Introduction
Colorectal cancer remains one of the most common
malignant cancer worldwide and one of the leading causes of
cancer-related deaths. Although the most effective treatment for
colorectal cancer is surgery, which suppresses recurrence, patients
with stage II/III colorectal cancer receive adjuvant chemotherapy
after curative surgery (1).
5-Fluorouracil (5-FU) is one of the main chemotherapeutic agents
for treating cancer, and oral fluoropyrimidines are widely used as
postoperative adjuvant chemotherapy in Japan (2). However, factors that are predictive of
patient prognosis in colorectal cancer remain unclear.
To establish adjuvant chemotherapy as precision
medicine, it is necessary to clarify the relationship between the
expression levels of enzymes affected by 5-FU or those that
metabolise 5-FU or influence its effects on the one hand and the
prognosis after adjuvant chemotherapy on the other hand.
Thymidylate synthase (TS) is a target enzyme of 5-FU, and patients
with advanced colorectal cancer and low TS mRNA or protein
expression levels in primary tumours have been reported to respond
better to 5-FU therapy than those with high TS levels (3–8). In
contrast, low TS expression has been reported as a marker of poor
prognosis after 5-FU-based adjuvant chemotherapy (9–12).
Dihydropyrimidine dehydrogenase (DPD) is a 5-FU degrading enzyme,
and among patients with advanced head and neck cancer, those with
low DPD activity have experienced higher responses to 5-FU therapy
(13). In advanced colorectal
cancer, it has been reported that more accurate predictions of 5-FU
therapy have been achieved by measuring tumour DPD expression
level, in addition to those of TS and thymidine phosphorylase (TP)
(14–19). TP, also known as platelet-derived
endothelial cell growth factor, is a known angiogenesis factor
related to the metabolism of 5-FU. Similar to the findings for TS,
high TP mRNA expression levels in metastatic colorectal cancer have
been associated with poor antitumour effects of 5-FU (20,21). On
the other hand, it has also been reported that colon cancer
patients with high TP expression had a good prognosis after
adjuvant chemotherapy (7,22).
Folylpolyglutamate synthase (FPGS) is an enzyme that
polymerizes glutamic acid and reduces folate. Reduced folic acid
administered in vivo passes through the cell membrane as
mono-glutamate, and inside the cell, FPGS polymerises
mono-glutamate into polyglutamic acid, which is retained in the
cell. γ-glutamyl hydrolase (GGH) is an enzyme that hydrolyses
polyglutamate and reverses glutamate polymerisation activity of
FPGS. GGH gene expression levels are inversely proportional to
tumour tissue methylenetetrahydrofolate levels in patients with
colorectal cancer. Therefore, the FPGS and GGH ratio affects the
antitumour effect of 5-FU (23).
Dihydrofolate reductase (DHFR) is an enzyme that reduces
dihydrofolic acid, and the target of this enzyme, methotrexate, is
a folic acid antagonist. The level of DHFR mRNA expression in
tumour tissues has been reported to affect the strength of 5-FU
anticancer effects (24).
In the present study, we performed adjuvant
chemotherapy using oral fluoropyrimidines in patients with stage
II/III colorectal cancer and investigated the relationship between
expression levels of genes influencing 5-FU effects and
prognosis.
Patients and methods
Patients and clinical samples
A total of 63 patients with colorectal cancer who
underwent surgical treatment at the Yamaguchi University and
affiliated hospitals between October 2008 and March 2012 were
enrolled in this study. The inclusion criteria for this study
specified histologically confirmed adenocarcinoma of the colon and
rectum; Eastern Cooperative Oncology Group Performance Status Scale
(ECOG-PS) 0 or 1; preserved organ function; underwent curative
surgery; and possibility of administration of DPD inhibitor within
8 weeks after surgery. The exclusion criteria ruled out distant
metastases and other cancer diagnosis. Written informed consent was
obtained from all patients according to the Guidelines of the
Medical Ethics Committee of the Yamaguchi University School of
Medicine and approval was provided by Institutional Review Board of
Yamaguchi University Hospital and the affiliated hospitals. This
study is conducted in compliance with the principles of the
Declaration of Helsinki and is registered in the University
Hospital Medical Information Network Clinical Trials Registry in
Japan (no. UMIN000003252). Patient samples were used in accordance
with the Helsinki Declaration after written informed consent from
all patients had been obtained.
Adjuvant chemotherapy regimens
Adjuvant chemotherapy using oral fluoropyrimidines
was started within 6 weeks after surgery. The choice of the
chemotherapy regimen was made by the patient, in consultation with
the surgeon. In the uracil-tegafur (UFT)/leucovorin (LV) group, UFT
(300 mg/m2/day as tegafur) and LV (75 mg/day) were simultaneously
administered after meals, three times per day for 28 days, followed
by a 7-day rest. This cycle was repeated for five courses.
Following that treatment, UFT was administered after meals three
times per day for 18 months. In the tegafur/gimeracil/oteracil
(S-1) group, S-1 (80 mg/m2/day) was administered after meals twice
per day for 28 days, followed by a 14-day rest. This cycle was
repeated for four courses. Following that treatment, UFT was
administered after meals three times per day for 18 months.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Extracted fresh tissue specimens were fixed with 20%
formalin for 3–5 days at room temperature. Ten micrometre-thick
sections were obtained from the areas that were identified to have
the highest concentrations of tumour cells and mounted on uncoated
glass slides. For histological diagnosis, representative sections
were stained with haematoxylin-eosin (haematoxylin for 10 min and
eosin for 2 min) at room temperature. Before microdissection,
sections were deparaffinised in xylene for 10 min and hydrated with
100%, 95%, and, finally, 70% ethanol solutions. Sections were then
washed in water for 30 sec, stained with nuclear fast red (American
MasterTech Scientific, Lodi, CA) for 20 sec, and rinsed again in
water for 30 sec. Finally, samples were dehydrated with 70%, 95%,
and 100% ethanol solutions for 30 sec each, followed by xylene for
10 min. The slides were then completely air-dried. The sections of
interest were selectively isolated by laser capture microdissection
(P.A.L.M. Microsystem; Leica Microsystems GmbH, Wetzlar, Germany)
according to a standard procedure (25).
