Introduction
Chondrosarcoma is a malignant tumour originating
from cartilage tissue and is the second most common malignant bone
tumour after osteosarcomas worldwide (1,2).
Chondrosarcoma primarily occurs in individuals >50 years of age
and, in the majority of cases, affects the limbs and pelvis
(3). Pathological types can be
divided into conventional intramedullary chondrosarcoma, clear cell
chondrosarcoma, mesenchymal chondrosarcoma, juxtacortical
chondrosarcoma and myxoid chondrosarcoma (4,5).
Chondrosarcoma has a poor response to traditional chemotherapy and
radiotherapy, and surgical resection is currently the only
successful treatment available (6–8).
In recent years, second primary cancer types have
become more prominent, and the incidence and risk of second primary
cancer is increasing in countries such as the USA and the
Netherlands (9,10). This increase may be due to a
significant improvement in the survival times of those adults with
cancer or the use of commonly used cancer treatments such as
radiotherapy or chemotherapy (11–13). In
the present study, second primary chondrosarcoma (pCS) is defined
as a tumour that is different in location or histology from the
initial pCS and is not derived from the metastasis or recurrence of
the initial pCS. Chondrosarcoma rarely presents as a second primary
tumour (10).
To the best of our knowledge, no studies have
evaluated the prognosis or treatment differences between the
initial pCS and the second pCS thus far. The purpose of the present
study was to analyse the difference in prognosis between the
initial and second pCS, and the difference in treatment outcomes on
the basis of a population-level study of chondrosarcoma.
Materials and methods
Data source and patients
The Surveillance Epidemiology and End Results (SEER)
database (https://seer.cancer.gov/) of the
National Cancer Institute (Bethesda, MD, USA) was used as the data
source in the present study. In total, 3,055 patients with initial
and second pCS were identified using the International
Classification of Diseases for Oncology (ICD-O-3) (http://codes.iarc.fr/) histology codes
9220/3,9221/3,9231/3,9240/3,9242/3 and 9243/3. The patients had
been diagnosed between January 1, 2004, and December 31, 2015.
SEER*Stat software (version 8.3.5; National Cancer Institute) was
used to access the SEER 18 Regs Custom Data (with an additional
treatment field) Nov 2017 Sub (1973–2015 varying) database using
the client-server mode of SEER*Stat. Patients with unknown survival
time, unknown American Joint Committee on Cancer (AJCC) stage or
unknown Tumour-Node-Metastasis (TNM) stage (14), as well as those diagnosed at autopsy,
<18 years of age or having a non-initial visit to hospital and
non-osteoarticular chondrosarcoma were excluded. The National
Cancer Institute's SEER database covers ~30% of the population in
the USA, and collects information such as demographics, tumour
histology, tumour stage at diagnosis, treatment information and
survival time (15). Finally, 1,655
eligible patients were identified.
The second pCS may be synchronous or metachronous.
The definitions of the second pCS met the following criteria: i)
Synchronous cancer is a second pCS diagnosed simultaneously or
within 6 months of diagnosis of the initial pCS (9); ii) metachronous cancer is a diagnosis
of second pCS ≥6 months following the diagnosis of an initial pCS;
iii) the primary site differs between the initial pCS and the
second pCS; iv) histology is different if the primary site is the
same as the primary site of the initial primary cancer.
Study variables
Overall survival (OS) and cancer-specific survival
(CSS) were the primary outcomes in the present study. The patients
in the present study were categorized into two groups: Initial pCS
and second pCS. The following variables were extracted for
analysis: Year of diagnosis, age at diagnosis, sex, ethnicity,
marital status, area of geographical state, urban-rural residence,
laterality, primary site, tumour grade, histological type, AJCC
stage, extent of disease (for T and M stages), regional nodes
positive (for N stage) and treatment methods (surgery, radiotherapy
or chemotherapy).
Statistical analysis
The baseline data of demographics and
clinicopathological characteristics of the initial and second pCS
were compared using χ2 tests. Kaplan-Meier curves and
log-rank tests were used for OS and CSS. Univariable analysis was
performed using the log-rank test to determine the factors
associated with all-cause mortality and cancer-specific mortality.
Furthermore, univariable and multivariable Cox regression analyses
were used to determine the factors associated with all-cause
mortality and cancer-specific mortality. All statistical analyses
were performed using SPSS statistics software (version 20; IBM
Corp., Armonk, NY, USA). P≤0.05 was considered to indicate a
statistically significant result.
Results
Demographic and clinical
characteristics of the initial and second pCS
In total, 1,655 eligible patients with
chondrosarcoma who were diagnosed between January 1, 2004, and
December 31, 2015, were included in the cohort of the present
study, with data obtained from the SEER database between January 1,
2004, and December 31, 2015. Among them, 1,455 (87.9%) patients had
initial pCS and 200 (12.1%) patients had second pCS. The clinical
characteristics and the χ2 test for comparison of the
initial and second pCS are presented in Table I. The data presented in Table I indicates that the incidence of
chondrosarcoma increases with the year of diagnosis. χ2
test revealed significant differences between the initial and
second pCS in certain variables, including year of diagnosis
(P=0.048), age at diagnosis (P<0.001), tumour AJCC stage
(P=0.024) and M-stage (P=0.044). Patients with second pCS were more
frequently diagnosed at 61–80 years of age (52.5 vs. 43.1%;
P<0.001) compared with patients with initial pCS. Furthermore,
the majority of patients with initial and second pCS chose to
receive surgical treatment (92.0 and 91.0% respectively), and
markedly less patients chose to receive radiotherapy (14.8 and
15.0%, respectively) or chemotherapy (8.0 vs. 5.5%,
respectively).
 | Table I.Characteristics of patients stratified
by initial and second pCS. |
Table I.
Characteristics of patients stratified
by initial and second pCS.
