Introduction
As a type of tumor originating from glial cells of
the spine or brain, glioma is one of the most common types of
central nervous system tumors, accounting for ~80% of all malignant
brain tumors (1). Glioma tumors
arising in different parts of the central nervous system may have
different consequences (2,3). Unlike other types of malignancy,
gliomas metastasize through the cerebrospinal fluid, but not the
blood circulation to cause drop metastases to the spinal cord
(4). Following the establishment of
metastasis, treatment outcomes and prognosis are markedly poor
(4). In spite of the advances in
understanding the development of glioma, the pathogenesis of this
disease remains unclear (5).
Therefore, in-depth investigation of the molecular mechanism of the
occurrence of glioma may lead to improved treatment and
prevention.
Genetic factors serve central roles in the
pathogenesis of glioma. Certain genetic diseases, including
tuberous sclerosis complex and neurofibromatosis type 1 and 2 are
associated with the occurrence of glioma (6). Transforming growth factor β (TGF-β)
signaling is considered to be a central factor in glioma
development owing to its role in regulating cancer cell
proliferation, migration and invasion (7,8). TGF-β
inhibits tumor growth at early stages of tumor development, and
promotes tumor metastasis at advance stages of the majority of
cancer types (9), particularly in
glioma (7,8). Long non-coding RNAs (lncRNAs) are a
type of non-coding RNA of >200 nucleotides in length that serve
pivotal roles in the pathogenesis of various types of cancer
(10). LncRNAs are also key factors
in glioma (11). TGF-β signaling has
been demonstrated to be regulated by lncRNAs (12). Growth-arrest-associated lncRNA 1
(GASL1) has been identified as a potential tumor suppressor in
liver cancer (13). Downregulation
of GASL1 was observed in liver cancer, and overexpression of GASL1
inhibited osteosarcoma cell proliferation by inactivating a
transcription factor involved in cell cycle progression (13). The involvement of GASL1 in other
malignancies remains unknown. Preliminary data obtained from
microarray revealed altered expression of GASL1 in glioma tissues
compared with in healthy tissues (data not shown). In the present
study, the effect of altered GASL1 expression on the proliferation
of glioma cells and the role of TGF-β1 in this process was
investigated.
Materials and methods
Patient and healthy control
samples
A retrospective study was performed to review the
clinical data of 62 patients diagnosed with glioma in The Second
Hospital of Hebei Medical University (Hebei, China). All the
patients were diagnosed through pathological examination and
treated between March 2010 and March 2013. Patient specimens
including tumor tissues, paired adjacent healthy tissues and serum
samples were obtained from the specimen library of the hospital.
The patients that were included in the study met the following
criteria: i) Diagnosis of glioma by pathological examination; ii)
first time being treated for glioma; iii) completion of the whole
treatment procedure; iv) completion of follow-up; and v) complete
clinical data available. The exclusion criteria were as follows: i)
Other malignancies diagnosed; ii) other severe diseases diagnosed;
iii) prior treatment or transferred to other hospitals during
treatment; or iv) mortality due to other causes during follow-up.
The 62 patients included 32 males and 30 females, and the ages
ranged between 33 and 72 years, with a mean age of 51.2±5.9 years.
Serum samples of 52 healthy volunteers were also obtained from the
specimen library of The Second Hospital of Hebei Medical
University. The healthy controls received routine physiological
examinations during the same period. The controls including 28
males and 24 females, were aged between 31 and 70 years, with a
mean age of 50.5±6.6 years. No significant differences in age, sex
and other basic clinical data were identified between the patient
group and control group.
Quantification of TGF-β1 by ELISA
Serum TGF-β1 was detected using the human TGF-β 1
ELISA kit (RAB0460; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany).
All experiments were performed according to the manufacturers
protocol.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) was used to extract
total RNA, and tissues were ground in liquid nitrogen before adding
TRIzol® to achieve complete cell lysis. Total RNA was
transcribed into cDNA using Reverse Transcriptase AMV kit
(Sigma-Aldrich; Merck KGaA) and the following temperature
conditions: 50°C for 20 min and 80°C for 10 min. The qPCR mix was
prepared using SYBR® Green Real-Time PCR Master mix
(Thermo Fisher Scientific, Inc.). Primers used in PCR were as
follows: GASL1 forward, 5′-CTGAGGCCAAAGTTTCCAAC-3′ and reverse,
5′-CAGCCTGACTTTCCCTCTTCT-3′; TGF-β1 forward,
5′-GGACACCAACTATTGCTTCAG-3′ and reverse, 5′-TCCAGGCTCCAAATGTAGG-3′;
β-actin forward, 5′-GACCTCTATGCCAACACAGT-3′ and reverse,
5′-AGTACTTGCGCTCAGGAGGA-3′. PCR thermocycling conditions were as
follows: 40 sec at 95°C, followed by 40 cycles of 20 sec at 95°C
and 40 sec at 57°C. GASL1 and TGF-β1 expression was normalized to
β-actin endogenous control using the 2−ΔΔCq method
(14).
Western blot analysis
Total protein was extracted using
radioimmunoprecipitation solution (Thermo Fisher Scientific, Inc.)
and bicinchoninic acid assay was performed to determine protein
concentration. SDS-PAGE (12% gel) was performed with 25-µg
protein/lane. Proteins were transferred onto polyvinylidene
difluoride membranes, and blocked with 5% skimmed milk in TBS with
0.2% Tween at room temperature for 2 h. Next, membranes were
incubated with primary antibodies against TGF-β1 (rabbit
anti-human; 1:1,500; ab92486; Abcam, Cambridge, MA, USA) and GAPDH
(rabbit anti-human; 1:1,200; ab9485; Abcam) at 4°C overnight.