Blinded tissue samples for subsequent extractions
were placed in a 0.5-ml thin-walled tube containing 400 µl of 4 M
dithiothreitol-GITC/sarc (4 M guanidinium isothiocyanate, 50 mM
Tris-HCl, pH 7.5, 25 mM EDTA) (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA; no. 15577-018). The samples
were homogenised, and an additional 60 µl of GITC/sarc solution was
added. The samples were heated at 92°C for 30 min and then
transferred to a 2-ml centrifuge tube. Fifty microliters of 2 M
sodium acetate pH 4.0 was added, followed by 600 µl of freshly
prepared phenol/chloroform/isoamyl alcohol (250:50:1) mixture. The
tubes were vortexed for 15 sec, placed on ice for 15 min, and then
centrifuged at 13,000 × g for 8 min in a chilled (8°C) centrifuge.
The upper aqueous phase was carefully removed and placed in a
1.5-ml centrifuge tube. Glycogen (10 µl) and 300–400 µl of
isopropanol were added, and the samples were vortexed for 10–15
sec. The tubes were chilled at −20°C for 30–45 min to precipitate
RNA. The samples were then centrifuged at 13,000 × g for 7 min at
8°C. The supernatant was discarded, and 500 µl of 75% ethanol was
added. The tubes were again centrifuged at 13,000 × g for 6 min in
a chilled (8°C) centrifuge. The supernatant was then carefully
poured off, so as not to disturb the RNA pellet, and the samples
were quick-spun for 15 sec at 13,000 × g. The remaining ethanol was
removed, and the samples were left to air-dry for 15 min. The
pellet was resuspended in 50 µl of 5 mM Tris. Finally, cDNA was
prepared based on the method by Lord et al (26). For cDNA synthesis, 20 µl 5X Moloney
murine leukemia virus (MMLV) buffer [containing 250 mmol/l Tris-HCl
(pH 8.3), 375 mmol/l KCl, and 15 mmol/l MgCl2; Thermo
Fisher Scientific, Inc.], 10 µl dithiothreitol (100 mmol/l; Thermo
Fisher Scientific, Inc.), 10 µl dNTP (each 10 mmol/l; Amersham
Pharmacia Biotech), 0.5 µl random hexamers [50 OD dissolved in 550
µl of 10 mmol/l Tris-HCl (pH 7.5), and 1 mmol/l EDTA; Amersham
Pharmacia Biotech, Piscataway, NJ, USA], 2.5 µl bovine serum
albumin [3 mg/ml in 10 mmol/l Tris-HC1 (pH 7.5); Amersham Pharmacia
Biotech], 2.5 µl RNAse inhibitor (5 × 1,000 units; Amersham
Pharmacia Biotech), and 5 µl MMLV reverse transcriptase (200 U/µl;
Thermo Fisher Scientific, Inc.), added to a total volume of 50.5
µl.
Quantification of six genes of interest and an
internal reference gene encoding β-actin was performed using the
fluorescence-based real-time detection method by a ABI PRISM 7900
Sequence detection system (Perkin-Elmer Applied Biosystems; Thermo
Fisher Scientific, Inc.). PCR reaction mixture consisted of 1,200
nM of each primer; 200 nM of the probe; 0.4 U of AmpliTaq gold
polymerase; 200 nM of dATP, dCTP, dGTP and dTTP; and 3.5 mM of
MgCl2 and 1X TaqMan® buffer A, containing a
reference dye. The final volume of the reaction mixture was 20 µl
(all reagents from Perkin-Elmer Applied Biosystems; Thermo Fisher
Scientific, Inc.). Cycling conditions were 50°C for 2 min, 95°C for
10 min, and 46 cycles of 95°C for 15 sec and 60°C for 1 min. The
primers and probes used listed in Table
I. TaqMan® measurements yield Cq values that are
inversely proportional to the amount of cDNA in the tube. For
example, a higher Cq value means that more PCR cycles are required
to reach a certain level of cDNA detection. Gene expression values
(relative mRNA levels) are expressed as ratios (differences between
the Ct values) between the levels of gene of interest and that of
the internal reference gene (β-actin). The reference gene provides
a baseline measurement for the amount of RNA isolated from a
specimen. Taiho Pharmaceutical, Co., Ltd. (Tokyo, Japan) quantified
gene expression by RT-qPCR.
 | Table I.Sequences of the primers for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Sequences of the primers for reverse
transcription-quantitative polymerase chain reaction.
| Gene symbol | Gene Name | Gene ID | F-Primer sequence,
5′-3′ | R-Primer sequence,
5′3′ | Probe sequence,
5′-3′ |
|---|
| TS (TYMS) | Thymidylate
Synthase | NM_001071.1 |
GCCTCGGTGTGCCTTTCA |
CCCGTGATGTGCGCAAT |
TCGCCAGCTACGCCCTGCTCA |
| DPD (DPYD) | Dihydropyrimidine
dehydrogenase | NM_000110.3 |
AGGACGCAAGGAGGGTTTG |
GTCCGCCGAGTCCTTACTGA |
CAGTGCCTACAGTCTCGAGTCTGCCAGTG |
| TP (TYMP) | Thymidine
phosphorylase | NM_001953.2 |
CCTTGGATAAGCTGGAGTCTATTCC |
CCTGGTCCAGCAGCACTTG |
TCAATGTCATCCAGAGCCCAGAGCAGAT |
| FPGS | Folylpolyglutamate
synthetase | NM_004957.4 |
GGCTGGAGGAGACCAAGGAT |
CATGAGTGTCAGGAAGCGGA |
CAGCTGTGTCTCCATGCCCCCCTAC |
| GGH | γ-glutamyl
hydrolase | NM_003878.1 |
GTGGCAATGCCGCTGAA |
CAACTCAGTAGGAAAATTCTGGAACA |
TTCACTGGAGGTCAATTGCACAGCAGA |
| DHFR | Dihydrofolate
reductase | NM_000791.3 |
GTCCTCCCGCTGCTGTCA |
GCCGATGCCCATGTTCTG |
TTCGCTAAACTGCATCGTCGCTGTGTC |
| ACTB | Actin, β | NM_001101.2 |
GAGCGCGGCTACAGCTT |
TCCTTAATGTCACGCACGATTT |
ACCACCACGGCCGAGCGG |
Statistical analysis
Relapse-free survival (RFS) was defined as the time
from the day of the surgery to the documented disease recurrence or
death. Kaplan-Meier curves were used to estimate survival, and they
were compared using the log-rank test. Each biomarker cut-off value
was set at the median point. Differences between groups were
analysed using the Student's t-test or χ2 test. The Cox
proportional hazards regression model was used to identify
variables associated with RFS. All statistical analyses were
performed using SPSS (version 20; IBM Corp., Armonk, NY, USA) with
a significance level of α=0.05 (P<0.05).
