | 3 |
| 8 | 4 |
|
| + | 35 | 9 |
| 33 | 10 |
|
| Progesterone
receptor |
|
| 0.336 |
|
| 1.000 |
| − | 18 | 3 |
| 16 | 5 |
|
| + | 25 | 9 |
| 25 | 9 |
|
| Human epidermal
receptor 2 |
|
| 0.183 |
|
| 1.000 |
| − | 26 | 10 |
| 27 | 9 |
|
| + | 17 | 2 |
| 14 | 5 |
|
| Lymphatic vessel
invasion |
|
| 0.320 |
|
| 0.346 |
| − | 24 | 4 |
| 23 | 5 |
|
| + | 19 | 7 |
| 18 | 8 |
|
| Vascular
invasion |
|
| 1.000 |
|
| 1.000 |
| − | 27 | 7 |
| 26 | 8 |
|
| + | 16 | 4 |
| 15 | 5 |
|
Serum VEGF levels in patients with CRP ≥0.12 mg/dl
(median, 472.0 ng/ml; range, 170.0–1,100.0 ng/ml) were
significantly higher compared with those in patients with CRP
<0.12 mg/dl (median, 220.0 ng/ml; range, 74.0–1,460.7 ng/ml)
(P=0.011). The Ki-67 labeling index of patients with CRP ≥0.12
mg/dl (median, 35.3%; range, 3.1–80.0%) was significantly higher
compared with that in patients with CRP <0.12 mg/dl (median,
16.4%; range, 1.0–78.2%) (P=0.035). The BMI of patients with CRP
≥0.12 mg/dl (median, 24.2 kg/m2; range, 16.4–29.6
kg/m2) was significantly higher compared with that in
patients with CRP <0.12 mg/dl (median, 21.5 kg/m2;
range, 20.8–40.3 kg/m2) (P=0.001; Fig. 2). When patients were categorized
according to IL-6 expression level (cutoff, 10.0 pg/ml), there were
no statistically significant differences among parameters. Serum
expression levels of galectin-3, sICAM-1, RBP, TTR, TF, NLR and
LMR, and the production of IL-12 and IL-17, were not significantly