Subsequently, membranes were incubated with horseradish
peroxidase-conjugated anti-rabbit IgG secondary antibody (1:1,000;
MBS435036; MyBioSource, Inc., San Diego, CA, USA) for 4 h at room
temperature. Pierce enhanced chemiluminescence western blotting
substrate (Thermo Fisher Scientific, Inc.) was added to develop the
signal, which was detected using a MYECL™ Imager (Thermo Fisher
Scientific, Inc.). Expression of TGF-β1 was normalized to GAPDH
endogenous control using ImageJ software (version 1.6; National
Institutes of Health, Bethesda, MD, USA).
Cell culture and transfection
The present study included two human glioma cell
lines, Hs 683 (ATCC® HTB-138™) and CCD-25Lu
(ATCC® CCL-215™). The two cell lines were purchased from
the American Type Culture Collection (ATCC; Manassas, VA, USA).
Cells were cultured with ATCC-formulated Eagles minimum essential
medium (cat. no. 30-2003; ATCC) containing 10% fetal bovine serum
(Sangon Biotech Co., Ltd., Shanghai, China). GASL1 short hairpin
(sh)RNA (5′-GACGTGTCAGGACCTTCGT-3′) and negative control shRNA were
synthesized by Shanghai GenePharma Co., Ltd. (Shanghai, China). PCR
was performed to obtain an EcoRI/EcoRI fragment
containing full-length GASL1 cDNA (performed by Sangon Biotech Co.,
Ltd.). The GASL1 cDNA was amplified by PCR using the cDNA generated
from RT-PCR as a template. The sequences of the primers were as
follows: Forward, 5′-GAATTAGGGTGCGTCACCGGAGCAG-3′ and reverse,
5′-AATTCGGTTTTTCTTTCTTAGTTTATTT-3′. The PCR mix was prepared using
Phusion® High-Fidelity DNA Polymerase kit (New England
BioLabs, Inc., Ipswich, MA, USA). The thermocycling conditions were
30 sec at 98°C, followed by 40 cycles of 10 sec at 98°C, 10 sec at
55°C and 70 sec at 72°C. This fragment was inserted into
pIRSE2-EGFP vector (Clontech Laboratories, Inc., Mountainview, CA,
USA) to construct a GASL1 expression vector.
Lipofectamine® 2000 reagent (cat. no. 11668-019;
Invitrogen; Thermo Fisher Scientific, Inc.) was used to transfect
10 nM vector and 50 nM shRNA into 5×105 cells. Negative
control shRNA and empty pIRSE2-EGFP vector were used as negative
controls. Untransfected cells were used as controls. Overexpression
and knockdown were confirmed by RT-qPCR, as mentioned before. All
subsequent experiments were performed on samples in which
expression of GASL1 was ≥200% for overexpression or ≤50% for
knockdown at 24 h after transfections compared with expression in
the control group.
Cell proliferation assay
A Cell Counting Kit-8 (CCK-8) assay was performed to
evaluate cell proliferation. In cases of TGF-β1 treatment, cells
were treated with TGF-β1 (Sigma-Aldrich; Merck KGaA) at a dose of
10 ng/ml for 12 h at 37°C before use. Hs 683 and CCD-25Lu cells
were collected during exponential phase to make cell suspensions
with a density of 4×104 cells/ml. Of this,
4×103 cells were added to each well of a 96-well plate.
Cells were cultured for 24, 48, 72 and 96 h in an incubator at 37°C
with 5% CO2, at which point 10 µl CCK-8 solution
(Sigma-Aldrich; Merck KGaA) was added. Following culture for a
further 4 h, optical density values were determined at 450 nm using
a Fisherbrand™ accuSkan™ GO UV/Vis microplate spectrophotometer
(Thermo Fisher Scientific, Inc.) to assess cell proliferation. OD
value of control group was set to 100%, and all other groups and
other time points were normalized to this value.
Statistical analysis
Statistical analysis was performed using SPSS
(version 19.0; IBM Corp., Armonk, NY, USA). GASL1 and TGF-β1
expression levels were recorded as the mean ± standard deviation,
and comparisons between two groups and among multiple groups were
performed by Students t-test and one-way analysis of variance
(ANOVA) followed by Tukeys post hoc test, respectively. The
χ2 test was performed to analyze the association between
GASL1 expression and clinical data of patients with glioma.
