Introduction
Philadelphia chromosome-positive acute lymphoblastic
leukemia (Ph+ ALL) has the well-known mutation t(9;22)(q34q11) that
causes the fusion of BCR and ABL1 genes (1). Ph+ frequency is less than 5% in ALL
pediatric patients; although in this subgroup, the BCR-ABL oncogene
encodes to a particular 190 kDa protein (p190) with deregulated
tyrosine kinase activity that confers resistance to drugs and poor
prognosis (2). BCR-ABL participates
in leukemia initiation and progression by interacting with several
signaling pathways; one of the most relevant is NF-κB activation
(3). NF-κB is a transcription factor
which regulates diverse genes involved in cell transformation,
proliferation, inflammation, angiogenesis, invasiveness, and
survival (4). NF-κB activation
dependent of BCR-ABL signaling is modulated by CK2; this
interaction enhances NF-κB nuclear translocation and
transactivation, which modifies gene expression (5,6).
Vincristine, daunorubicin, and imatinib are some of
the essential chemotherapy drugs for Ph+ ALL patients (7). Vincristine is a cell cycle specific
drug that binds to tubulin, causing depolymerization of
microtubules resulting in metaphase arrest and apoptosis induction
(8). Daunorubicin is an
anthracycline that inhibits topoisomerase II, inducing DNA damage
and apoptosis (9). Imatinib is an
essential drug used in Ph+ leukemias as target therapy, due to its
inhibitory action of BCR-ABL signaling. A significant improvement
in patient's outcome has been reported when standard chemotherapy
is combined with imatinib (10).
Despite the advances in treatment, standard chemotherapy
effectiveness remains limited; the cure rate in Ph+ ALL pediatric
patients using chemotherapy alone is only 30%, most of them
commonly showed a reduced response and frequently relapse due to
adverse reactions, toxicity or drug resistance development
(11). Consequently, an adequate
therapy where standard drugs are combined with multitarget
compounds which develop minimal toxicity is of particular interest
to improve treatment effectiveness. Curcumin (diferuloylmethane) is
a therapeutic component of turmeric, which is obtained from the
rhizome Curcuma longa; it has been used widely by some Asian
countries in cooking and traditional medicine (12). Curcumin induces apoptosis, inhibits
cellular transformation, proliferation, and invasion in a variety
of cancer models in vitro and in vivo (13). The study of curcumin has become
relevant due to its antitumoral potential (14). In vitro experiments have shown low
toxicity of curcumin in normal cells (15). Furthermore, no adverse effects have
been observed in clinical trials (16). Recent research had reported the
anti-tumoral properties of curcumin due to the inhibition of
proteins kinases and transcription factors, such as NF-κB (17). Besides being involved in the tumoral
process, NF-κB downstream pathways also promote resistance to
chemotherapy drugs, which reduces treatment effectiveness (18). Different studies showed that curcumin
modulates the expression of several NF-κB regulated genes
associated with tumoral processes, leading to a subsequent
suppression of pro-tumoral pathways (19). According to the previous data,
curcumin is capable to improve treatment efficacy. However, in
human Ph+ ALL models, the therapeutic effect of curcumin and the
molecular pathways involved in tumoral suppression remain
unclear.
Due to the effect of curcumin in multiple protein
kinases, we speculate that it can modulate BCR-ABL associated
pathways, conducting to an increment of treatment efficiency. The
purpose of this study was to evaluate the effect of vincristine,
imatinib, and daunorubicin in combination with curcumin in the
human OP-1 cell line. We determined the effect of the drugs alone
and in combination with curcumin on cell viability, apoptosis
degree, NF-κB activation, and gene expression.
Materials and methods
Reagents
Curcumin was acquired from Sigma-Aldrich (St. Louis,
MO., USA). Vincristine, imatinib, and daunorubicin were provided by
PiSa Farmacéutica (Guadalajara, México). Curcumin was dissolved in
DMSO at a concentration of 10 µM and stored at −20°C.
Chemotherapeutic drugs were dissolved in distilled water at a stock
concentration, aliquoted and stored at −20°C. Each stock solution
was diluted in culture medium to the final concentration before
use.
Cell culture and treatment
OP-1 human cell line (RRID: CVCL_DG77) was gently
provided by St. Jude Children's Research Hospital (Memphis, TN,
USA), it was cultured in RPMI 1640 medium supplemented with 2 mM
Glutamine, 100 U/ml penicillin-100 g/ml streptomycin and 15% FBS
(Thermo Fisher Scientific, Inc., Waltham, MA, USA) in a humidified
incubator at 37°C with an atmosphere of 5% CO2. Afterward, 1×106
OP-1 cells were treated at 12, 24 and 48 h with chemotherapy drugs
alone and in combination with curcumin at concentration ranges of;
curcumin (5–30 µM), vincristine (0.5–4 µg/ml), imatinib (0.5–5 µM),
and daunorubicin (0.2–3 µg/ml), DMSO was used as the vehicle. Each
evaluation was performed in triplicate, and the quality criteria
for the experiments validation included coefficients of variation
smaller than 10%.
Viability assay
To determine the cytotoxic effect of curcumin and
chemotherapy drugs combination 7 ADD assay (Beckman Coulter, Inc.,
Brea, CA, USA) was performed. After the exposure to experimental
treatments, cells were washed with PBS, centrifuged at 100 × g for
1 min and resuspended in PBS at a concentration of 1×106 cells/ml.
Next, 100 µl of the solution was transferred to cytometry tubes
and, 15 µl of anti-CD45/anti-CD19 and 100 µl of 7-ADD were added to
each tube, homogenized and incubated for 20 min at room temperature
in the dark. Subsequently, cells were resuspended in PBS. Briefly,
20,000 events were acquired in Gallios 10 Flow Cytometer (Beckman
Coulter, Inc.) and analyzed in Flowing Software (Cell Imaging Core,
FIN). Cells with 7ADD positive stain were considered non-viable,
while live viable cells did not show 7ADD stain. Unstained cells
were used as assay controls to exclude cell debris and
auto-fluorescence. The IC50 of each drug was obtained from the
dose-response curve using Compusyn software 1.0 (Combosyn Inc.,
Paramus, NJ, USA).
Apoptosis assay
To evaluate apoptosis, FITC Annexin V/Propidium
Iodide Apoptosis Detection Kit (Pharmigen BD, Biosciences, San Jose
CA, USA) was used. After experimental treatments exposure, cells
were washed with cold PBS and resuspended in 1X Binding Buffer at a
concentration of 1×106 cells/ml. 100 µl of the solution was
transferred to cytometry tubes and, 5 µl of Annexin V FITC and 5 µl
of Propidium Iodide (PI) were added, 15 µl anti-CD45/anti-CD19 were
also used to select cell population, briefly, cells were
homogenized with vortex, and incubated at room temperature for 15
min in the dark. Then 400 µl of 1X Binding Buffer was added. 20,000
cells were analyzed in Gallios 10 flow cytometer (Beckman Coulter,
Inc) and data were interpreted in Flowing Software (Cell Imaging
Core, Turku, Finland). The assay was evaluated according to the
next criteria: Annexin V(−)/PI(−)=live-viable cells; Annexin
V(+)/PI(−)=early apoptosis stage; Annexin V(+)/PI(+)=late apoptosis
stage; Annexin V(−)/PI(+)=necrotic cells. Untreated cells
stimulated with 1 mM Camptotepcin (5 h, 37°C) were used as positive
controls; untreated and unstimulated cells were used as negative
controls; unstained cells, unstained treated with curcumin cells,
stained cells with Annexin V alone and stained with PI alone were
used as compensation controls.
NF-κB detection
To determine the activation/transactivation
potential of NF-κB after treatment; phosphorylated p65 subunit was
analyzed using the Phospho-Epitopes Exposure kit (Beckman Coulter,
Inc) and anti-NF-κB p65pS529-PE (Miltenyi Biotec GmbH, Bergisch
Gladback, Germany). After experimental treatments, cells were fixed
with PerFix Fixative Reagent an incubated for 10 min at room
temperature. Next, cells were permeabilized using Perfix
Permeabilizing reagent for 5 min at 37°C in a water bath.
Thereafter, a premixed of 50 µl staining reagent, 2 µl anti-NF-κB
p65pS529-PE (dilution 1:50), 15 µl anti-CD45/anti-CD19 were added
to each tube and immediately incubated at room temperature for 30
min in the dark. Once the incubation time has passed, cells were
washed with 3 ml 1X wash reagent, centrifuged at 300 × g for 5 min
and the supernatant was completely discarded by aspiration. The
cells pellet was resuspended in 0.5 ml of the 1X wash reagent. Data
acquisition was carried out in Gallios 10 Flow Cytometer (Beckman
Coulter, Inc) considering 20,000 events and analyzed in Flowing
Software (Cell Imaging Core, FIN). Stained cells with anti-NF-κBp65
were considered active. To calculate the activation percentage of
NF-κB, a negative control absent from the anti-NF-κBp65 was used;
cells stimulated with TNF were considered as positive control.
Gene expression
Changes in the expression of BCR-ABL1 and genes
regulated by NF-κB were evaluated. Genes were selected considering
its particular role in ALL processes as described below:
Proliferation, CCND1, TNF, and MYC; antiapoptosis BCL2, BIRC5, and
PTGS; invasivity ALOX, NOS2, and VEGFA; drug resistance MDR1; NF-κB
inhibitor NFKBIA. After treatment, the cells were washed with PBS
and centrifuged at 100 × g for 2 min at room temperature, next RNA
isolation was performed using TRIzol® Reagent (Thermo
Fisher Scientific, Inc.) according to manufacturer's instructions.
Isolated RNA was treated with DNase1 amplification grade (Thermo
Fisher Scientific, Inc.) and reverse transcription PCR (RT-PCR) was
performed using 1 µg total RNA and the High Capacity cDNA Reverse
Transcription kit (Thermo Fisher Scientific, Inc.) in a GeneAmp PCR
System 9700 thermal cycler (Thermo Fisher Scientific, Inc.) with
the following conditions 25°C/25 min, 37°C/120 min, 85°C/5 min, and
infinite hold at 4°C. Real-Time quantitative PCR (qRT-PCR) was
performed using 1 µl of cDNA, TaqMan® Gene Expression
Master Mix and TaqMan® Gene Expression Assays with FAM
MGB fluorophore-quencher system for each gene (Thermo Fisher
Scientific, Inc.), the experimental samples were evaluated in
duplicate. The next TaqMan assays were used in this study: BCRABL1,
Hs03024844_ft; CCND1, Hs00765553_m1; TNF, Hs99999043_m1; MYC,
Hs00153408_m1; BCL2, Hs00608023_m1; BIRC5, Hs00153353_m1; PTGS2,
Hs00153133_m1; ALOX5, Hs01095330_m1; NOS2, Hs01075529_m1; VEGFA,
Hs00900055_m1; MDR1, Hs00184500_m1; NFKBIA, Hs00153283_m1; GUSB,
Hs00939627_m1; cat. no. 4331182 (Thermo Fisher Scientific, Inc.).
qRT-PCR was performed in a 7900 HT Fast Real-Time PCR System linked
to SDS 2.4 software (Thermo Fisher Scientific, Inc.), cycling
conditions were: 50°C/2 min, 95°C/10 min, 95°C/15 sec and 60°C/1
min (40 cycles). Gene expression was calculated by relative
quantification using the 2ΔΔCq method (20), β-glucuronidase (GUSB) housekeeping
gene was evaluated as constitutive control and chemotherapy drugs
alone groups as a calibrator.
Combinations analysis
Interaction effect of the chemotherapy drugs and
curcumin was evaluated by the isobologram method using the Compusyn
Software (Compusyn, Inc. Paramus, NJ, USA). The combination index
(CI) of each treatment was calculated according to the Chou &
Talalay's median effect equation, based on the dose-effect curve
for experimental combination and their respective IC50 (21). The CI value indicates the interaction
of treatment combinations as follows: CI<0.9, synergism; CI
between 0.9–1.1, additive; CI>1.1, antagonism. Dose reduction
index (DRI) was calculated according to the CI of each combination
treatment, DRI indicates the level that a drug can be reduced and
will continue producing a similar individual effect in a
synergistic combination (22).
Statistical analysis
Significant differences were analyzed using the SPSS
Software (SPSS, Inc., Chicago, IL, USA). Data were reported as the
mean value ± standard deviation. Comparison between groups was
statistically evaluated using one-way ANOVA. When intragroup
variance was similar Tukey's test was performed, Dunnett's T3 was
used when the variance was different. P<0.05 was considered to
indicate a statistically significant difference.