associated with the expression of IL-6 or CRP.
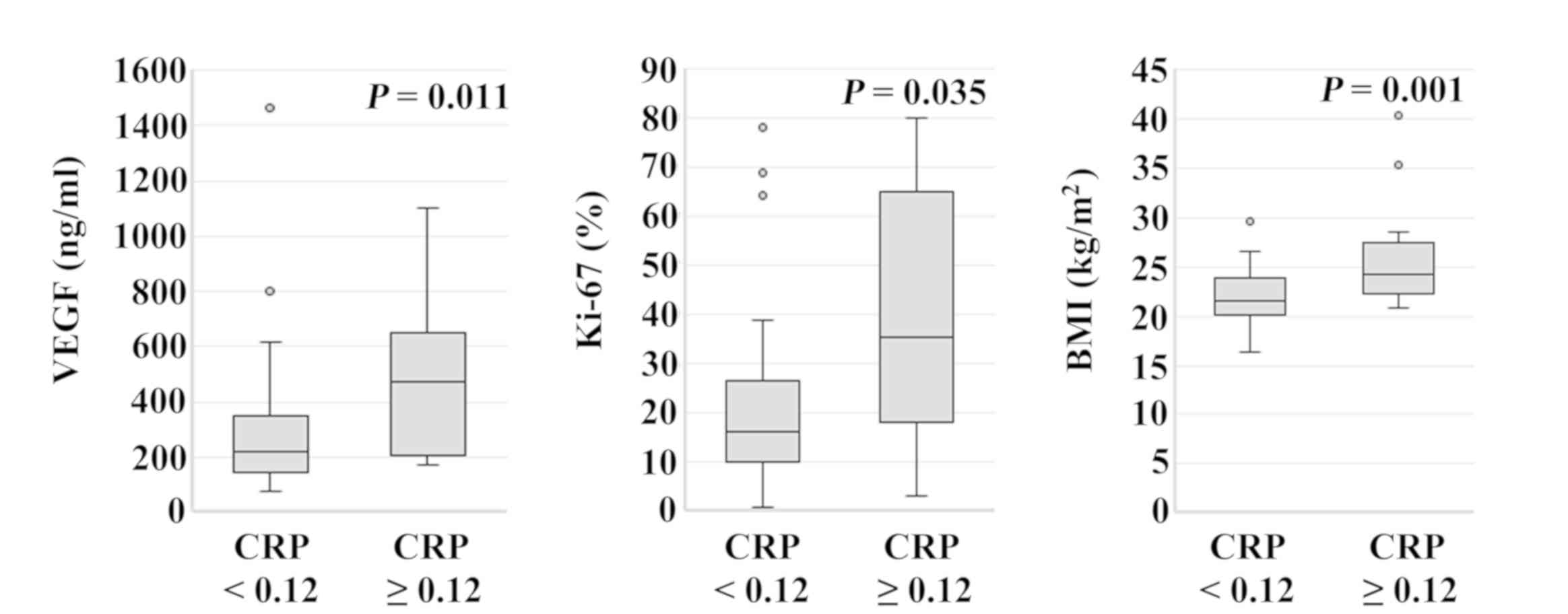 | Figure 2.Association between CRP expression
level and patient parameters. Serum VEGF levels in patients with
CRP ≥0.12 mg/dl (median, 472.0 ng/ml; range, 170.0–1,100.0 ng/ml)
were significantly higher compared with those in patients with CRP
<0.12 mg/dl (median, 220.0 ng/ml; range, 74.0–1,460.7 ng/ml)
(P=0.011). Ki-67 labeling index in patients with CRP ≥0.12 mg/dl
(median, 35.3%; range, 3.1–80.0%) was significantly higher compared
with that of patients with CRP <0.12 mg/dl (median, 16.4%;
range, 1.0–78.2%) (P=0.035). BMI of patients with CRP ≥0.12 mg/dl
(median, 24.2 kg/m2; range, 16.4–29.6 kg/m2)
was significantly higher compared with that of patients with CRP
<0.12 mg/dl (median, 21.5 kg/m2; range, 20.8–40.3
kg/m2) (P=0.001). CRP, C-reactive protein; VEGF,
vascular endothelial growth factor; BMI, body mass index. |
OS and RFS
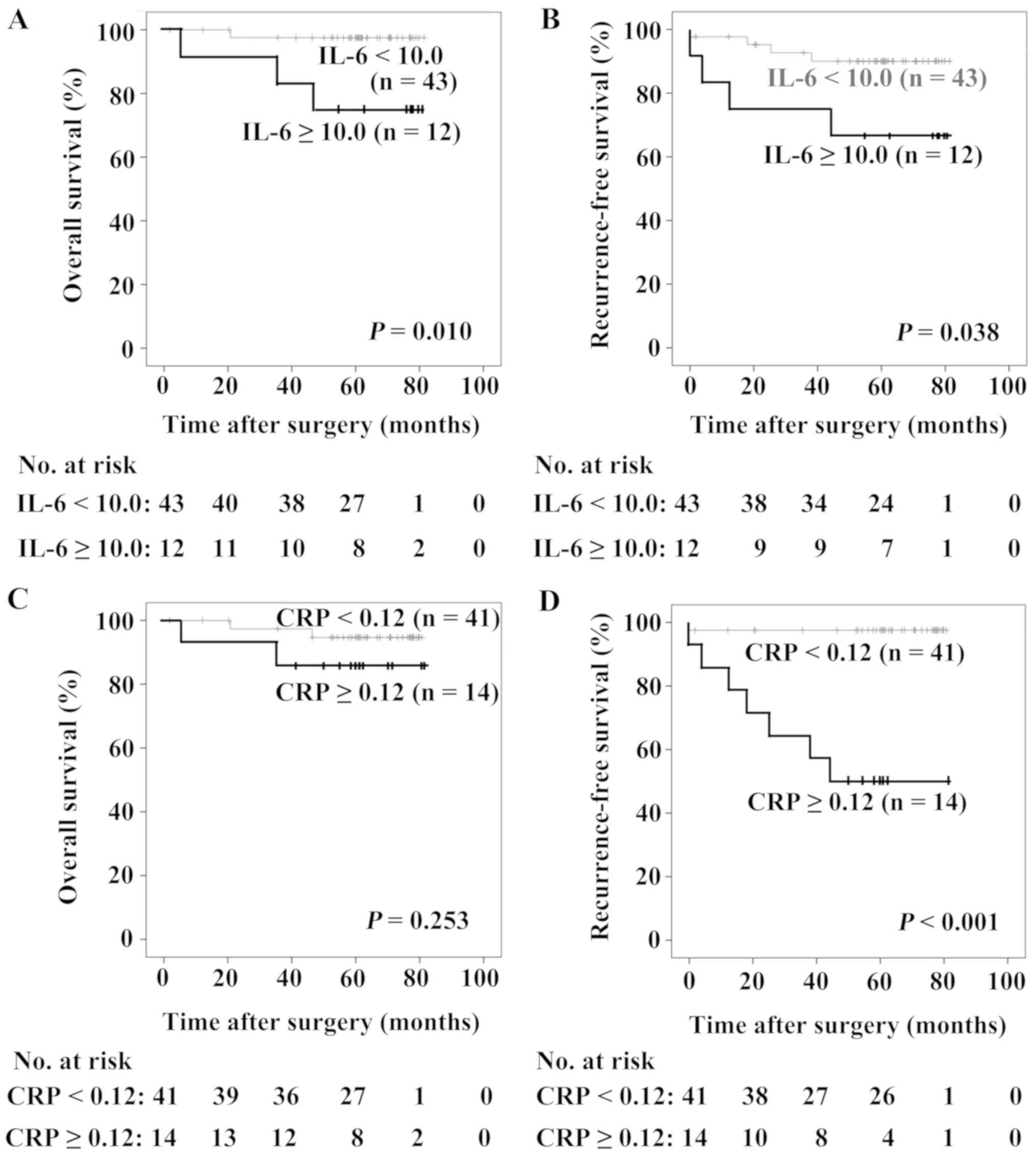
As revealed in Fig. 3A
and B, patients with IL-6 ≥10.0 pg/ml possessed poorer OS and
RFS compared with those with IL-6 <10.0 pg/ml (P=0.010 and
P=0.038, respectively). There were no statistically significant
differences in OS between patients with different levels of CRP
(P=0.253; Fig. 3C), whereas patients
with CRP ≥0.12 mg/dl exhibited a poorer RFS compared with those
with CRP <0.12 mg/dl (P<0.001; Fig. 3D). Differences in the serum