Pearsons correlation coefficient was used to analyze TGF-β1 and
GASL1 expression for potential correlations. Receiver operating
characteristic (ROC) curve analysis was performed to evaluate the
diagnostic value of serum GASL1 for glioma with patients as true
positive cases and controls as true negative cases. The 62 patients
with glioma were divided into high and low level expression groups
(n=31) according to the median serum level of GASL1. Kaplan-Meier
method was used to plot survival curves and the two groups were
compared using a log-rank test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Expression of GASL1 in tumor tissues
and paired normal tissues of 62 patients with glioma
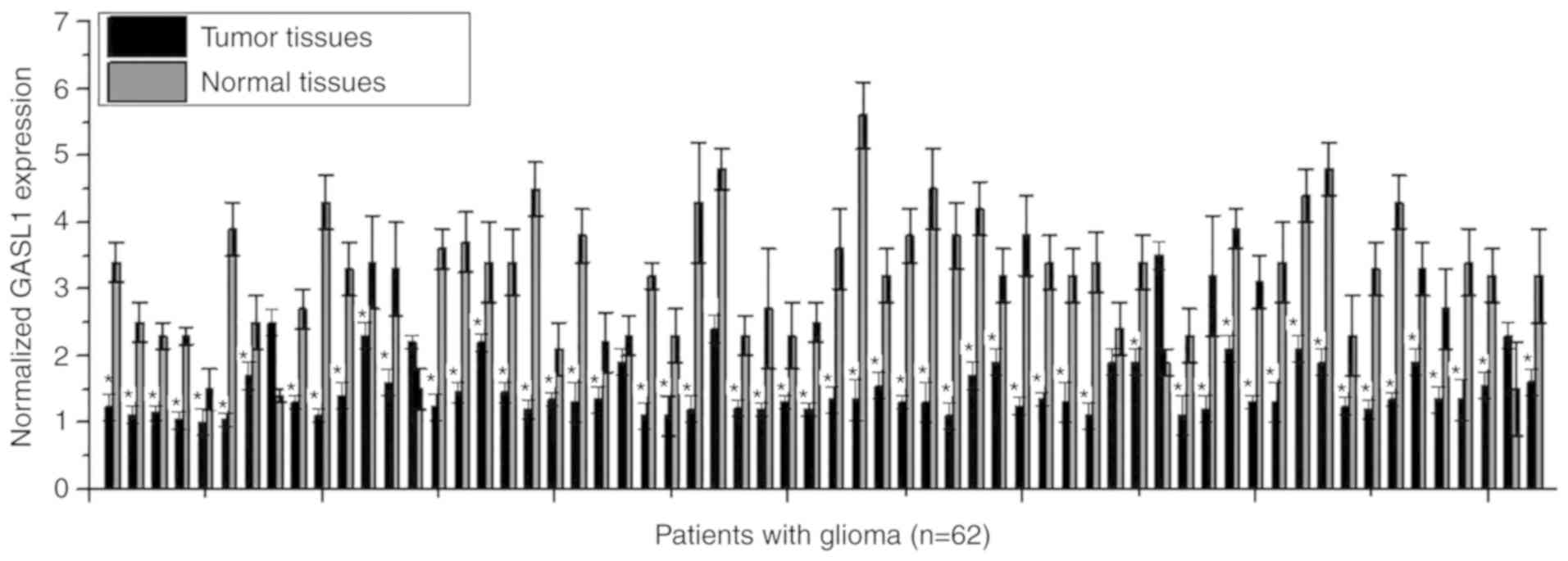
GASL1 expression in tumor tissues and paired normal
tissues of 62 patients with glioma was detected using RT-qPCR. The
results demonstrated that a majority of patients with glioma
(56/62, 90.3%) had significantly decreased expression of GASL1 in
tumor tissues compared with expression in paired adjacent healthy
tissues (P<0.05; Fig. 1),
indicating that downregulation of GASL1 may be involved in the
pathogenesis of glioma.
Comparison of serum level of GASL1 in
patients with glioma and in healthy controls, and analysis of the
diagnostic value of serum GASL1
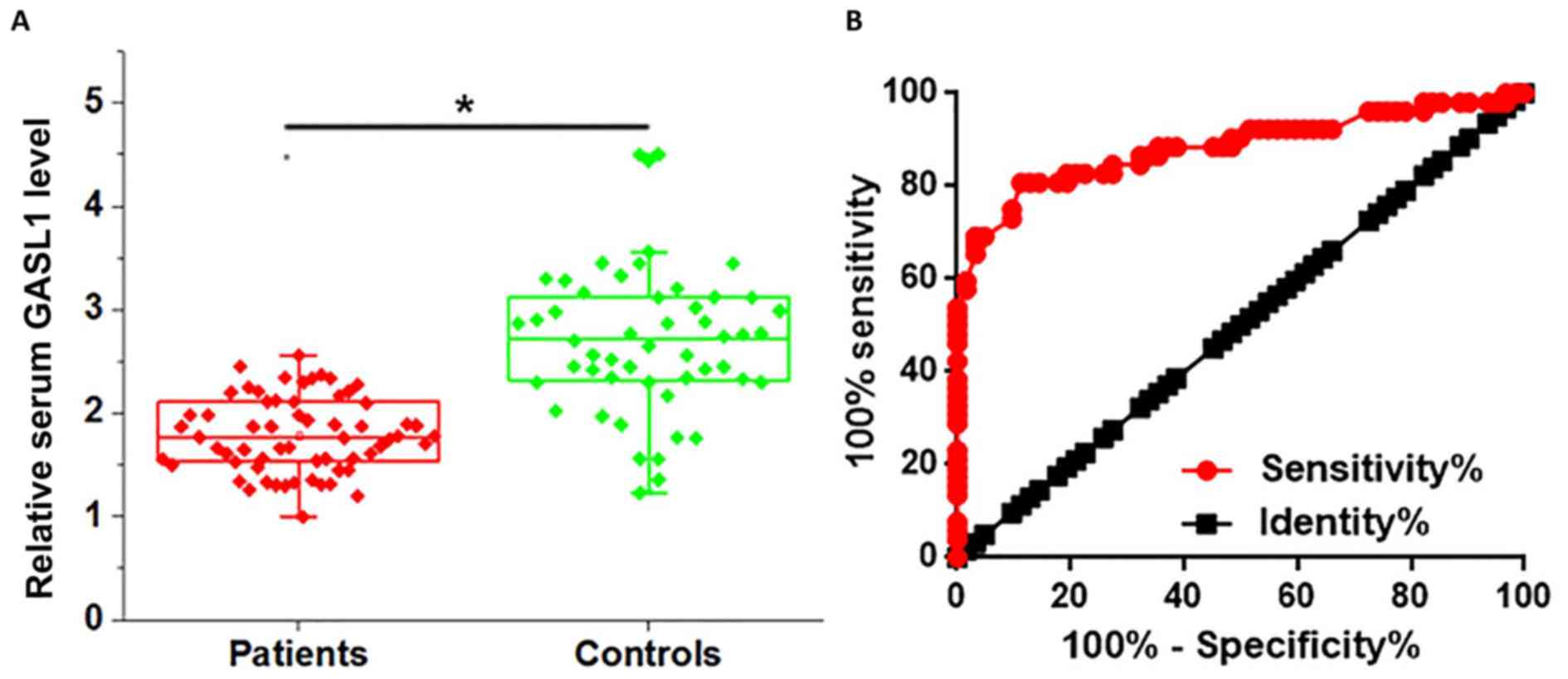
Serum levels of GASL1 in patients with glioma and in
healthy controls were also determined using RT-qPCR. As presented
in Fig. 2A, the serum level of GASL1
was significantly lower in patients with glioma compared with that