Results
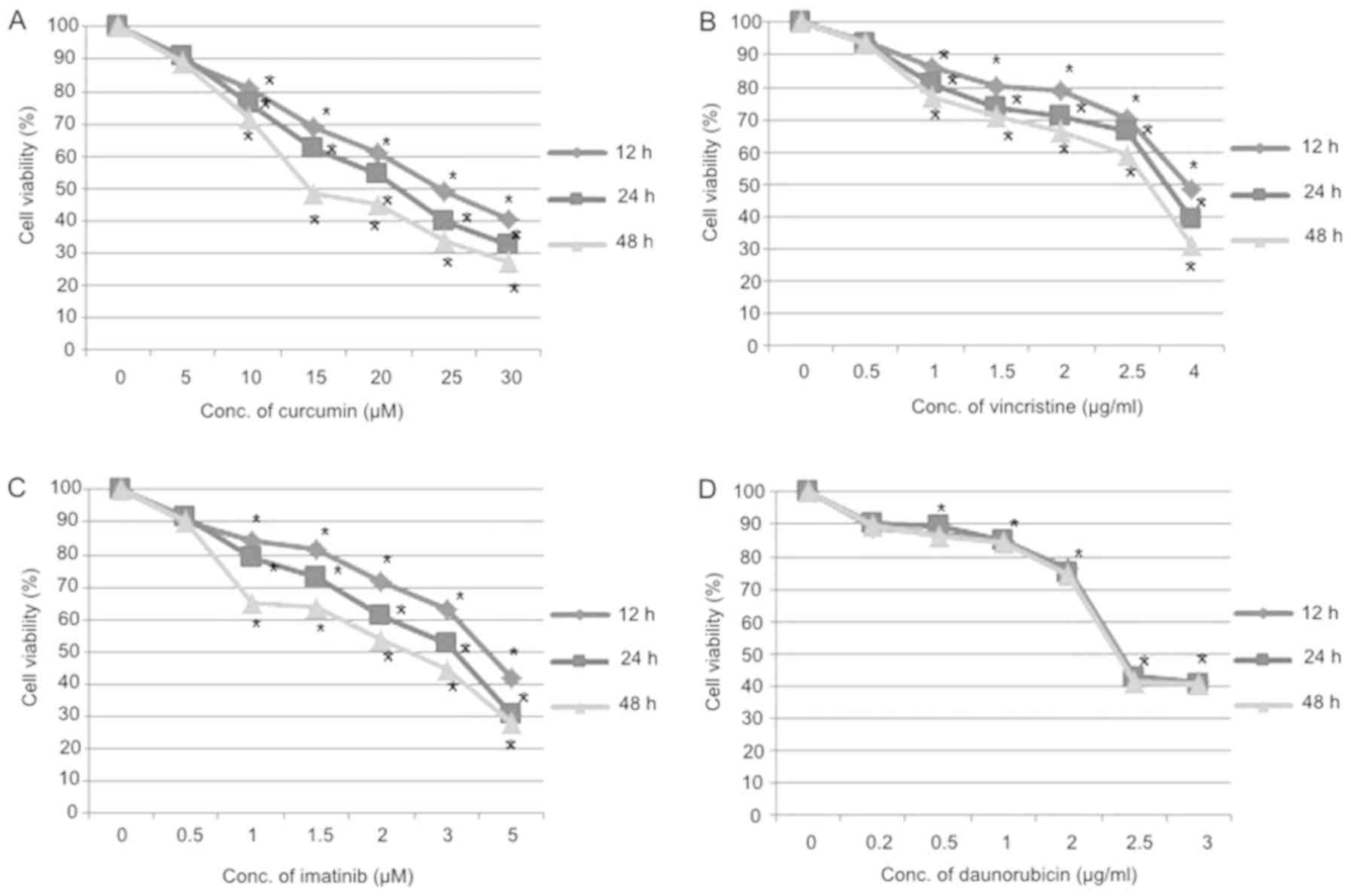
Cytotoxic effect of treatments
The cytotoxic effect of imatinib, vincristine, and
daunorubicin alone and in combination with curcumin in OP-1 cell
line was determined by the 7ADD assay. After 12, 24 and 48 h of
treatment, IC50 values for the chemotherapy drugs, were
respectively as follows: curcumin, 24.6, 21.5 and 14.7 µM;
vincristine, 4, 3.4 and 3 µg/ml; imatinib, 4.2, 3.2 and 2.4 µM;
daunorubicin, 2.4, 2.4 and 2.3 µg/ml. This data showed that
curcumin, vincristine, and imatinib induce cytotoxicity to OP-1
cell line in a dose-and time-dependent manner (P<0.05), while
daunorubicin showed cytotoxic effect just in a dose-dependent
manner (P<0.05; Fig. 1).
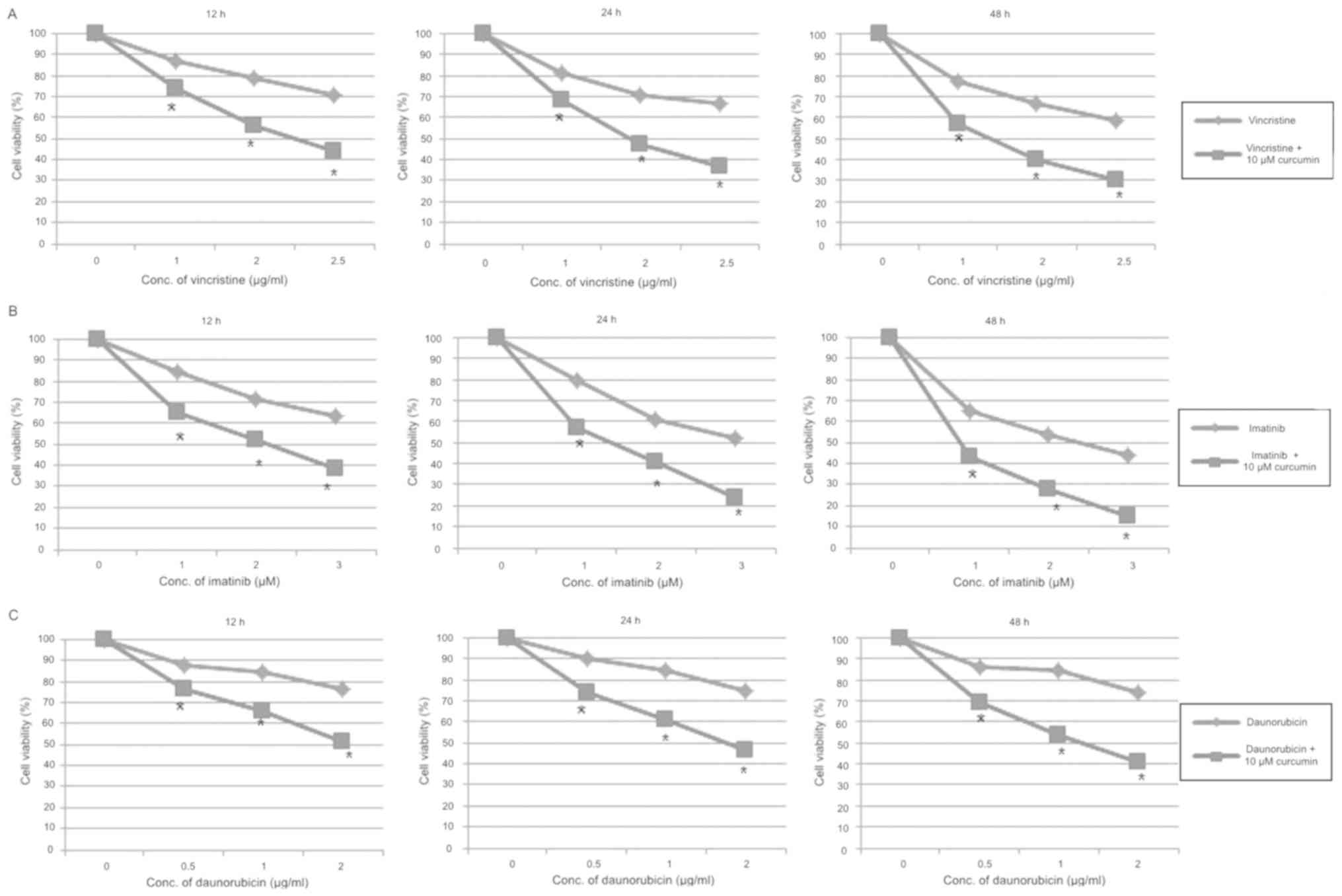
According to these results and in-vitro assays parameters, three
concentrations of each chemotherapy drug were selected and combined
with 10 µM of curcumin for similar treatment. The cytotoxic effect
was found to be greater for each combination compared to individual
chemotherapy drugs effect (P<0.05; Fig. 2).
Evaluation of treatment
interaction
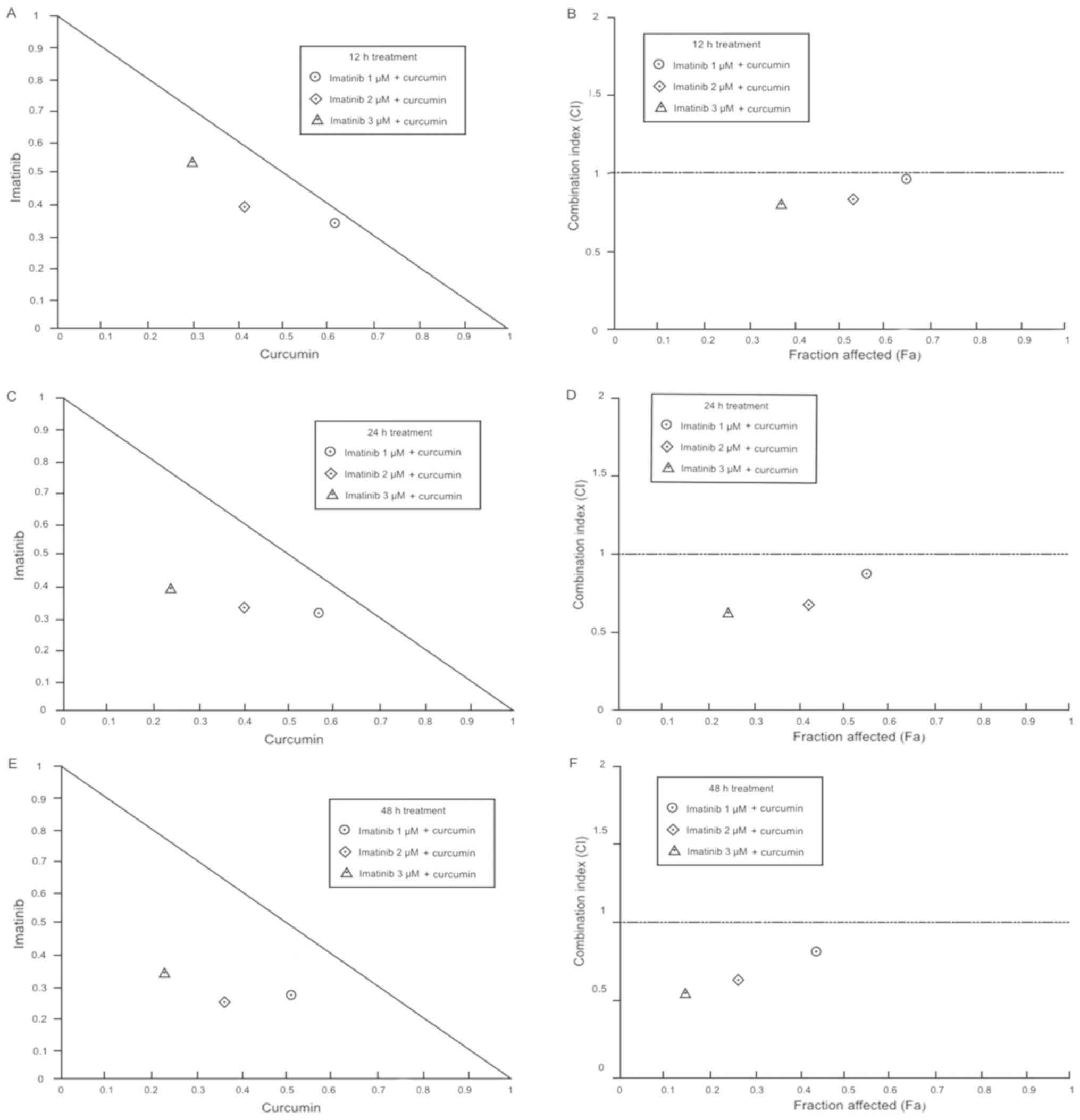
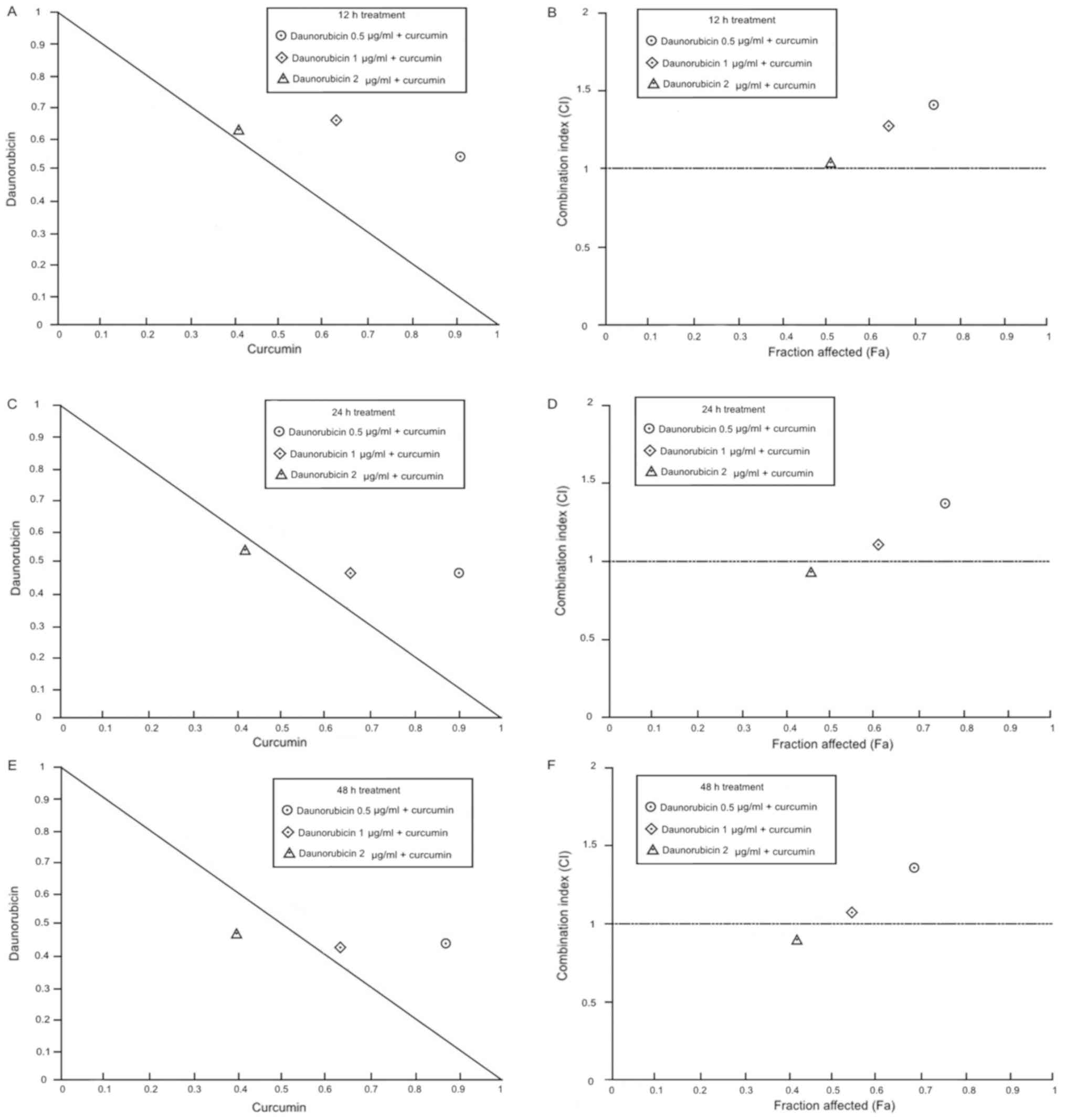
To evaluate the effect of combined treatments,
combination index (CI) values were generated from the IC50 ratio of
individual drugs and its combination with curcumin; CI values
indicate the interaction degree between curcumin with chemotherapy
drugs. This data was represented in normalized isobolograms and
fractional affected-combination index plots (Fa-CI) using Compusyn
Software 1.0 (Combosyn Inc., Paramus, NJ, USA).
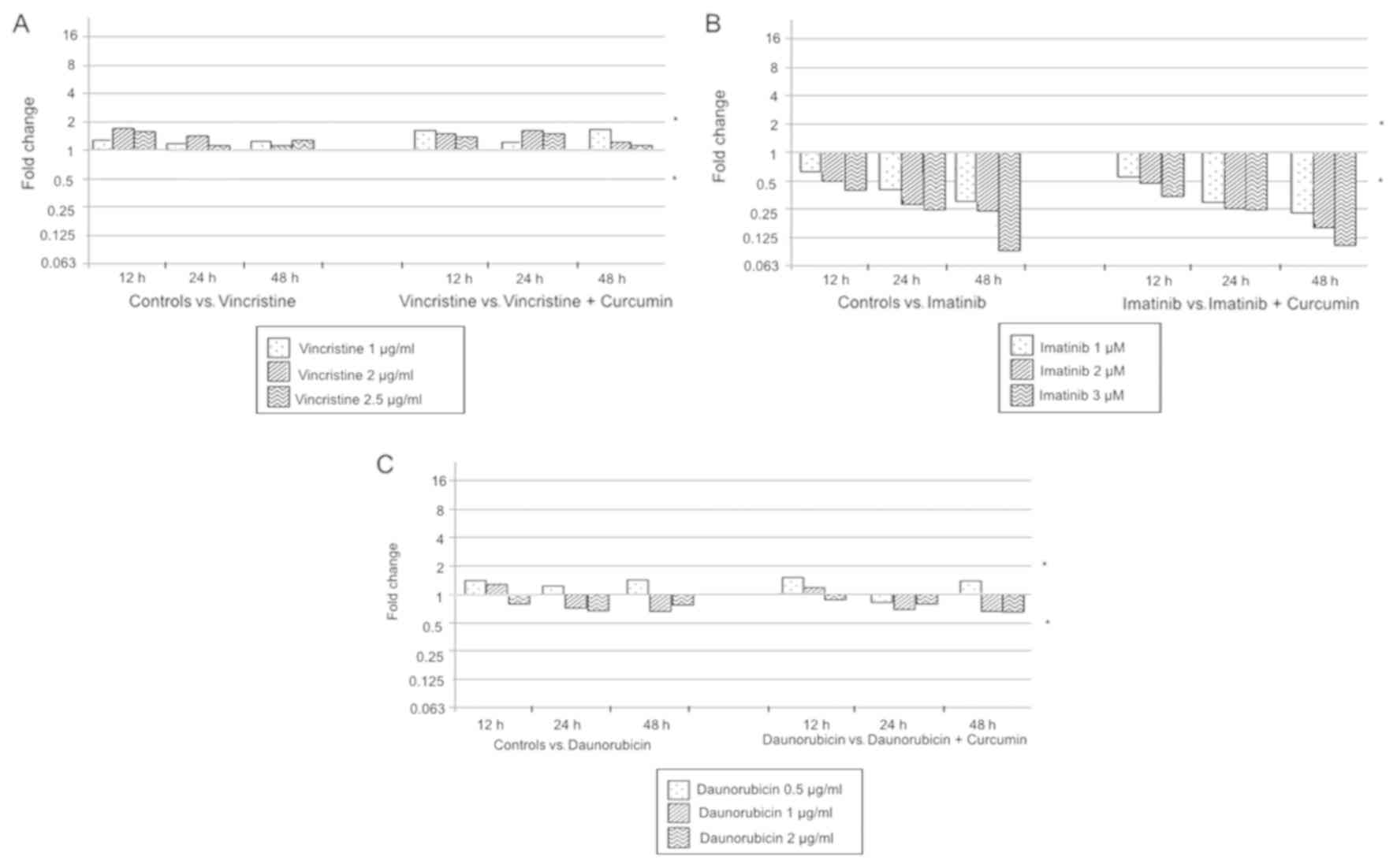
The combination of vincristine with 10 µM curcumin
at 12 h, showed a dose-dependent pattern; 1 µg/ml vincristine +
