Introduction
Renal carcinoma in children is rare compared with
that in adults. It has been reported that the incidence of renal
carcinoma in people under the age of 20 years is <0.5%, and a
great difference exists between the renal carcinoma in children and
the renal carcinoma in adults in terms of epidemiology, clinical
manifestations, pathological type and prognosis (1). Wilms tumor (WT), a malignant tumor of
the kidneys, is common in children with an incidence of about seven
in a million, second only to neuroblastoma in the primary malignant
solid tumor of the abdomen in children (2). According to previous data, as a
malignant solid mixed tumor is derived from undifferentiated
post-renal embryos, WT has a very rapid growth. Blastema cells,
immature epithelial tissues, and mesenchymal cells are the
components of tumor cells with typical lesions (3), the pathogenesis of which is not clear
yet. Therefore, searching for specific target genes and studying
the significance of their expression in WT is of great
importance.
Highly homologous to the partial sequence of p53
gene, the p73 gene is located at 1p36.2~36.3 and has been proven to
be closely related to the occurrence and development of various
tumors with overexpression (4).
Lowly expressed in normal tissues and excessively expressed in
tumor tissues, the p73 is closely related to the proliferation and
migration of tumor cells and the prognosis of patients (5). Moreover, it has unique functions in the
metabolic regulation (6),
neurodevelopment and differentiation (7), as well as the regulation of
spermatogenesis and male fertility (8). Many studies have discovered the
overexpression of p73 in tumors, such as bladder, ovarian, gastric,
lung, thymic epithelial, breast, esophageal and liver cancers
(9–11). The study by Mancini et al
(12) has found that the expression
level of p73, the tumor suppressor gene, has a certain influence on
the grade and prognosis of patients with cystic renal cell
carcinoma.
Considering that there are only a few studies on the
expression of p73 in the WT in children, in the present study the
expression level of p73 mRNA in blood was analyzed, as detected by
reverse transcription-quantitative PCR (RT-qPCR), to provide a
theoretical basis for the clinical targeted therapy.
Subjects and methods
General information
Fifty patients (29 males and 21 females, aged from
13 to 65 months) diagnosed with WT in the People's Hospital of
Rizhao (Rizhao, China), from July 2013 to January 2015, were
enrolled as the study group, including 15 cases in stage I, 17
cases in stage II, 11 cases in stage III, and 7 cases in stage IV,
according to the clinical stage standardized by the NWTS-5 (US WT
Research Collaboration Group). In the study group there were 12
cases of unfavorable histopathology (UH) and 38 cases of favorable
histopathology (FH), according to the pathological classification.
Also, there were 10 patients with lymphatic metastasis, 13 patients
with vascular invasion, 5 patients with failed treatment and 45
patients with successful treatment, according to the treatment
outcome. Twenty healthy children (13 males and 7 females, aged from
15 to 68 months) with similar age and sex, who received health
examinations in the same hospital during the same period, were
enrolled as the control group.
The inclusion criteria were: children confirmed by
pathology as WT patients; patients with complete clinical and
pathological data; patients with no chemotherapy or radiotherapy;
patients who received examination of liver and kidney function,
blood routine examination and other necessary examinations before
operation; patients with normal body mass index.
The exclusion criteria were: patients with other
malignant tumors; patients with severe congenital heart disease;
patients with liver dysfunction and severe organ diseases; patients
with autoimmune diseases; patients who did not cooperate with the
medical examinations; patients whose family members refused to sign
the informed consent.
The study was approved by the Ethics Committee of
the People's Hospital of Rizhao and the research subjects family
members signed a complete informed consent form after receiving
details on the experimental contents.
Serum collection
After diagnosis, 2 ml of peripheral blood were
collected from WT children, with an empty stomach in the morning,
and placed in an anticoagulation tube to be sent to the laboratory;
and 2 ml of fasting venous blood were taken from the children of
the control group in the morning, on the day of the physical
examination. After coagulation for 60 min at 20–25°C, the blood
samples of the two groups were centrifuged at 2,600 × g for 10 min
at 4°C to obtain the supernatant liquid that was kept at −80°C to
be tested (repeated freezing and thawing was avoided as far as
possible).