in healthy controls (P<0.05). Receiver operating characteristic
(ROC) curve analysis was performed to evaluate the diagnostic value
of serum GASL1 for glioma. As presented in Fig. 2B, the area under the curve was
0.8838, with standard error of 0.03429 and 95% confidence interval
of 0.8166 to 0.9511. Therefore, serum GASL1 may serve as a
potential diagnostic biomarker for glioma.
Prognostic value of serum GASL1 for
glioma
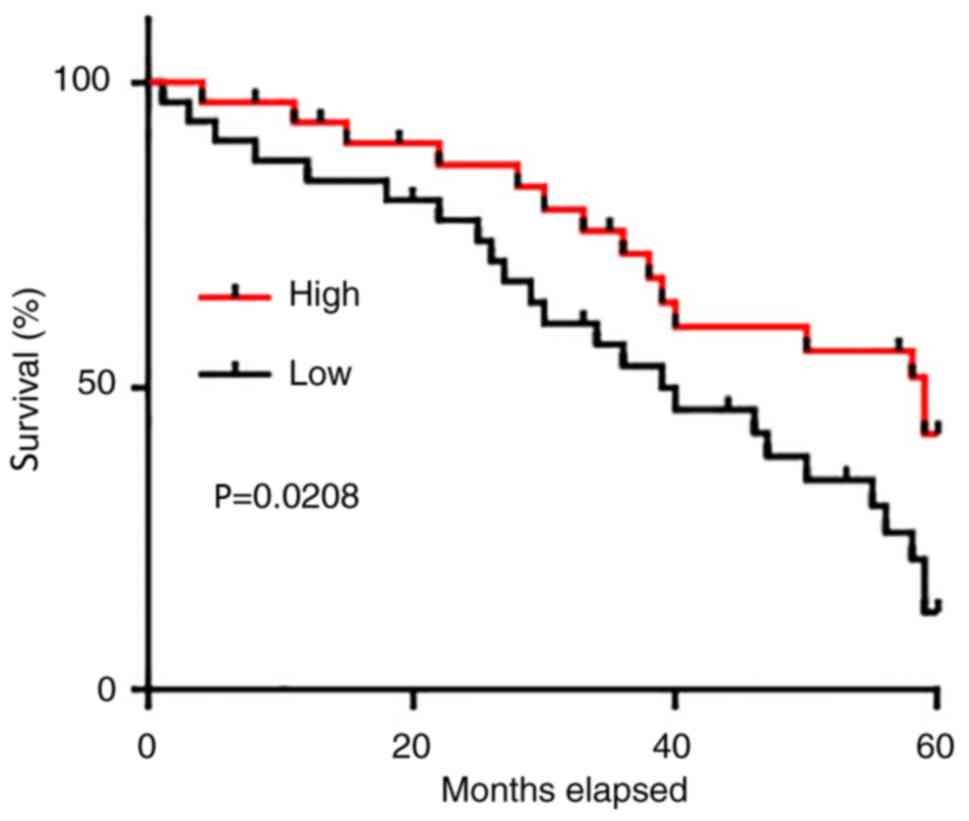
The 62 patients with glioma were divided into high
and low level expression groups (n=31) according to the median
serum level of GASL1. All patients were followed up for 5 years or
until their mortality to determine the survival rate. The
Kaplan-Meier method was used to plot survival curves and the two
groups were compared using a log-rank test. As presented in
Fig. 3, the overall survival rate of
patients with a low serum level of GASL1 was significantly worse
compared with that of patients with a high serum level of
GASL1.
Association between serum levels of
GASL1 and patients clinicopathological data
Potential associations between serum levels of GASL1
and patients clinicopathological data were analyzed using a
χ2 test. As presented in Table I, no significant correlations were
identified between serum levels of GASL1 and patients age, sex,
smoking habit, drinking habit and existence of distant tumor
metastasis. In contrast, there was a significant inverse
correlation between levels of serum GASL1 and tumor size.
 | Table I.Serum GASL1 level and
clinicopathological data of 62 patients with glioma. |
Table I.
