Introduction
PIM2 proto-oncogene, serine/threonine kinase (PIM2)
was first identified in mice with lymphoma. PIM2 belongs to the
proviral integration of Moloney virus (Pim) family of
serine/threonine kinases, which also includes PIM1 and PIM3
(1). PIM2 serves an essential role
in cell cycle regulation and cell proliferation and is involved in
the malignant phenotypes of different types of cancer cells; in
particular, PIM2 phosphorylates a wide range of cellular proteins
and it is highly expressed in different types of tumors, including
solid tumors (tumors of the prostate (2) and digestive system) (3) and hematological diseases including
leukemia, lymphoma (4) and multiple
myeloma (5). A previous study
demonstrated that the PIM2 expression level was increased in cell
lines derived from solid tumors (the A549 and H1299 lung cancer
cell lines) and hematopoietic malignancies (the K562 leukemia cell
line and RPMI-8226 multiple myeloma cell line) (6). The aforementioned study revealed that
the downregulation of PIM2 resulted in cell cycle arrest in the
G0/G1 phase. PIM2 regulates the cell cycle by
inhibiting cyclin-dependent kinase 2 (CDK2) and phosphorylated
retinoblastoma protein (pRb) expression levels via upregulation of
cyclin dependent kinase inhibitor 1A (CDKN1A), which is a negative
modulator of cell cycle progression. In addition, downregulation of
PIM2 led to the downregulation of nuclear factor κβ (NF-κβ) which
serves several roles in inflammatory responses, cell proliferation
and tumorigenesis (6). PIM2
activates apoptosis inhibitor 5 (API-5) to inhibit apoptosis in
hepatocellular carcinoma cells via the NF-κβ signaling pathway
(7). Thus, NF-κβ may be a key
downstream target of PIM2, which is consistent with the results of
other studies (4). PIM2 may serve a
role in the pathogenesis of myelodysplastic syndromes (MDS), a
group of clonal malignant hematopoietic disorders characterized by
ineffective hematopoiesis and an increased risk of malignant
transformation. MDS are stem-cell disorders in which blockade of
hematopoietic stem cell (HSC) maturation may give rise to different
diseases, including acute myeloid leukemia (AML) (8,9).
Isocitrate dehydrogenase (IDH) mutations occur in a
small proportion of patients with acute AML (10) and MDS (11). A previous study revealed an adverse
prognostic effect for IDH1 mutants in MDS (12). The IDH family consists of three
catalytic isozymes: IDH1, IDH2 and IDH3 (13). Overexpression of IDH1 mutants in
cultured U-87MG cells suppressed the activity of wild-type IDH1 via
the formation of heterodimers, resulting in decreased levels of the
enzyme product, α-ketoglutarate (α-KG), which is essential for
prolyl hydroxylase (PHD) activity, which promotes hypoxia inducible
factor 1 subunit α (HIF1A) degradation (14). The aforementioned results were
consistent with those of a previous study, which revealed that the
expression of the IDH1 R132H mutant increased HIF1A levels in cells
and increased cell proliferation (14). Furthermore, it was demonstrated that
the IDH1 R132H mutant activated the NF-κβ signaling pathway, which
is frequently dysregulated in cancer (15). Wang et al (16) reported that IDH1 mutants promoted the
proliferation of glioma cells via activation of NF-κβ in a
HIF1A-dependent manner. HIF1A expression was correlated with poor
overall survival and worse disease progression in MDS (17). In addition, the correlation analysis
revealed that the expression of HIF1A was associated with the
percentage of bone marrow blasts; i.e., the group exhibiting HIF1A
expression had a greater percentage of bone marrow blasts (17). The results obtained in these studies
suggest that there may be a correlation between HIFIA and PIM2 in
the pathogenesis of MDS. The aim of the current study was to
investigate the association between PIM2 and the IDH1/HIF1A
signaling pathway in regulating the proliferation of HSCs in
MDS.
Materials and methods
Subjects
The study included 77 participants (35 males and 42
females). A total of 23 healthy donors, 36 patients with MDS and 18
patients with AML were enrolled at the Tianjin Medical University
General Hospital (Tianjin, China) between July 2017 and April 2018.
According to the World Health Organization (WHO) (18), the MDS cases included 1 case of
single lineage dysplasia (SLD), 1 case of 5q-syndrome, 5 cases of
ring sideroblasts (RS), 5 cases of multilineage dysplasia (MLD), 7
cases of excess blasts-1 (EB-1) and 17 cases of excess blasts-2
(EB-2). The patients with MDS were divided into two groups
according to the blast count: Group A, which included patients with
<5% blasts (SLD, MLD, RS and 5q-syndrome) and group B, which
included patients with 5–19% blasts (EB-1 and EB-2) (Table I). The present study was approved by
the Ethics Committee of Tianjin Medical University General
Hospital. Written informed consent was obtained from each
patient.
 | Table I.Characteristics of the patients and
healthy donors in the present study. |
Table I.
Characteristics of the patients and
healthy donors in the present study.
|
|
|
| Sex |
|---|
|
|
|
|
|
|---|
| Category | n | Mean age (range),
years | Male | Female |
|---|
| Patients with
MDS | 36 | 59.4 (30–84) | 19 | 17 |
| World Health
Organisation Classification (2016) |
|
|
|
|
|
SLD | 1 | 49 | 1 | 0 |
|
MLD | 5 | 63.6 (59–67) | 4 | 1 |
| RS | 5 | 67.4 (56–84) | 4 | 1 |
|
5q-syndrome | 1 | 63 | 0 | 1 |
|
EB-1 | 7 | 51.9 (30–66) | 2 | 5 |
|
EB-2 | 17 | 59.1 (44–75) | 8 | 9 |
|
Patients with AML | 18 | 51.9 (20–78) | 10 | 8 |
| Healthy
donors | 23 | 47.7 (23–79) | 6 | 17 |
Isolation of CD34+ cells by
magnetic absorption cell sorting
Bone marrow samples (10 ml) were collected from the
patients and healthy donors. Mononuclear cells were isolated using
lymphocyte separation medium (Beijing Solarbio Science &
Technology Co., Ltd., Beijing, China) and washed twice. A total of
1×108 cells were resuspended in 300 µl autoMACS Running
Buffer (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany).