Experimental reagents and
instruments
RNAiso Plus kit (Takara Biotechnology Co., Ltd.,
Dalian, China); Two-Step Reverse Transcription kit (Takara Bio,
Inc., Otsu, Japan); SYBR-Green qPCR Master Mix kit (Thermo Fisher
Scientific, Inc., Waltham, MA, USA); UV spectrophotometry
instrument (Bio-Rad Laboratories, Inc., Hercules, CA, USA); RT-qPCR
kit and StepOnePlus real-time PCR instrument (both from Thermor
Fisher Scientific, Inc.).
Primers
The primers of this study were designed by the
Primer Premier 5.0 design software (Premier Biosoft, Inc., Palo
Alto, CA, USA) and were generated by Tianjin Saier Biotechnology
Co., Ltd. (Tianjin, China). The sequences of the reference gene
β-actin (13) and the p73 are shown
in Table I.
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Genes | Forward primers | Reverse primers |
|---|
| p73 |
5′-AGCCACTTGTCACTCAGAACAG-3′ |
5′-TCTTACACGAAAAACACGGATG-3′ |
| β-actin |
5′-GATGAGATTGGCATGGCTTT-3′ |
5′-CACCTTCACCGTTCCAGTTT-3′ |
Experimental procedures
The specimens were dissolved at 4°C overnight, and
were transferred to a new centrifuge tube after mixing by
oscillation. Total RNA was extracted using the RNAiso Plus kit in
strict accordance with the manufacturer's instructions; the purity
and concentration of the RNA were measured by UV spectrophotometry;
the RNA integrity was detected by the agarose gel electrophoresis.
Then the method of Two-Step reverse transcription was performed to
transfer RNA to cDNA: i) first step: removal of the genomic DNA.
The DNase I digestion of the template before the reverse
transcription was performed to eliminate the pollution of the
genome. The reaction system was 10 µl in total, with 1 µg of RNA,
and the reaction conditions were: 42°C for 2 min. Then, the system
was quickly put on ice for cooling and kept at 4°C. ii) Second
step: reverse transcription reaction. The above extracted total RNA
was reversely transcribed into cDNA, and then frozen at −20°C. The
reaction system was 20 µl in total, 37°C for 15 min, afterwards
85°C for 5 sec, and then the system was kept at 4°C. qPCR was
subsequently performed using the SYBR-Green qPCR Master Mix.
RT-qPCR: the sample was kept on ice away from light, and the
reaction system was 20 µl in total, with 3 µl of the template, 0.8
µl (10 µM) of the forward primer and 0.8 µl (10 µM) of the reverse
primer of p73, and 0.8 µl (10 µM) of the forward primer and 0.8 µl
(10 µM) of the reverse primer of β-actin. The reaction conditions
were 40 cycles of 95°C for 30 sec, 95°C for 5 sec, 60°C for 34 sec,
and the dissolution curve: 95°C for 15 sec, 60°C for 1 min, 95°C
for 15 sec, 60°C for 15 sec. The product of p73 was 124 bp in
length and the product of β-actin was 100 bp in length. The
configuration and operation of the above reaction systems were
strictly in accordance with the respective protocols. Three
independent experiments were performed to record the Cq value and
the number of cycles required for the fluorescence signal to reach
the set threshold from the background, and the data were analyzed
using the formula: Relative quantity (RQ) = 2−ΔCq
(14). The control group in this
experiment served as the internal control.
Follow-up and observation
indicators
After the end of treatment, follow-up for patients
with WT was carried out by patients revisiting the hospital and
telephone surveys, during which prompt treatment and strengthened
examinations were provided for those patients with reoccurrence of
the disease. The final follow-up time and situation were recorded
for patients lost to follow-up and the date of death was recorded
in detail for patients who died after the treatment, for the
completion of the prognostic data. Follow-up deadline was February
1, 2018. Survival time was recorded from the first day of diagnosis
after admission to the day of death or follow-up deadline. The
expression of p73 protein in each group, its relationship with the
clinicopathological features and the clinical stages, as well as
the survival rates were observed.
Statistical analysis
Statistical analysis of the experimental data was
carried out using SPSS 17.0 statistical software (Tianjin KSoft
Tech. Co., Ltd., Tianjin, China). Enumeration data were expressed
as n (%) and their comparison between groups was made using
Chi-square (χ2) test. Measurement data were expressed as
mean ± SD and their comparison between groups was made using
t-test. Comparisons of the mean of multiple groups were made using
one-way analysis of variance (ANOVA) with Least Significant
Difference post hoc test. The correlation between the clinical
staging and p73 expression in the peripheral blood was analyzed by
Spearmans correlation coefficient. Survival curves were generated
based on the Kaplan-Meier analysis and log-rank test was used for
their comparison. P<0.05 was considered to indicate a
statistically significant difference.