curcumin exerted a nearly additive effect, while 2 and 2.5 µg/ml
vincristine + curcumin yielded slight and moderate synergism
respectively. Similarly, combinations at 24 h treatment, exhibit a
dose-dependent pattern; 1 µg/ml vincristine + curcumin resulted in
a nearly additive effect, however, 2 and 2.5 µg/ml vincristine +
curcumin effects were enhanced, pointing to moderate synergism and
complete synergism respectively. At 48 h treatment, the effects
significantly increase compared to 12 and 24 h, and showed a
dose-dependent pattern as well; all vincristine doses showed a
synergistic effect; 1, 2 and 2.5 µg/ml of vincristine + curcumin
had a slight, moderate and complete synergism respectively. In
general, the combination of vincristine + curcumin produces a more
potent effect than each agent alone; this combination exhibited a
dose-and time-dependent pattern, shifting from nearly additive
interaction to synergism (Table I;
Fig. 3).
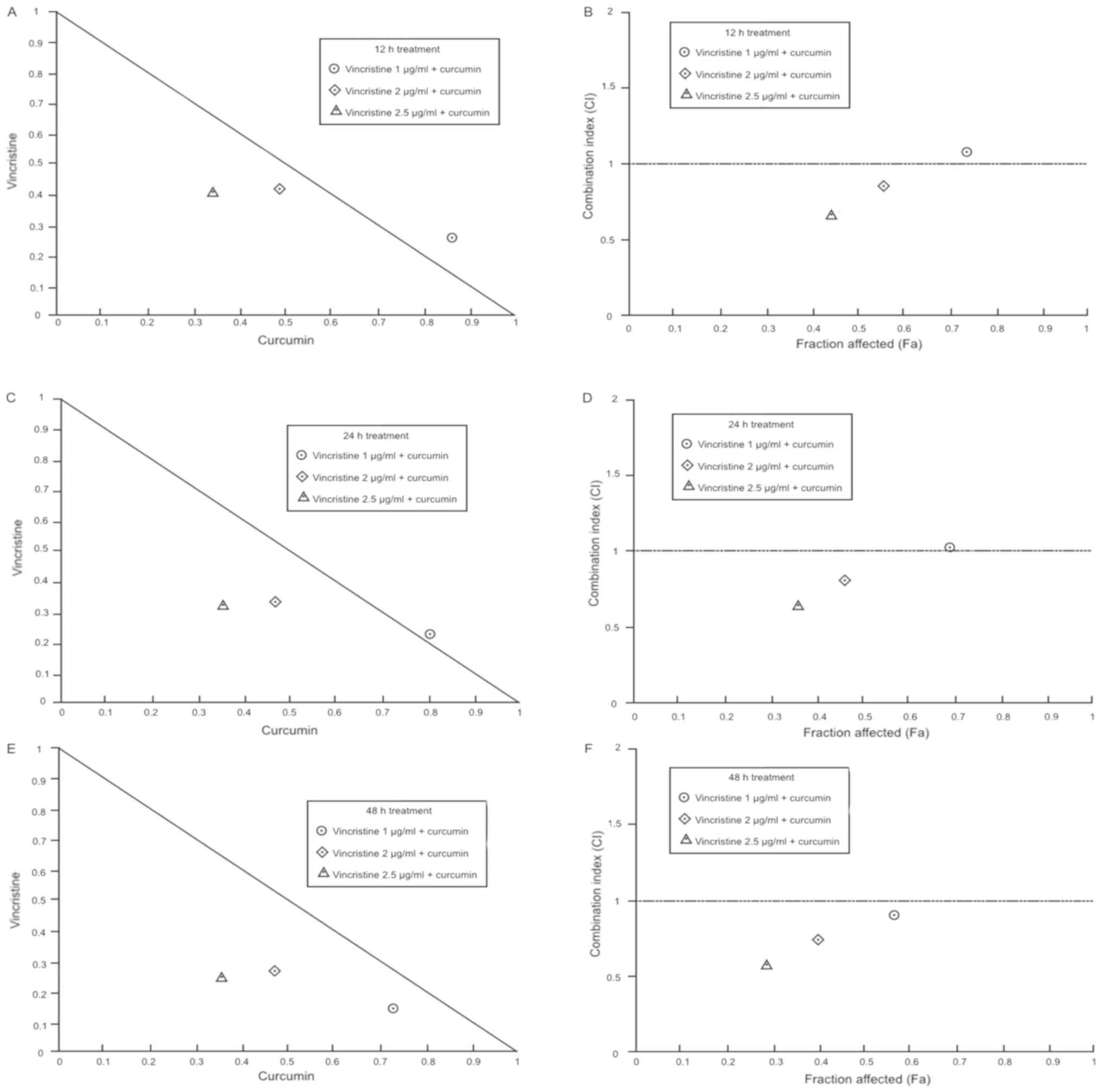 | Figure 3.Analysis of vincristine + curcumin
interaction. Combination indexes between vincristine + curcumin
experiments in OP-1 cells are represented in normalized
isobolograms at (A) 12 h, (C) 24 h, and (E) 48 h treatment
exposition, the diagonal line represents additive effect, values
below diagonal line indicates synergism, and values above diagonal
line indicate antagonism. Additionally, fractional
affected-combination index plots (Fa-CI) at (B) 12 h, (D) 24 h and
(F) 48 h are presented, the horizontal line indicates additive
effect, values below the line indicate synergism, and values above
the line indicate antagonism. Each point represents the mean value
of triplicated experiments. |
 | Table I.Chemotherapy drugs/curcumin
combination treatments interaction analysis. |
Table I.