Hemopoietic stem and progenitor cells were purified from
mononuclear cells with the CD34 MicroBeads kit (Miltenyi Biotec
GmbH) according to the manufacturer's protocol. Bone marrow
mononuclear cells were cultured with the CD34 MicroBeads
(monoclonal mouse anti-human CD34 antibodies; isotype, mouse IgG1)
and Fc receptor blocking reagent (human IgG) from the kit, for 30
min at 4°C in the dark. CD34+ cells were obtained using
a magnetic absorption cell sorter, and the purity of the
CD34+ cells was analyzed using the CytExpert Pro
analysis software (version 2.0; Beckman Coulter, Inc., Brea, CA,
USA).
Cell culture and transfection
The MDS cell line SKM-1 was obtained from the
National Institute of Biomedical Innovation, Osaka, Japan (19). The AML cell line KG-1 was obtained
from the American Type Culture Collection (Manassas, VA, USA).
These two cell lines were cultured in RPMI 1640 medium (Boehringer
Ingelheim, Ingelheim am Rhein, Germany) containing 10%
heat-inactivated fetal calf serum (Boehringer Ingelheim), 100 µg/ml
penicillin (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) and 100 U/ml streptomycin (Gibco; Thermo Fisher Scientific,
Inc.) in a humidified atmosphere at 37.5°C and 5% CO2.
PIM2 si-RNA was transfected into the MDS cell line SKM-1 to silence
PIM2 expression. The transfections were performed using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol with
silencer-validated PIM2 siRNA (Silencer™ Select validated siRNA;
Thermo Fisher Scientific, Inc.). A total of three PIM2-specific
siRNAs were tested in the current study. The sequences were the
following: siRNA-1 sense, 5′-CCAGCTCCACCTTCGACACC-3′ and antisense,
5′-CCGGTAGTGGTCCTCATCAG-3′; siRNA-2 sense,
5′-ACCGTCACTATGGACCAGC-3′ and antisense, 5′-TTCAGAGCTGGACTACATCC-3′
and siRNA-3 sense, 5′-GUGCCAAACUCAUUGAUUUTT-3′ and antisense,
5′-AAAUCAAUGAGUUUGGCACTT-3′. siRNA-3 resulted in the greatest
knock-down efficiency and was selected for subsequent
experimentation. A scrambled siRNA sequence was used as a control
and had the following sequence: Sense, 5′-AUCCGCGCGAUAGUACGUATT-3′
and antisense, 5′-UACGUACUAUCGCGCGGAUTT-3′. The siRNAs were diluted
to 20 µM with diethyl pyrocarbonate-treated water and aliquoted
into a six-well plate. The siRNA master mix, which consisted of 5
µl siRNA (20 µM), 5 µl Lipofectamine 2000 and 100 µl Opti-MEM™ I
Reduced Serum Medium (Gibco; Thermo Fisher Scientific, Inc.), was
gently agitated and incubated for 15 min at room temperature to
allow complex formation between the siRNA and lipids. The medium
was removed from the wells and 1,900 µl of fresh RPMI 1640 culture
medium was added to each well. The siRNA mixture (110 µl per well)
was added drop by drop while gently swirling the plate. Cells were
cultured for 48 h at 37.5°C and harvested for analysis. The
interference efficiency was assessed by western blotting.
Proliferation assay
Cells were seeded into 96-well plates at a density
of 1.5×105 cells/ml in 200 µl complete medium and
incubated at 37°C in 5% CO2 for 1.5 h. A total of 20 µl
Cell Counting Kit-8 (CCK-8; Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan) reagent was added to the wells and incubated for
1.5 h at 37°C, and the optical density (OD) was read at a
wavelength of 450 nm. This test was repeated three times with three
replicates per sample. Cell proliferation was calculated using the
following equation: Proliferation (%)=(OD450 of the
experimental group/OD450 of the control group)
×100%.
Cell cycle analysis by flow
cytometry
Cell cycle analysis was performed using a
FACSCalibur C6 flow cytometer (BD Biosciences, San Jose, CA, USA).
Cells were fixed in 70% ethanol at 4°C for at least 4 h and then
stained with PI/RNase staining buffer (20 µg/ml propidium iodide
(PI) containing 10 µg/ml RNase; BD Biosciences, San Jose, CA, USA)
for 30 min at room temperature. The DNA distributions in the cells
were analyzed by Modifit (v.4.0; Verity Software House, Inc.,
Topsham, ME, USA) to determine the proportions of cells in each
phase of the cell cycle.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the CD34+
cells, and the SKM-1 and KG-1 cells using TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.). Total RNA (1
µg) was reverse transcribed using the SuperScript First-Strand
Synthesis system (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. qPCR was subsequently
performed using SYBR® Premix Ex Taq™ II (Tiangen
Biotech, Co., Ltd., Beijing, China) and the Thermal Cycler Dice
Real Time system (Tiangen Biotech Co., Ltd.) in a 96-well plate
according to the manufacturer's protocol. The primers used were
synthesized by Sangon Biotech, Co., Ltd., (Shanghai, China) and are
presented in Table II. The
optimized parameters for PCR were: 95°C for 2 min, 94°C for 10 sec,
61.5°C for 30 sec and 72°C for 40 sec (40 cycles). The expression
level of each gene was calculated by the 2−∆∆Cq method
(20) using GAPDH as an internal
control.
 | Table II.Primers used for reverse
transcription-quantitative polymerase chain reaction. |
Table II.