Results
Patients
From October 2008 and March 2012, a total of 63
patients met our inclusion criteria and were included for analysis.
There were 45 men and 18 women, with a mean age of 68.0±9.3 years.
The median follow-up period for these patients was 73.2 months
(5.4–105.2 months). A total of 42 patients received adjuvant
chemotherapy with UFT/LV, and 21 patients received adjuvant
chemotherapy with S-1.
Patients in the recurrence and no-recurrence groups
had significantly different gender ratio (P=0.04) and
tumour-node-metastasis (TNM) stage (P=0.001; Table II).
 | Table II.Patient characteristics and
clinicopathological parameters. |
Table II.
Patient characteristics and
clinicopathological parameters.
| Clinicopathological
variable | Recurrence, n=14 | No-recurrence,
n=49 | P-value |
|---|
| Age | 65.4±10.5 | 68.7±9.0 | 0.29 |
| Sex |
|
| 0.04 |
| Male | 13 | 32 |
|
|
Female | 1 | 17 |
|
| ECOG-PS |
|
| 0.81 |
| 0 | 13 | 43 |
|
| 1 | 1 | 5 |
|
| 2 | 0 | 1 |
|
| Tumor of
location |
|
| 0.13 |
|
Right | 3 | 19 |
|
|
Left | 4 | 19 |
|
|
Rectum | 7 | 11 |
|
| Size of tumor,
cm | 5.4±2.1 | 5.8±2.1 | 0.52 |
| Histological
grade |
|
| 0.56 |
|
Tub1 | 2 | 13 |
|
|
Tub2 | 10 | 32 |
|
| Muci,
poor | 2 | 4 |
|
| Lymphatic
invasion |
|
| 0.05 |
|
Present | 14 | 38 |
|
|
Absent | 0 | 11 |
|
| Venous
invasion |
|
| 0.06 |
|
Present | 10 | 21 |
|
|
Absent | 4 | 28 |
|
| T stage |
|
| 0.42 |
| 2 | 2 | 3 |
|
| 3 | 7 | 33 |
|
| 4 | 5 | 13 |
|
| TNM stage |
|
| 0.001 |
| 2 | 1 | 29 |
|
| 3a | 8 | 16 |
|
| 3b | 5 | 4 |
|
| Adjuvant
chemotherapy |
|
| 0.83 |
|
UFT/LV | 9 | 33 |
|
|
S-1 | 5 | 16 |
|
Comparison of the expression levels of
genes modulating 5-FU effects in primary colorectal cancer tissues
from the recurrence and no-recurrence groups
To investigate the expression levels of six genes
modulating 5-FU effects in 63 primary colorectal cancer tissue
samples, RT-qPCR was performed. The association between respective
expression levels in the primary colorectal tumours of recurrence
group and those of no-recurrence group was then investigated as