| Characteristics | All patients | Initial pCS | Second pCS | P-value |
|---|
| Total, n (%) | 1,655 | 1,455 (87.9) | 200 (12.1) |
|
| Median survival,
months | 41.00 | 42.00 | 34.50 |
|
| Year of diagnosis, n
(%) |
|
|
| 0.048 |
|
2004–2006 | 343 (20.7) | 299 (20.5) | 44 (22.0) |
|
|
2007–2009 | 399 (24.1) | 363 (24.9) | 36 (18.0) |
|
|
2010–2012 | 427 (25.8) | 362 (24.9) | 65 (32.5) |
|
|
2013–2015 | 486 (29.4) | 431 (29.6) | 55 (27.5) |
|
| Age at diagnosis in
years, n (%) |
|
|
| <0.001 |
|
18–40 | 392 (23.7) | 382 (26.3) | 10 (5.0) |
|
|
41–60 | 691 (41.8) | 627 (43.1) | 64 (32.0) |
|
|
61–80 | 490 (29.6) | 385 (26.5) | 105 (52.5) |
|
|
>80 | 82 (5.0) | 61 (4.2) | 21 (10.5) |
|
| Sex, n (%) |
|
|
| 0.724 |
|
Female | 742 (44.8) | 650 (44.7) | 92 (46.0) |
|
| Male | 913 (55.2) | 805 (55.3) | 108 (54.0) |
|
| Ethnicity, n (%) |
|
|
| 0.882 |
|
Caucasian | 1,444 (87.3) | 1,269 (87.2) | 175 (87.5) |
|
|
African-American | 111 (6.7) | 99 (6.8) | 12 (6.0) |
|
|
Other | 100 (6.0) | 87 (6.0) | 13 (6.5) |
|
| Marital status, n
(%) |
|
|
| 0.265 |
|
Married | 957 (57.8) | 831 (57.1) | 126 (63.0) |
|
|
Single | 622 (37.6) | 555 (38.1) | 67 (33.5) |
|
|
Unknown | 76 (4.6) | 69 (4.7) | 7 (3.5) |
|
| State, n (%) |
|
|
| 0.751 |
|
West | 875 (52.9) | 763 (52.4) | 112 (56.0) |
|
|
Northeast | 300 (18.1) | 267 (18.4) | 33 (16.5) |
|
|
South | 334 (20.2) | 294 (20.2) | 40 (20.0) |
|
|
Midwest | 146 (8.8) | 131 (9.0) | 15 (7.5) |
|
| Urban-rural
residence, n (%) |
|
|
| 0.650 |
|
Metropolitan | 1,472 (88.9) | 1,296 (89.1) | 176 (88.0) |
|
|
Non-metropolitan | 183 (11.1) | 159 (10.9) | 24 (12.0) |
|
| Laterality, n
(%) |
|
|
| 0.907 |
|
Left | 633 (38.2) | 554 (38.1) | 79 (39.5) |
|
|
Right | 676 (40.8) | 597 (41.0) | 79 (39.5) |
|
|
Other | 346 (20.9) | 304 (20.9) | 42 (21.0) |
|
| Primary site, n
(%) |
|
|
| 0.542 |
|
Appendicular | 803 (48.5) | 710 (48.8) | 93 (46.5) |
|
|
Axial | 852 (51.5) | 745 (51.2) | 107 (53.5) |
|
| Grade, n (%) |
|
|
| 0.505 |
|
Well-differentiated | 575 (34.7) | 513 (35.3) | 62 (31.0) |
|
|
Moderately differentiated | 684 (41.3) | 603 (41.5) | 81 (40.5) |
|
| Poorly
differentiated | 230 (13.9) | 198 (13.6) | 32 (16.0) |
|
|
Undifferentiated | 141 (8.5) | 119 (8.2) | 22 (11.0) |
|
|
Unknown | 25 (1.5) | 22 (1.5) | 3 (1.5) |
|
| Histological type,
n (%) |
|
|
| 0.633 |
|
Chondrosarcoma, NOS | 1,353 (81.8) | 1,191 (81.9) | 162 (81.0) |
|
|
Juxtacortical
chondrosarcoma | 21 (1.3) | 19 (1.3) | 2 (1.0) |
|
| Myxoid
chondrosarcoma | 102 (6.2) | 93 (6.4) | 9 (4.5) |
|
|
Mesenchymal
chondrosarcoma | 18 (1.1) | 16 (1.1) | 2 (1.0) |
|
| Clear
cell chondrosarcoma | 11 (0.7) | 10 (0.7) | 1 (0.5) |
|
|
Dedifferentiated
chondrosarcoma | 150 (9.1) | 126 (8.7) | 24 (12.0) |
|
| AJCC stage, n
(%) |
|
|
| 0.024 |
| I | 1,213 (73.3) | 1,072 (73.7) | 141 (70.5) |
|
| II | 307 (18.5) | 257 (17.7) | 50 (25.0) |
|
|
III | 18 (1.1) | 16 (1.1) | 2 (1.0) |
|
| IV | 117 (7.1) | 110 (7.6) | 7 (3.5) |
|
| T-stage, n (%) |
|
|
| 0.593 |
| T1 | 1,020 (61.6) | 903 (62.1) | 117 (58.5) |
|
| T2 | 608 (36.7) | 528 (36.3) | 80 (40.0) |
|
| T3 | 27 (1.6) | 24 (1.6) | 3 (1.5) |
|
| N-stage, n (%) |
|
|
| 0.359 |
| N0 | 1,636 (98.9) | 1,437 (98.8) | 199 (99.5) |
|
| N1 | 19 (1.1) | 18 (1.2) | 1 (0.5) |
|
| M-stage, n (%) |
|
|
| 0.044 |
| M0 | 1,552 (93.8) | 1,358 (93.3) | 194 (97.0) |
|
| M1 | 103 (6.2) | 97 (6.7) | 6 (3.0) |
|
| Surgery, n (%) |
|
|
| 0.617 |
| No | 134 (8.1) | 116 (8.0) | 18 (9.0) |
|
|
Yes | 1,521 (91.9) | 1,339 (92.0) | 182 (91.0) |
|
| Radiotherapy, n
(%) |
|
|
| 0.934 |
| No | 1,410 (85.2) | 1,240 (85.2) | 170 (85.0) |
|
|
Yes | 245 (14.8) | 215 (14.8) | 30 (15.0) |
|
| Chemotherapy, n
(%) |
|
|
| 0.207 |
| No | 1,527 (92.3) | 1,338 (92.0) | 189 (94.5) |
|
|
Yes | 128 (7.7) | 117 (8.0) | 11 (5.5) |
|
Univariate survival analyses of
factors associated with all-cause mortality and cancer-specific
mortality
Univariate survival analyses of patients with
initial and second pCS according to various clinicopathological
variables (Table II). Among the
1,655 initial and second pCS patients, 390 (23.6%) were categorized
as cases of all-cause mortality and 228 (13.8%) succumbed to
chondrosarcoma. Univariate analyses revealed that year of
diagnosis, age at diagnosis, sex, tumour grade, histological type,
AJCC stage, TNM stage, surgery, radiotherapy and chemotherapy were
associated with all-cause mortality and chondrosarcoma-associated
mortality (all P<0.05). In addition, patients with second pCS
had a higher rate of all-cause mortality compared with patients
with initial pCS (P<0.001), but there was no significant
difference between initial and second pCS in terms of
chondrosarcoma-associated mortality (P=0.734).
 | Table II.Univariate survival analyses of the
1,655 patients with chondrosarcoma according to various
clinicopathological variables. |
Table II.