Primers used for reverse
transcription-quantitative polymerase chain reaction.
|
| Primer sequence
(5′→3′) |
|---|
|
|
|
|---|
| Gene | Forward | Reverse |
|---|
| Hypoxia inducible
factor 1 subunit α |
ACGTTCCTTCGATCAGTTGTCACC |
GGCAGTGGTAGTGGTGGCATTAG |
| Isocitrate
dehydrogenase [NADP(+)] 1, cytosolic |
TCAGTGGCGGTTCTGTGGTAGAG |
CATCCTTGGTGACTTGGTCGTTGG |
| Pim-2
proto-oncogene, serine/threonine kinase |
TTGGGAAGGAATGGTAGATG | CAGGAGAACAAACAG
CAAGC |
| GAPDH |
GGAGCGAGATCCCTCCAAAAT |
GGCTGTTGTCATACTTCTCATGG |
Western blotting
Western blot analysis was used to evaluate the PIM2,
CDKN1A, CDK2, IDH1 and HIF1A protein concentrations in the SKM-1
cell extracts 48 h after siRNA transfection. Cells were lysed with
a lysis buffer (20 mM Tris-HCl, pH 8.0, 150 mM NaCl, 2 mM EDTA, 100
mM NaF, 1% NP40, 1 µg/ml leupeptin, 1 µg/ml anti-pain and 1 mM
phenyl methyl sulfonyl fluoride), and the protein concentration was
determined using a bicinchoninic acid assay (Pierce; Thermo Fisher
Scientific, Inc.). The extracted proteins (30 µg/lane) were
separated via SDS-PAGE on an 8% gel. The separated proteins were
subsequently transferred to polyvinylidene difluoride membranes
(BioRad Laboratories, Inc., Hercules, CA, USA). PVDF membranes were
blocked using a solution containing 5% skimmed milk in double
distilled water (95%) and incubated for 1 h at room temperature.
The membranes were incubated with primary antibodies against CDKN1A
(1:1,000; cat. no. ab2947), CDK2 (1:1,000; cat. no. ab2546), IDH1
(1:1,000; cat. no. ab172964), HIF1-α (1:1,000; cat. no. ab179483),
PIM2 (1:1,000; cat. no. ab97475) (all Abcam, Cambridge, UK) and
GAPDH (1:1,000; cat. no. 5174; Cell Signaling Technology, Inc.,
Danvers, MA, USA) overnight at 4°C. The membranes were washed with
Tris-buffered saline containing Tween-20 and incubated with
horseradish peroxidase-conjugated anti-rabbit IgG sheep antibody
(1:2,000; cat. no. ab6721; Abcam) or horseradish
peroxidase-conjugated anti-mouse IgG sheep antibody (1:2,000; cat.
no. ab6785; Abcam) diluted in PBS for 1 h at room temperature.
Protein bands were visualized with the Immobilon Western
Horseradish Peroxidase Chemiluminescence kit (EMD Millipore,
Billerica, MA, USA).
Statistical analysis
All experiments were performed at least three times
in triplicate for each group. SPSS software (v.19; IBM Corp.,
Armonk, NY, USA) was used for statistical analysis. Multiple groups
were analyzed using a one-way analysis of variance (ANOVA) followed
by the Tukey's test. The Student's t-test was used to compare two
groups. The Spearman's correlation test was used for correlation
analysis between PIM2 and IDH-1 or HIF1A. The data are presented as
the mean ± standard deviation. P<0.05 was considered to indicate
a statistically significant difference.
Results
PIM2 expression is reduced in the
CD34+ cells of patients with MDS and the MDS cell line,
compared with patients with AML and the AML cell line
Patients with MDS were stratified into two groups
according to the WHO 2016 classification: Group A (SLD, MLD, RS,
and 5q-syndrome, n=12) and group B (EB-1 and EB-2, n=24). The ratio
of the 2−ΔΔCq values of PIM2 to GAPDH was reported as
the relative PIM2 mRNA level. The comparisons of the PIM2 mRNA
level in the different groups were performed by a one-way ANOVA
followed by the Tukey's test. The expression levels of PIM2
transcripts were significantly increased in patients with MDS and
de novo AML compared with healthy donors (P<0.0001;
Fig. 1A). The PIM2 expression level
was reduced in patients with MDS compared with patients with AML.
Furthermore, there was a significant difference in the relative
PIM2 mRNA levels between groups A and B among patients with MDS
(P=0.0325; Fig. 1B). The expression
level of PIM2 was also measured in the MDS cell line SKM-1 and the
AML cell line KG-1 using RT-qPCR (Fig.
1C) and western blotting (Fig.
1D). Expression of PIM2 was detected in MDS cell lines;
however, the levels of mRNA expression were significantly decreased
compared with the AML cell line (P=0.036).