presented in Fig. 1. TP expression
in recurrence group tumours was significantly lower than that in
no-recurrence group tumours (P=0.03; Fig. 1C). Expression levels of other studied
genes were not statistically different between recurrence and
no-recurrence groups (Fig. 1).
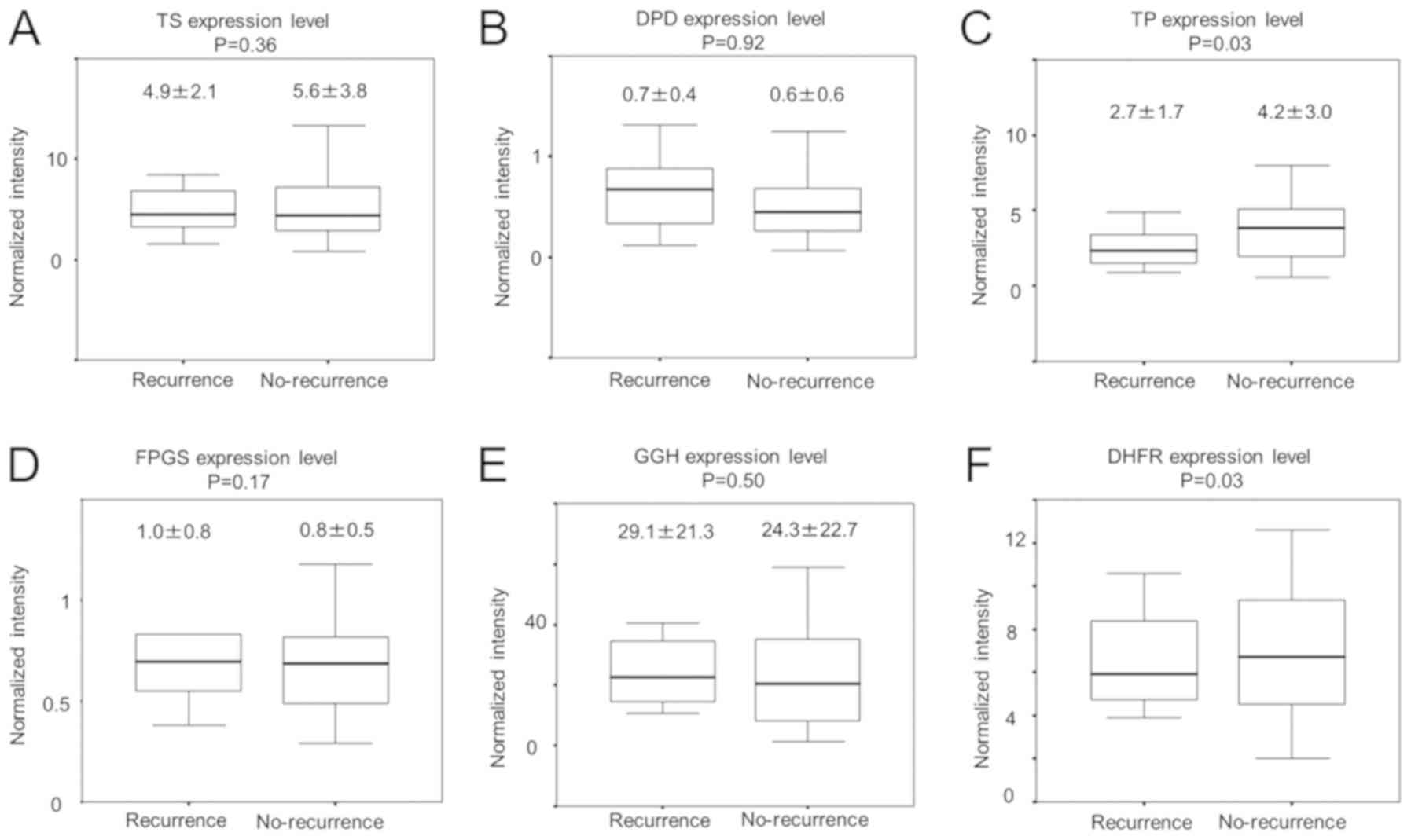 | Figure 1.Comparison of the expression levels of
genes known to influence 5-FU effects in primary colorectal cancer
tissues, according to recurrence status. Expression levels of genes
encoding (A) TS, (B) DPD, (C) TP, (D) FPGS, (E) GGH and (F) DHFR
are compared in recurrence and no-recurence groups. TS, thymidylate
synthase; DPD, dihydropyrimidine dehydrogenase; TP, thymidine
phosphorylase; FPGS, folylpolyglutamate synthetase; GGH, γ-glutamyl
hydrolase; DHFR, dihydrofolate reductase. |
The association between the expression of each of
the six genes of interest and clinicopathological parameters was
then investigated. No significant correlations of the expression
levels of these genes with any of the investigated
clinicopathological parameters, including age, gender, tumour
location, histological grade, invasion depth, lymphatic metastasis,
lymphatic invasion, venous invasion or TNM stage were found (data
are not shown).
Association between expression levels
of genes modulating 5-FU effects and RFS
To investigate whether expression levels of genes
modulating 5-FU effects in primary colorectal cancer tissues were
associated with RFS following adjuvant chemotherapy with oral
fluoropyrimidines, Kaplan-Meier analyses were performed as
presented in Fig. 2. Kaplan-Meier
analysis revealed that patients with low TP expression level
experienced significantly shorter 5-year RFS (65.2 vs. 90.2%,
log-rank P=0.03) than those with high TP expression level (Fig. 2C), whilst expression levels of other
five genes examined did not affect RFS (Fig. 2).
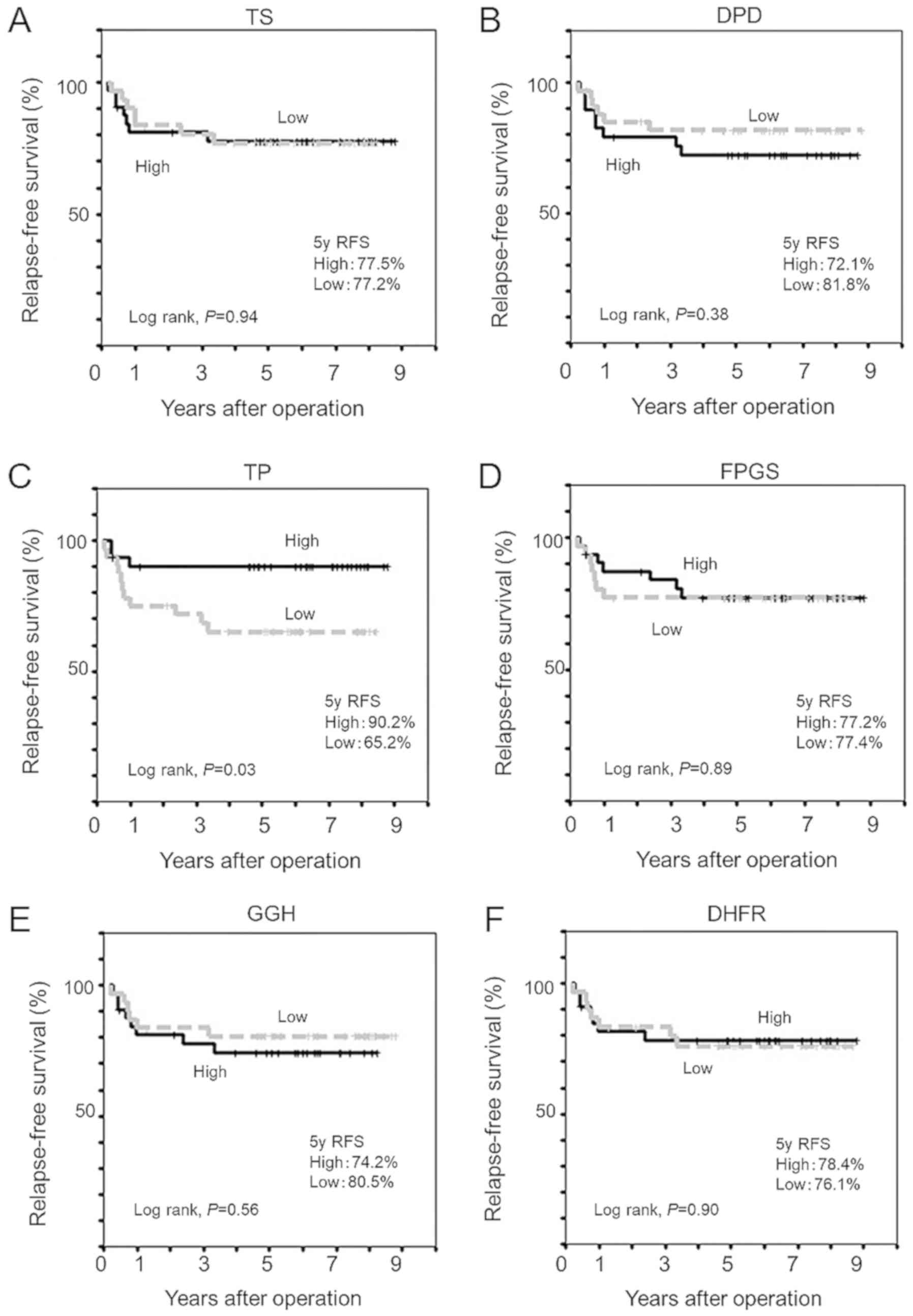 | Figure 2.Kaplan-Meier estimates of relapse-free
survival curves for colorectal cancer patients following adjuvant
chemotherapy using a dihydropyrimidine dehydrogenase inhibitor.