Univariate survival analyses of the
1,655 patients with chondrosarcoma according to various
clinicopathological variables.
|
| All-cause |
|
Chondrosarcoma-associated |
|
|---|
|
|
|
|
|
|
|---|
|
Characteristics | Succumbed, n
(%) | Alive, n (%) | P-value | Succumbed, n
(%)a | Censored, n
(%) | P-value |
|---|
| Total patients in
study | 390 (23.6) | 1,265 (76.4) |
| 228 (13.8) | 1,427 (86.2) |
| Year of
diagnosis |
|
| <0.001 |
|
| <0.001 |
|
2004–2006 | 120 (30.8) | 223 (17.6) |
| 68 (29.8) | 275 (19.3) |
|
|
2007–2009 | 109 (27.9) | 290 (22.9) |
| 62 (27.2) | 337 (23.6) |
|
|
2010–2012 | 109 (27.9) | 318 (25.1) |
| 69 (30.3) | 358 (25.1) |
|
|
2013–2015 | 52 (13.3) | 434 (34.3) |
| 29 (12.7) | 457 (32.0) |
|
| Age at diagnosis,
years |
|
| <0.001 |
|
| <0.001 |
|
18–40 | 38 (9.7) | 354 (28.0) |
| 27 (11.8) | 365 (25.6) |
|
|
41–60 | 142 (36.4) | 549 (43.4) |
| 92 (40.4) | 599 (42.0) |
|
|
61–80 | 167 (42.8) | 323 (25.5) |
| 93 (40.8) | 397 (27.8) |
|
|
>80 | 43 (11.0) | 39 (3.1) |
| 16 (7.0) | 66 (4.6) |
|
| Sex |
|
| <0.001 |
|
| 0.041 |
|
Female | 144 (36.9) | 598 (47.3) |
| 88 (38.6) | 654 (45.8) |
|
|
Male | 246 (63.1) | 667 (52.7) |
| 140 (61.4) | 773 (54.2) |
|
| Ethnicity |
|
| 0.390 |
|
| 0.862 |
|
Caucasian | 339 (86.9) | 1,105 (87.4) |
| 201 (88.2) | 1,243 (87.1) |
|
|
African-American | 31 (7.9) | 80 (6.3) |
| 15 (6.6) | 96 (6.7) |
|
|
Other | 20 (5.1) | 80 (6.3) |
| 12 (5.3) | 88 (6.2) |
|
| Marital status |
|
| 0.352 |
|
| 0.491 |
|
Married | 220 (56.4) | 737 (58.3) |
| 135 (59.2) | 822 (57.6) |
|
|
Single | 156 (40.0) | 466 (36.8) |
| 86 (37.7) | 536 (37.6) |
|
|
Unknown | 14 (3.6) | 62 (4.9) |
| 7 (3.1) | 69 (4.8) |
|
| State |
|
| 0.469 |
|
| 0.309 |
|
West | 209 (53.6) | 666 (52.6) |
| 129 (56.6) | 746 (52.3) |
|
|
Northeast | 65 (16.7) | 235 (18.6) |
| 35 (15.4) | 265 (18.6) |
|
|
South | 75 (19.2) | 259 (20.5) |
| 40 (17.5) | 294 (20.6) |
|
|
Midwest | 41 (10.5) | 105 (8.3) |
| 24 (10.5) | 122 (8.5) |
|
| Urban-rural
residence |
|
| 0.836 |
|
| 0.684 |
|
Metropolitan | 348 (89.2) | 1,124 (88.9) |
| 201 (88.2) | 1,271 (89.1) |
|
|
Non-metropolitan | 42 (10.8) | 141 (11.1) |
| 27 (11.8) | 156 (10.9) |
|
| Laterality |
|
| 0.941 |
|
| 0.224 |
|
Left | 152 (39.0) | 481 (38.0) |
| 94 (41.2) | 539 (37.8) |
|
|
Right | 158 (40.5) | 518 (40.9) |
| 96 (42.1) | 580 (40.6) |
|
|
Other | 80 (20.5) | 266 (21.0) |
| 38 (16.7) | 308 (21.6) |
|
| Primary site |
|
| 0.887 |
|
| 0.532 |
|
Appendicular | 188 (48.2) | 615 (48.6) |
| 115 (50.4) | 688 (48.2) |
|
|
Axial | 202 (51.8) | 650 (51.4) |
| 113 (49.6) | 739 (51.8) |
|
| Grade |
|
| <0.001 |
|
| <0.001 |
|
Well-differentiated | 61 (15.6) | 514 (40.6) |
| 26 (11.4) | 549 (38.5) |
|
|
Moderately differentiated | 133 (34.1) | 551 (43.6) |
| 76 (33.3) | 608 (42.6) |
|
| Poorly
differentiated | 87 (22.3) | 143 (11.3) |
| 56 (24.6) | 174 (12.2) |
|
|
Undifferentiated | 89 (22.8) | 52 (4.1) |
| 60 (26.3) | 81 (5.7) |
|
|
Unknown | 20 (5.1) | 5 (0.4) |
| 10 (4.4) | 15 (1.1) |
|
| Histological
type |
|
| <0.001 |
|
| <0.001 |
|
Chondrosarcoma, NOS | 242 (62.1) | 1,111 (87.7) |
| 137 (60.1) | 1,216 (85.2) |
|
|
Juxtacortical
chondrosarcoma | 2 (0.5) | 19 (1.5) |
| 2 (0.9) | 19 (1.3) |
|
| Myxoid
chondrosarcoma | 29 (7.4) | 73 (5.8) |
| 20 (8.8) | 82 (5.7) |
|
|
Mesenchymal
chondrosarcoma | 10 (2.6) | 8 (0.6) |
| 7 (3.1) | 11 (0.8) |
|
| Clear
cell chondrosarcoma | 3 (0.8) | 8 (0.6) |
| 0 (0.0) | 11 (0.8) |
|
|
Dedifferentiated
chondrosarcoma | 104 (26.7) | 46 (3.6) |
| 62 (27.2) | 88 (6.2) |
|
| AJCC stage |
|
| <0.001 |
|
| <0.001 |
| I | 164 (42.1) | 1,049 (82.9) |
| 75 (32.9) | 1,138 (79.7) |
|
| II | 126 (32.3) | 181 (14.3) |
| 78 (34.2) | 229 (16.0) |
|
|
III | 6 (1.5) | 12 (0.9) |
| 3 (1.3) | 15 (1.1) |
|
| IV | 94 (24.1) | 23 (1.8) |
| 72 (31.6) | 45 (3.2) |
|
| T-stage |
|
| <0.001 |
|
| <0.001 |
| T1 | 151 (38.7) | 869 (68.7) |
| 80 (35.1) | 940 (65.9) |
|
| T2 | 226 (57.9) | 382 (30.2) |
| 141 (61.8) | 467 (32.7) |
|
| T3 | 13 (3.3) | 14 (1.1) |
| 7 (3.1) | 20 (1.4) |
|
| N-stage |
|
| <0.001 |
|
| <0.001 |
| N0 | 378 (96.9) | 1,258 (99.4) |
| 220 (96.5) | 1,416 (99.2) |
|
| N1 | 12 (3.1) | 7 (0.6) |
| 8 (3.5) | 11 (0.8) |
|
| M-stage |
|
| <0.001 |
|
| <0.001 |
| M0 | 304 (77.9) | 1,248 (98.7) |
| 161 (70.6) | 1,391 (97.5) |
|
| M1 | 86 (22.1) | 17 (1.3) |
| 67 (29.4) | 36 (2.5) |
|
| Surgery |
|
| <0.001 |
|
| <0.001 |
| No | 74 (19.0) | 60 (4.7) |
| 44 (19.3) | 90 (6.3) |
|
|
Yes | 316 (81.0) | 1,205 (95.3) |
| 184 (80.7) | 1,337 (93.7) |
|
| Radiotherapy |
|
| <0.001 |
|
| <0.001 |
| No | 293 (75.1) | 1,117 (88.3) |
| 166 (72.8) | 1,224 (87.2) |
|
|
Yes | 97 (24.9) | 148 (11.7) |
| 62 (27.2) | 183 (12.8) |
|
| Chemotherapy |
|
| <0.001 |
|
| <0.001 |
| No | 308 (79.0) | 1,219 (96.4) |
| 170 (74.6) | 1,357 (95.1) |
|
|
Yes | 82 (21.0) | 46 (3.6) |
| 58 (25.4) | 70 (4.9) |
|
| Group |
|
| <0.001 |
|
| 0.734 |
| Initial
pCS | 316 (81.0) | 1,139 (90.0) |
| 202 (88.6) | 1,253 (87.8) |
|
| Second
pCS | 74 (19.0) | 126 (10.0) |
| 26 (11.4) | 174 (12.2) |
|
Survival
The median survival time among the entire cohort was
41.00 months, with patients with initial pCS experiencing a longer
median survival time (42.00 months), and patients with second pCS
experiencing a shorter median survival time (34.50 months).