Results
Comparison of clinical general
data
No statistical difference was detected in the
comparison of the general clinical baseline data between the two
groups including sex, age, body mass index, hemoglobin (Hb),
platelet (PLT) count, white blood cell (WBC) count, red blood cell
(RBC) count, alanine transaminase (ALT) and aspartate transaminase
(AST) (P>0.05) (Table II).
 | Table II.Comparison of the baseline data
between the study and the control group [n (%)]/(mean ± SD). |
Table II.
Comparison of the baseline data
between the study and the control group [n (%)]/(mean ± SD).
| Clinical data | Study group
(n=50) | Control group
(n=20) | χ2/t | P-value |
|---|
| Sex |
|
| 0.292 | 0.589 |
| Male | 29 (58.0) | 13 (65.0) |
|
|
|
Female | 21 (42.0) | 7 (35.0) |
|
|
| Age (years) |
|
| 1.072 | 0.301 |
| ≤3 | 31 (62.0) | 15 (75.0) |
|
|
|
>3 | 19 (38.0) | 5 (25.0) |
|
|
| Body mass index
(kg/m2) | 14.5±7.09 | 14.1±6.86 | 0.689 | 0.058 |
| Hb (gm/dl) | 11.48±1.69 | 11.67±1.87 | −1.102 | 0.499 |
| PLT (×109/l) | 151.89±20.98 | 156.18±21.65 | −1.564 | 0.577 |
| WBC (×109/l) | 7.38±2.51 | 7.15±2.32 | 0.602 | 0.304 |
| RBC (×1012/l) | 4.32±0.49 | 4.28±0.43 | 0.185 | 0.356 |
| ALT (U/l) | 21.97±10.04 | 21.36±9.86 | 1.996 | 0.226 |
| AST (U/l) | 18.87±7.01 | 18.12±6.46 | 2.542 | 0.145 |
Comparison of the expression levels of
p73 in the peripheral blood of the two groups
As shown in Table
III, the expression level of p73 in the blood of the study
group (4.04±0.79) was significantly higher than that of the control
group (1.57±1.12) and the difference was statistically significant
(t=11.44, P<0.01).
 | Table III.Comparison of p73 expression levels in
the blood of the study and the control group (mean ± SD). |
Table III.
Comparison of p73 expression levels in
the blood of the study and the control group (mean ± SD).
| Group | No. of cases | p73 |
|---|
| Study group | 50 | 4.04±0.79 |
| Control group | 20 | 1.57±1.12 |
| t |
| 11.44 |
| P-value |
| <0.01 |
Relationship between the expression
levels of p73 in the peripheral blood and the clinicopathological
features of WT patients
According to Table
IV, the differences of the expression levels of p73 in the
peripheral blood among WT patients of different age, sex, or
vascular invasion situation were not statistically significant
(P>0.05); while patients with different tumor size, pathological
type, lymph node metastasis situation, or treatment outcome had
significantly different p73 expression levels in the peripheral
blood (P<0.05).
 | Table IV.Relationship between the expression
levels of p73 in the peripheral blood and the clinicopathological
features of WT patients (mean ± SD). |
Table IV.
Relationship between the expression
levels of p73 in the peripheral blood and the clinicopathological
features of WT patients (mean ± SD).