Chemotherapy drugs/curcumin
combination treatments interaction analysis.
|
|
|
|
|
| DRI |
|---|
|
|
|
|
|
|
|
|---|
| Combination
treatment | Exposition
time | Fa | CI | Interaction
degree | Chemoterapy
drug | Curcumin |
|---|
| Vincristine 1 µg/ml
+curcumin | 12 h | 0.74 | 1.09 | Nearly
additive | 3.9 | 1.1 |
| Vincristine 2 µg/ml
+curcumin | 12 h | 0.56 | 0.87 | Slight
synergism | 2.5 | 2.1 |
| Vincristine 2.5
µg/ml +curcumin | 12 h | 0.44 | 0.73 | Moderate
synergism | 2.4 | 2.9 |
| Vincristine 1 µg/ml
+curcumin | 24 h | 0.68 | 1.02 | Nearly
additive | 4.2 | 1.3 |
| Vincristine 2 µg/ml
+curcumin | 24 h | 0.47 | 0.77 | Moderate
synergism | 3.1 | 2.2 |
| Vincristine 2.5
µg/ml +curcumin | 24 h | 0.36 | 0.66 | Synergism | 3.1 | 2.9 |
| Vincristine 1 µg/ml
+curcumin | 48 h | 0.57 | 0.88 | Slight
synergism | 6.7 | 1.4 |
| Vincristine 2 µg/ml
+curcumin | 48 h | 0.40 | 0.76 | Moderate
synergism | 3.6 | 2.0 |
| Vincristine 2.5
µg/ml +curcumin | 48 h | 0.29 | 0.64 | Synergism | 3.6 | 2.7 |
| Imatinib 1 µM
+curcumin | 12 h | 0.65 | 0.95 | Nearly
additive | 2.6 | 1.5 |
| Imatinib 2 µM
+curcumin | 12 h | 0.52 | 0.80 | Moderate
synergism | 2.7 | 2.3 |
| Imatinib 3 µM
+curcumin | 12 h | 0.38 | 0.79 | Moderate
synergism | 1.9 | 3.5 |
| Imatinib 1 µM
+curcumin | 24 h | 0.57 | 0.90 | Slight
synergism | 3.2 | 1.7 |
| Imatinib 2 µM
+curcumin | 24 h | 0.41 | 0.73 | Moderate
synergism | 2.9 | 2.5 |
| Imatinib 3 µM
+curcumin | 24 h | 0.24 | 0.62 | Synergism | 2.5 | 4.1 |
| Imatinib 1 µM
+curcumin | 48 h | 0.43 | 0.80 | Moderate
synergism | 3.5 | 1.9 |
| Imatinib 2 µM
+curcumin | 48 h | 0.28 | 0.66 | Synergism | 3.3 | 2.7 |
| Imatinib 3 µM
+curcumin | 48 h | 0.15 | 0.58 | Synergism | 2.8 | 4.3 |
| Daunorubicin 0.5
µg/ml +curcumin | 12 h | 0.76 | 1.44 | Moderate
antagonism | NA | NA |
| Daunorubicin 1
µg/ml +curcumin | 12 h | 0.66 | 1.22 | Sligth
antagonism | 1.6 | 1.5 |
| Daunorubicin 2
µg/ml +curcumin | 12 h | 0.51 | 1.02 | Nearly
additive | 1.6 | 2.4 |
| Daunorubicin
0.5µg/ml +curcumin | 24 h | 0.74 | 1.40 | Moderate
antagonism | NA | NA |
| Daunorubicin 1
µg/ml +curcumin | 24 h | 0.60 | 1.10 | Nearly
additive | 2.0 | 1.6 |
| Daunorubicin 2
µg/ml +curcumin | 24 h | 0.46 | 0.97 | Nearly
additive | 1.9 | 2.2 |
| Daunorubicin 0.5
µg/ml +curcumin | 48 h | 0.69 | 1.37 | Moderate
antagonism | NA | NA |
| Daunorubicin 1
µg/ml +curcumin | 48 h | 0.54 | 1.06 | Nearly
additive | 2.6 | 1.5 |
| Daunorubicin 2
µg/ml +curcumin | 48 h | 0.41 | 0.93 | Nearly
additive | 2.3 | 1.9 |
Imatinib + 10 µM curcumin at 12 h produces the next
results; 1 µM imatinib + curcumin exhibit a nearly additive effect,
whereas 2 and 3 µM imatinib + curcumin showed a moderate synergism.
Imatinib + 10 µM curcumin at 24 h exposition produces synergistic
effects at different degrees; 1 µM imatinib + curcumin, slight
synergism; 2 µM imatinib + curcumin, moderate synergism; 3 µM
imatinib + curcumin, synergism. Imatinib + curcumin after the 48 h
exposition also generates a synergistic effect in all combinations;
1 µM imatinib + curcumin showed a moderate synergism, while 2 and 3
µM imatinib + curcumin produced a complete synergism effect. In
general, imatinib + curcumin effect was synergistic and increases
in a dose-and time-dependent manner (Table I; Fig.
4).
The combination of daunorubicin with 10 µM curcumin
resulted in a moderate antagonism effect when 0.5 µg/ml
daunorubicin was used at all exposition times. Combination of 1
µg/ml daunorubicin + curcumin generated a slight antagonism at 12
h, while the rest of the combinations produced a nearly additive
effect. Although, 2 µg/ml daunorubicin + curcumin produces a nearly
additive effect at all exposition times. According to these
results, daunorubicin + curcumin treatment showed an additive
effect only at doses greater than 1 µg/ml daunorubicin and exposed
for more than 24 h (Table I;
Fig. 5).
Experimental induction of
apoptosis
In order to evaluate the apoptosis degree induced by
chemotherapy drugs alone compared to chemotherapy drugs + curcumin
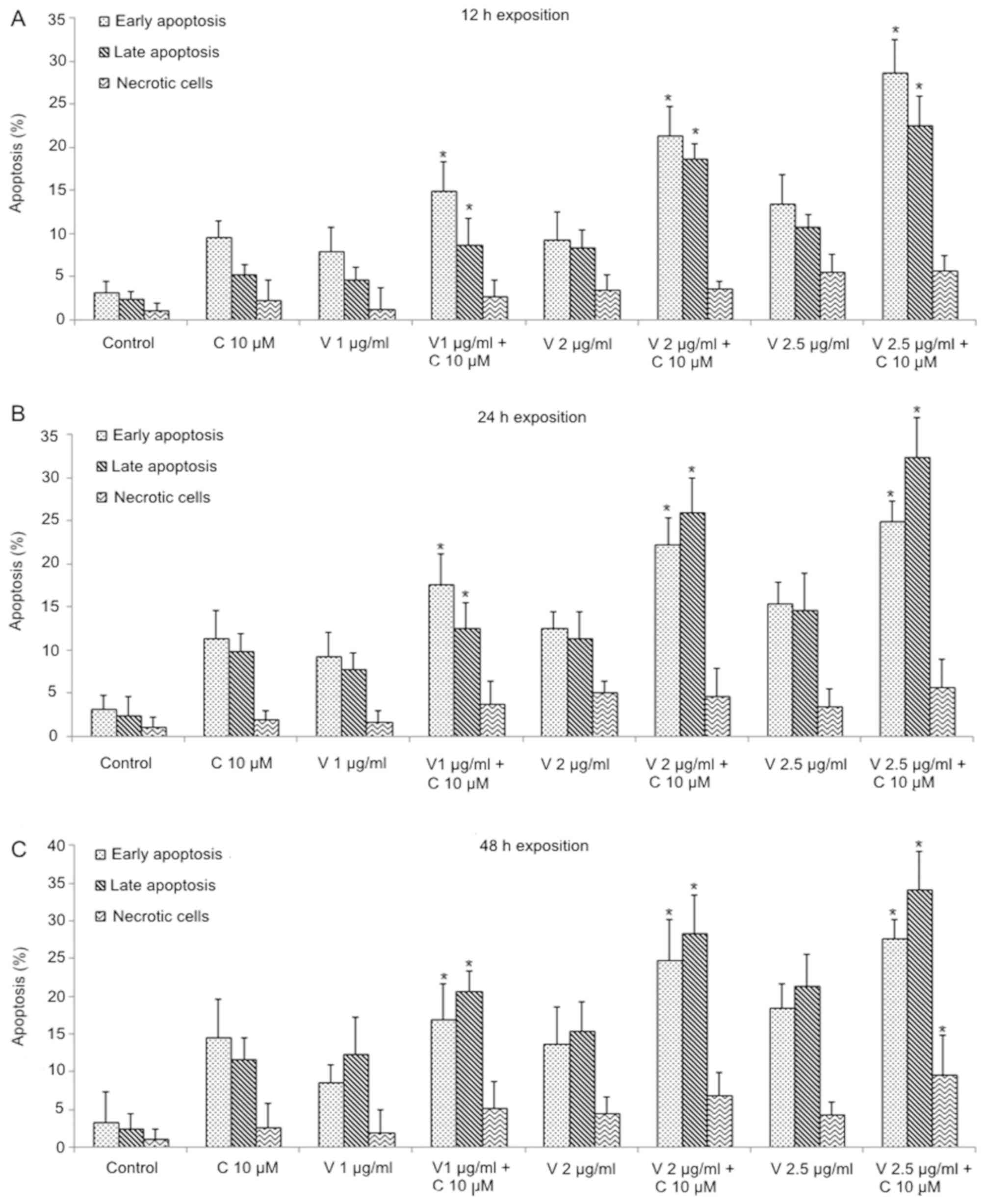
in OP-1 cells; FITC Annexin V/PI assay was performed. Vincristine +
curcumin combinations induced a significant increment of apoptotic
cells compared to both agents alone (P<0.05). An early apoptotic
stage was principally observed in this combination at 12 h, whereas
at 24 and 48 h exposition, late apoptotic cells stage showed a
significant increase (P<0.05; Fig.
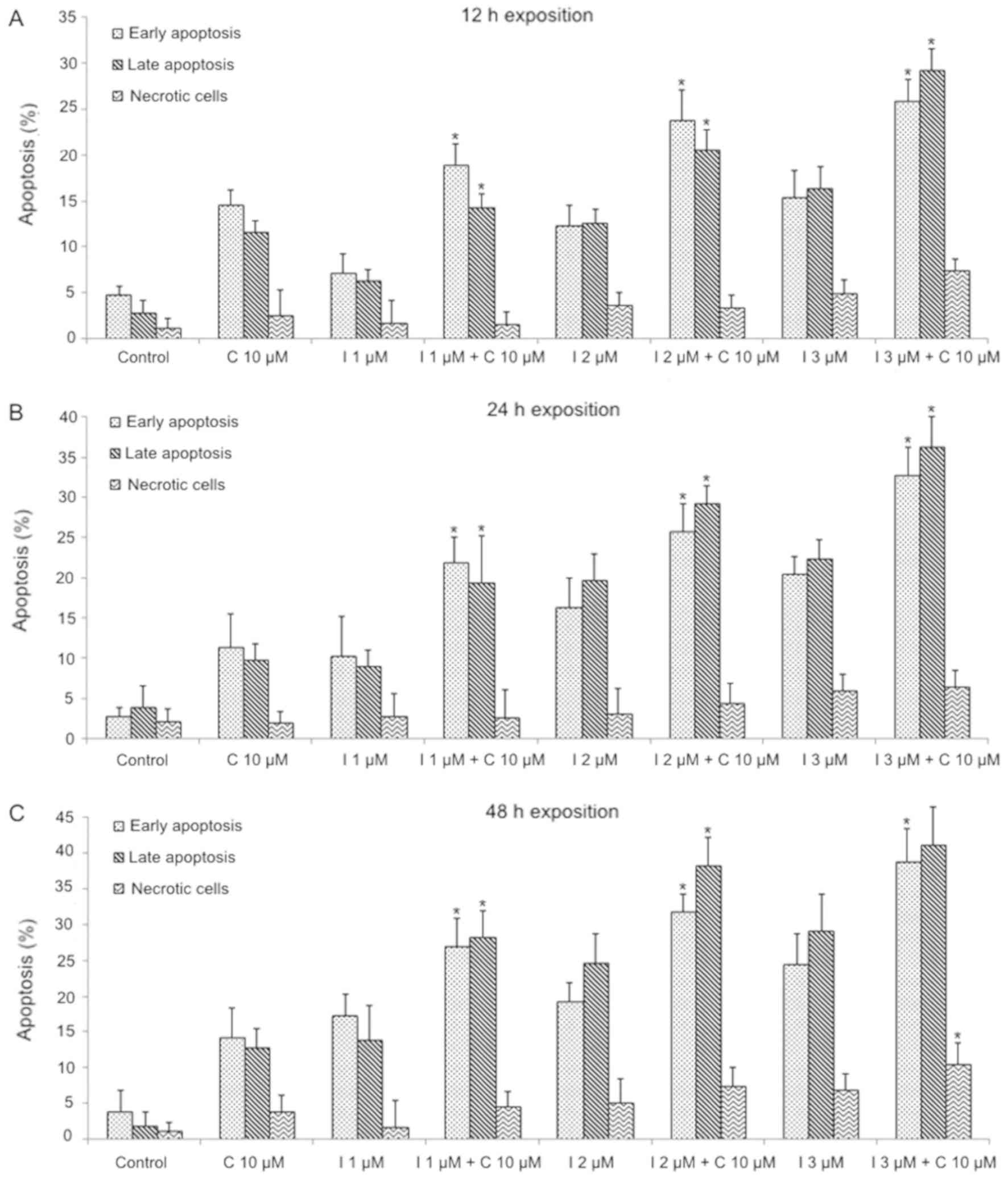
6). The combination of imatinib + curcumin produced a
significative increase on apoptosis, compared to each agent alone
(P<0.05); the apoptosis degree increments significantly from
early apoptosis to late apoptosis in a dose-dependent manner
(P<0.05), 3 µM Imatinib + curcumin induced a high percentage of
late apoptosis in all exposition times; moreover, necrotic cells
significantly increase in the combination compared to controls at
48 h (P<0.05; Fig. 7).
