Introduction
Retinoblastoma RB) is the most common intraocular
malignancy in infants and young children worldwide (1,2). RB
usually occurs in children <3 years of age and can affect one or
both eyes (3). It is reported that
the incidence of RB globally is ~1 in 15,000–20,000 live births,
with as many as 9.32 cases per million children between 0 and 5
years of age worldwide annually (3).
Identification of the pathological features
associated with RB is considered the gold standard for diagnosis
(4). For those children who are
suspected to have RB, clinicians rely mainly on clinical
manifestations and auxiliary examinations to establish a diagnosis
(5). Ultrasound and computed
tomography examination can indicate the presence of intraocular
tumors and the possible calcification points in the lesions, thus
providing a basis for the clinical diagnosis of RB (6). Despite the increasing sophistication of
examination methods, there remain cases of RB that cannot be
diagnosed due to the clinical manifestations that are markedly
similar to other ocular disorders (1). In a laboratory diagnosis,
neuron-specific enolase (NSE) is a tumor marker widely used in the
clinical setting to determine the presence of RB (7); however, this marker also has a certain
value for diagnosing neuroblastoma, non-small cell lung cancer and
other malignant tumors, leading to a high misdiagnosis and false
positive rate when used to diagnose RB (8). Lactate dehydrogenase and
carcinoembryonic antigen have also been used to assist in the
diagnosis of RB, but the sensitivity and specificity of these
laboratory indicators are poor (9).
Therefore, identification of a more sensitive and specific
molecular biology index is required in order to improve the early
diagnosis rate of RB and save the lives of children.
MicroRNA (miRNA) is a non-coding endogenous small
molecule of RNA 18–25 nucleotides in length (10). It binds to the target mRNA either
completely or incompletely, and regulates the expression of
downstream genes at the transcriptional or translational level,
thus participating in embryonic development, cell proliferation,
differentiation, apoptosis and fat metabolism (10). With the in-depth study of tumors and
associated miRNAs, circulating miRNAs have attracted increasing
attention. It has been revealed that miRNA expression can be
detected in serum, cerebrospinal fluid and a number of other bodily
fluids and may derive from the active release of apoptotic,
necrotic or live tumor cells, although the precise origin of
circulating miRNAs has not yet been universally identified
(11–13). The presence of circulating miRNAs
within bodily fluids alongside proteins and other particles makes
them resistant to degradation via ribonucleases (13). Even under extreme conditions, such as
strongly acidic, strongly alkaline or repeated freezing and
thawing, expression of miRNA remains stable. This important
characteristic of miRNA suggests that serum or serum miRNAs may
have objective conditions as tumor markers for application in the
clinical setting (13,14).
Abnormal expression of miR-338-5p has been widely
observed in various types of cancer (15,16). In
patients with colorectal cancer, upregulated serum miR-338-5p was
suggested to be a potential circulating marker (15). Additionally, miR-338-5p was revealed
as being increased in melanoma tissues and glioma (17,18).
However, to the best of our knowledge, the possibility of whether
miR-338-5p is dysregulated in RB has never been investigated. The
aim of the present study was to evaluate the expression of
miR-338-5p in RB, thereby revealing whether it could be used as a
potential biomarker to screen patients with RB from healthy
controls.
Materials and methods
Patient samples
Peripheral blood (4 ml) was collected from 65
patients with RB (male/female: 34/31; mean age: 3.8±1.7 years; age
range: 2.2–5.8 years) and 65 healthy controls (male/female: 29/36;
mean age: 3.5±1.6 years; 1.9–6.1 years) at Peking University Third
Hospital (Beijing, China) between December 2016 and November 2017.
Inclusion criteria were as follows: Patients who did not receive
any treatment prior to surgery, patients whose tissue sections were
diagnosed by the chief physician and patients with complete
clinical data. Exclusion criteria were as follows: Patients with a
history of mental disease and a family history of mental disease,
or patients with complicated severe heart, lung, liver and renal
dysfunction. According to the International Classification Standard
for Retinoblastoma (ICRB) (19),
patients with RB were divided into stages A, B and C (12 patients)
or stages D and E (53 patients) (Table
I). Written informed consent was obtained from each patient.
The experimental protocol was preapproved by the Medical Ethics
Committee of Peking University Third Hospital.
 | Table I.Clinical features of the patients with
RB and healthy control participants. |
Table I.
Clinical features of the patients with
RB and healthy control participants.
| Variable | Patients (n=65) | Controls (n=65) | P-value |
|---|
| Average age, months;
mean ± SD | 24.6±16.5 | 27.92±12.03 | 0.196a |
| Sex, n (%) |
|
|
<1.000b |
| Male | 39 (60.0) | 31 (47.7) |
|
|
Female | 26 (40.0) | 34 (52.3) |
|
| Laterality, n
(%) |
|
|
|
|
Unilateral | 45 (69.2) | N/A |
|
|
Bilateral | 20 (30.8) | N/A |
|
| IIRC clinical stage,
n |
|
|
|
| Stages
A-C | 12 | N/A |
|
| Stages
D-E | 53 | N/A |
|
| NSE level, ng/ml;
mean ± SD | 27.4±7.0 | 10.6±3.5 |
<0.0001a |
Cell culture
A human retinoblastoma cell line Y79 was purchased
from the American Type Culture Collection (Manassas, VA, USA). Y79
cells were cultured for 24 h to 70% confluence in RPMI-1640 medium
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (FBS; HyClone; GE
Healthcare Life Sciences, Logan, UT, USA), 100 U/ml penicillin and
100 mg/ml streptomycin at 37°C under normoxic conditions of 100%
humidity, 95% air and 5% CO2.