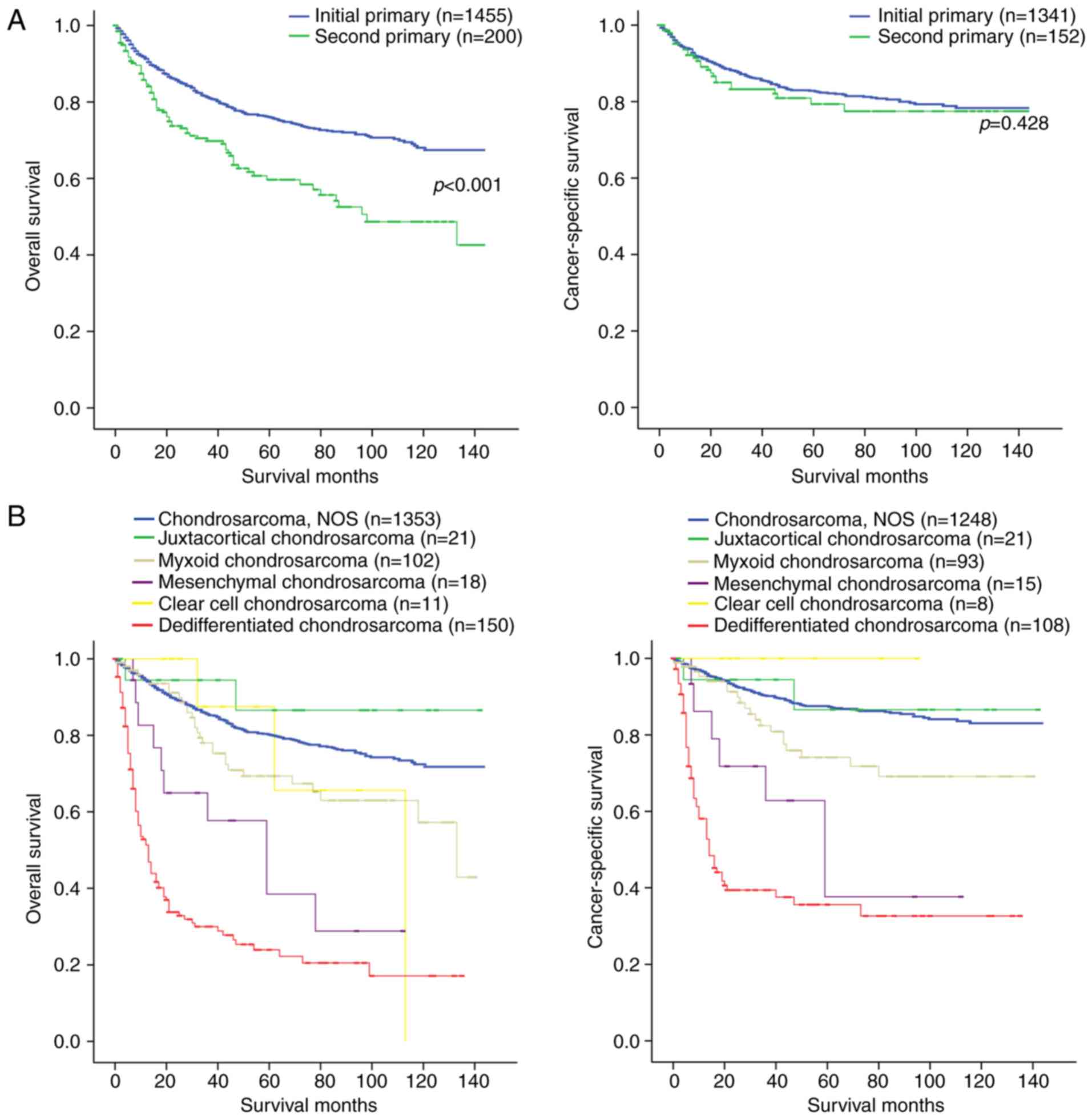
Fig. 1A presents the OS and CSS of
the initial and second pCS. The OS rate of patients with initial
pCS was significantly higher compared with that of the patients
with second pCS (log-rank test, P<0.001), but the CSS rate of
the patients with initial pCS was not significantly different from
that of the patients with second pCS (log-rank test, P=0.428). In
addition, it was also revealed that among the pathological types of
chondrosarcoma, dedifferentiated chondrsarcoma exhibited the worst
OS and CSS rates (Fig. 1B). Patients
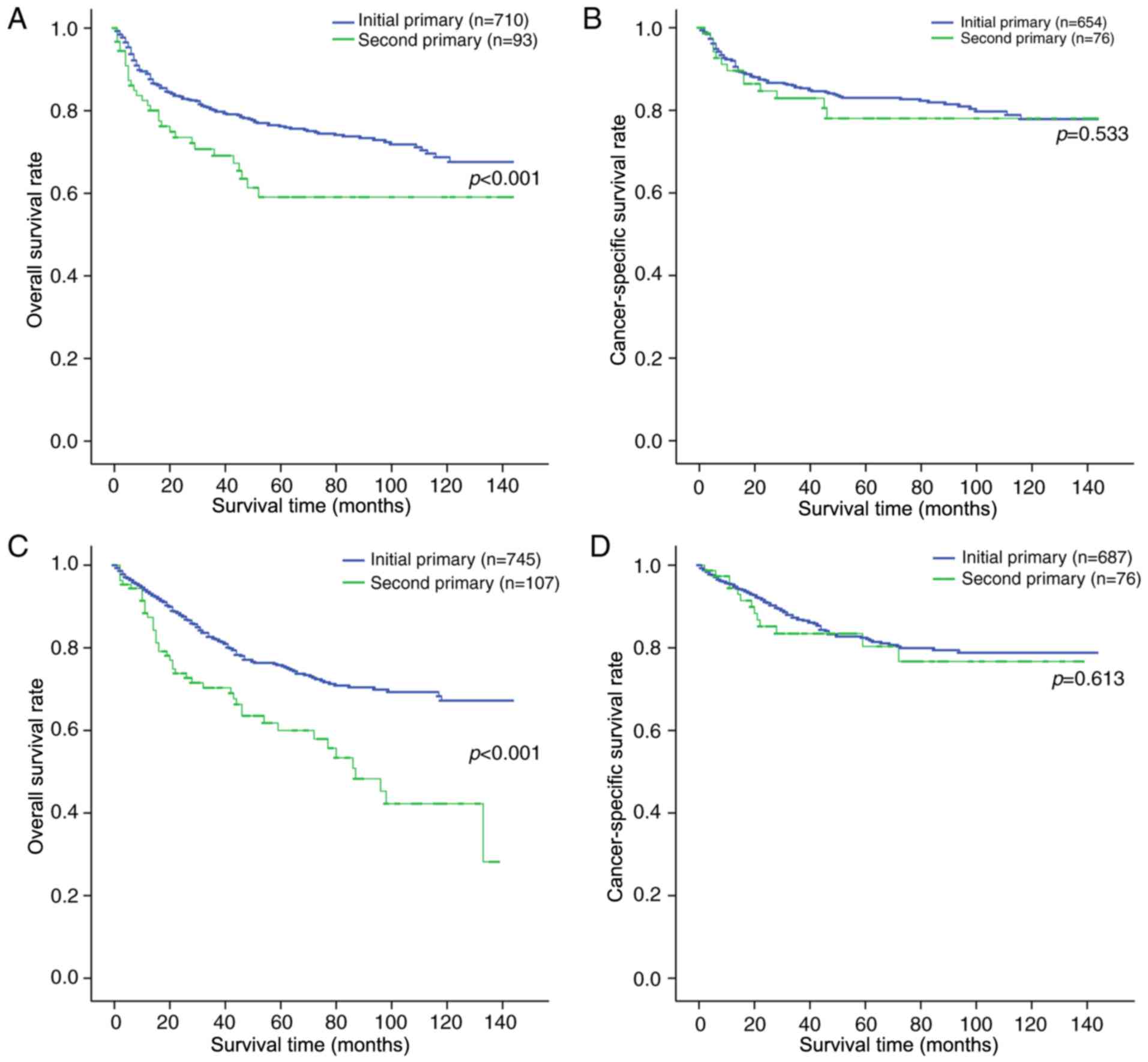
were stratified by primary sites and laterality, and in doing so,
it was revealed that the OS rate of the patients with initial pCS
was also higher compared with that of the patients with second pCS.
However, there was no significant difference between the initial
and second pCS in terms of chondrosarcoma-associated mortality
(Fig. 2).
Risk factors for all-cause mortality
and cancer-specific mortality
Univariate and multivariate Cox regression were used
to analyse the factors associated with all-cause mortality and
cancer-specific mortality (Table
III). Age at diagnosis, sex, histological type, AJCC stage, T
stage, surgery and radiotherapy were associated with all-cause
mortality and chondrosarcoma-cause mortality (all P<0.05) in
univariate and multivariate Cox regression. In the multivariate Cox
regression analysis among patients with initial and second pCS, age