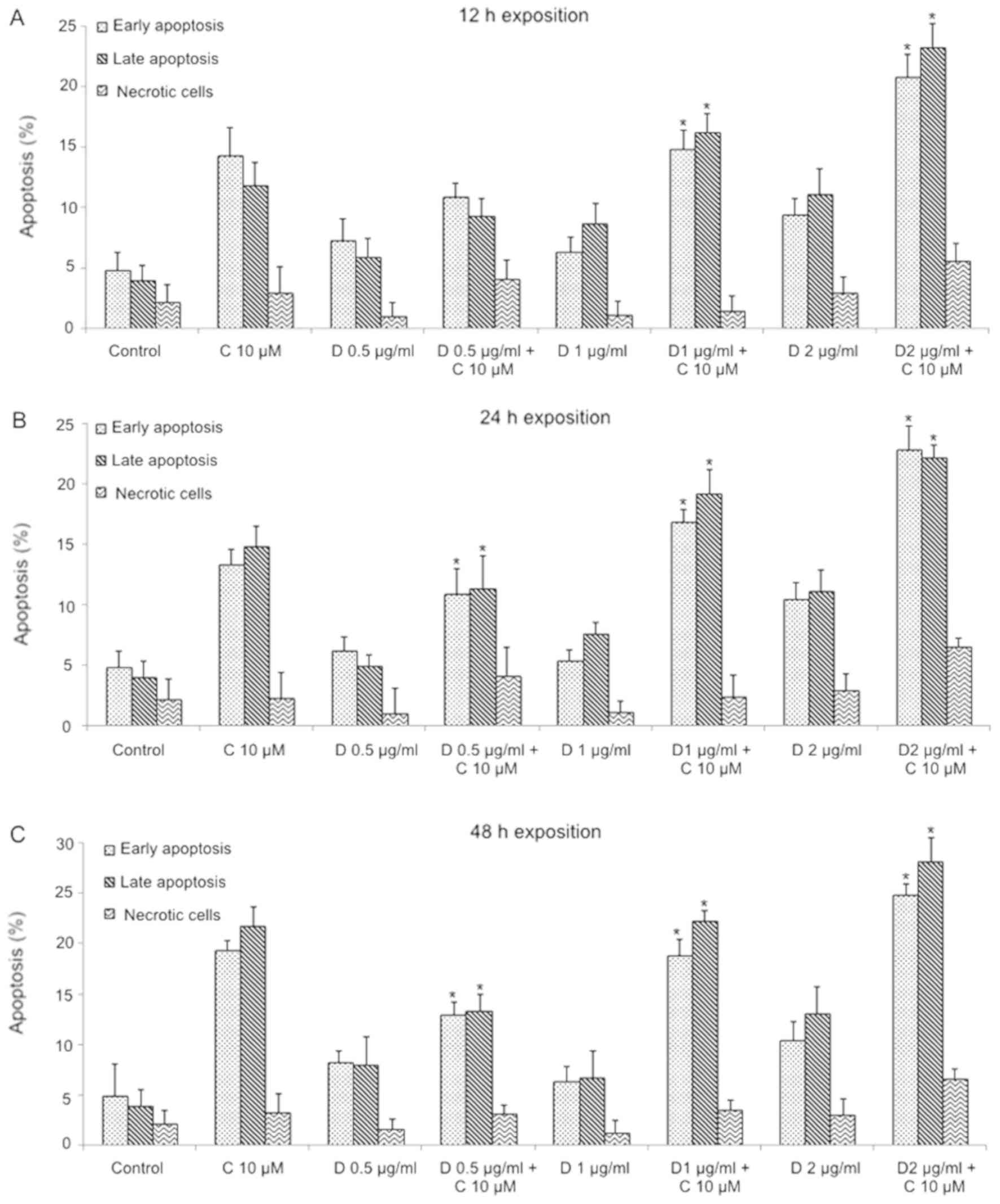
Daunorubicin + curcumin combination showed a significant increase
in apoptosis than daunorubicin alone using 1 and 2 µg/ml
daunorubicin + curcumin (P<0.05), this increment did not change
due to exposition time (Fig. 8).
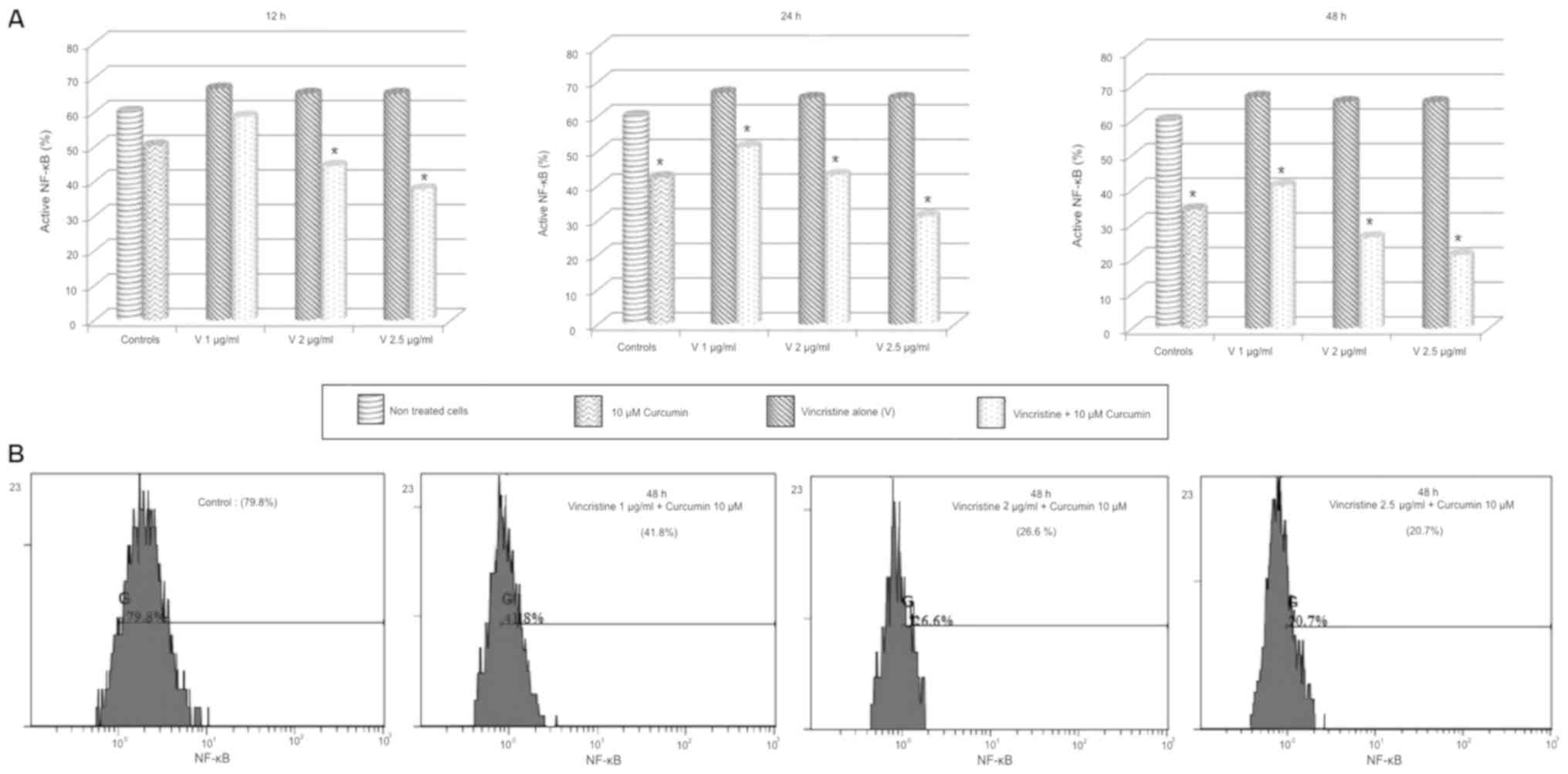
NF-κB activity after treatment
To determine the activation/transactivation
potential of NF-κB in OP-1 cells after exposition to chemotherapy
drugs alone and in combination with curcumin, phosphorylated p65
subunit in ser529 was evaluated. 10 µM curcumin significantly
reduced NF-κB activity at 24 and 48 h in all experiments
(P<0.05), while individual treatment of vincristine showed a
higher NF-κB activity compared to control groups; this increment
was not statistically significant and it did not change with dose
or exposition time increments. Combination of 1 µg/ml of
vincristine + curcumin decreased the NF-κB activity compared to
vincristine alone at 24 and 48 h, these changes were statistically
significative in a dose-and time-dependent manner (P<0.05). In
all experiments, 2 and 2.5 µg/ml vincristine + curcumin reduced the
NF-κB activity compared to vincristine alone in a dose-and
time-dependent manner (P<0.01; Fig.
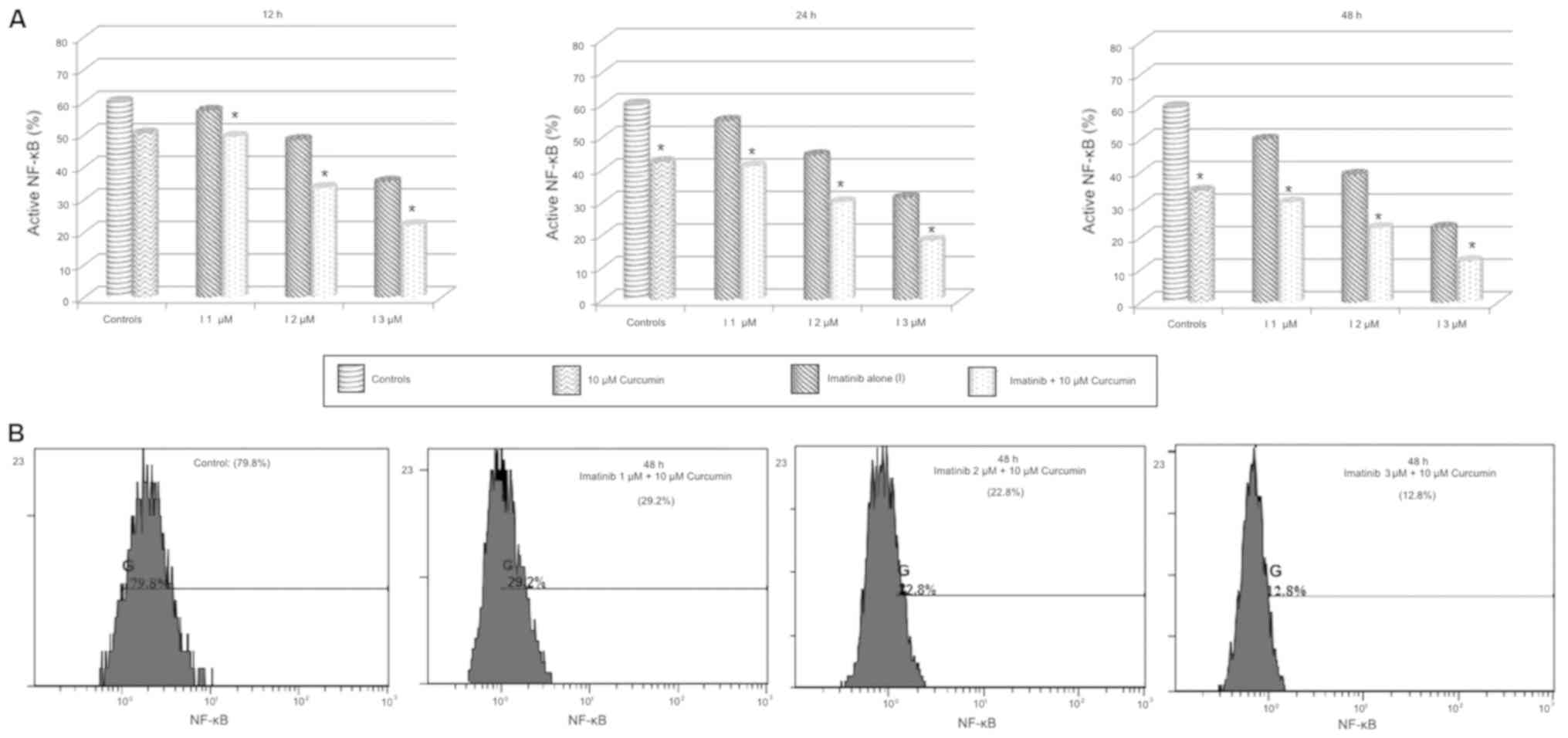
9). Imatinib alone treatment significantly reduced NF-κB
activity in a dose-and time-dependent manner (P<0.05), the
combination of imatinib + curcumin compared to imatinib alone
showed a significantly reduced activity of NF-κB in all
experiments, these changes exhibit a dose-and time-dependent
pattern (P<0.05–0.01; Fig. 10).