expression levels of galectin-3, VEGF, sICAM-1 and G-CSF did not
significantly affect the prognosis of patients with breast
cancer.
Cox proportional hazards model
The following prognostic factors were analyzed using
a Cox proportional hazards model: pathological T factor (T1 + T2
vs. T3 + T4), pathological N factor (N0 vs. ≥ N1), TNM stage (I +II
vs. III + IV), triple-negative subtype (no vs. yes), CRP (<0.12
mg/dl vs. ≥0.12 mg/dl) and serum IL-6 expression level (<10.0
pg/ml vs. ≥10.0 pg/ml). Triple-negative subtype (hazard ratio,
8.795; 95% confidence interval, 1.230–62.875; P=0.030) and serum
IL-6 expression level (hazard ratio, 10.785; 95% confidence
interval, 1.122–103.693; P=0.039) were significantly associated
following univariate analysis. Triple-negative subtype (hazard
ratio, 11.739; 95% confidence interval, 1.415–97.362; P=0.023) and
serum IL-6 expression level were independent prognostic factors for
the OS of patients with breast cancer. With regard to RFS, the
pathological N factor (hazard ratio, 5.598; 95% confidence
interval, 1.129–27.748; P=0.035) and CRP level (hazard ratio,
23.865; 95% confidence interval, 2.930–194.408; P=0.003) showed
statistical significance following univariate analysis, and the CRP
expression level was an independent prognostic factor for RFS in
patients with breast cancer (hazard ratio, 18.571; 95% confidence
interval, 2.240–153.949; P=0.007; Table III).
 | Table III.Cox proportional hazards model. |
Table III.