41–60 years [vs. age 18–40 years; HR, 1.80; 95% confidence interval
(CI), 1.22–2.65; P=0.003], age 61–80 years (vs. age 18–40 years;
HR, 2.91; 95% CI, 1.97–4.30; P<0.001), age >80 years (vs. age
18–40 years; HR, 4.04; 95% CI, 2.49–6.57; P<0.001), male (vs.
female; HR, 1.50; 95% CI, 1.21–1.85; P<0.001), moderately
differentiated (vs. well-differentiated; HR, 1.41; 95% CI,
1.03–1.93; P=0.033), undifferentiated (vs. well-differentiated; HR,
2.00; 95% CI, 1.09–3.68; P=0.026), mesenchymal chondrosarcoma (vs.
chondrosarcoma, NOS; HR, 2.11; 95% CI, 1.03–4.33; P=0.042),
dedifferentiated chondrosarcoma (vs. chondrosarcoma, NOS; HR, 3.00;
95% CI, 2.20–4.08; P<0.001), AJCC stage IV (vs. AJCC stage I;
HR, 5.67; 95% CI, 3.64–8.82; P<0.001), T2 stage (vs. T1 stage;
HR, 1.81; 95% CI, 1.46–2.25; P<0.001), surgery (vs. no surgery;
HR, 0.39; 95% CI, 0.29–0.52; P<0.001), radiotherapy (vs. no
radiotherapy; HR, 1.31; 95% CI, 1.03–1.67; P=0.030) and second pCS
(vs. initial pCS; HR, 1.72; 95% CI, 1.31–2.26; P<0.001) were
associated with a significant increase in all-cause mortality.
However, initial and second pCS were still not associated with
improved chondrosarcoma-associated survival on multivariable
survival analysis (P=0.294).
 | Table III.Risk factors for survival: Outcome is
all-cause mortality and cancer-specific mortality. |
Table III.
Risk factors for survival: Outcome is
all-cause mortality and cancer-specific mortality.