| Clinicopathological
features | No. of cases | p73 | t | P-value |
|---|
| Sex |
|
| 1.611 | 0.112 |
|
Male | 29 | 3.34±0.95 |
|
|
|
Female | 21 | 3.21±1.07 |
|
|
| Age (years) |
| ≤3 | 31 | 3.37±0.89 | −0.110 | 0.052 |
|
>3 | 19 | 3.29±1.21 |
|
|
| Tumor size
(cm) |
|
| −3.439 | 0.009a |
|
≤10 | 35 | 2.98±0.87 |
|
|
|
>10 | 15 | 3.96±0.52 |
|
|
| Pathological
type |
|
| −2.213 | 0.008a |
| FH | 38 | 2.95±0.87 |
|
|
| UH | 12 | 3.86±0.52 |
|
|
| Lymph node
metastasis |
|
| 7.808 | 0.004a |
|
Yes | 10 | 4.87±0.23 |
|
|
| No | 40 | 3.14±0.87 |
|
|
| Vascular
invasion |
|
| 0.624 | 0.052 |
|
Yes | 13 | 3.35±0.37 |
|
|
| No | 37 | 3.46±0.67 |
|
|
| Treatment
outcome |
|
| −1.182 | 0.032a |
|
Failure | 5 | 4.63±0.42 |
|
|
|
Success | 45 | 3.01±1.01 |
|
|
Comparison of p73 expression levels in
the peripheral blood of patients with WT in different clinical
stages
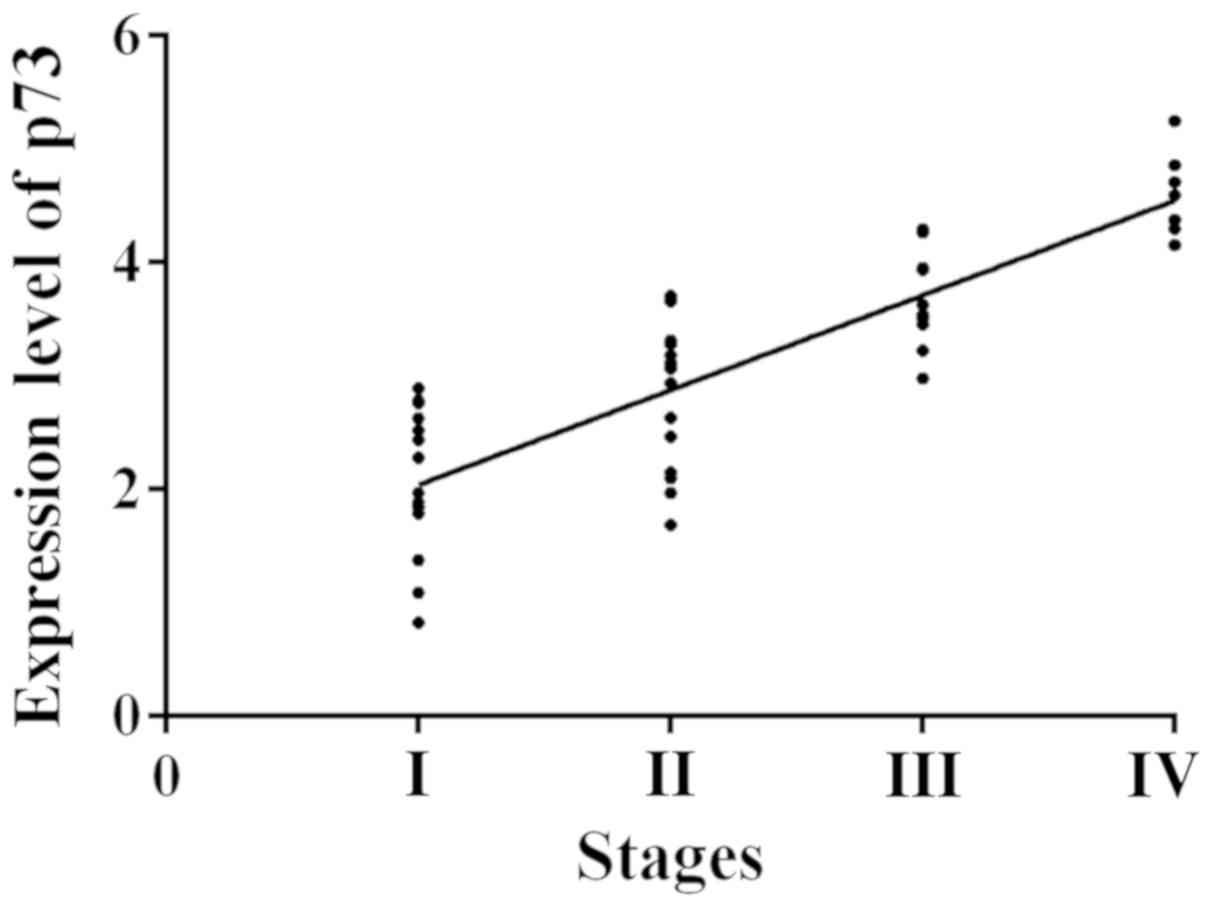
The clinical stages ranged from I to IV. According
to the specific expression level of p73 in blood (Table V), the expression level of p73 in the
peripheral blood of WT patients was in a progressive increase with
the change of clinical stage. The expression levels of p73 among
different clinical stages were significantly different (F=23.54,
P<0.01). The Spearmans correlation analysis showed that the
correlation between the expression level of p73 and the clinical
stages is positive (r=0.7230, P<0.01) (Fig. 1).
 | Table V.Comparison of p73 expression in the
peripheral blood of patients with WT in different clinical stages
(mean ± SD). |
Table V.