Cox proportional hazards model.
| A, Overall
survival |
|---|
|
|---|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Category | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| T | 5.121 | 0.721–36.369 | 0.102 | – | – | – |
| N | 1.729 | 0.243–12.278 | 0.584 | – | – | – |
| TNM stage | 2.886 | 0.406–20.485 | 0.289 | – | – | – |
| HG | 7.198 | 0.652–79.503 | 0.107 | – | – | – |
| TN | 8.795 | 1.230–62.875 | 0.030 | 11.739 |
1.415–97.362 | 0.023 |
| IL-6 | 10.785 | 1.122–103.693 | 0.039 | 13.230 |
1.285–136.214 | 0.030 |
| CRP | 2.970 | 0.41–21.100 | 0.277 | – | – | – |
|
| B,
Recurrence-free survival |
|
|
| Univariate
analysis | Multivariate
analysis |
|
|
|
|
|
Category | HR | 95% CI | P-value | HR | 95% CI | P-value |
|
| T | 3.355 | 0.801–14.053 | 0.098 | 2.499 | 0.583–10.711 | 0.218 |
| N | 5.598 | 1.129–27.748 | 0.035 | 3.429 | 0.677–17.364 | 0.136 |
| TNM stage | 0.993 | 0.200–4.920 | 0.993 | – | – | – |
| HG | 3.172 | 0.708–14.206 | 0.131 | – | – | – |
| TN | 2.702 | 0.543–13.456 | 0.225 | – | – | – |
| IL-6 | 3.637 | 0.733–18.040 | 0.114 | – | – | – |
| CRP | 23.865 | 2.930–194.408 | 0.003 | 18.571 | 2.240–153.949 | 0.007 |
Discussion
The prognostic impact of preoperative serum IL-6
expression level has been reported previously, but based only on
the results of Kaplan-Meier analysis (26). Furthermore, the prognostic impact of
serum IL-6 expression level has only been observed in patients with
metastatic disease (21–23). Although the prognostic impact of
preoperative CRP has been reported (16,17),
other studies have described controversial results (18–20).
Thus, the present study aimed to evaluate the prognostic impact of
preoperative CRP and IL-6 expression levels in patients with breast
cancer. It was revealed that preoperative expression levels of IL-6
and CRP effected OS and RFS, respectively.
IL-6 is secreted by various cell types, including
breast cancer cells. The response to IL-6 in such cells depends on
the expression of ERs (24). The
ER-positive MCF-7 cell line does not secrete IL-6, whereas
ER-negative MD-MBA-231 cells do (32,33).
However, Fontanini et al (34) reported that expression levels of IL-6
correlated with ER expression. Ravishankaran et al (25) reported that serum IL-6 expression
levels correlated with the extent of tumor invasion, lymph node
metastasis, distant metastasis and TNM staging. In the present
study, IL-6 did not correlate with such tumor statuses.
In the present study, CRP expression level
correlated with VEGF expression, Ki-67 labeling index and BMI.
Expression levels of VEGF in breast cancer have been reported to
correlate with poorer RFS (35).
Although serum VEGF expression level was reportedly increased in
patients with breast cancer, compared with healthy controls
(36), numerous studies have
suggested that it has no prognostic value (37,38).
Highly proliferative tumors, including triple-negative breast
cancer, show enhanced angiogenesis and reportedly exhibit high
levels of VEGF expression (39).
Additionally, the Ki-67 labeling index has been associated with OS
in breast cancer (40). In the
present study, serum expression levels of VEGF and the Ki-67
labeling index had no significant association with OS or RFS;
however, a significant correlation with higher CRP expression
indicates that they may contribute to poor RFS. Furthermore,
increased risk of breast cancer was associated with increased body
weight and obesity in women, particularly in postmenopausal
patients, and a significant link was observed between CRP
expression levels and BMI (41). The
suggested association between elevated CRP expression level, VEGF,
Ki-67 and BMI warrants further investigation.
Limitations of the present study include its
relatively small patient cohort and short observational period.
Also, lifestyle factors that may influence IL-6 and/or CRP
expression levels, including smoking, menopausal status, and other
comorbidities (including diabetes and cardiovascular disease), were
not taken into account.