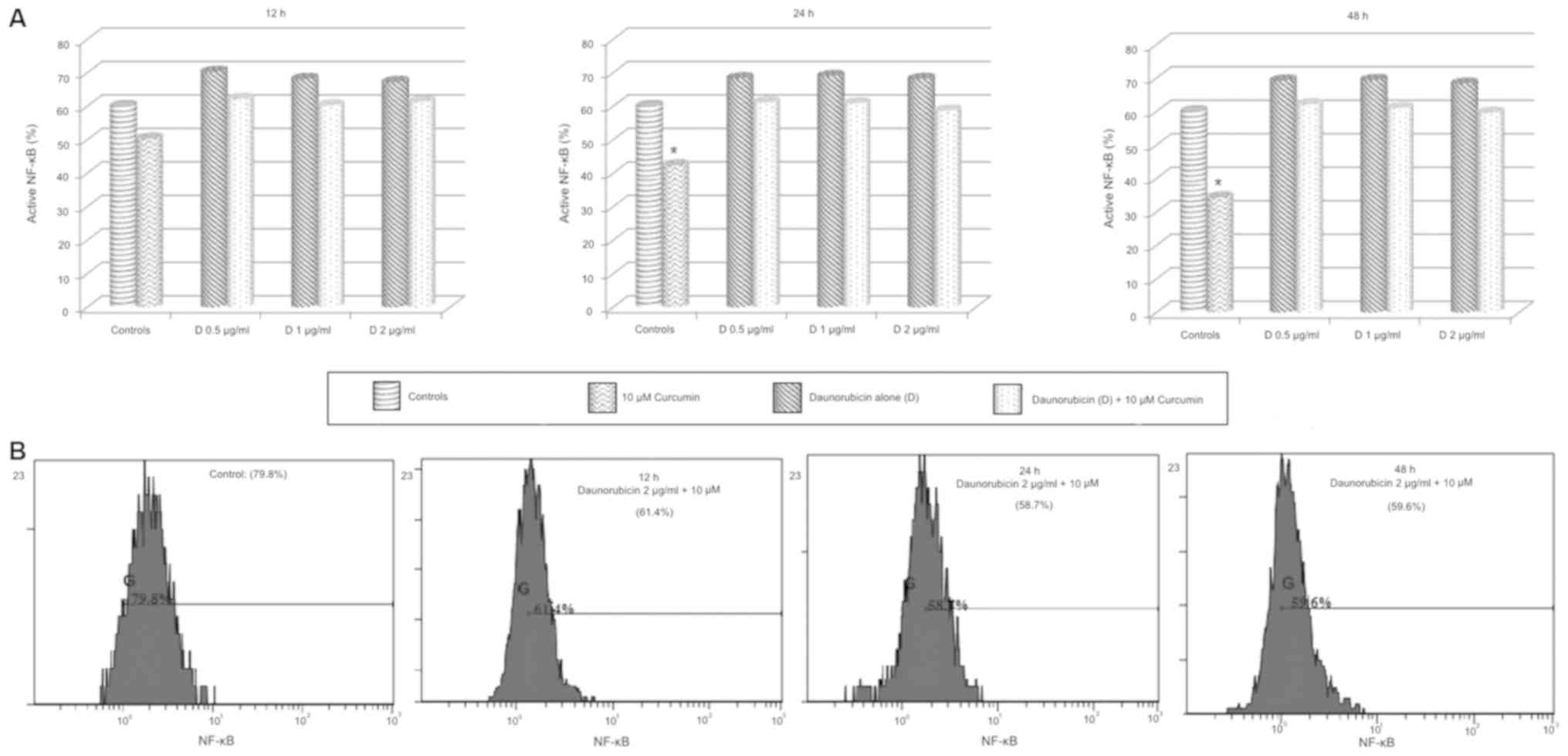
Daunorubicin alone treatment increased NF-κB activity compared to
control groups, although this increment was not significative. As
well, daunorubicin + curcumin treatment did not produce any
significative change of NF-κB activity (Fig. 11).
Expression of BCR-ABL1 fusion gene in
experimental conditions
BCR-ABL1 gene was detected in OP-1 cell line and its
expression level was measured after each experimental treatment.
Relative gene expression level significantly decreased only in
imatinib-treated cells in a dose and time-dependent manner.
Vincristine and daunorubicin did not induce any change in BCR-ABL1
expression level compared to controls. As well no significant
differences were observed when each chemotherapy drug was combined
with curcumin (Fig. 12).
Effect of combination treatments on
the expression of NF-κB regulated genes
Gene expression was evaluated in OP-1 cells after
the exposition to chemotherapy drugs alone as the calibrator and
chemotherapy drugs + curcumin as the target group.
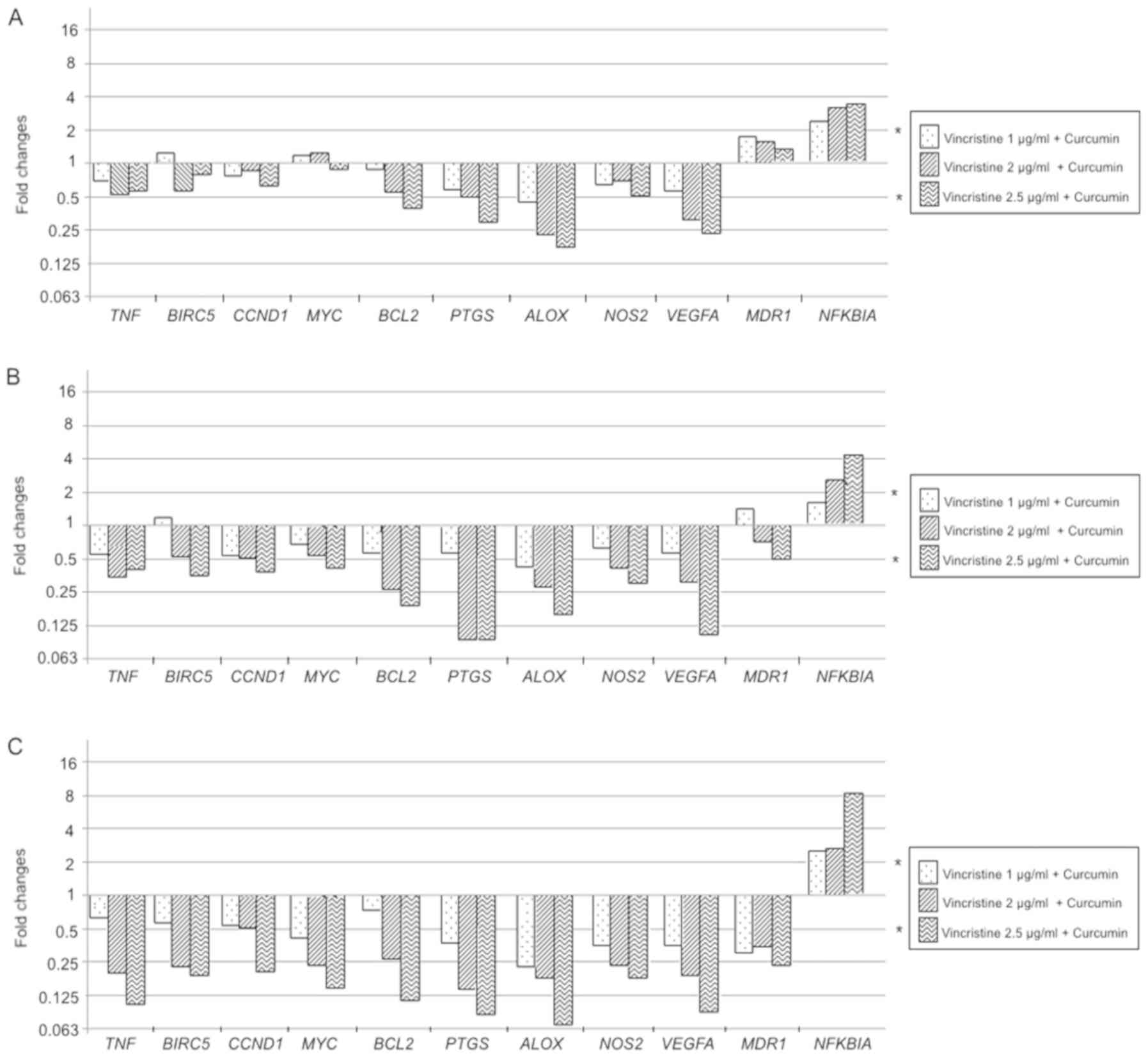
In the evaluation of vincristine + curcumin compared
to vincristine alone treatments, following results are reported; at
12 h exposition BCL2, PTGS, ALOX, VEGFA significantly reduce its
expression, while NFKBIA increment it; these changes showed a
dose-dependent pattern (P<0.05). At the 24 h exposition, a
decrease in the expression of BIRC5, CCND1, MYC, BCL2, PTGS2, ALOX,
NOS2, and VEGFA in a dose-dependent manner is observed, as well TNF
showed an expression decrement, but it was not dependent of
vincristine dose (P<0.05). Similarly to 12 h experiments, NFKBIA
showed an increased expression in a dose-dependent manner at 24 h
(P<0.05). When experiments were performed at 48 h exposition
MDR1 showed a downregulated expression not associated to
vincristine dose (P<0.01), NFKBIA continues showing a
dose-dependent overexpression (P<0.01), and the expression of
all other genes significantly decreased in a dose-dependent manner
(P<0.01). In general, vincristine + curcumin modulated
expression of selected genes in a dose-and time-dependent manner
(Fig. 13).