Serum GASL1 level and
clinicopathological data of 62 patients with glioma.
| Characteristics | Cases, n | High GASL1, n | Low GASL1, n | χ2 | P-value |
|---|
| Age, years |
|
|
| 0.58 | 0.45 |
|
>50 | 29 | 13 | 16 |
|
|
|
<50 | 33 | 18 | 15 |
|
|
| Sex |
|
|
| 1.03 | 0.31 |
| Male | 32 | 14 | 18 |
|
|
|
Female | 30 | 17 | 13 |
|
|
| Alcohol
consumption |
|
|
| 0.28 | 0.60 |
| Yes | 22 | 12 | 10 |
|
|
| No | 40 | 19 | 21 |
|
|
| Smoking |
|
|
| 0.62 | 0.43 |
| Yes | 23 | 10 | 13 |
|
|
| No | 39 | 21 | 18 |
|
|
| Primary tumor
diameter, cm |
|
|
| 6.51 | 0.01 |
|
>2 | 28 | 9 | 19 |
|
|
|
<2 | 34 | 22 | 12 |
|
|
| Tumor distant
metastasis |
|
|
| 0.60 | 0.44 |
| Yes | 25 | 11 | 14 |
|
|
| No | 37 | 20 | 17 |
|
|
Interactions between GASL1 and TGF-β1
in glioma cells
The data in Table I
indicate that GASL1 may be involved in the growth of glioma. It has
been reported that activation of TGF-β promotes growth of glioma
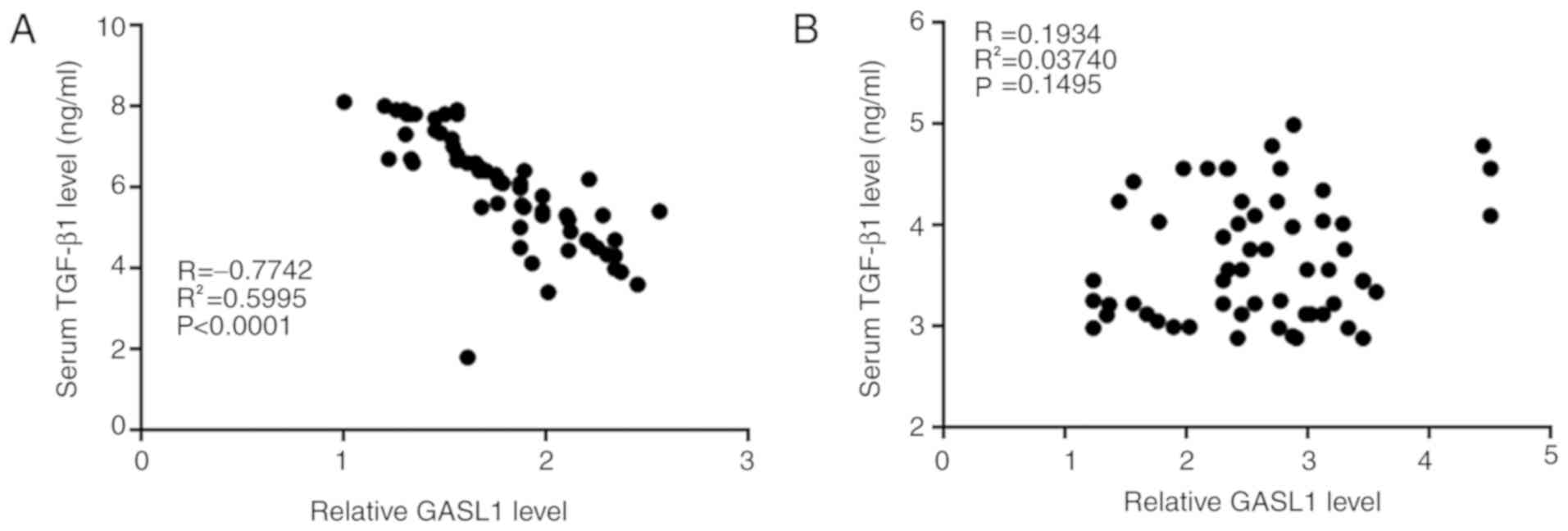
(8). In the present study, Pearsons