For ROC curve analysis, 10.0 pg/m IL-6 l and 0.12
mg/dl CRP were determined as threshold values to predict OS and
RFS, respectively. Patients with IL-6 ≥10.0 pg/ml exhibited poorer
OS compared with those with IL-6 <10.0 pg/ml, and patients with
CRP ≥0.12 mg/dl exhibited poorer RFS compared with those with CRP
<0.12 mg/dl. In patients with breast cancer, serum IL-6 and CRP
expression levels were independent prognostic factors for OS and
RFS, respectively. Specifically, CRP is indicated to be a superior
prognostic marker compared with established prognostic factors,
including T factor, N factor and histological grade, supporting a
possible association between inflammation and recurrence in breast
cancer.
In conclusion, in patients with invasive breast
cancer, preoperative serum IL-6 expression levels and
triple-negative subtype may be independent prognostic factors for
OS, while for RFS, preoperative CRP expression level may be a more
accurate prognostic factor compared with those already established.
Particular attention should be paid to patients with higher
preoperative IL-6 and/or CRP expression levels during preoperative
follow-up for breast cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets generated from the present study are
available from the corresponding author upon reasonable
request.
Authors' contribution
TS, MS and TO contributed to the conception, design,
and integrity of this study. KG, YM, MN, KT and NA performed data
acquisition, analysis, and interpretation. TS and MS drafted and
critically revised the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Fukushima Medical University (approval no. 1095) and
written informed consent was obtained from all enrolled patients.
Additionally, all patient data were treated in accordance with the
local privacy regulations.
Patient consent for publication
All patients provided written informed consent.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ER
|
estrogen receptor
|
|
PgR
|
progesterone receptor
|
|
HER2
|
the human epidermal receptor 2
|
|
IL
|
interleukin
|
|
CRP
|
C-reactive protein
|
|
VEGF
|
vascular endothelial growth factor
|
|
G-CSF
|
granulocyte colony-stimulating
factor
|
|
sICAM
|
soluble intercellular adhesion
molecule
|
|
BMI
|
body mass index
|
|
RBP
|
retinol binding protein
|
|
TTR
|
transthyretin
|
|
TF
|
transferrin
|
|
NLR
|
neutrophil-to-lymphocyte ratio
|
|
LMR
|
lymphocyte-to-monocyte ratio
|
|
PBMCs
|
Peripheral blood mononuclear cells
|
|
ROC
|
receiver operating characteristic
|
|
OS
|
overall survival
|
|
RFS
|
recurrence-free survival
|
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kwa M, Makris A and Esteva FJ: Clinical
utility of gene-expression signatures in early stage breast cancer.
Nat Rev Clin Oncol. 14:595–610. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Goldhirsch A, Winer EP, Coates AS, Gelber
RD, Piccart-Gebhart M, Thürlimann B, Senn HJ, Albain KS, André F,
Bergh J, et al Panel members, : Personalizing the treatment of
women with early breast cancer: Highlights of the St Gallen
International Expert Consensus on the Primary Therapy of Early
Breast Cancer 2013. Ann Oncol. 24:2206–2223. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sanpaolo P, Barbieri V and Genovesi D:
Prognostic value of breast cancer subtypes on breast cancer
specific survival, distant metastases and local relapse rates in
conservatively managed early stage breast cancer: A retrospective
clinical study. Eur J Surg Oncol. 37:876–882. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Millar EK, Graham PH, O'Toole SA, McNeil
CM, Browne L, Morey AL, Eggleton S, Beretov J, Theocharous C, Capp
A, et al: Prediction of local recurrence, distant metastases, and
death after breast-conserving therapy in early-stage invasive
breast cancer using a five-biomarker panel. J Clin Oncol.
27:4701–4708. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tillett WS and Francis T: Serological
reactions in pneumonia with a non-protein somatic fraction of
pneumococcus. J Exp Med. 52:561–571. 1930. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Eklund CM: Proinflammatory cytokines in
CRP baseline regulation. Adv Clin Chem. 48:111–136. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fang Y, Xu C, Wu P, Zhang LH, Li DW, Sun
JH, Li WF and Liao ZS: Prognostic role of C-reactive protein in
patients with nasopharyngeal carcinoma: A meta-analysis and
literature review. Medicine (Baltimore). 96:e84632017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zheng Z, Zhou L, Gao S, Yang Z, Yao J and
Zheng S: Prognostic role of C-reactive protein in hepatocellular
carcinoma: A systematic review and meta-analysis. Int J Med Sci.