Comparison of p73 expression in the
peripheral blood of patients with WT in different clinical stages
(mean ± SD).
| Clinical stage | No. of cases | p73 |
|---|
| I | 15 | 2.05±0.98 |
| II | 17 |
2.99±0.65a |
| III | 11 |
3.76±0.43a,b |
| IV | 7 |
4.48±0.33a–c |
| F |
| 23.54 |
| P-value |
| <0.01 |
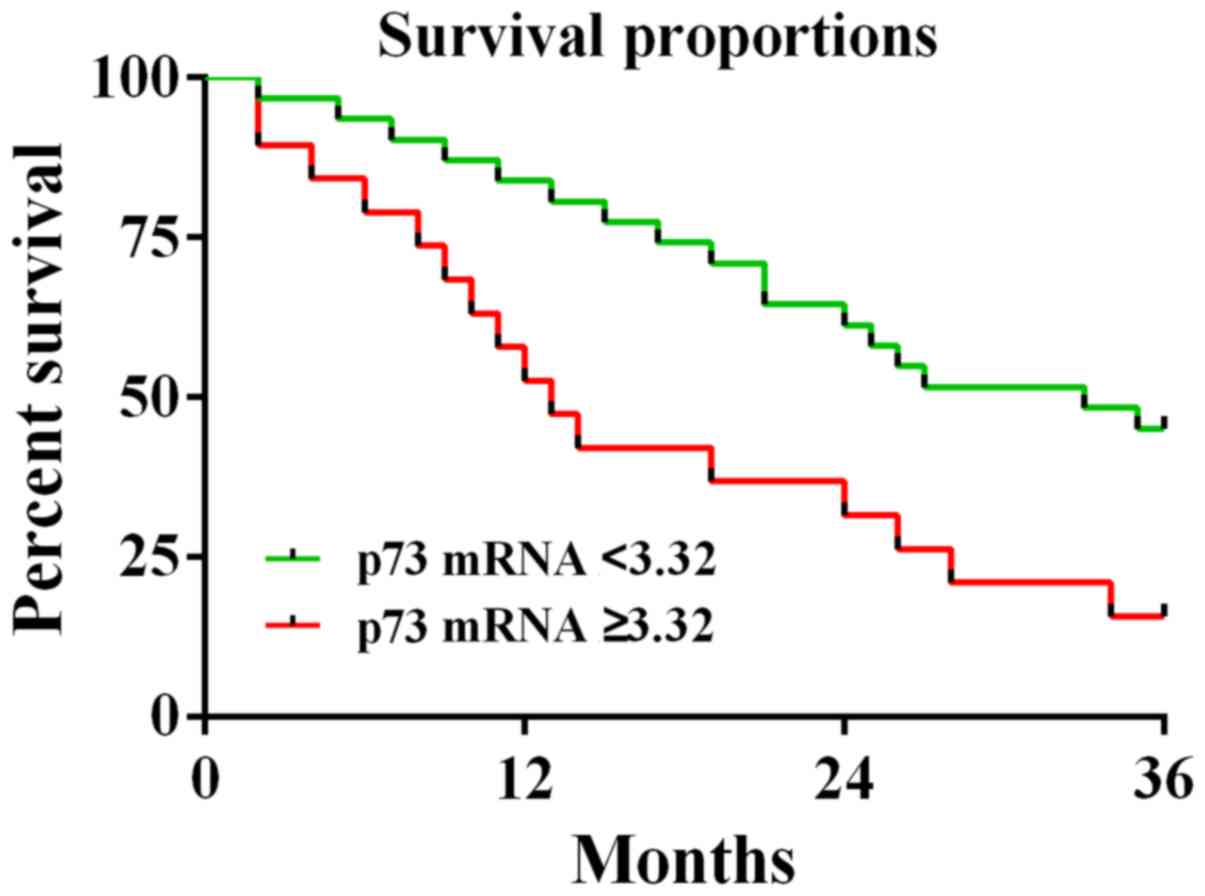
Relationship between the p73
expression level in the peripheral blood and the survival rate of
children with WT
According to the survival data of children in the
study group and taking the mean value of the expression of p73
(3.32) as the boundary, the patients with p73 expression value
<3.32 were considered as the low-expression group (31 children;
median survival time, 30 months) and the patients with p73
expression value ≥3.32 were considered as the high-expression group
(19 children; median survival time, 13.5 months) (Fig. 2). The 3-year follow-up revealed the
relationship between the high expression of p73 and the low
survival rate of patients. The survival rate of the patients in the
high-expression group (15.8%) was significantly lower than that of
the low-expression group (45.2%).