|
| All-cause
mortality | Cancer-specific
mortality |
|---|
|
|
|
|
|---|
|
| Univariate Cox
regression | Multivariate Cox
regression | Univariate Cox
regression | Multivariate Cox
regression |
|---|
|
|
|
|
|
|
|---|
|
Characteristics | Hazard ratio (95%
CI) | P-value | Hazard ratio (95%
CI) | P-value | Hazard ratio (95%
CI) | P-value | Hazard ratio (95%
CI) | P-value |
|---|
| Age at diagnosis,
years |
|
18–40 | Reference |
| Reference |
| Reference |
| Reference |
|
|
41–60 | 2.34
(1.63–3.34) | <0.001 | 1.80
(1.22–2.65) | 0.003 | 2.16
(1.41–3.32) | <0.001 | 1.86
(1.14–3.02) | 0.013 |
|
61–80 | 4.47
(3.14–6.35) | <0.001 | 2.91
(1.97–4.30) | <0.001 | 3.77
(2.45–5.78) | <0.001 | 2.79
(1.71–4.56) | <0.001 |
|
>80 | 8.52
(5.50–13.21) | <0.001 | 4.04
(2.49–6.57) | <0.001 | 5.60
(3.01–10.39) | <0.001 | 3.50
(1.81–6.80) | <0.001 |
| Sex |
|
Female | Reference |
| Reference |
| Reference |
| Reference |
|
|
Male | 1.46
(1.19–1.80) | <0.001 | 1.50
(1.21–1.85) | <0.001 | 1.40
(1.07–1.83) | 0.014 | 1.40
(1.06–1.85) | 0.017 |
| Grade |
|
Well-differentiated | Reference |
| Reference |
| Reference |
| Reference |
|
|
Moderately differentiated | 1.95
(1.44–2.64) | <0.001 | 1.41
(1.03–1.93) | 0.033 | 2.65
(1.69–4.13) | <0.001 | NA | 0.128 |
| Poorly
differentiated | 4.51
(3.25–6.26) | <0.001 | 1.51
(0.84–2.71) | 0.169 | 7.14
(4.49–11.38) | <0.001 | NA | 0.532 |
|
Undifferentiated | 10.62
(7.65–14.73) | <0.001 | 2.00
(1.09–3.68) | 0.026 | 17.51
(11.04–27.78) | <0.001 | NA | 0.297 |
|
Unknown | 15.53
(9.34–25.82) | <0.001 | 0.95
(0.49–1.85) | 0.878 | 25.74
(12.37–53.56) | <0.001 | NA | 0.583 |
| Histological
type |
|
Chondrosarcoma, NOS | Reference |
| Reference |
| Reference |
| Reference |
|
|
Juxtacortical
chondrosarcoma | 0.51
(0.13–2.04) | 0.340 | 0.71
(0.18–2.90) | 0.636 | 0.86
(0.21–3.47) | 0.830 | 1.10
(0.27–4.50) | 0.900 |
| Myxoid
chondrosarcoma | 1.61
(1.09–2.36) | 0.016 | 1.19
(0.80–1.76) | 0.400 | 2.02
(1.26–3.23) | 0.003 | 1.72
(1.06–2.77) | 0.027 |
|
Mesenchymal
chondrosarcoma | 3.61
(1.92–6.80) | <0.001 | 2.11
(1.03–4.33) | 0.042 | 4.74
(2.22–10.14) | <0.001 | 2.37
(0.97–5.80) | 0.058 |
| Clear
cell chondrosarcoma | 1.45
(0.46–4.53) | 0.523 | 1.72
(0.54–5.45) | 0.360 | NA | NA | NA | NA |
|
Dedifferentiated
chondrosarcoma | 7.84
(6.21–9.92) | <0.001 | 3.00
(2.20–4.08) | <0.001 | 9.68
(7.14–13.13) | <0.001 | 3.43
(2.41–4.87) | <0.001 |
| AJCC stage |
| I | Reference |
| Reference |
| Reference |
| Reference |
|
| II | 3.90
(3.09–4.92) | <0.001 | 1.53
(0.89–2.61) | 0.124 | 5.49
(4.00–7.54) | <0.001 | 2.91
(2.03–4.16) | <0.001 |
|
III | 2.46
(1.09–5.55) | 0.031 | 1.11
(0.35–3.53) | 0.863 | 2.86
(0.90–9.08) | 0.074 | 1.98
(0.42–9.34) | 0.391 |
| IV | 14.12
(10.88–18.32) | <0.001 | 5.67
(3.64–8.82) | <0.001 | 24.47
(17.57–34.09) | <0.001 | 4.26
(1.69–10.79) | 0.002 |
| T-stage |
| T1 | Reference |
| Reference |
| Reference |
| Reference |
|
| T2 | 3.13
(2.54–3.85) | <0.001 | 1.81
(1.46–2.25) | <0.001 | 3.82
(2.91–5.03) | <0.001 | 1.94
(1.44–2.60) | <0.001 |
| T3 | 4.03
(2.29–7.11) | <0.001 | 2.20
(0.98–4.90) | 0.055 | 4.40
(2.03–9.53) | <0.001 | 1.43
(0.50–4.10) | 0.502 |
| N-stage |
| N0 | Reference |
| Reference |
| Reference |
| Reference |
|
| N1 | 3.95
(2.22–7.03) | <0.001 | NA | 0.167 | 5.19
(2.56–10.52) | <0.001 | NA | 0.260 |
| M-stage |
| M0 | Reference |
| Reference |
| Reference |
| Reference |
|
| M1 | 10.69
(8.33–13.71) | <0.001 | NA | 0.085 | 15.86
(11.79–21.34) | <0.001 | 3.13
(1.23–7.99) | 0.017 |
| Surgery |
| No | Reference |
| Reference |
| Reference |
| Reference |
|
|
Yes | 0.25
(0.19–0.32) | <0.001 | 0.39
(0.29–0.52) | <0.001 | 0.22
(0.16–0.30) | <0.001 | 0.54
(0.36–0.81) | 0.003 |
| Radiotherapy |
| No | Reference |
| Reference |
| Reference |
| Reference |
|
|
Yes | 2.16
(1.71–2.71) | <0.001 | 1.31
(1.03–1.67) | 0.030 | 2.53
(1.89–3.39) | <0.001 | 1.47
(1.08–2.01) | 0.015 |
| Chemotherapy |
| No | Reference |
| Reference |
| Reference |
| Reference |
|
|
Yes | 4.44
(3.47–5.67) | <0.001 | NA | 0.943 | 6.19
(4.58–8.35) | <0.001 | NA | 0.827 |
| Group |
| Initial
pCS | Reference |
| Reference |
| Reference |
| Reference |
|
| Second
pCS | 1.87
(1.45–2.41) | <0.001 | 1.72
(1.31–2.26) | <0.001 | 1.18
(0.78–1.77) | 0.429 | NA | 0.294 |
Benefits of different treatment for
initial and second pCS
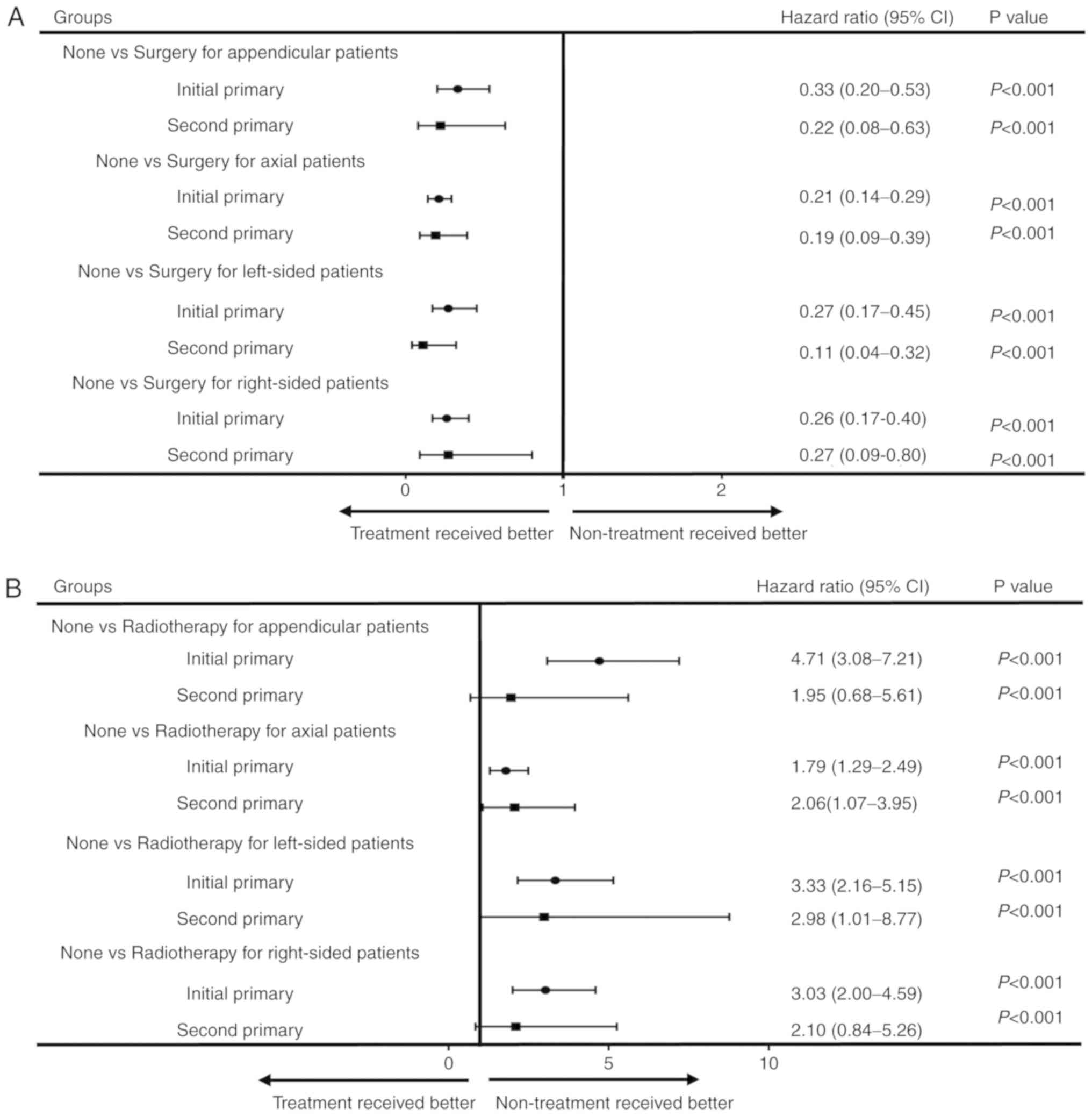
To analyse the benefit of different treatments for