10:653–664. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stevens L, Pathak S, Nunes QM,
Pandanaboyana S, Macutkiewicz C, Smart N and Smith AM: Prognostic
significance of pre-operative C-reactive protein and the
neutrophil-lymphocyte ratio in resectable pancreatic cancer: A
systematic review. HPB (Oxford). 17:285–291. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Woo HD, Kim K and Kim J: Association
between preoperative C-reactive protein level and colorectal cancer
survival: A meta-analysis. Cancer Causes Control. 26:1661–1670.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hu Q, Gou Y, Sun C, Ding W, Xu K, Gu B,
Xia G and Ding Q: The prognostic value of C-reactive protein in
renal cell carcinoma: A systematic review and meta-analysis. Urol
Oncol. 32:50.e1–50.e8. 2014. View Article : Google Scholar
|
|
14
|
Luo Y, Fu SJ, She DL, Xiong HU and Yang
LI: Preoperative C-reactive protein as a prognostic predictor for
upper tract urothelial carcinoma: A systematic review and
meta-analysis. Mol Clin Oncol. 3:924–928. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu ZQ, Chu L, Fang JM, Zhang X, Zhao HX,
Chen YJ and Xu Q: Prognostic role of C-reactive protein in prostate
cancer: A systematic review and meta-analysis. Asian J Androl.
16:467–471. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Allin KH, Nordestgaard BG, Flyger H and
Bojesen SE: Elevated pre-treatment levels of plasma C-reactive
protein are associated with poor prognosis after breast cancer: A
cohort study. Breast Cancer Res. 13:R552011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sicking I, Edlund K, Wesbuer E, Weyer V,
Battista MJ, Lebrecht A, Solbach C, Grinberg M, Lotz J, Hoffmann G,
et al: Prognostic influence of pre-operative C-reactive protein in
node-negative breast cancer patients. PLoS One. 9:e1113062014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Frydenberg H, Thune I, Lofterød T,
Mortensen ES, Eggen AE, Risberg T, Wist EA, Flote VG, Furberg AS,
Wilsgaard T, et al: Pre-diagnostic high-sensitive C-reactive
protein and breast cancer risk, recurrence, and survival. Breast
Cancer Res Treat. 155:345–354. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Al Murri AM, Wilson C, Lannigan A, Doughty
JC, Angerson WJ, McArdle CS and McMillan DC: Evaluation of the
relationship between the systemic inflammatory response and
cancer-specific survival in patients with primary operable breast
cancer. Br J Cancer. 96:891–895. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tibau A, Ennis M and Goodwin PJ:
Post-surgical highly sensitive C-reactive protein and prognosis in
early-stage breast cancer. Breast Cancer Res Treat. 141:485–493.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Salgado R, Junius S, Benoy I, Van Dam P,
Vermeulen P, Van Marck E, Huget P and Dirix LY; Salgado R1, :
Junius S, Benoy I, Van Dam P, Vermeulen P, Van Marck E, Huget P and
Dirix LY: Circulating interleukin-6 predicts survival in patients
with metastatic breast cancer. Int J Cancer. 103:642–646. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Knüpfer H and Preiss R: Significance of
interleukin-6 (IL-6) in breast cancer (Review). Breast Cancer Res
Treat. 102:129–135. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dethlefsen C, Højfeldt G and Hojman P: The
role of intratumoral and systemic IL-6 in breast cancer. Breast
Cancer Res Treat. 138:657–664. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Al-Youzbaki WB, Al-Youzbaki NB and Telfah
MM: Tissue polypeptide antigen & interleukin-6: Are their serum
levels a predictor for response to chemotherapy in breast cancer?
Pak J Med Sci. 30:1108–1112. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ravishankaran P and Karunanithi R:
Clinical significance of preoperative serum interleukin-6 and
C-reactive protein level in breast cancer patients. World J Surg
Oncol. 9:182011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Babaei Z, Moslemi D, Parsian H, Khafri S,
Pouramir M and Mosapour A: Relationship of obesity with serum
concentrations of leptin, CRP and IL-6 in breast cancer survivors.