Discussion
WT, a malignant tumor common in children 2–3 years
of age, has common clinical symptoms, such as abdominal masses,
irritability, weight loss and anhelation, and a certain mortality
rate (15,16). The development in medical technology
has offered surgical treatment as the main method for treating WT
in children. It has good efficacy, but causes large trauma and many
adverse reactions harmful to the recovery of children (17). Therefore, the study of the molecular
mechanism of WT is particularly important. Since the molecular
research on the development mechanism of WT is still unclear, the
‘nephrogenic rest’ theory (18) has
been confirmed to a certain degree, which suggests that the
embryonic renal tissue in some individual renal tissues may be the
pre-neoplastic lesions of the WT. Worldwide studies on many genes
have been found to be related to the occurrence and development of
WT, such as WT1, WT2, WTX, WT3, WT4 (FWT1), p53, p16, tumor
synechia and other molecular genes (19,20), but
studies on their mechanism of action are rare. This study found
that p73 plays an important role in the WT in children.
As part of the p53 family, with different kinds of
promoter transcription and alternative splicing, p73 can produce
>10 different subtypes, collectively called DNp73 or ΔTAp73
(21), that play an important role
in the expression of human tumors. The DNp73 is different from p53
in function due to the significant difference in their structures,
while the ΔTAp73 has similar functions as p53 in inhibiting tumor
and promoting apoptosis (22). p73
gene is characterized by the dual feature of tumor suppressor and
carcinogenesis because of the co-expression and mutual intervention
of different subtypes of p73 and p53 (21), proved by related studies to be
possibly involved in the proliferation, metastasis, and invasion of
tumor cells. The mechanism of action of p73 may be as follows: i)
as one of the direct transcriptional target genes of E2F-1 which
mainly regulates cell proliferation and is overexpressed in various
cell lines, p73 can have induced expression by E2F-1 (23). The study by Ozono et al
(24) has shown that the ERE73s
located in the TAP73 promoter can detect the abnormal expression of
E2F in the normal human fibroblasts, likely in joint action with
the TAP73 to induce the increase of DNp73 mRNA expression in the
body. ii) DNp73, a subtype of p73, is involved in the
proliferation, metastasis, and invasion of tumor cells, mainly by
stimulating the expression of Cola1 and PAI-1 receptors in the
TGF-β signaling pathway (25).
Certain relations between the occurrence, development, and
prognosis of various malignant tumors and the p73 are recognized
(26,27).
In the present study, the study and control groups
were not obviously different in the clinical general data,
according to the comparison between the two groups (P>0.05),
protecting this experiment from the interference by some basic
clinical indicators. The analysis of the relationship between the
expression level of p73 in the peripheral blood and the
clinicopathological features of WT revealed the differences of the
p73 expression levels among patients with different tumor sizes,
pathological types, lymph node metastasis, and treatment outcomes
(P<0.05), suggesting the important role of p73 in the occurrence
and development of WT (28). This
study found that the expression level of p73 in the peripheral
blood of the study group (4.04±0.79) was much higher than that of
the control group (1.57±1.12), indicating the high concentration of
p73 in the patients serum. Related studies (29) have pointed out that the p73 gene is
highly expressed in tumors in the main form of DNp73. In addition,
some studies (30) have confirmed
the frequent increase in the expression of the subtype, DNp73 in
cervical cancer, but the present study aimed at the WT. Due to the
significantly higher expression of p73 in the peripheral blood of
patients in the study group, compared with that in the control
group, the assumption was drawn that the expression of p73 in the
peripheral blood of patients with WT was mainly in the form the
DNp73, and the overexpression of p73 in tumor patients was also
indirectly indicated (4). The
present study revealed the significantly positive correlation
between the expression level of p73 and the clinical stage by
comparing the expression of p73 in the peripheral blood of patients
in different clinical stages (r=0.7230, P<0.01), suggesting that
p73 is of great significance for the clinical stage of WT in
children. The expression level of p73 in patients progressively
increased with the increase of tumor malignancy, indicating that
p73 may be involved in the proliferation, invasion and metastasis
of tumor cells (22). Analysis of
the relationship between the expression of p73 in the peripheral
blood of patients with WT and the survival rate of patients showed
that the survival rate of the high-expression group is
significantly lower than that of the low-expression group
(P<0.05), presenting the relationship between the low survival
rate of patients and the high expression of p73. Also, in
literature it has been stated that patients with highly expressed
p73 have low survival rate (25).