Relapse-free survival according to expression levels of genes
encoding (A) TS, (B) DPD, (C) TP, (D) FPGS, (E) GGH and (F) DHFR.
TS, thymidylate synthase; DPD, dihydropyrimidine dehydrogenase; TP,
thymidine phosphorylase; FPGS, folylpolyglutamate synthetase; GGH,
γ-glutamyl hydrolase; DHFR, dihydrofolate reductase. |
Recurrence risk factor
In the univariate Cox proportional hazards
regression analyses, the following variables were significantly
associated with worse RFS: venous invasion [present; hazard ratio
(HR)=3.22, 95% confidence interval (CI): 1.01–10.3; P=0.049], TNM
stage (3b; HR=5.19, 95% CI: 1.73–15.6; P=0.003), and TP expression
(low; HR=3.86, 95% CI: 1.08–13.9; P=0.04; Table III). Furthermore, in the
multivariate analyses based on the stepwise Cox model, venous
invasion (present; HR=6.51, 95% CI: 1.55–27.4; P=0.01), TNM stage
(3b; HR=6.18, 95% CI: 1.36–28.2; P=0.02) and TP (low; HR=9.61, 95%
CI: 1.81–51.0; P=0.04) remained significantly associated with worse
RFS (Table III). We performed
power analysis and Power was 0.34 in Cox proportional hazards
regression of this study.
 | Table III.Univariate and multivariate analyses
of clinicopathological factors for correlations with relapse-free
survival rate following adjuvant chemotherapy using oral
fluoropyrimidines. |
Table III.
Univariate and multivariate analyses
of clinicopathological factors for correlations with relapse-free
survival rate following adjuvant chemotherapy using oral
fluoropyrimidines.
| Clinicopathological
variable | n (%) | Univariate
analysis, HR (95% CI) | P-value | Multivariate
analysis, HR (95% CI) | P-value |
|---|
| Age |
|
≥70 | 28 (44.4) | 0.71
(0.24–2.13) | 0.550 |
|
|
| Sex |
|
Male | 45 (71.4) | 5.51
(0.72–42.2) | 0.100 | 1.9
(0.21–16.9) | 0.570 |
| ECOG-PS |
| ≥1 | 7
(11.1) | 0.67
(0.09–5.09) | 0.690 |
|
|
| Tumor of
location |
|
Rectum | 18 (28.6) | 2.79
(0.98–7.98) | 0.060 | 1.93
(0.64–5.80) | 0.240 |
| Size of tumor |
| ≥5 | 43 (68.3) | 0.8
(0.27–2.38) | 0.680 |
|
|
| Histological
grade |
|
Poor | 6 (9.5) | 2.4
(0.54–10.8) | 0.250 |
|
|
| Lymphatic
invasion |
|
Present | 52 (82.5) | NE |
|
|
|
| Venous
invasion |
|
Present | 31 (49.2) | 3.22
(1.01–10.3) | 0.049 | 6.51
(1.55–27.4) | 0.010 |
| T stage |
| ≥4 | 18 (28.6) | 1.47
(0.49–4.38) | 0.490 |
|
|
| TNM stage |
| 3b | 9
(14.3) | 5.19
(1.73–15.6) | 0.003 | 6.18
(1.36–28.2) | 0.020 |
| Adjuvant
chemotherapy |
|
S-1 | 21 (33.3) | 1.02
(0.34–3.06) | 0.970 |
|
|
| TS |
|
Low | 31 (49.2) | 1.04
(0.34–2.74) | 0.940 |
|
|
| DPD |
|
High | 29 (46.0) | 1.61
(0.56–4.63) | 0.380 |
|
|
| TP |
|
Low | 32 (50.8) | 3.86
(1.08–13.9) | 0.040 | 9.61
(1.81–51.0) | 0.008 |
| FPGS |
|
Low | 31 (49.2) | 1.07
(0.38–3.06) | 0.890 |
|
|
| GGH |
|
Low | 31 (49.2) | 0.73
(0.25–2.11) | 0.560 |
|
|
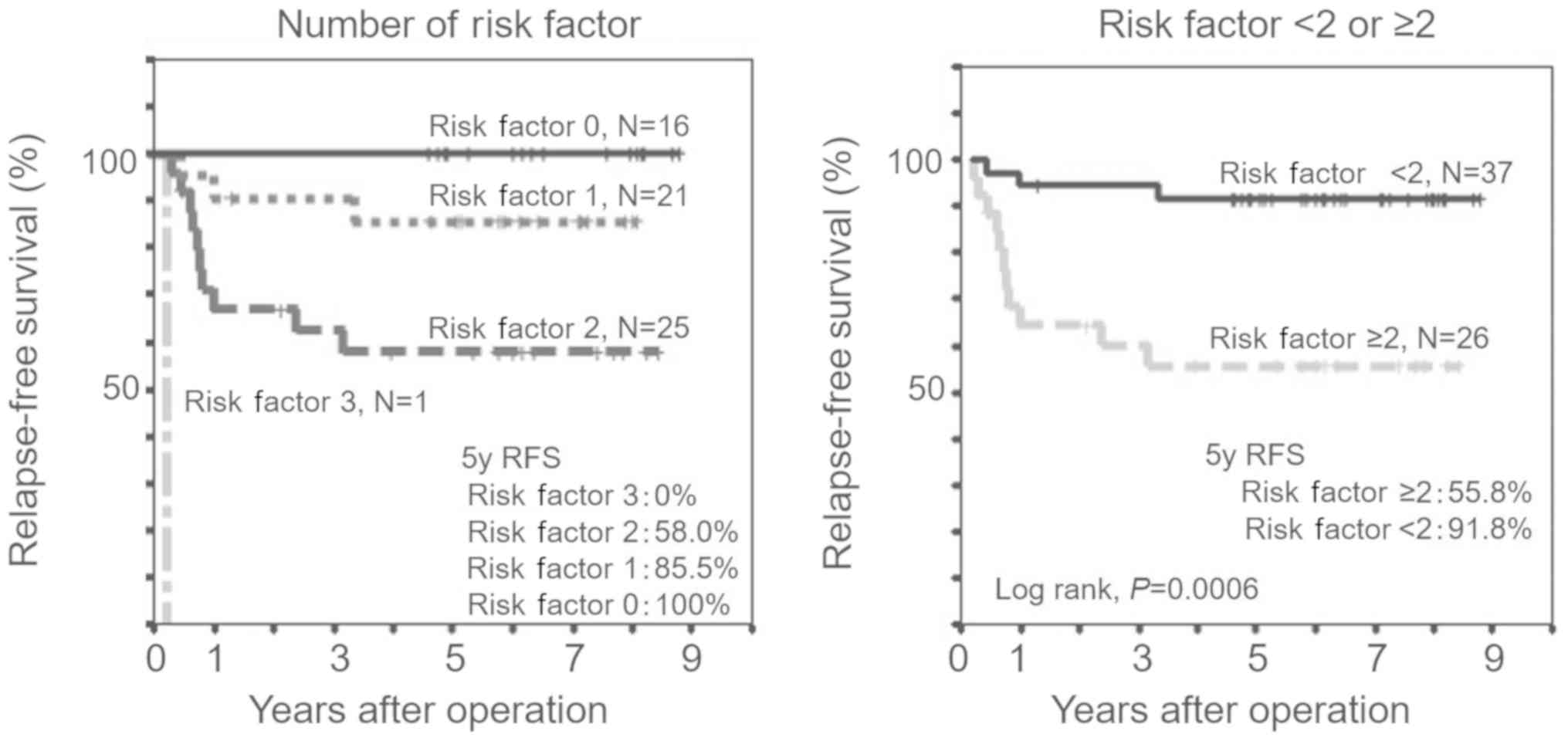
Recurrence risk characteristic
strata
The three significant predictors from previous
elimination analyses, namely venous invasion, TNM stage and TP
expression level, were selected to generate the recurrence risk
prediction model. The association between the number of risk
features and RFS was also clearly indicated by the Kaplan-Meier
curves (Fig. 3A). In addition, a
clear inflection point towards worse outcomes was observed when
patients had two or more risk characteristics. The patients with
two or more risk characteristics had significantly shorter 5-year
RFS (55.8 vs. 91.8%, log-rank P=0.0006) than those with one or no
risk characteristics (Fig. 3B).
Discussion
This study was designed to evaluate the relationship
between expression levels of genes that are known to modulate 5-FU
effects and prognosis in patients with stage II/III colorectal