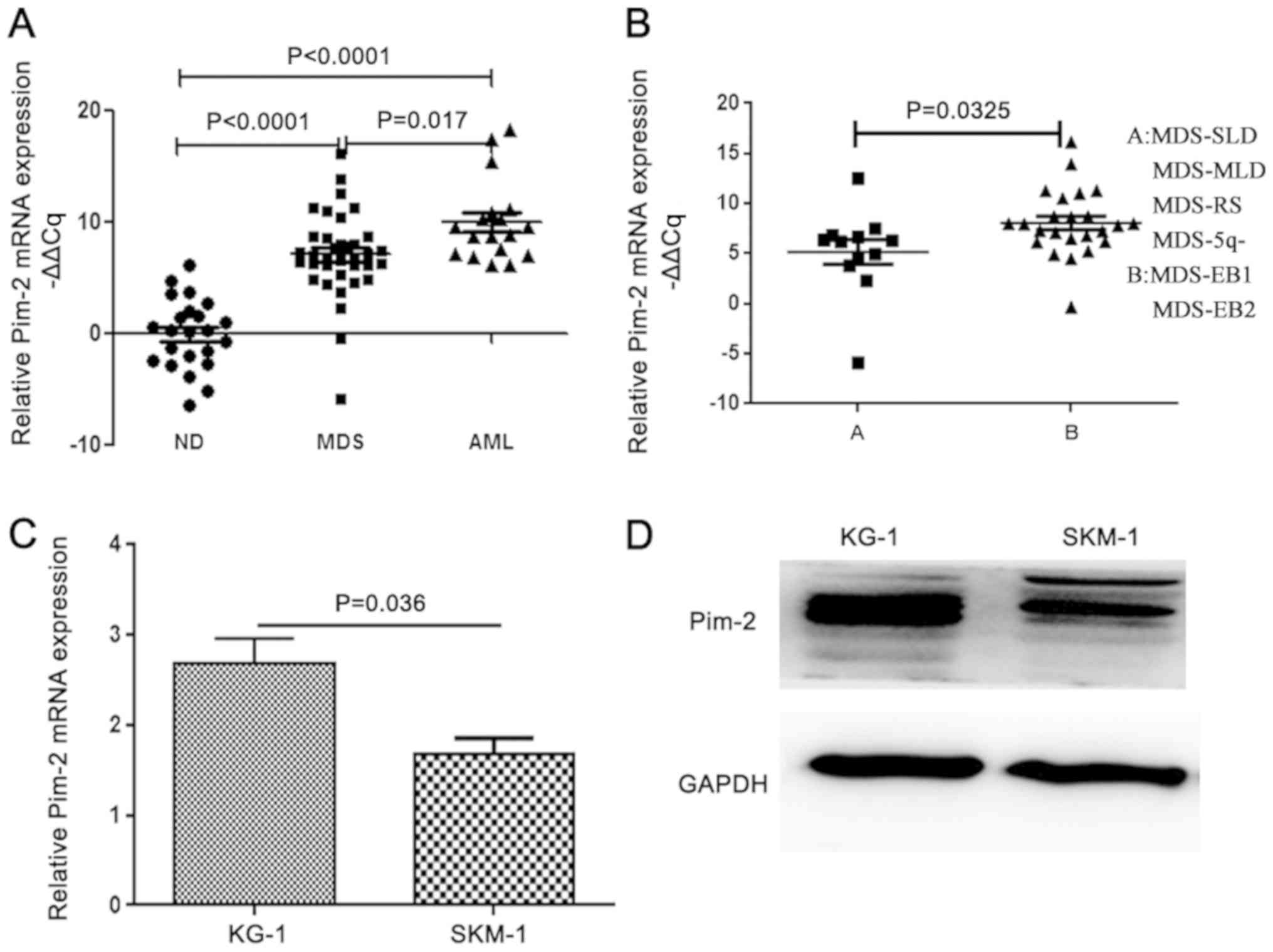 | Figure 1.PIM2 expression is decreased in the
CD34+ cells of patients with MDS and an MDS cell line,
compared with patients with AML and an AML cell line, respectively.
(A) RT-qPCR analysis of the expression level of PIM2 in
CD34+ cells obtained from the bone marrow of healthy
donors and newly diagnosed patients with MDS/AML. (B) RT-qPCR
analysis of the expression level of PIM2 in CD34+ cells
obtained from the bone marrow of newly diagnosed patients with MDS
(A and B groups). (C) RT-qPCR analysis of the expression level of
PIM2 in an MDS cell line (SKM-1) and an AML cell line (KG-1). (D)
Western blot analysis of the PIM2 expression levels in the KG-1 and
SKM-1 cell lines. RT-qPCR, reverse transcription-quantitative
polymerase chain reaction; MDS, myelodysplastic syndrome; AML,
acute myeloid leukemia; PIM2, Pim-2 proto-oncogene,
serine/threonine kinase; SLD, single lineage dysplasia; RS, ring
sideroblasts; MLD, multilineage dysplasia; EB, excess blasts; ND,
healthy donors. |
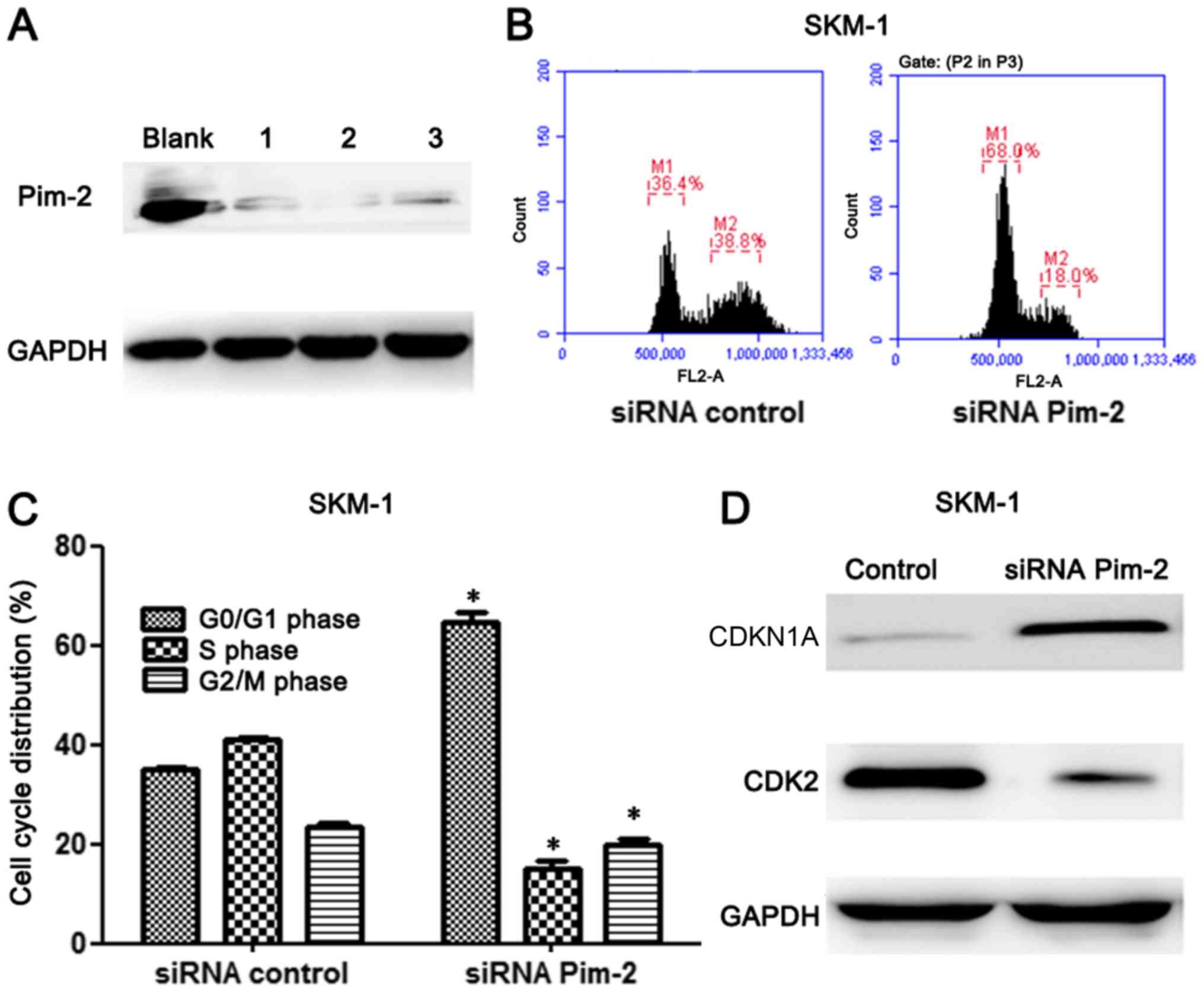
Downregulation of PIM2 kinase
expression induces cell cycle arrest at the
G0/G1 phase
To investigate the role of PIM2 in MDS, PIM2 was
knocked down in SKM-1 cells. The degree of PIM2 expression
knockdown by siRNA was assessed by western blotting. The
PIM2-specific siRNAs markedly decreased the protein expression
levels (Fig. 2A), and the third
siRNA was chosen for further experiments, as it exhibited good
efficiency in several cell lines (data not shown).
Cell cycle changes following inhibition of PIM2 were
analyzed by flow cytometry. Cells in the
G0/G1, S and G2/M phases were separated based
on linear fluorescence intensity following staining with PI. The
cell cycle analysis demonstrated a significant increase in the
percentage of cells in the G0/G1 cell cycle
phase after transfection with PIM2 siRNA compared with the
percentage Pleasin the control cells (P<0.05; Fig. 2B and C; Table III). There was a significant
decrease in the percentage of cells in the S phase and G2/M phase
in the PIM2-silenced cells compared with the control cells
(P<0.05). Therefore, downregulation of PIM2 led to an increased
number of cells in the G0/G1 phase. A
previous study revealed that the inhibition of CDK2 and pRb
expression via upregulation of CDKN1A resulted in PIM2