However, the absolute relationship between the high expression of
p73 in patients with WT and the poor survival rate in patients has
not been verified by literature.
The results of the present study have certain
reliability in consideration of its comprehensive analysis of the
clinical baseline data of the two groups, the relationship between
the expression level of p73 in the peripheral blood and the
clinicopathological features of WT, the comparison of the
expression levels of p73 in the peripheral blood between the two
groups, the comparison of the expression of p73 in the peripheral
blood among patients with different clinical stages, and the
relationship between the expression of p73 in the peripheral blood
of WT patients, and the survival rate of patients. However, this
study was retrospective, which brought certain limitations. Thus,
more research subjects are needed in a future study to better serve
clinical treatment.
In summary, the finding that p73 is highly expressed
in WT in children provides guiding significance for the clinical
diagnosis and prognosis of WT in children.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors contributions
YD drafted the manuscript. YD and XG were
responsible for the serum collection and PCR. XL and JL collected
and analyzed the general data of patients. NL and CX contributed to
the follow-up and the analysis of the observation indicators. The
final version was read and approved by all authors.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the People's Hospital of Rizhao (Rizhao, China).
Patients who participated in this study, had complete clinical
data. Signed informed consents were obtained from the parents of
the child patients/or the guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Saltzman AF, Carrasco A Jr, Weinman J,
Meyers ML and Cost NG: Initial imaging for pediatric renal tumors:
An opportunity for improvement. J Urol. 199:1330–1336. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bozlu G and Çıtak EÇ: Evaluation of renal
tumors in children. Turk J Urol. 44:268–273. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tian F, Yourek G, Shi X and Yang Y: The
development of Wilms tumor: From WT1 and microRNA to animal models.
Biochim Biophys Acta. 1846:180–187. 2014.PubMed/NCBI
|
|
4
|
Zhang Y, Young A, Zhang J and Chen X: P73
tumor suppressor and its targets, p21 and PUMA, are required for
Madin-Darby canine kidney cell morphogenesis by maintaining an
appropriate level of epithelial to mesenchymal transition.
Oncotarget. 6:13994–14004. 2015.PubMed/NCBI
|
|
5
|
Kurian JJ, Jehangir S and Korula A:
Multiloculated cystic renal tumors of childhood: Has the final word
been spoken? J Indian Assoc Pediatr Surg. 23:22–26. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cutruzzolà F, Avigliano L and Candi E: p73
keeps metabolic control in balance. Cell Cycle. 13:179–180. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Killick R, Niklison-Chirou M, Tomasini R,
Bano D, Rufini A, Grespi F, Velletri T, Tucci P, Sayan BS, Conforti
F, et al: p73: A multifunctional protein in neurobiology. Mol
Neurobiol. 43:139–146. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Inoue S, Tomasini R, Rufini A, Elia AJ,
Agostini M, Amelio I, Cescon D, Dinsdale D, Zhou L, Harris IS, et
al: TAp73 is required for spermatogenesis and the maintenance of
male fertility. Proc Natl Acad Sci USA. 111:1843–1848. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ye B, Wang X, Yang Z, Sun Z, Zhang R, Hu
Y, Lu Y and Du J: p53 and p73 expression in esophageal carcinoma
correlate with clinicopathology of tumors. Hepatogastroenterology.
59:2192–2195. 2012.PubMed/NCBI
|
|
10
|
Arisawa A, Watanabe Y, Tanaka H, Takahashi
H, Matsuo C, Fujiwara T, Fujiwara M, Fujimoto Y and Tomiyama N:
Comparative study of pulsed-continuous arterial spin labeling and
dynamic susceptibility contrast imaging by histogram analysis in
evaluation of glial tumors. Neuroradiology. 60:599–608. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Moll UM and Slade N: p63 and p73: roles in
development and tumor formation. Mol Cancer Res. 2:371–386.