cancer after adjuvant chemotherapy with oral fluoropyrimidines.
First, we investigated the relationship between the expression
levels of the chosen six genes and recurrence status. Our data
showed that only tumour TP expression in the recurrence group was
significantly lower than that in the no-recurrence group.
Additionally, RFS was significantly shorter in patients with low TP
expression than in those with high TP expression.
TP has angiogenic activity and is one of the key
metabolic enzymes of 5-FU (21,27).
There have been contradictory reports about the relationship
between TP expression and colorectal cancer prognosis (27). It has been reported that patients
with high TP expression have poor prognosis (20,21). On
the other hand, it has also been demonstrated that TP amplifies the
sensitivity to anticancer agents (18,22). The
reason with such different reports is because TP has dual roles in
cancer tissues. TP associated a promotion of angiogenesis and
metastasis. However, high TP expression in tumor tissue may
increase concentration of 5-FU in cancer tissues thorough
angiogenesis and may contribute to the sensitivity of the DPD
inhibitor through promoting phosphorylation of 5-FU. Recent
meta-analysis indicated that low TP expression was associated with
poor prognosis in 5-FU-based adjuvant chemotherapy (28). Consistent with this, our findings
suggest that patients with low levels of TP mRNA in primary tumours
had worse RFS than those with high levels of TP when adjuvant
chemotherapy with oral fluoropyrimidine was administered. For
patients with high TP levels, the recurrence rate may improve with
the DPD inhibitor. Patients with low TP may not be monotherapy but
may need doublet therapy, such as oxaliplatin or irinotecan.
However, the effect of combination therapy with TP has not been
studied. Further research is necessary in the future. The reason
why patients with low TP expression have poor prognosis is still
unclear. Further research is necessary to reveal why TP is a
prognostic factor.
In this study, we also examined the impact of other
genes influencing 5-FU effects, namely those encoding TS, DPD,
FPGS, GGH and DHFR. No significant associations of their expression
levels with prognosis or clinical outcomes were found.
Our most significant finding was that the number of
high risk factors present form strata, which incrementally
associate with recurrence in patients with stage II/III colon
cancer, which received adjuvant chemotherapy with oral
fluoropyrimidines. The venous invasion, TNM stage, and tumour TP
expression were identified as significant predictive factors for
RFS by multivariate analysis in the present prospective study. For
patients with no risk factors or only one of them, the 5-year RFS
rate was 91.8%, which indicated successful suppression of the
recurrence by the treatment. In contrast, for the patients with two
or three risk factors, the 5-year RFS rate was 55.8%. These
patients may potentially benefit from receiving adjuvant
chemotherapy, including oxaliplatin.
The current study had some limitations. First, we
included a relatively small number of patients. Next, TP expression
was inferred from mRNA levels in this study. TP activity and
protein expression levels have not been investigated. The
possibility is that expression levels of the protein does not
accord with expression of the RNA. Another possibility is that the
RT-PCR assessment is based on a small portion of the tumor field,
but the whole field. It was wished I examined an evaluation in
western blotting and immunohistochemistry. It is necessary to
examine an evaluation in western blotting and immunohistochemistry.