downregulation and G0/G1 arrest in lung
cancer and hematopoietic malignancies (6). The western blot results obtained in
SKM-1 cells in the current study are consistent with the findings
of previous studies. CDK2 was markedly downregulated, whereas
CDKN1A was markedly upregulated following treatment with PIM2 siRNA
(Fig. 2D). The CDKN1A protein, as a
member of the CDK interacting protein/kinase inhibitory protein
family of CDK2 inhibitors, binds to and inhibits CDK2/cyclin
complexes during the G1 phase (21,22).
Therefore, PIM2 may serve an important role in the regulation of
the cell cycle.
 | Table III.Percentage of SKM-1 cells in each
cell cycle phase. |
Table III.
Percentage of SKM-1 cells in each
cell cycle phase.
| siRNA |
G0/G1 phase, % | S phase, % | G2/M phase, % |
|---|
| Control | 35.07±1.35 | 41.30±0.30 | 23.63±1.22 |
| PIM2 | 64.70±3.90 | 15.10±2.72 | 20.20±1.90 |
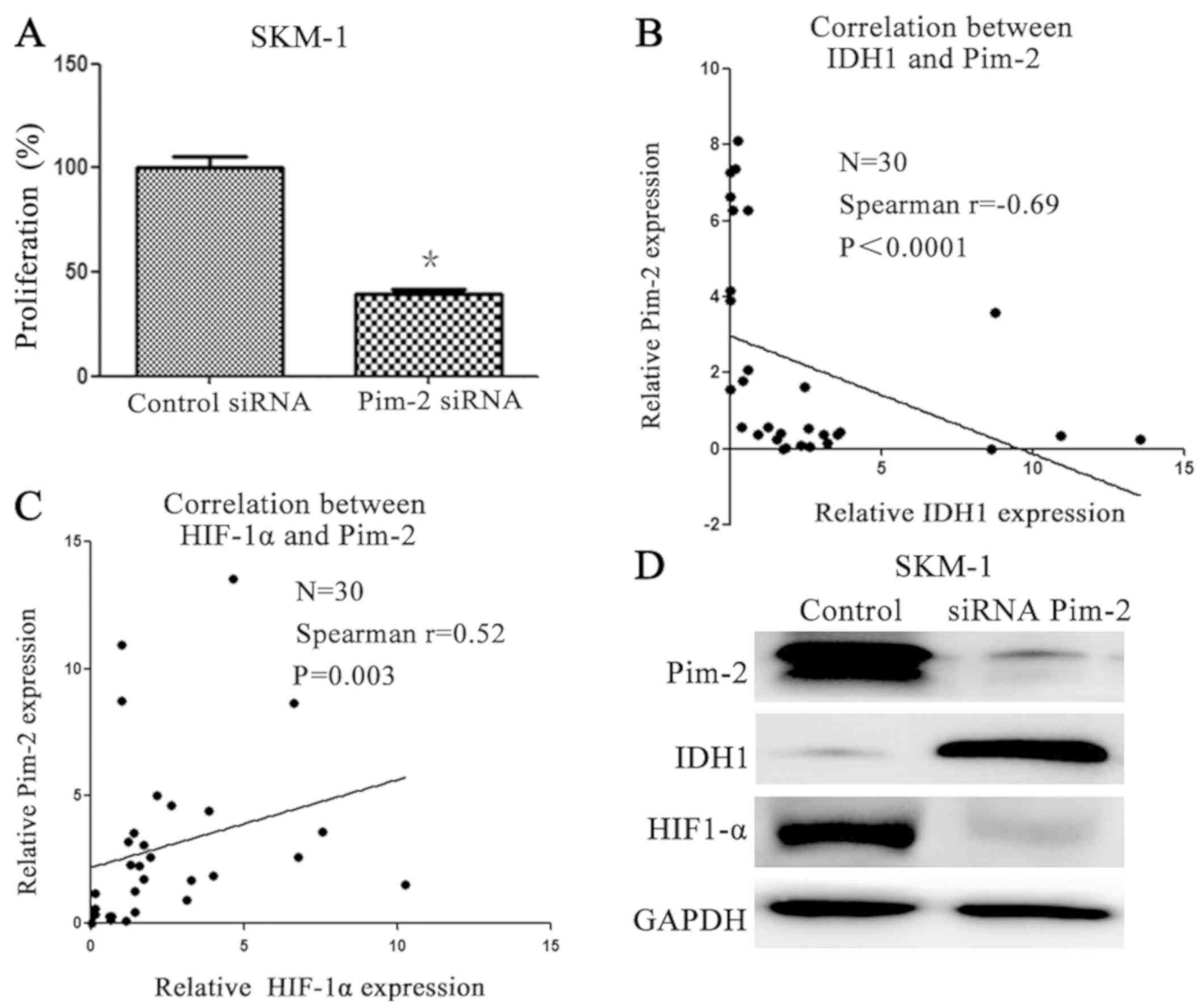
PIM2 promotes cell proliferation via
the IDH1/HIF1A signaling pathway
To determine whether downregulation of PIM2 by siRNA
had an inhibitory effect on MDS cell growth, cell proliferation was
assessed by the CCK-8 assay. Cell proliferation was significantly
reduced in PIM2 siRNA-transfected cells when compared with control
siRNA-transfected cells (P<0.05; Fig.
3A; Table IV). These data
suggested that PIM2 may serve a role in the proliferation of MDS
HSCs. To explore the underlying molecular mechanisms, the
expression levels of IDH1 and HIF1A in bone marrow CD34+
cells obtained from patients with MDS were investigated. The
Spearman's correlation test indicated that PIM2 expression is
negatively correlated with IDH1 (Fig.
3B) and positively correlated with HIF1A in patients with MDS
(Fig. 3C). To further explore the
molecular mechanisms, the levels of IDH1 and HIF1A proteins in an
MDS cell line following treatment with PIM2 siRNA were analyzed by
western blotting. IDH1 was markedly downregulated, whereas HIF1A
was markedly upregulated following transfection with PIM2 siRNA
(Fig. 3D).
 | Table IV.Effect of PIM2 silencing on the
proliferation of SKM-1 cells. Cell proliferation was determined by
the Cell Counting Kit-8 assay. |
Table IV.
Effect of PIM2 silencing on the
proliferation of SKM-1 cells. Cell proliferation was determined by
the Cell Counting Kit-8 assay.