2004.PubMed/NCBI
|
|
12
|
Mancini P, Angeloni A, Risi E, Orsi E and
Mezi S: Standard of care and promising new agents for
triple-negative metastatic breast cancer. Cancers (Basel).
6:2187–2223. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Murphy AJ, Pierce J, de Caestecker C,
Taylor C, Anderson JR, Perantoni AO, de Caestecker MP and Lovvorn
HN III: SIX2 and CITED1, markers of nephronic progenitor
self-renewal, remain active in primitive elements of Wilms tumor. J
Pediatr Surg. 47:1239–1249. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ishibashi K, Haber T, Breuksch I, Gebhard
S, Sugino T, Kubo H, Hata J, Koguchi T, Yabe M, Kataoka M, et al:
Overriding TKI resistance of renal cell carcinoma by combination
therapy with IL-6 receptor blockade. Oncotarget. 8:55230–55245.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cutruzzula P, Cahn D, Kivlin D, Tong C,
Edwards D and Amster M: A review of translocation t(6;11) renal
cell carcinoma tumors in the adult patient. Curr Urol. 10:69–71.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schultz KAP, Rednam SP, Kamihara J, Doros
L, Achatz MI, Wasserman JD, Diller LR, Brugières L, Druker H,
Schneider KA, et al: Pten, dicer1, fh, and their associated tumor
susceptibility syndromes: Clinical features, genetics, and
surveillance recommendations in childhood. Clin Cancer Res.
23:e76–e82. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Al-Hussain T, Ali A and Akhtar M: Wilms
tumor: An update. Adv Anat Pathol. 21:166–173. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zitzmann F, Mayr D, Berger M, Stehr M, von
Schweinitz D, Kappler R and Hubertus J: Frequent hypermethylation
of a CTCF binding site influences Wilms tumor 1 expression in Wilms
tumors. Oncol Rep. 31:1871–1876. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Franken J, Lerut E, Van Poppel H and
Bogaert G: p53 immunohistochemistry expression in Wilms tumor: A
prognostic tool in the detection of tumor aggressiveness. J Urol.
189:664–670. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Melino G, De Laurenzi V and Vousden KH:
p73: Friend or foe in tumorigenesis. Nat Rev Cancer. 2:605–615.
2002. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Song DJ, Yue LF, Zhang D, Yang HY, Fan YX,
Yue M, Pei H and Wang JX: Relationship between mRNA expression and
promoter methylation status of p73 gene in peripheral blood among
children with Wilms' tumor. Zhongguo Dang Dai Er Ke Za Zhi.
15:638–643. 2013.(In Chinese). PubMed/NCBI
|
|
23
|
McKeon F and Melino G: Fog of war: The
emerging p53 family. Cell Cycle. 6:229–232. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ozono E, Komori H, Iwanaga R, Tanaka T,
Sakae T, Kitamura H, Yamaoka S and Ohtani K: Tumor suppressor TAp73
gene specifically responds to deregulated E2F activity in human
normal fibroblasts. Genes Cells. 17:660–672. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fuchs J: Surgical concepts in the
treatment of Wilms tumor: An update. Urologe A. 54:1784–1791.
2015.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Al-Daghmin A, Alhamss S, Al-Qasem K,
Al-Najjar H, Al-Smadi K, Olaimat A and Al-Halbouni L: Patterns of
management of translocation renal cell carcinoma. Turk J Urol.
44:467–472. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pan X, Quan J, Zhao L, Li W, Wei B, Yang S
and Lai Y: Xp11.2 translocation renal cell carcinoma with TFE3 gene
fusion: A case report. Mol Clin Oncol. 8:83–85. 2018.PubMed/NCBI
|
|
28
|
Yong M, Yang L, Suyila Q, Han W, Yuan H,
Zhao C and Su X: Expression and clinical implications of P53, P63,
and P73 protein in malignant tumor of the parotid gland. Turk J Med
Sci. 44:875–882. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ishimoto O, Kawahara C, Enjo K, Obinata M,
Nukiwa T and Ikawa S: Possible oncogenic potential of DeltaNp73: A
newly identified isoform of human p73. Cancer Res. 62:636–641.
2002.PubMed/NCBI
|
|
30
|
Inoue K and Fry EA: Alterations of p63 and
p73 in human cancers. Subcell Biochem. 85:17–40. 2014. View Article : Google Scholar : PubMed/NCBI
|