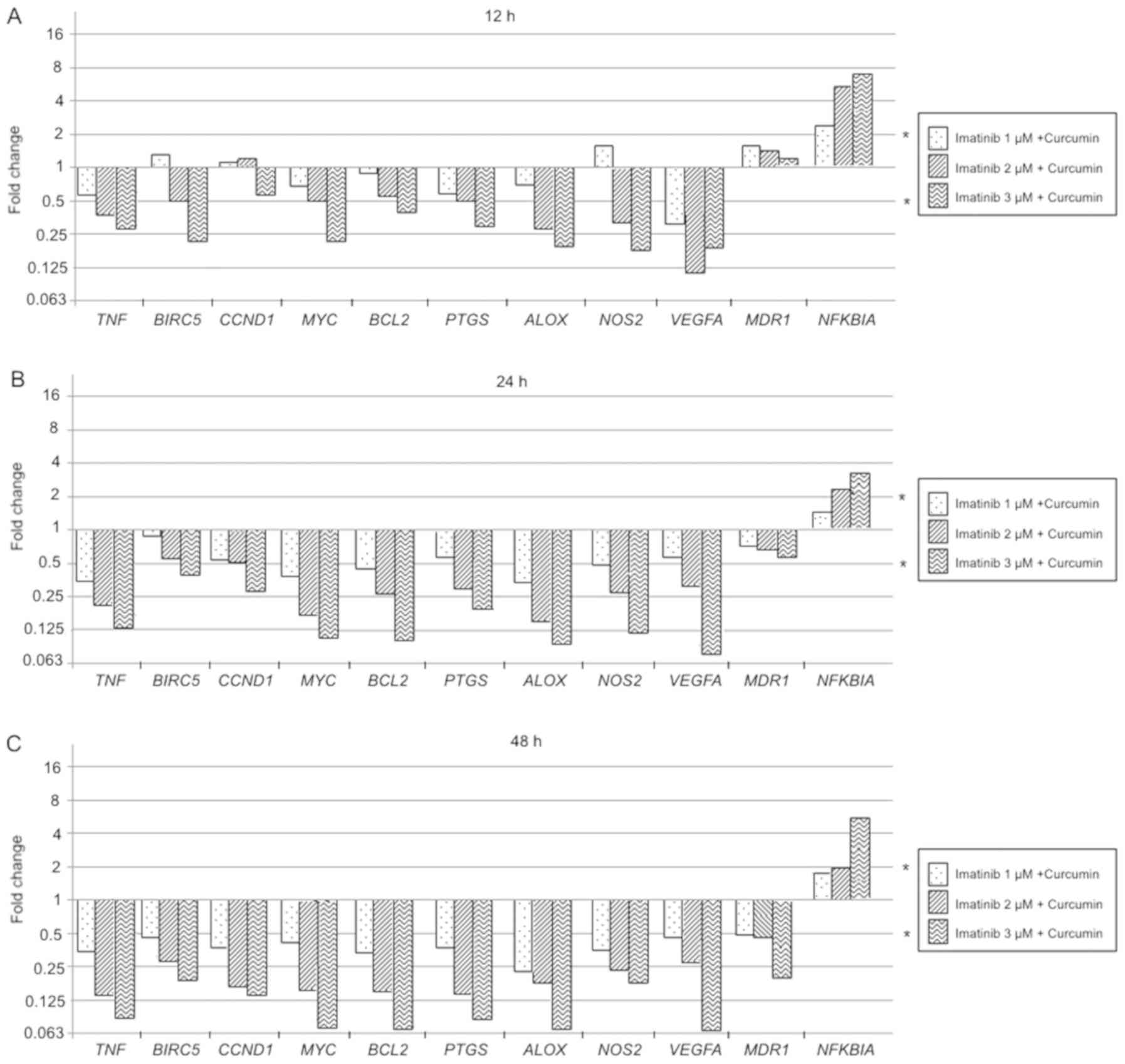
Imatinib + curcumin compared to imatinib alone
treatment effect on genes expression is reported below: At 12 h
exposition TNF, BIRC5, MYC, BCL2, PTGS2, ALOX, and NOS2 were
significantly downregulated in a dose-dependent manner (P<0.05),
VEGFA showed a significant decrement of gene expression that was
not related to imatinib dose (P<0.05), while NFKBIA was
overexpressed according to imatinib dose increment (P<0.05).
After 24 h of exposure, MDR1 and NFKBIA showed a dose-dependent
significant overexpression (P<0.05), but NFKBIA overexpression
was smaller compared to 12 h exposure. The rest of the evaluated
genes showed a significant expression reduction in a dose-and time
dependent manner (P<0.05). The 48 h evaluation showed a
significant decrease of MDR1 expression in a dose-and time
dependent-manner (P<0.01), NFKBIA was overexpressed just at 3 µM
imatinib (P<0.05), in all the rest of the evaluated genes the
expression was downregulated in a dose-dependent manner
(P<0.01). In general, imatinib combined with curcumin produce
changes in the expression of evaluated genes in a dose-and
time-dependent manner (Fig.
14).
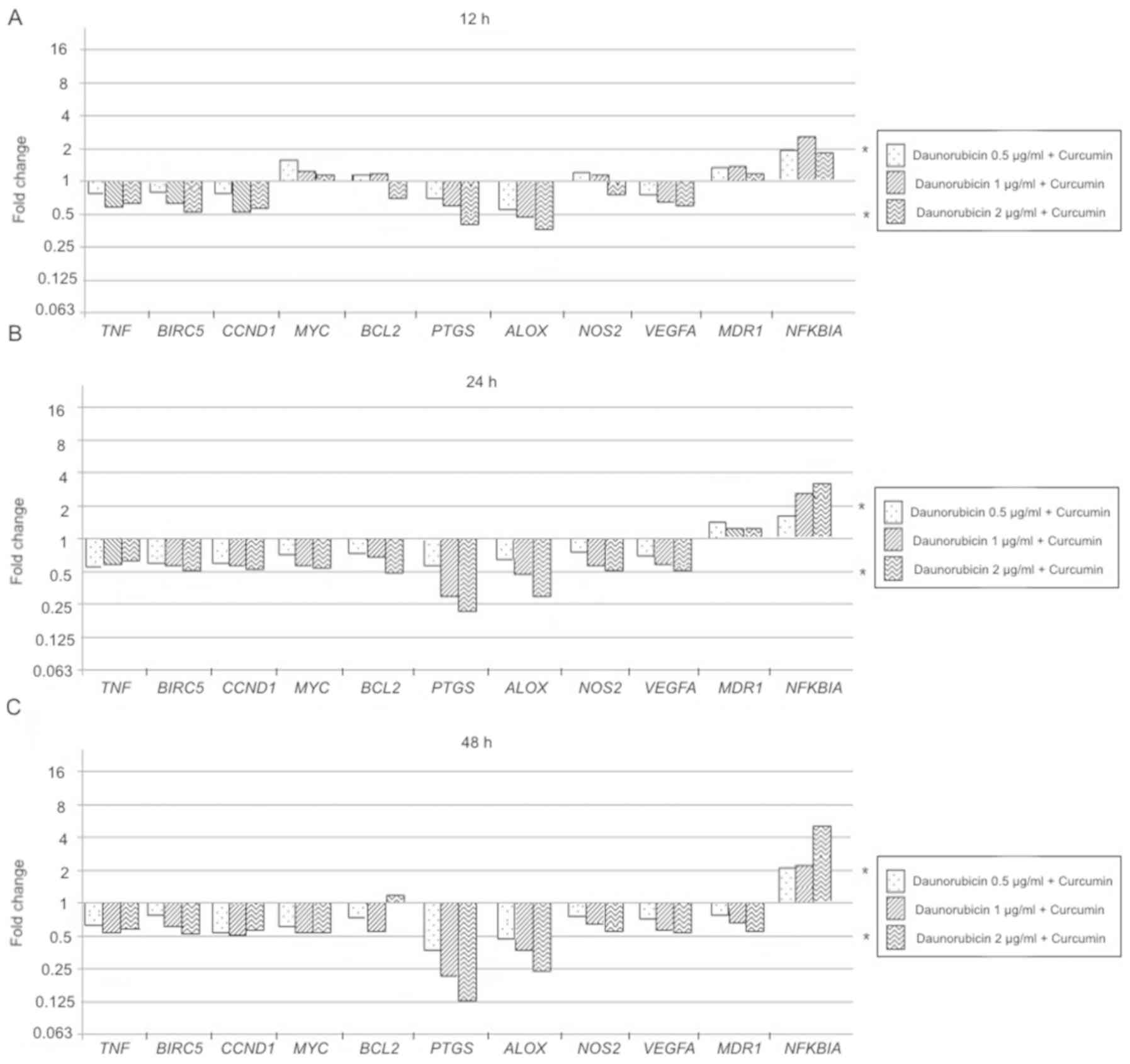
Daunorubicin + curcumin treatment effect compared to
daunorubicin alone, showed the next results: the 12 h evaluation
resulted in a significant decrease of PTGS and ALOX expression at 1
and 1–2 µg/ml respectively (P<0.05), while NFKBIA showed an
overexpression only in treatment with 1 µg/ml daunorubicin +
curcumin (P<0.05). Similarly, experiments at 24 and 48 h
treatment exposition only showed significant decrement of
expression in PTGS and ALOX in a dose-dependent manner (P<0.05),
while NFKBIA was significantly overexpressed according to
daunorubicin dose increment (P<0.05; Fig. 15).
Discussion
In the current study, the effect of curcumin in
combination with chemotherapeutic drugs in OP-1 cell line was
investigated. Curcumin antitumoral properties have been evaluated
in different types of cancer cells (23), and a vital role in NF-κB activity
modulation has been reported (24).
Our results show that curcumin potentiates the anti-leukemia effect
of vincristine and imatinib in a dose and time-dependent manner,
while curcumin + daunorubicin effect did not significantly change.
The decrease in NF-κB activity caused by the experimental
treatments was associated with the anti-leukemia effect increment.
We could not find any evaluation performed in a Ph+ ALL model,
similar to those made in this study.
The results revealed that each tested agent induces
cytotoxicity and its combination with curcumin increment it
significantly. Previously, this cytotoxic effect was reported in
ALL, considering only the dose of the drugs (25–28) but
not the exposition of time as we did.
Vincristine + curcumin combination produce an effect
in the range of nearly additive to synergistic. We did not find any
other report of this interaction in a Ph+ ALL model, but we suggest
that curcumin could prolong cellular accumulation of vincristine in
cancer cells (29), increasing the
anti-leukemia effect. Similarly, imatinib + curcumin exhibit an
interaction effect varying from nearly additive to synergistic.
Previously, was demonstrated the efficacy of this combination in
ALL (25) cells in vitro and in
vivo, but they did not perform interaction analysis as we did. In
daunorubicin + curcumin combination, interaction response from
moderate antagonistic to nearly additive was observed. We suppose
that daunorubicin anti-leukemia activities strongly coincide with
those generated by curcumin (30) so
that an enhancing effect is not produced, and instead an
antagonistic/additive effect is observed. Again, previous reports
of this interaction in Ph+ ALL model are not available.
Our results reveal a significant apoptosis induction
increment produced by chemotherapeutic drugs + curcumin compared to
each individual agent. In agreement with our study, Guo et
al (2015) reported an apoptosis increase using 1 µM imatinib +
curcumin (25). However, they did
not evaluate the drug dose and exposition time effect. Furthermore,
we report for the first time a pro-apoptotic effect of
vincristine/daunorubicin + curcumin in a Ph+ ALL model using
Annexin V/PI staining. Previously, caspase-3 activation was
measured to report that the combination of vincristine/daunorubicin
with curcumin augments apoptosis in REH cells (28).
Considering that curcumin is involved in the
suppression of NF-κB activity by inhibiting different kinases,
Ser529p65 phosphorylation was evaluated. OP-1 cells treated with
vincristine + curcumin exhibit a decrease in NF-κB activation
compared to vincristine alone. Pimentel-Gutiérrez et al
(2016) reported an increment of NF-κB activation in REH cells
treated with vincristine (28), but
unlike our study, they evaluated the Ser536p65 phosphorylation,
that is not associated with the BCR-ABL activity. We suggest that
the NF-κB activity decrease observed in our results is principally
due to curcumin activity and exposition time; this can be explained
by the findings reported in Das & White study (1997), where
they also observed NF-κB activation (31). Imatinib + curcumin induce a
significant decrease in NF-κB activation compared to imatinib
alone. Our results are in accordance with the data reported by
Demiray et al in adenoid cystic carcinoma (32). We could not find any study which
evaluates the imatinib + curcumin effect on NF-κB activity in a Ph+
ALL model. Daunorubicin + curcumin did not produce any significant
change in NF-κB activities compared to daunorubicin alone; thus we
suggest curcumin activities could be masked by daunorubicin
functions.
The BCR-ABL1 gene expression level is a gold
standard tool used in diagnosis and follow-up of pediatric patients
(6). We performed such measurement
in each OP-1 cells experimental conditions that result as expected:
a higher level of expression in untreated cells and decreased gene
expression only in imatinib-treated cells. Clearly, curcumin did
not induce significant changes in BCR-ABL1 gene expression, but its
addition provided increased treatment effectivity based on evidence
obtained from NF-κB activation due to BCR-ABL presence, as well as
the rest of biological parameters measured (NF-κB regulated genes
expression, proliferation, and apoptosis). These explain the
synergistic effect of imatinib + curcumin combination, and its
difference with vincristine or daunorubicin combination treatments.