correlation analysis revealed a significant negative correlation
between serum GASL1 and TGF-β1 in patients with glioma (Fig. 4A), but not in healthy controls
(Fig. 4B). In addition, the effects
of GASL1 overexpression and knockdown on the expression of TGF-β1
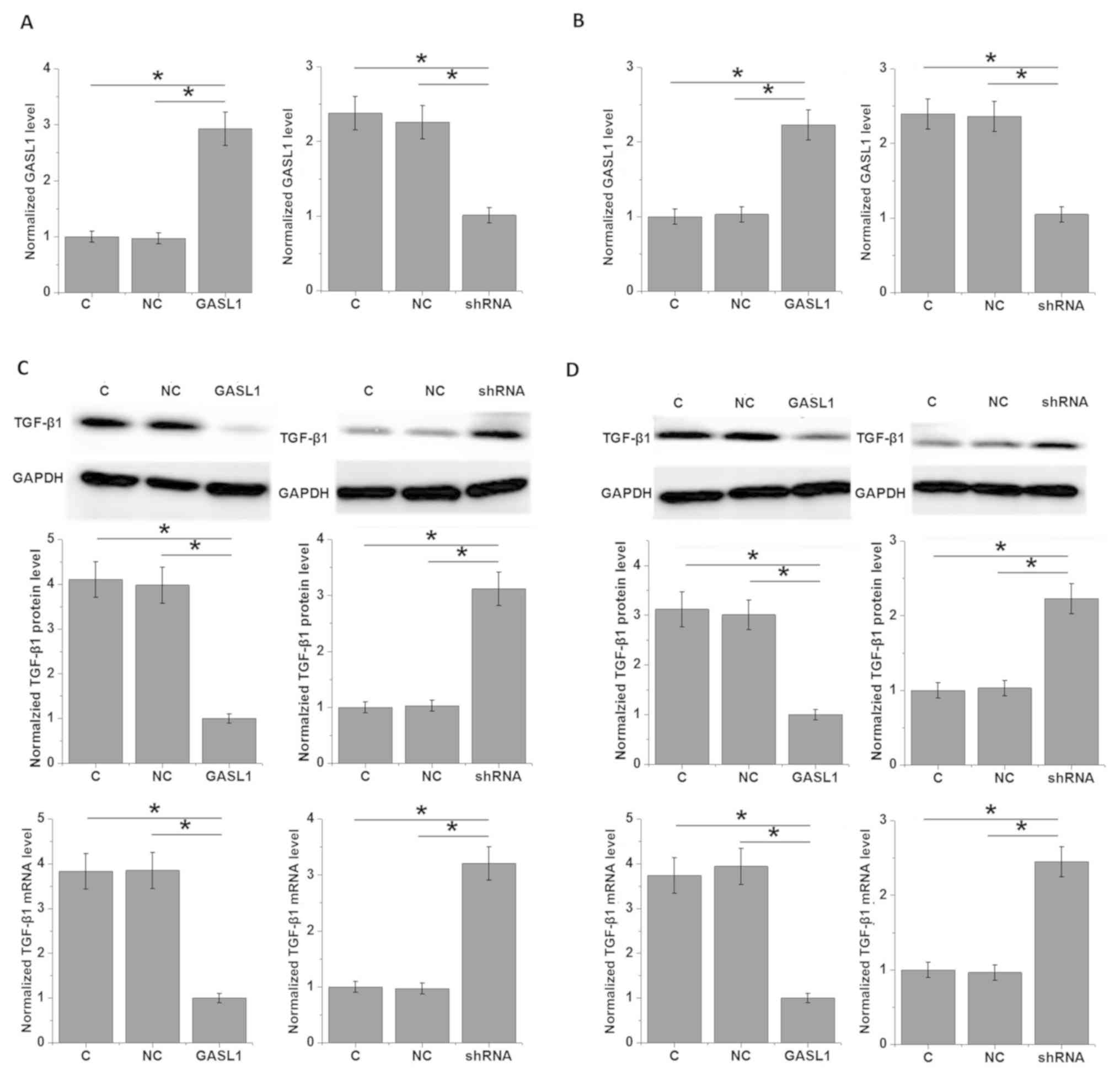
in glioma cell lines were examined. GASL1 overexpression and
shRNA-mediated knockdown of GASL1 were successfully achieved in two
glioma cell lines, Hs 683 (Fig. 5A)
and CCD-25Lu (Fig. 5B). GASL1
overexpression significantly inhibited and shRNA silencing
significantly promoted the expression TGF-β1 in cells of glioma
cell lines Hs 683 (Fig. 5C) and
CCD-25Lu (Fig. 5D). However,
treatment with TGF-β1 (5, 10 and 50 ng/ml; Sigma-Aldrich) for 12 h
had no significant effect on GASL1 expression (data not shown).
Therefore, GASL1 may be an upstream inhibitor of TGF-β1.
Effects of GASL1 overexpression and
knockdown and TGF-β1 treatment on cell proliferation
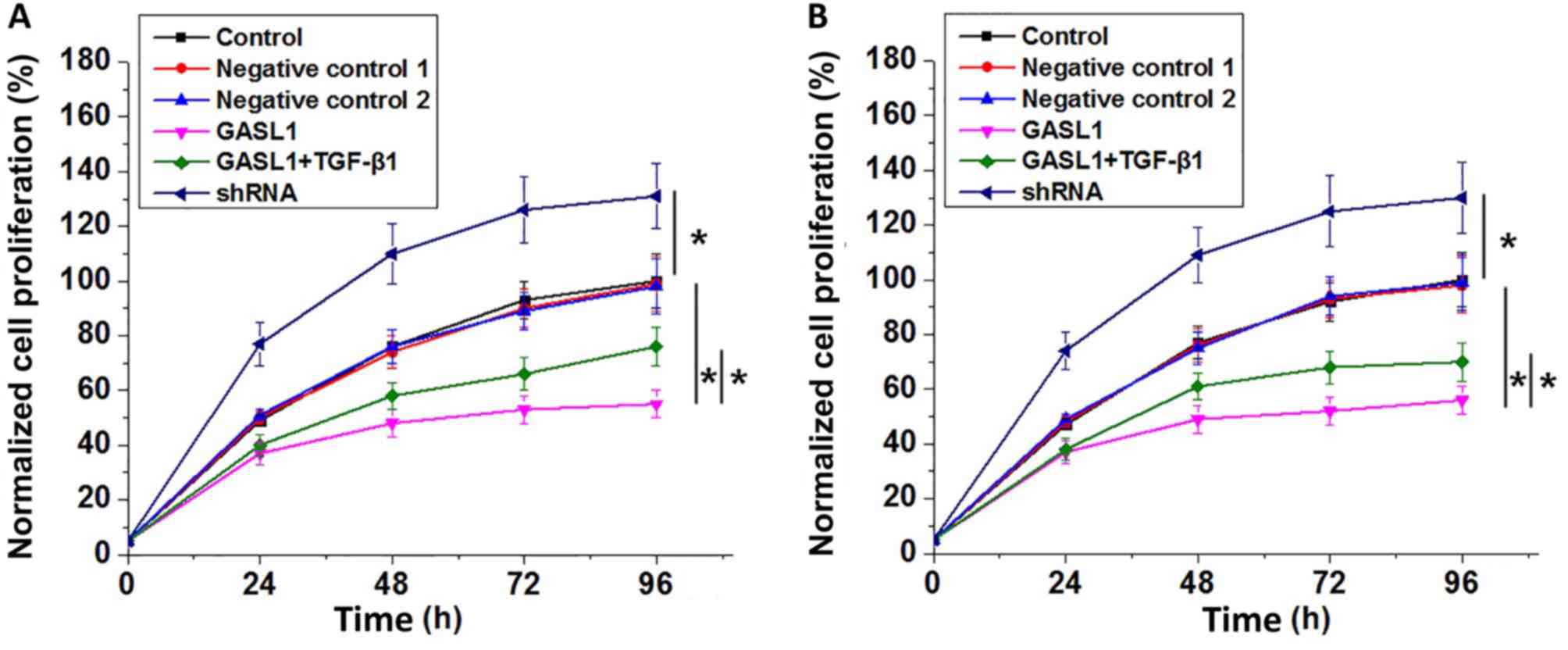
As presented in Fig.