J Egypt Natl Canc Inst. 27:223–229. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Honjo Y, Nangia-Makker P, Inohara H and
Raz A: Down-regulation of galectin-3 suppresses tumorigenicity of
human breast carcinoma cells. Clin Cancer Res. 7:661–668.
2001.PubMed/NCBI
|
|
28
|
Song YK, Billiar TR and Lee YJ: Role of
galectin-3 in breast cancer metastasis: Involvement of nitric
oxide. Am J Pathol. 160:1069–1075. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nangia-Makker P, Wang Y, Raz T, Tait L,
Balan V, Hogan V and Raz A: Cleavage of galectin-3 by matrix
metalloproteases induces angiogenesis in breast cancer. Int J
Cancer. 127:2530–2541. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen C, Duckworth CA, Zhao Q, Pritchard
DM, Rhodes JM and Yu LG: Increased circulation of galectin-3 in
cancer induces secretion of metastasis-promoting cytokines from
blood vascular endothelium. Clin Cancer Res. 19:1693–1704. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Van Eckyen E: Breast tumours. TNM
classification of malignant tumours. 8th. Brieley JD, Gospodarowicz
MK and Wittekind C: John Wiley and Sons, Ltd.; West Sussex: pp.
151–158. 2017
|
|
32
|
Faggioli L, Costanzo C, Merola M,
Bianchini E, Furia A, Carsana A and Palmieri M: Nuclear factor
kappa B (NF-kappa B), nuclear factor interleukin-6 (NFIL-6 or C/EBP
beta) and nuclear factor interleukin-6 beta (NFIL6-beta or C/EBP
delta) are not sufficient to activate the endogenous interleukin-6
gene in the human breast carcinoma cell line MCF-7. Comparative
analysis with MDA-MB-231 cells, an interleukin-6-expressing human
breast carcinoma cell line. Eur J Biochem. 239:624–631. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Robinson EK, Sneige N and Grimm EA:
Correlation of interleukin 6 with interleukin 1alpha in human
mammary tumours, but not with oestrogen receptor expression.
Cytokine. 10:970–976. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fontanini G, Campani D, Roncella M,
Cecchetti D, Calvo S, Toniolo A and Basolo F: Expression of
interleukin 6 (IL-6) correlates with oestrogen receptor in human
breast carcinoma. Br J Cancer. 80:579–584. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Toi M, Inada K, Suzuki H and Tominaga T:
Tumor angiogenesis in breast cancer: Its importance as a prognostic
indicator and the association with vascular endothelial growth
factor expression. Breast Cancer Res Treat. 36:193–204. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Heer K, Kumar H, Read JR, Fox JN, Monson
JR and Kerin MJ: Serum vascular endothelial growth factor in breast
cancer: Its relation with cancer type and estrogen receptor status.
Clin Cancer Res. 7:3491–3494. 2001.PubMed/NCBI
|
|
37
|
Byrne GJ, McDowell G, Agarawal R, Sinha G,
Kumar S and Bundred NJ: Serum vascular endothelial growth factor in
breast cancer. Anticancer Res 27 (5B). 3481–3487. 2007.
|
|
38
|
Hodorowicz-Zaniewska D, Kibil W, Małek A,
Szpor J, Kulig J and Sztefko K: Evaluation of serum concentrations
of vascular endothelial growth factor (VEGF) in breast cancer
patients. Pol J Pathol. 63:255–260. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Greenberg S and Rugo HS: Triple-negative
breast cancer: Role of antiangiogenic agents. Cancer J. 16:33–38.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Elston CW and Ellis IO: Pathological
prognostic factors in breast cancer. I. The value of histological
grade in breast cancer: Experience from a large study with
long-term follow-up. Histopathology. 19:403–410. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Dossus L, Jimenez-Corona A, Romieu I,
Boutron-Ruault MC, Boutten A, Dupré T, Fagherazzi G,
Clavel-Chapelon F and Mesrine S: C-reactive protein and
postmenopausal breast cancer risk: Results from the E3N cohort
study. Cancer Causes Control. 25:533–539. 2014. View Article : Google Scholar : PubMed/NCBI
|