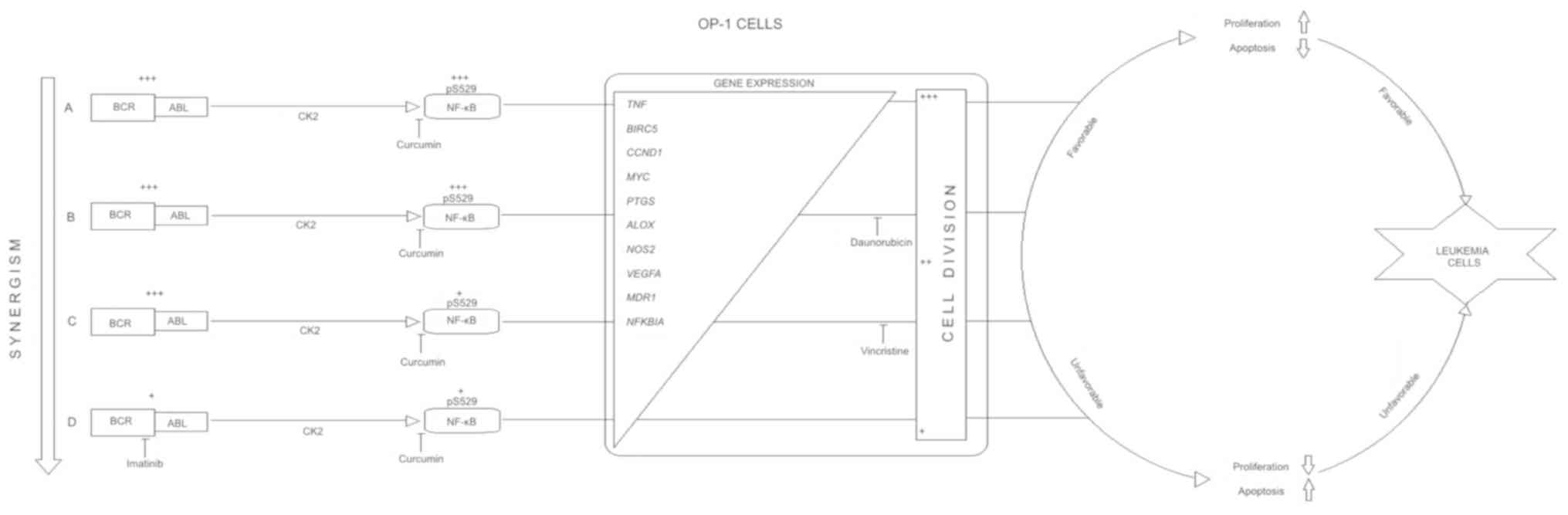
This information is described in an integrated proposal diagram in
Fig. 16.
Vincristine/imatinib + curcumin induce significant
decrease in the expression of CCND1, TNF, MYC, BCL2, BIRC5, PTGS,
ALOX, NOS2, VEGFA, and MDR1 genes; these decreases are explained
due to the NF-κB activity inhibition. As well, both combinations
induce NFKBIA overexpression; we proposed that while NF-κB activity
decreases the NKBIA gene, increments its molecular activity.
Daunorubicin + curcumin induces only significant downregulation of
ALOX and PTGS genes and overexpression of NFKBIA gene; according to
the NF-κB activity status in this combination, we suggest that
these changes could be associated only to curcumin effect.
In conclusion, curcumin potentiates the
anti-leukemia effect of vincristine and imatinib in a dose-and
time-dependent manner. Otherwise, the combination of curcumin with
daunorubicin did not produce any improvement (Fig. 16). The described results are of
clinical importance and suggest that curcumin could be a promising
agent. The treatment efficacy could be potentiated in Ph+ ALL with
bad prognosis by combining these drugs with curcumin. Further
studies are necessary to evaluate curcumin effect in combination
with more chemotherapy drugs of ALL standard scheme. Finally,
further studies are necessary to assess if the anti-leukemia effect
of chemotherapy drugs-curcumin interactions would be observed in
alternative fusion-genes cell line models related to leukemia.
Acknowledgements
The authors would like to thank the St. Jude
Children's Research Hospital (Memphis, TN, USA) for providing the
OP-1 cell line and Dr Fernando Sánchez-Zubieta (Pediatric
Hematology and Oncology Service, Pediatric Division, Civil Hospital
of Guadalajara, Jalisco, México).
Funding
The present study was partially supported by the
National Council of Science and Technology (CONACYT, México, Grant
no. 375707-305844-2014), PhD Molecular Biology Doctoral Program
(University of Guadalajara, Grant no. 208522618-2014) and Medical
Genetics Residency Program (University of Guadalajara, Grant no.
237181-2017).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
UFSB participated in study design, cell culture and
all performed experiments, analyzed and interpreted the results and
was a major contributor in writing the manuscript. LBM participated
in study design, analysis and interpretation of the results, was
involved in drafting the manuscript and revising it critically for
important intellectual content. LMM participated in cell culture
and all performed experiments, and contributed to the analysis and
the interpretation of the experimental data. ETA, participated in
experimental design, analyzed and interpreted the results. SABJ
participated in interpreting the results, and drafting the
manuscript. CCBB performed flow cytometry experiments. JRCR made
substantial contribution to the conception of the study, and was
involved in drafting the manuscript and revising it critically for
important intellectual content. ACR coordinated the study, made
substantial contribution to its conception and design, analysis and
interpretation of the experimental data, was involved in drafting
the manuscript and revising it critically for important
intellectual content given final approval of the version to be
published.
Ethics approval and consent to
participate
The present study was performed in accordance with
the declaration of Helsinki and was approved by the local ethics
committee O.P.D. Civil Hospital of Guadalajara and University of
Guadalajara (Guadalajara, Jalisco, México).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Thomas DA: Philadelphia chromosome
positive acute lymphocytic leukemia: A new era of challenges.
Hematology Am Soc Hematol Educ Program. 435–443. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mishra S, Zhang B, Cunnick JM, Heisterkamp
N and Groffen J: Resistance to imatinib of bcr/abl p190
lymphoblastic leukemia cells. Cancer Res. 66:5387–5393. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cilloni D and Saglio G: Molecular
pathways: BCR-ABL. Clin Cancer Res. 18:930–937. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hoffman A and Baltimore D: Circuitry of
nuclear factor kappaB signaling. Immunol Rev. 210:171–186. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mishra S, Pertz V, Zhang B, Kaur P,
Shimada H, Groffen J, Kazimierczuk Z, Pinna LA and Heisterkamp N:
Treatment of P190 Bcr/Abl lymphoblastic leukemia cells with
inhibitors of the serine/threonine kinase CK2. Leukemia.
21:178–180. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Morotti A, Carrà G, Panuzzo C, Crivellaro
S, Taulli R, Guerrasio A and Saglio G: Protein kinase CK2: A
targetable BCR-ABL partner in philadelphia positive leukemias. Adv
Hematol. 2015:6125672015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fielding AK: How I treat Philadelphia
chromosome-positive acute lymphoblastic leukemia. Blood.
116:3409–3417. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Thomas DA, Sarris AH, Cortes J, Faderl S,
O'Brien S, Giles FJ, Garcia-Manero G, Rodrigues MA, Cabanillas F
and Cantarjian H: Phase II study of sphingosomal vincristine in
patients with recurrent or refractory adult acute lymphocytic
leukemia. Cancer. 106:120–127. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stock W, Johnson J, Yu D, Bennett D, Sher
D, Stone R, Kolitz J, Powell B, Wetzler M, Vardiman J, et al:
Daunorubicin dose intensification during treatment of adult acute
lymphoblastic leukemia (ALL): Final results from cancer and
leukemia group B study 19802. Blood. 106:18332005.
|
|
10
|
Lyseng-Williamson K and Jarvis B:
Imatinib. Drugs. 61:1765–1776. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Soverini I, De Benedittis C, Papayannidis
C, Paolini S, Venturi C, Iacobucci I, Luppi M, Bresciani P,
Salvucci M, Russo D, et al: Drug resistance and BCR-ABL kinase
domain mutations in Philadelphia chromosome-positive acute
lymphoblastic leukemia from the imatinib to the second-generation
tyrosine kinase inhibitor era: The main changes are in the type of
mutations, but not in the frequency of mutation involvement.
Cancer. 120:1002–1009. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chattopadhyay I, Biswas K, Bandyopadhyay U
and Banerjee RK: Turmeric and curcumin Biological actions and
medicinal applications. Curr Sci. 87:44–50. 2004.
|
|
13
|
Ravindran J, Prasad S and Aggarwal BB:
Curcumin and cancer cells: How many ways can curry kill tumor cells
selectively? AAPS J. 11:495–510. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kunnumakkara AB, Anand P and Aggarwal BB:
Curcumin inhibits proliferation, invasion, angiogenesis and
metastasis of different cancers through interaction with multiple
cell signaling proteins. Cancer Lett. 269:199–225. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kunwar A, Barik A, Mishra B, Rathinasamy
K, Pandey R and Priyadarsini K: Quantitative cellular uptake,
localization and cytotoxicity of curcumin in normal and tumor
cells. Biochim Biophys Acta. 1780:673–679. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF,
Shen TS, Ko JY, Lin JT, Lin BR, Ming-Shiang W, et al: Phase I
clinical trial of curcumin, a chemopreventive agent, in patients
with high-risk or pre-malignant lesions. Anticancer Res.
21:2895–2900. 2001.PubMed/NCBI
|
|
17
|
Singh S and Aggarwal BB: Activation of
transcription factor NF-kappa B is suppressed by curcumin
(diferuloylmethane) [corrected]. J Biol Chem. 270:24995–25000.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zeligs KP, Neuman MK and Annunziata CM:
Molecular pathways: The balance between cancer and the immune
system challenges the therapeutic specificity of targeting nuclear
factor-κB signaling for cancer treatment. Clin Cancer Res.
22:4302–4308. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xia Y, Shen S and Verma I: NF-κB, an
active player in human cancers. Cancer Immunol Res. 2:823–830.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak K and Schmittgen T: Analysis of
relative gene expression data using realtime quantitative PCR and
the 2(Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chou TC: Theoretical basis, experimental
design, and computerized simulation of synergism and antagonism in
drug combination studies. Pharmacol Rev. 68:621–681. 2006.
View Article : Google Scholar
|
|
22
|
Chou J and Chou TC: Computerized
simulation of dose reduction index (DRI) in synergistic drug
combinations. Pharmacologist. 30:A2311988.
|
|
23
|
Rahmani AH, Al Zohairy MA, Aly SM and Khan
MA: Curcumin: A potential candidate in prevention of cancer via
modulation of molecular pathways. Biomed Res Int. 2014:7616082014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen J, Wang FL and Chen WD: Modulation of
apoptosis-related cell signaling pathways by curcumin as a strategy
to inhibit tumor progression. Mol Biol Rep. 41:4583–4594. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Guo Y, Li Y, Shan Q, He G, Lin J and Gong
Y: Curcumin potentiates the anti-leukemia effects of imatinib by
downregulation of the AKT/mTOR pathway and BCR/ABL gene expression
in Ph+ acute lymphoblastic leukemia. Int J Biochem Cell Biol.
65:1–11. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gupta M, Kumar A and Dabadghao S:
Resistance of bcr-abl-positive acute lymphoblastic leukemia to
daunorubicin is not mediated by mdr1 gene expression. Am J Hematol.
71:172–176. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kang MH, Kang YH, Szymanska B,
Wilczynska-Kalak U, Sheard MA, Harned TM, Lock RB and Reynolds CP:
Activity of vincristine, L-ASP, and dexamethasone against acute
lymphoblastic leukemia is enhanced by the BH3-mimetic ABT-737 in
vitro and in vivo. Blood. 110:2057–2066. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pimentel-Gutiérrez HJ, Bobadilla-Morales
L, Barba-Barba CC, Ortega-De-La-Torre C, Sánchez-Zubieta FA,
Corona-Rivera JR, González-Quezada BA, Armendáriz-Borunda JS,
Silva-Cruz R and Corona-Rivera A: Curcumin potentiates the effect
of chemotherapy against acute lymphoblastic leukemia cells via
downregulation of NF-κB. Oncol Lett. 12:4117–4124. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang J, Wang F, Li F, Zhang W, Shen Y,
Zhou D and Guo S: A multifunctional poly(curcumin) nanomedicine for
dual-modal targeted delivery, intracellular responsive release,
dual-drug treatment and imaging of multidrug resistant cancer
cells. J Mater Chem B. 4:2954–2962. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Langevin PB and Atlee JL: Chemotherapeutic
agents. Complications Anesth. 2:110–118. 2007. View Article : Google Scholar
|
|
31
|
Das K and White C: Activation of NF-kappaB
by antineoplastic agents. Role of protein kinase C. J Biol Chem.
272:14914–14920. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Demiray M, Sahinbas H, Atahan S, Demiray
H, Selcuk D, Yildirim I and Atayoglu A: Successful treatment of
c-kit-positive metastatic adenoid cystic carcinoma (ACC) with a
combination of curcumin plus imatinib: A case report. Complement
Ther Med. 27:108–113. 2016. View Article : Google Scholar : PubMed/NCBI
|