all-cause mortality, patients with initial and second pCS were
stratified by primary site and laterality, and multivariate
analysis was used. For patients with appendicular or axial
chondrosarcoma, those with initial and second pCS who underwent
surgery had lower all-cause mortality (all P<0.05; Fig. 3A) compared with patients who did not
receive surgery (Fig. 3A).
Similarly, for left-sided or right-sided chondrosarcoma patients,
those with initial and second pCS who underwent surgery had lower
all-cause mortality rate (all P<0.05) compared with patients
without surgery. However, the use of radiotherapy or chemotherapy
did not reduce the all-cause mortality rate (Fig. 3B and C).
Discussion
The incidence of chondrosarcoma accounts for ~20% of
all primary bone sarcomas (16).
Above the age of 40 years, the incidence rate of chondrosarcoma
gradually increases, and the incidence rate in men is higher than
that in women. Rozeman et al (17) reported that common types of
chondrosarcoma accounted for 85% of cases, dedifferentiation for
10%, interstitial type for 2% and clear cell type for 1%. Giuffrida
et al (18) analysed 2,890
cases of chondrosarcoma between 1973 and 2003, and revealed that
the highest 5-year survival rate was that of clear cell type
chondrosarcoma (100%), followed by common chondrosarcoma (70%), and
the lowest was that of dedifferentiated chondrosarcoma (0%). The
results from the present study also revealed that dedifferentiated
chondrosarcoma has the worst prognosis.
Second pCS is a rare occurrence, and, to the best of
our knowledge, there are currently no studies that have assessed
the prognosis and treatment differences between the initial pCS and
the second pCS. Therefore, the present study is the first to report
differences in prognosis and treatment between initial pCS and the
second pCS.
The present study revealed that the proportion of
the four age stages (18–40, 41–60, 61–80 and >80 years) of the
patients with initial pCS were 26.3, 43.1, 26.5 and 4.2%,
respectively, while the proportion of the patients with second pCS
in the four age stages was 5.0,32.0,52.5 and 10.5%, respectively.
It was demonstrated that patients with second pCS were at an older
age when diagnosed compared with patients with initial pCS. The
difference may be due to a significant improvement in survival for
patients with cancer.
As one of the multiple primary malignancies (MPMs),
the second primary cancer has received greater attention. MPMs are
rarely encountered in different organs and tissues in the same
patient, and the incidence of MPMs ranges from 0.7–11.0%, as
determined through the statistical analysis of several national
cancer registries (10,19,20). The
most common subsequent types of cancer are squamous cell skin
cancer, colorectal cancer and breast cancer (13). Patients with MPMs exhibit a worse
5-year OS rate compared with patients with a single malignancy
(21). This may be associated with
genetic, environmental and immunological factors, as well as the
application of radiotherapy or chemotherapy (22,23).
The risk of developing a subsequent cancer in
patients with MPMs was 1.4 to 3.0 times higher compared to the
general population (13). There are
a number of studies that have focused on second primary tumours. By
investigating 2,462 patients with hepatocellular carcinoma who
underwent liver transplantation, Heo et al (24) revealed that patients with
hepatocellular carcinoma who had received a liver transplantation
had a longer life expectancy and higher risk of second primary
cancer compared with the general population (standardized incidence
ratios, 2.79; 95% CI, 2.27–3.38). Chen et al (25) demonstrated that the prognosis of
patients with second primary colorectal cancer was worse than that
of patients with initial primary colorectal cancer, and the
therapeutic benefit on colorectal cancer prognosis was generally
similar for the patients with initial and second primary colorectal
cancer. The present study revealed similar results; it was
identified that patients with second pCS had a worse prognosis
compared with patients with initial pCS, and the treatment benefits
were similar for patients with initial and second pCS. Surgery can
significantly reduce all-cause mortality, while the use of
radiotherapy or chemotherapy does not reduce all-cause
mortality.