6, analysis of cell proliferation data by one-way ANOVA with a
post-hoc Tukeys test showed that GASL1 overexpression significantly
inhibited and GASL1 significantly promoted the proliferation of two
human glioma cell lines, Hs 683 (Fig.
6A) and CCD-25Lu (Fig. 6B). In
addition, treatment with TGF-β1 at a dose of 10 ng/ml significantly
decreased the inhibitory effect of GASL1 overexpression on cell
proliferation in the two cell lines.
Discussion
GASL1 is an lncRNA that was identified as a tumor
suppressor gene in osteosarcoma (12). The key result of the present study is
that GASL1 may also serve a role as a tumor suppressor gene in
glioma by inhibiting cancer cell proliferation. The action of GASL1
in glioma may be achieved through the downregulation of TGF-β1. In
addition, the results of the present study also revealed that
circulating GASL1 in the serum of patients with glioma may have
diagnostic and prognostic value for glioma.
Previous studies in the last several decades have
indicated that the occurrence, development and progression of
glioma are accompanied by changes in expression patterns of a large
set of genes, and certain genes may act as tumor suppressors or
oncogenes (6). LncRNAs are
potentially key factors in different types of malignancy including
glioma (9,10). Expression of the lncRNA ADAMTS9
antisense RNA 2 (ADAMTS9-AS2) is significantly decreased in glioma
tissues compared with that in paired adjacent normal cells, and
downregulation of ADAMTS9-AS2 increased the migration of glioma
cells (15). In contrast, H19 is an
oncogenic lncRNA with increased expression in glioma (16). A previous study reported that GASL1
is downregulated in liver cancer (13), revealing its role as a potential
tumor suppressor gene in this disease. In the present study, GASL1
in tumor tissues was decreased compared with that in paired
adjacent healthy tissues, indicating that GASL1 may also be a tumor
suppressor gene in glioma.
Circulating biomarkers are widely used in the
diagnosis and prognosis of different types of cancer (17). The main challenge in the treatment of
glioma is the presence of distant tumor metastasis, and early and
accurate diagnosis remains the key factor in influencing the
survival rate of patients (18). In
the present study, levels of circulating GASL1 in serum of patients
with glioma were identified to be significantly lower compared with
in healthy controls. ROC curve analysis indicated that decreased
serum GASL1 may be a useful biomarker to distinguish patients with
glioma from healthy controls. In addition, survival curve
comparison also indicated that low serum levels of GASL1 were
associated with a poor postoperative survival rate. Therefore,
circulating GASL1 may serve as a potential diagnostic and
prognostic biomarker for glioma. However, GASL1 is a novel lncRNA
lacking known expression patterns in other diseases. Therefore, a
combination of multiple biomarkers should be used to improve the
accuracy of diagnosis and prognosis.
The results of the present study also demonstrated
that serum GASL1 is significantly associated with tumor size, but
not with tumor metastasis. In vitro investigation revealed
that GASL1 can inhibit the proliferation of glioma cells. TGF-β
signaling serves a central role in the pathogenesis of various
types of cancer (9,19,20). As
a double-edged sword in cancer biology, TGF-β signaling induces
cancer cell migration and invasion, but also exerts
anti-proliferative effects (9).
However, it is generally considered that TGF-β signaling
accelerates the proliferation rate of glioma cells (8). In the present study, GASL1 was
identified as a potential upstream inhibitor of TGF-β1. In
addition, TGF-β1 treatment significantly decreased the inhibitory
effects of GASL1 overexpression on glioma cell proliferation. This
partial rescue of cell proliferation by TGF-β has been reported
previously (21). GASL1 may inhibit
the growth of glioma by downregulating TGF-β1 expression. However,
the effect of GASL1 on the regulation of TGF-β1 expression may not
be direct, as no significant correlation was identified between
serum GASL1 and TGF-β1 in healthy controls. Therefore, there may be
glioma-specific factors that mediate the association between GASL1
and TGF-β1.