Moreover, our study included several adjuvant chemotherapy
regimens, which were not randomised.
In conclusion, the present study revealed that low
TP expression predicts poor colorectal cancer prognosis. Moreover,
we succeeded in stratifying recurrence risk by identifying
combinations of parameters such as venous invasion, TNM stage and
tumour expression of TP, a 5-FU metabolizing enzyme, which were
associated with poor prognosis in colorectal cancer.
Acknowledgements
The authors thank Dr T. Kato (Yamaguchi Rosai
Hospital, Sanyo-Onoda, Japan), Dr A. Seyama (Syuto General
Hospital, Yanai, Japan), Dr T. Takahashi (Yamaguchi Saiseikai
Yamaguchi General Hospital, Yamaguchi, Japan), Dr K. Fujioka
(Sanyo-Onoda Municipal Hospital, Sanyo-Onoda, Japan), Dr M. Orita
(Hikari Municipal Hikari General Hospital, Hikari, Japan), Dr Y.
Minami (Yamaguchi Saiseikai Shimonoseki General Hospital,
Shimonoseki, Japan), Dr T. Kuga (Nagato General Hospital, Nagato,
Japan), Dr S. Noshima (Yamaguchi Prefectural Grand Medical Center,
Hofu, Japan), Dr S. Kobayashi (Ehime Rosai Hospital, Niihama,
Japan), Dr M. Harada (Hikari Municipal Yamato General Hospital,
Hikari, Japan) and Dr N. Akiyama (Tokuyama Central Hospital,
Syunan, Japan) for their assistance in acquiring samples and
collecting information from the patients with colorectal
cancer.
Funding
The study was supported by Taiho Pharmaceutical,
Co., Ltd. (Tokyo, Japan).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
EH and KH contributed to the design and conception
of the present study. NK, YT, YS and AS analyzed and interpreted
the patient data.
Ethics approval and consent to
participate
Written informed consent was obtained from all
patients, according to the Guidelines of the Medical Ethics
Committee of the Yamaguchi University School of Medicine
(Yamaguchi, Japan) and approval was provided by the Institutional
Review Board of Yamaguchi University Hospital (Ube, Japan) and the
affiliated hospitals. The present study was conducted in compliance
with the principles of the Declaration of Helsinki and is
registered in the University Hospital Medical Information Network
Clinical Trials Registry in Japan (no. UMIN000003252).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
5-FU
|
5-fluorouracil
|
|
DHFR
|
dihydrofolate reductase
|
|
DPD
|
dihydropyrimidine dehydrogenase
|
|
FPGS
|
folylpolyglutamate synthetase
|
|
GGH
|
γ-glutamyl hydrolase
|
|
HR
|
hazard ratio
|
|
RFS
|
relapse-free survival
|
|
TNM
|
tumour-node-metastasis
|
|
TP
|
thymidine phosphorylase
|
|
TS
|
thymidylate synthase
|
References
|
1
|
Twelves C, Wong A, Nowacki MP, Abt M,
Burris H III, Carrato A, Cassidy J, Cervantes A, Fagerberg J,
Georgoulias V, et al: Capecitabine as adjuvant treatment for stage
III colon cancer. N Engl J Med. 352:2696–2704. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shimada Y, Hamaguchi T, Mizusawa J, Saito
N, Kanemitsu Y, Takiguchi N, Ohue M, Kato T, Takii Y, Sato T, et
al: Randomised phase III trial of adjuvant chemotherapy with oral
uracil and tegafur plus leucovorin versus intravenous fluorouracil
and levofolinate in patients with stage III colorectal cancer who
have undergone Japanese D2/D3 lymph node dissection: Final results
of JCOG0205. Eur J Cancer. 50:2231–2240. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Leichman CG, Lenz H-J, Leichman L,
Danenberg K, Baranda J, Groshen S, Boswell W, Metzger R, Tan M and
Danenberg PV: Quantitation of intratumoral thymidylate synthase
expression predicts for disseminated colorectal cancer response and
resistance to protracted-infusion fluorouracil and weekly
leucovorin. J Clin Oncol. 15:3223–3229. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lenz H-J, Hayashi K, Salonga D, Danenberg
KD, Danenberg PV, Metzger R, Banerjee D, Bertino JR, Groshen S,
Leichman LP, et al: p53 point mutations and thymidylate synthase
messenger RNA levels in disseminated colorectal cancer: An analysis
of response and survival. Clin Cancer Res. 4:1243–1250.
1998.PubMed/NCBI
|
|
5
|
Bathe OF, Franceschi D, Livingstone AS,
Moffat FL, Tian E and Ardalan B: Increased thymidylate synthase
gene expression in liver metastases from colorectal carcinoma:
Implications for chemotherapeutic options and survival. Cancer J
Sci Am. 5:34–40. 1999.PubMed/NCBI
|
|
6
|
Paradiso A, Simone G, Petroni S, Leone B,
Vallejo C, Lacava J, Romero A, Machiavelli M, De Lena M, Allegra
CJ, et al: Thymidilate synthase and p53 primary tumour expression
as predictive factors for advanced colorectal cancer patients. Br J
Cancer. 82:560–567. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fujii R, Seshimo A and Kameoka S:
Relationships between the expression of thymidylate synthase,
dihydropyrimidine dehydrogenase, and orotate
phosphoribosyltransferase and cell proliferative activity and
5-fluorouracil sensitivity in colorectal carcinoma. Int J Clin
Oncol. 8:72–78. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ciaparrone M, Quirino M, Schinzari G,
Zannoni G, Corsi DC, Vecchio FM, Cassano A, La Torre G and Barone
C: Predictive role of thymidylate synthase, dihydropyrimidine
dehydrogenase and thymidine phosphorylase expression in colorectal
cancer patients receiving adjuvant 5-fluorouracil. Oncology.
70:366–377. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Johnston PG, Fisher ER, Rockette HE,
Fisher B, Wolmark N, Drake JC, Chabner BA and Allegra CJ: The role
of thymidylate synthase expression in prognosis and outcome of
adjuvant chemotherapy in patients with rectal cancer. J Clin Oncol.