| Parameter | Control siRNA | PIM2 siRNA | P-value |
|---|
|
Proliferation,% | 100±17.17 | 39.23±7.84 | <0.0001 |
Discussion
The current study revealed that the expression level
of PIM2 was increased in cells from patients with MDS compared with
the PIM2 levels in cells from healthy donors. Furthermore, the
expression level of PIM2 was decreased in the CD34+
cells of patients with MDS and in an MDS cell line, compared with
patients with AML and the AML cell lines, respectively. PIM2 may
serve a role in the pathogenesis of hematological malignancies
(3). The PIM2 expression level was
increased in patients with AML and acute lymphoblastic leukemia
(ALL) (4). The results obtained in
the present study suggest that PIM2 expression is increased in MDS.
PIM2 expression was increased in patients with EB-1 and EB-2
compared with patients with other types of MDS. The downregulation
of PIM2 in SKM-1 cells induced cell cycle arrest at the
G0/G1 phase and was associated with changes
in the expression of cell cycle-associated proteins, CDK2 and
CDKN1A. This result is consistent with results obtained in a
previously published study, which revealed that the inhibition of
CDK2 and pRb expression via the upregulation of CDKN1A mediated the
effects of PIM2 downregulation on G0/G1
arrest in lung cancer and hematopoietic malignancies. The current
study demonstrated that PIM2 is required for the proliferation of
MDS cells as the downregulation of PIM2 inhibited their
proliferation. A previous study in AML cell lines suggested that
NF-κβ may be a downstream factor in the PIM2 signaling pathway
(6). Furthermore, PIM2 activated
API-5 to inhibit apoptosis in hepatocellular carcinoma cells via
the NF-κβ signaling pathway (7).
The present study aimed to investigate the action of
PIM2 in the pathogenesis of MDS. MDS is a stem-cell disorder in
which maturation blockade of HSCs results in the disease, similar
to the pathology in AML (8,9). The present study analyzed the
expression level of PIM2 in CD34+ cells extracted from
the bone marrow of healthy donors and patients with MDS and AML by
RT-qPCR. These data suggested that PIM2 transcripts were
significantly increased in patients with MDS and de novo AML
compared with healthy donors, and the transcript levels were
increased in patients with AML compared with patients with MDS.