The present study identified a negative correlation
between GASL1 and TGF-β1 in serum samples of patients with glioma,
but not in those of healthy controls. However, the potential
correlation between expression of GASL1 and TGF-β1 in tumor tissues
of patients with glioma was not investigated owing to a lack of
tumor tissues. Future studies are required to perform this
analysis.
In conclusion, GASL1 expression was significantly
decreased in glioma tissue compared with that in normal tissue.
Serum GASL1 may serve as a potential diagnostic and prognostic
biomarker for glioma. GASL1 overexpression inhibited glioma cell
proliferation and downregulated TGF-β1 expression, but TGF-β1
treatment decreased the effect of GASL1 overexpression on
proliferation. Therefore, lncRNA GASL1 may inhibit growth of glioma
by inactivating the TGF-β signaling pathway.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors contributions
YH, BJ, LC, MW and XH were responsible for the
conception and design of the study. YH and BJ performed the
experiments. YH, LC and MW analyzed and interpreted the data. YH
drafted the article. BJ, LC, MW and XH were responsible for the
revision of the manuscript.
Ethics approval and consent to
participate
The protocol of the present study was approved by
the Ethics Review Committee of The Second Hospital of Hebei Medical
University (Hebei, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Goodenberger ML and Jenkins RB: Genetics
of adult glioma. Cancer Genet Cytogenet. 205:613–621. 2012.
View Article : Google Scholar
|
|
2
|
Omuro A and DeAngelis LM: Glioblastoma and
other malignant gliomas: A clinical review. JAMA. 310:1842–1850.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mishra MV, Andrews DW, Glass J, Evans JJ,
Dicker AP, Shen X and Lawrence YR: Characterization and outcomes of
optic nerve gliomas: A population-based analysis. J Neurooncol.
107:591–597. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stark AM, van de Bergh J, Hedderich J,
Mehdorn HM and Nabavi A: Glioblastoma: Clinical characteristics,
prognostic factors and survival in 492 patients. Clin Neurol
Neurosurg. 114:840–845. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ichimura K: Molecular pathogenesis of IDH
mutations in gliomas. Brain Tumor Pathol. 29:131–139. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Radner H, El-Shabrawi Y, Eibl RH, Brüstle
O, Kenner L, Kleihues P and Wiestler OD: Tumor induction by ras and
myc oncogenes in fetal and neonatal brain: Modulating effects of
developmental stage and retroviral dose. Acta Neuropathol.
86:456–465. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kaminska B, Kocyk M and Kijewska M:
TGF-beta signaling and its role in glioma pathogenesis. Adv Exp Med
Biol. 986:171–187. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bruna A, Darken RS, Rojo F, Ocaña A,
Peñuelas S, Arias A, Paris R, Tortosa A, Mora J, Baselga J and
Seoane J: High TGF-beta Smad activity confers poor prognosis in
glioma patients and promotes cell proliferation depending on the
methylation of the PDGF-B gene. Cancer Cell. 11:147–160. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Akhurst RJ and Derynck R: TGF-beta
signaling in cancer-a double-edged sword. Trends Cell Biol.
11:S44–S51. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang G, Lu X and Yuan L: LncRNA: A link
between RNA and cancer. Biochim Biophys Acta. 1839:1097–1109. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kiang KM, Zhang XQ and Leung GK: Long
non-coding RNAs: The key players in glioma pathogenesis. Cancers
(Basel). 7:1406–1424. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yuan JH, Yang F, Wang F, Ma JZ, Guo YJ,
Tao QF, Liu F, Pan W, Wang TT, Zhou CC, et al: A long noncoding RNA
activated by TGF-β promotes the invasion-metastasis cascade in
hepatocellular carcinoma. Cancer Cell. 25:666–681. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gasri-Plotnitsky L, Ovadia A, Shamalov K,
Nizri-Megnaji T, Meir S, Zurer I, Cohen CJ and Ginsberg D: A novel
lncRNA, GASL1, inhibits cell proliferation and restricts E2F1
activity. Oncotarget. 8:23775–23786. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yao J, Zhou B, Zhang J, Geng P, Liu K, Zhu
Y and Zhu W: A new tumor suppressor LncRNA ADAMTS9-AS2 is regulated
by DNMT1 and inhibits migration of glioma cells. Tumour Biol.
35:7935–7944. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shi Y, Wang Y, Luan W, Wang P, Tao T,
Zhang J, Qian J, Liu N and You Y: Long non-coding RNA H19 promotes
glioma cell invasion by deriving miR-675. PLoS One. 9:e862952014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pritchard CC, Kroh E, Wood B, Arroyo JD,
Dougherty KJ, Miyaji MM, Tait JF and Tewari M: Blood cell origin of
circulating microRNAs: A cautionary note for cancer biomarker
studies. Cancer Prev Res (Phila). 5:492–497. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lei B, Xiang W, Yu M, Yu L and Qi S: Case
report glioma progress and extracranial systemic multiple
metastasis: A case report. Int J Clin Exp Med. 9:16883–16886.
2016.
|
|
19
|
Derynck R, Akhurst RJ and Balmain A:
TGF-beta signaling in tumor suppression and cancer progression. Nat
Genet. 29:117–129. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bellam N and Pasche B: TGF-β signaling
alterations and colon cancer. Cancer Treat Res. 155:85–103. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li Z, Dong M, Fan D, Hou P, Li H, Liu L,
Lin C, Liu J, Su L, Wu L, et al: LncRNA ANCR down-regulation
promotes TGF-β-induced EMT and metastasis in breast cancer.
Oncotarget. 8:67329–67343. 2017.PubMed/NCBI
|