Furthermore, in terms of demographic and clinical
characteristics, χ2 tests revealed a significant
difference in the age at diagnosis for patients with initial pCS
and those patients with second pCS; patients with second pCS were
diagnosed at an older age compared with patients with initial
pCS.
The present study does have certain limitations.
First, the research is based on the SEER database, a retrospective
dataset that therefore has inherent limitations. Secondly, the
patients’ physical condition was unclear; patients with excessive
comorbidities may pursue more conservative treatment. In addition,
the number of patients included in the present study was small, so
further prospective studies are necessary.
The results of the present study revealed that
patients with second pCS were more frequently diagnosed at an older
age and had a worse prognosis compared with patients with initial
pCS. For patients with initial and second pCS, surgery was the main
treatment method.
Acknowledgements
Not applicable.
Funding
The present study was supported by grant from the
Tongji University (grant no. 1501219143) and the National Natural
Science Foundation of China (grant no. 81001134.
Availability of data and materials
All the data generated or analyzed during the
present study are included in this published article.
Authors' contributions
WM, JF and JG designed the research. HY and MK
acquired the data. DW, XH and XY analyzed the results. WM wrote the
article. JF and JG revised and provided critical comments. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wagner MJ, Ricciotti RW, Mantilla J,
Loggers ET, Pollack SM and Cranmer LD: Response to PD1 inhibition
in conventional chondrosarcoma. J Immunother Cancer. 6:942018.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen JC, Huang C, Lee IN, Wu YP and Tang
CH: Amphiregulin enhances cell migration and resistance to
doxorubicin in chondrosarcoma cells through the MAPK pathway. Mol
Carcinog. 57:1816–1824. 2018. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sangma MM and Dasiah S: Chondrosarcoma of
a rib. Int J Surg Case Rep. 10:126–128. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Murphey MD, Walker EA, Wilson AJ,
Kransdorf MJ, Temple HT and Gannon FH: From the archives of the
AFIP: Imaging of primary chondrosarcoma: Radiologic-pathologic
correlation. Radiographics. 23:1245–1278. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Aigner T: Towards a new understanding and
classification of chondrogenic neoplasias of the
skeleton-biochemistry and cell biology of chondrosarcoma and its
variants. Virchows Arch. 441:219–230. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schoenfeld AJ, Hornicek FJ, Pedlow FX,
Kobayashi W, Raskin KA, Springfield D, DeLaney TF, Nielsen GP,
Mankin HJ and Schwab JH: Chondrosarcoma of the mobile spine: A
review of 21 cases treated at a single center. Spine (Phila Pa
1976). 37:119–126. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Staals EL, Bacchini P and Bertoni F:
Dedifferentiated central chondrosarcoma. Cancer. 106:2682–2691.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Grimer RJ, Gosheger G, Taminiau A, Biau D,
Matejovsky Z, Kollender Y, San-Julian M, Gherlinzoni F and Ferrari
C: Dedifferentiated chondrosarcoma: Prognostic factors and outcome
from a European group. Eur J Cancer. 43:2060–2065. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu L, de Vries E, Louwman M, Aben K,
Janssen-Heijnen M, Brink M, Coebergh JW and Soerjomataram I:
Prevalence of multiple malignancies in the Netherlands in 2007. Int
J Cancer. 128:1659–1667. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cheng HY, Chu CH, Chang WH, Hsu TC, Lin
SC, Liu CC, Yang AM and Shih SC: Clinical analysis of multiple
primary malignancies in the digestive system: A hospital-based
study. World J Gastroenterol. 11:4215–4219. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chrouser K, Leibovich B, Bergstralh E,
Zincke H and Blute M: Bladder cancer risk following primary and
adjuvant external beam radiation for prostate cancer. J Urol.
174:107–110. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Brumback RA, Gerber JE, Hicks DG and
Strauchen JA: Adenocarcinoma of the stomach following irradiation
and chemotherapy for lymphoma in young patients. Cancer.
54:994–998. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Frederick LG, Carolyn CC, April G. Fritz
CTR, Jatin PS and David PW: AJCC Cancer Staging Atlas. Springer
Berlin. 2012.
|
|
15
|
Martin AM, Cagney DN, Catalano PJ, Warren
LE, Bellon JR, Punglia RS, Claus EB, Lee EQ, Wen PY, Haas-Kogan DA,
et al: Brain metastases in newly diagnosed breast cancer: A
population-based study. JAMA Oncol. 3:1069–1077. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fromm J, Klein A, Baur-Melnyk A, Knösel T,
Lindner L, Birkenmaier C, Roeder F, Jansson V and Dürr HR: Survival
and prognostic factors in conventional central chondrosarcoma. BMC
Cancer. 18:8492018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rozeman LB, Hogendoorn PC and Bovee JV:
Diagnosis and prognosis of chondrosarcoma of bone. Expert Rev Mol
Diagn. 2:461–472. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Giuffrida AY, Burgueno JE, Koniaris LG,
Gutierrez JC, Duncan R and Scully SP: Chondrosarcoma in the United
States (1973 to 2003): An analysis of 2890 cases from the SEER
database. J Bone Joint Surg Am. 91:1063–1072. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Savary M, Monnier P, Pasche R, Brossard E,
Pasche P and Lang F: Multiple primary malignancies. Adv
Otorhinolaryngol. 46:165–175. 1991.PubMed/NCBI
|
|
20
|
Armstrong GT, Liu W, Leisenring W, Yasui
Y, Hammond S, Bhatia S, Neglia JP, Stovall M, Srivastava D and
Robison LL: Occurrence of multiple subsequent neoplasms in
long-term survivors of childhood cancer: A report from the
childhood cancer survivor study. J Clin Oncol. 29:3056–3064. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rosso S, De Angelis R, Ciccolallo L,
Carrani E, Soerjomataram I, Grande E, Zigon G and Brenner H;
EUROCARE Working Group, : Multiple tumours in survival estimates.
Eur J Cancer. 45:1080–1094. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Keller U, Grabenbauer G, Kuechler A,
Sprung CN, Müller E, Sauer R and Distel L: Cytogenetic instability
in young patients with multiple primary cancers. Cancer Genet
Cytogenet. 157:25–32. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Crocetti E, Buiatti E and Falini P;
Italian Multiple Primary Cancer Working Group, : Multiple primary
cancer incidence in Italy. Eur J Cancer. 37:2449–2456. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Heo J, Noh OK, Oh YT, Chun M and Kim L:
Second primary cancer after liver transplantation in hepatocellular
carcinoma: A nationwide population-based study. Hepatol Int.
11:523–528. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen Q, Zhao S, Song Y, Gao P, Sun J, Chen
X, Sun Y and Wang Z: Do patients with second primary colorectal
cancer hold the similar prognosis and therapeutic benefits as those
with initial primary colorectal cancer? Biomed Res Int.
2018:Article ID 6172670. 2018. View Article : Google Scholar
|