Furthermore, there was a significant difference between the two
groups of patients with MDS, PIM2 levels were increased in patients
with EB-1 and EB-2 compared with SLD, MLD, RS and 5q-. These data
suggested that PIM2 serves an important role in the pathogenesis of
MDS. Therefore, the present study tested this hypothesis.
Flow cytometry analysis revealed that a greater
percentage of SKM-1 cells transfected with PIM2 siRNA were in the
G0/G1 phase of the cell cycle compared with
control siRNA-transfected SKM-1 cells. There was a significant
decrease in the percentage of cells in S and G2/M phases in the
PIM2 siRNA-transfected cells compared with control
siRNA-transfected cells. This indicated that PIM2 may promote cell
cycle arrest at the G0/G1 phase.
To explore the molecular mechanisms underlying the
effects of PIM2 on the cell cycle, the current study investigated
the protein expression levels of the cell cycle regulator CDK2 and
its inhibitor CDKN1A (21,22) by western blotting. The results
revealed that CDK2 expression was downregulated and CDKN1A was
upregulated in the SKM-1 cells transfected with PIM2 siRNA. The
decreased level of CDK2 and increased level of CDKN1A suggested a
possible mechanism by which cell-cycle arrest is induced at the
G0/G1 phase following deregulation of PIM2
expression. Downregulation of PIM2 may serve a key role in the
G0/G1 arrest via upregulation of CDKN1A to
inhibit the expression of CDK2.
The CCK-8 assay revealed a significant decrease in
the proliferation rate of PIM2 siRNA-transfected SKM-1 cells
compared with the proliferation of control siRNA-transfected SKM-1
cells. The effect of PIM2 on the cell cycle suggested increased
cell proliferation; however, there may be another mechanism through
which PIM2 influenced the proliferation of HSCs. Kapelko-Slowik
et al (4) reported that the
expression of PIM2 altered the NF-κβ pathway, resulting in the
development of AML and ALL. A previous study on AML cell lines
suggested that the NF-κβ signaling pathway may serve a role in the
action of PIM2 on cell proliferation (6). The current study revealed that PIM2
expression is negatively correlated with IDH1 and positively
correlated with HIF1A in patients with MDS. To further confirm the
RT-qPCR results, protein expression in a PIM2 siRNA-transfected
cell line was analyzed by western blotting. IDH1 was markedly
downregulated and HIF1A was markedly upregulated in cells
transfected with PIM2 siRNA. A previous study revealed that
upregulated HIF1A was correlated with poor overall survival time
and disease progression (17). In
addition, correlation analysis revealed that the expression of
HIF1A was positively correlated with the percentage of bone marrow
blasts (17). This suggested that
HIF1A may accelerate the proliferation of HSCs. The overexpression
of IDH1 mutants in cultured U-87MG cells suppressed the activity of
wild-type IDH1 via the formation of heterodimers, resulting in a
decrease of the enzyme product, α-KG (14). α-KG is required for PHD activity,
which promotes HIF1A degradation (14). The forced overexpression of the IDH1
mutant activated NF-κβ in a HIF1A-dependent manner and was involved
in the regulation of cell proliferation (16). Previous studies have reported IDH1
mutants in patients with MDS and AML (12,23).
However, the MDS patients in the aforementioned studies had a low
frequency of IDH1 mutations, implying that different pathways may
result in the reduction of IDH1 expression. The results obtained in
the present study substantiated the initial hypothesis that PIM2
expression is negatively correlated with IDH1 and positively
correlated with HIF1A in MDS HSCs. PIM2 upregulation may lead to
increased expression of HIF1A via downregulation of IDH1. However,
a limitation of the current study was that the direct role of PIM2
in IDH1 expression was not investigated. A future study is required
to investigate the molecular mechanism, via chromatin
immunoprecipitation or co-immunoprecipitation assays, for
example.
Taken together, the present study demonstrated that
the expression level of PIM2 was reduced in patients with MDS
compared with patients with AML, but increased compared with the
healthy donor group. These results suggested that PIM2 upregulated
HIFA by downregulating IDH1, resulting in increased proliferation
of HSCs. Novel therapeutic agents that suppress PIM2 may be
effective for the treatment of MDS and hematopoietic malignancies,
and therefore future studies are required to elucidate the
underlying mechanisms behind PIM2 inhibitors and their effects on
MDS cells.
Acknowledgements
Not applicable.
Funding
The present study was funded by the National Natural
Science Foundation of China (grant nos. 81570106, 81400088 and
81400085), the Tianjin Municipal Natural Science Foundation (grant
nos. 14JCYBJC25400, 15JCYBJC24300, 16ZXMJSY00180, 18JCQNJC80400,
2018KJ043, 2018KJ045 and 20140118) and the Youth Incubation Fund of
Tianjin Medical University General Hospital (grant no. ZYYFY
2016006).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
ZL and RF designed the study. ZL and MT performed
the experiments, analyzed the data, generated the figures and
drafted the manuscript. MT contributed to cell culture and western
blotting. YW contributed to RT-qPCR and flow cytometry. KD and HL
collected the data of patients and healthy donors. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Tianjin Medical University General Hospital (ethical
no. IRB2017-YX-041). Written informed consent was obtained from
each patient for any study-specific experiments being
performed.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Narlik-Grassow M, Blanco-Aparicio C and
Carnero A: The PIM family of serine/threonine kinases in cancer.
Med Res Rev. 34:136–159. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ren K, Gou X, Xiao M, Wang M, Liu C, Tang
Z and He W: The over-expression of Pim-2 promote the tumorigenesis
of prostatic carcinoma through phosphorylating eIF4B. Prostate.