12:2640–2647. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Takenoue T, Nagawa H, Matsuda K, Fujii S,
Nita ME, Hatano K, Kitayama J, Tsuruo T and Muto T: Relation
between thymidylate synthase expression and survival in colon
carcinoma, and determination of appropriate application of
5-fluorouracil by immunohistochemical method. Ann Surg Oncol.
7:193–198. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Edler D, Glimelius B, Hallström M,
Jakobsen A, Johnston PG, Magnusson I, Ragnhammar P and Blomgren H:
Thymidylate synthase expression in colorectal cancer: A prognostic
and predictive marker of benefit from adjuvant fluorouracil-based
chemotherapy. J Clin Oncol. 20:1721–1728. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Donada M, Bonin S, Nardon E, De Pellegrin
A, Decorti G and Stanta G: Thymidilate synthase expression predicts
longer survival in patients with stage II colon cancer treated with
5-flurouracil independently of microsatellite instability. J Cancer
Res Clin Oncol. 137:201–210. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Etienne MC, Chéradame S, Fischel JL,
Formento P, Dassonville O, Renée N, Schneider M, Thyss A, Demard F
and Milano G: Response to fluorouracil therapy in cancer patients:
The role of tumoral dihydropyrimidine dehydrogenase activity. J
Clin Oncol. 13:1663–1670. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Salonga D, Danenberg KD, Johnson M,
Metzger R, Groshen S, Tsao-Wei DD, Lenz HJ, Leichman CG, Leichman
L, Diasio RB, et al: Colorectal tumors responding to 5-fluorouracil
have low gene expression levels of dihydropyrimidine dehydrogenase,
thymidylate synthase, and thymidine phosphorylase. Clin Cancer Res.
6:1322–1327. 2000.PubMed/NCBI
|
|
15
|
Lassmann S, Hennig M, Rosenberg R, Nährig
J, Schreglmann J, Krause F, Poignee-Heger M, Nekarda H, Höfler H
and Werner M: Thymidine phosphorylase, dihydropyrimidine
dehydrogenase and thymidylate synthase mRNA expression in primary
colorectal tumors-correlation to tumor histopathology and clinical
follow-up. Int J Colorectal Dis. 21:238–247. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tokunaga Y, Sasaki H and Saito T: Clinical
role of orotate phosphoribosyl transferase and dihydropyrimidine
dehydrogenase in colorectal cancer treated with postoperative
fluoropyrimidine. Surgery. 141:346–353. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ochiai T, Umeki M, Miyake H, Iida T,
Okumura M, Ohno K, Sakamoto M, Miyoshi N, Takahashi M, Tsumura H,
et al: Impact of 5-fluorouracil metabolizing enzymes on
chemotherapy in patients with resectable colorectal cancer. Oncol
Rep. 32:887–892. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mori T, Ohue M, Takii Y, Hashizume T, Kato
T, Kotake K, Sato T and Tango T: Factors predicting the response to
oral fluoropyrimidine drugs: A phase II trial on the
individualization of postoperative adjuvant chemotherapy using oral
fluorinated pyrimidines in stage III colorectal cancer treated by
curative resection (ACT-01 Study). Oncol Rep. 29:437–444. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Koda K, Miyauchi H, Kosugi C, Kaiho T,
Takiguchi N, Kobayashi S, Maruyama T and Matsubara H; Boso Clinical
Oncology Group, : Tumor 5-FU-related mRNA expression and efficacy
of oral fluoropyrimidines in adjuvant chemotherapy of colorectal
cancer. Anticancer Res. 36:5325–5331. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Metzger R, Danenberg K, Leichman CG,
Salonga D, Schwartz EL, Wadler S, Lenz HJ, Groshen S, Leichman L
and Danenberg PV: High basal level gene expression of thymidine
phosphorylase (platelet-derived endothelial cell growth factor) in
colorectal tumors is associated with nonresponse to 5-fluorouracil.
Clin Cancer Res. 4:2371–2376. 1998.PubMed/NCBI
|
|
21
|
Tokunaga Y, Hosogi H, Hoppou T, Nakagami
M, Tokuka A and Ohsumi K: Prognostic value of thymidine
phosphorylase/platelet-derived endothelial cell growth factor in
advanced colorectal cancer after surgery: Evaluation with a new
monoclonal antibody. Surgery. 131:541–547. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Saito S, Tsuno N, Nagawa H, Sunami E,
Zhengxi J, Osada T, Kitayama J, Shibata Y, Tsuruo T and Muto T:
Expression of platelet-derived endothelial cell growth factor
correlates with good prognosis in patients with colorectal
carcinoma. Cancer. 88:42–49. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Odin E, Wettergren Y, Nilsson S, Willén R,
Carlsson G, Spears CP, Larsson L and Gustavsson B: Altered gene
expression of folate enzymes in adjacent mucosa is associated with
outcome of colorectal cancer patients. Clin Cancer Res.
9:6012–6019. 2003.PubMed/NCBI
|
|
24
|
Satta T, Isobe K, Yamauchi M, Nakashima I,
Akiyama S, Itou K, Watanabe T and Takagi H: Establishment of drug
resistance in human gastric and colon carcinoma xenograft lines.
Jpn J Cancer Res. 82:593–598. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bonner RF, Emmert-Buck M, Cole K, Pohida
T, Chuaqui R, Goldstein S and Liotta LA: Laser capture
microdissection: Molecular analysis of tissue. Science.
278:1481–1483, 1483. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lord RV, Salonga D, Danenberg KD, Peters
JH, DeMeester TR, Park JM, Johansson J, Skinner KA, Chandrasoma P,
DeMeester SR, et al: Telomerase reverse transcriptase expression is
increased early in the Barrett's metaplasia, dysplasia,
adenocarcinoma sequence. J Gastrointest Surg. 4:135–142. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bronckaers A, Gago F, Balzarini J and
Liekens S: The dual role of thymidine phosphorylase in cancer
development and chemotherapy. Med Res Rev. 29:903–953. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Che J, Pan L, Yang X, Liu Z, Huang L, Wen
C, Lin A and Liu H: Thymidine phosphorylase expression and
prognosis in colorectal cancer treated with 5-fluorouracil-based
chemotherapy: A meta-analysis. Mol Clin Oncol. 7:943–952.
2017.PubMed/NCBI
|