73:1462–1469. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Asano J, Nakano A, Oda A, Amou H, Hiasa M,
Takeuchi K, Miki H, Nakamura S, Harada T, Fujii S, et al: The
serine/threonine kinase Pim-2 is a novel anti-apoptotic mediator in
myeloma cells. Leukemia. 25:1182–1188. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kapelko-Słowik K, Urbaniak-Kujda D,
Wołowiec D, Jaźwiec B, Dybko J, Jakubaszko J, Słowik M and
Kuliczkowski K: Expression of PIM-2 and NF-κB genes is increased in
patients with acute myeloid leukemia (AML) and acute lymphoblastic
leukemia (ALL) and is associated with complete remission rate and
overall survival. Postepy Hig Med Dosw (Online). 67:553–559. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ramachandran J, Santo L, Siu KT, Panaroni
C and Raje N: Pim2 is important for regulating DNA damage response
in multiple myeloma cells. Blood Cancer J. 6:e4622016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu Z, Liu H, Yuan X, Wang Y, Li L, Wang
G, Song J, Shao Z and Fu R: Downregulation of Pim-2 induces cell
cycle arrest in the G0/G1 phase via the p53-non-dependent p21
signaling pathway. Oncol Lett. 15:4079–4086. 2018.PubMed/NCBI
|
|
7
|
Ren K, Zhang W, Shi Y and Gong J: Pim-2
activates API-5 to inhibit the apoptosis of hepatocellular
carcinoma cells through NF-kappaB pathway. Pathol Oncol Res.
16:229–237. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Corey SJ, Minden MD, Barber DL, Kantarjian
H, Wang JC and Schimmer AD: Myelodysplastic syndromes: The
complexity of stem-cell diseases. Nat Rev Cancer. 7:118–129. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nimer SD: MDS: A stem cell disorder-but
what exactly is wrong with the primitive hematopoietic cells in
this disease? Hematology Am Soc Hematol Educ Program. 43–51. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Boissel N, Nibourel O, Renneville A,
Gardin C, Reman O, Contentin N, Bordessoule D, Pautas C, de Revel
T, Quesnel B, et al: Prognostic impact of isocitrate dehydrogenase
enzyme isoforms 1 and 2 mutations in acute myeloid leukemia: A
study by the acute leukemia french association group. J Clin Oncol.
28:3717–3723. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Thol F, Weissinger EM, Krauter J, Wagner
K, Damm F, Wichmann M, Göhring G, Schumann C, Bug G, Ottmann O, et
al: IDH1 mutations in patients with myelodysplastic syndromes are
associated with an unfavorable prognosis. Haematologica.
95:1668–1674. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Patnaik MM, Hanson CA, Hodnefield JM,
Lasho TL, Finke CM, Knudson RA, Ketterling RP, Pardanani A and
Tefferi A: Differential prognostic effect of IDH1 versus IDH2
mutations in myelodysplastic syndromes: A mayo clinic study of 277
patients. Leukemia. 26:101–105. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ramachandran N and Colman RF: Chemical
characterization of distinct subunits of pig heart DPN-specific
isocitrate dehydrogenase. J Biol Chem. 255:8859–8864.
1980.PubMed/NCBI
|
|
14
|
Zhao S, Lin Y, Xu W, Jiang W, Zha Z, Wang
P, Yu W, Li Z, Gong L, Peng Y, et al: Glioma-derived mutations in
IDH1 dominantly inhibit IDH1 catalytic activity and induce
HIF-1alpha. Science. 324:261–265. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dolcet X, Llobet D, Pallares J and
Matias-Guiu X: NF-kB in development and progression of human
cancer. Virchows Arch. 446:475–482. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang G, Sai K, Gong F, Yang Q, Chen F and
Lin J: Mutation of isocitrate dehydrogenase 1 induces glioma cell
proliferation via nuclear factor-κB activation in a
hypoxia-inducible factor 1-α dependent manner. Mol Med Rep.
9:1799–1805. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tong H, Hu C, Zhuang Z, Wang L and Jin J:
Hypoxia-inducible factor-1alpha expression indicates poor prognosis
in myelodysplastic syndromes. Leuk Lymphoma. 53:2412–2418. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Arber DA, Orazi A, Hasserjian R, Thiele J,
Borowitz MJ, Le Beau MM, Bloomfield CD, Cazzola M and Vardiman JW:
The 2016 revision to the world health organization classification
of myeloid neoplasms and acute leukemia. Blood. 127:2391–2405.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nakagawa T, Matozaki S, Murayama T,
Nishimura R, Tsutsumi M, Kawaguchi R, Yokoyama Y, Hikiji K, Isobe T
and Chihara K: Establishment of a leukaemic cell line from a
patient with acquisition of chromosomal abnormalities during
disease progression in myelodysplastic syndrome. Br J Haematol.
85:469–476. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Harper JW, Elledge SJ, Keyomarsi K,
Dynlacht B, Tsai LH, Zhang P, Dobrowolski S, Bai C, Connell-Crowley
L, Swindell E, et al: Inhibition of cyclin-dependent kinases by
p21. Mol Biol Cell. 6:387–400. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gartel AL, Serfas MS and Tyner AL:
p21-negative regulator of the cell cycle. Proc Soc Exp Biol Med Soc
Exp Biol Med. 213:138–149. 1996. View Article : Google Scholar
|
|
23
|
Lin CC, Hou HA, Chou WC, Kuo YY, Liu CY,
Chen CY, Lai YJ, Tseng MH, Huang CF, Chiang YC, et al: IDH
mutations are closely associated with mutations of DNMT3A, ASXL1
and SRSF2 in patients with myelodysplastic syndromes and are stable
during disease evolution. Am J Hematol. 89:137–144. 2014.
View Article : Google Scholar : PubMed/NCBI
|