Human normal retinal vascular endothelial cell line
ACBRI-181 is a commercially available cell line (Cell Systems,
Kirkland, WA, USA). For the culture of ACBRI-181 for 48 h, a
special medium was used, consisting of Complete Serum-Free (CSC)
medium with RocketFuel™, supplemented with 10% FBS
(HyClone; GE Healthcare Life Sciences), 100 U/ml penicillin and 100
mg/ml streptomycin at 37°C under normoxic conditions of 100%
humidity, 95% air and 5% CO2.
Cell transfection
The miR-338-5p mimic, miR-338-5p inhibitor and
miR-negative control (NC) were all purchased from GenePharma
(Shanghai, China). Increased or decreased miR-338-5p expression was
achieved by transfection of miR-338-5p mimic or miR-338-5p
inhibitors, respectively. Transfection was performed using the
instructions of HiPerfect Transfection reagent (Qiagen, Inc.,
Valencia, CA, USA), according to the manufacturer's protocol, and
each group of cells was harvested 24–48 h after transfection for
further assays.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The serum was collected from the blood samples of
the patients included in the study. For preparation of the serum
samples, the blood was centrifuged at 3,000 × g at 4°C for 15 min.
Total RNA was isolated from serum using RNAVzol reagent (Vigorous
Biotechnology Beijing Co., Ltd., Beijing, China) according to the
manufacturer's protocol. RNA purity was assessed at optical density
(OD)260/OD280 (values, 1.7–2.0). RNA (1 µg)
was reverse-transcribed into cDNA using Moloney Murine Leukemia
Virus reverse transcription enzyme (Applied Biosystems; Thermo
Fisher Scientific, Inc.) with specific primers, and amplified via
qPCR using a Rotor-Gene 3000 Real-Time PCR system (Corbett Life
Sciences; Qiagen, Inc., Valencia, CA, USA). The temperature
protocol used for RT was as follows: 72°C for 10 min, 42°C for 60
min, 72°C for 5 min and 95°C for 2 min. To quantify the relative
mRNA levels, qPCR was performed using SYBR® Green
Supermix (Bio-Rad Laboratories, Inc., Hercules, CA, USA) in an
iCycler iQ Real-Time PCR Detection system. The PCR amplifications
were performed in a 10 µl reaction system containing 5 µl SYBR
Green Supermix, 0.4 µl forward primer, 0.4 µl reverse primer, 2.2
µl double-distilled water and 2 µl template cDNA. Thermocycling
conditions were as follows: 95°C for 10 min followed by 40 cycles
of 95°C for 15 sec and 60°C for 1 min. Relative mRNA expression was
normalized to U6 using the 2−∆∆Cq method (20). Primer sequences are as follows:
miR-338-5p-RT,
5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACCACTC-3′; U6-RT,
5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACAAAATG-3′;
miR-338-5p, forward 5′-AACAATATCCTGGTGCTG-3′; U6, forward
5′-GCGCGTCGTGAAGCGTTC-3′; universal reverse primer,
5′-GTGCAGGGTCCGAGGT-3′.
Cell proliferation assay
Following transfection for 72 h, cells were seeded
into 96-well culture plates at a density of 2×104
cells/well. After 3–4 h when cells had adhered to each well, 100 µl
RPMI-1640 medium supplemented with 10% FBS, 100 U/ml penicillin and
100 mg/ml streptomycin and 10 µl Cell Containing Kit-8 (Dojindo
Laboratories, Kumamoto, Japan) were added. The culture medium was
placed in 5% CO2 at 37°C for 1, 2, 3, 4 and 5 days. A
microplate reader (Molecular Devices, LLC, Sunnyvale, CA, USA) was
used to determine the OD450 value.
Cell migration and invasion
assays
Cell migration assays were performed using Boyden
chambers (8 µm pore filter; Corning Inc., Corning, NY, USA). For
the cell invasion assay, the filter surfaces were precoated with
Matrigel (BD Biosciences, San Jose, CA, USA). In brief, ACBRI-181
or Y79 cells were seeded at a density of 1×105
cells/well for 24 h and then were transfected with NC or miR-338-5p
inhibitor in the upper chamber in 500 µl CSC medium or RPMI-1640
medium without FBS. Cell culture medium (600 µl) with 20% FBS was
plated in the lower chamber. After 48 h of incubation,
non-migratory and non-invasive cells were removed using cotton
swabs. The migratory or invasive cells located on the lower side of
the chamber were fixed in methanol for 30 min at 37°C and stained
with 0.5% crystal violet for 1 h at 37°C. Stained cells were
counted in five random fields using fluorescence microscopy
(magnification, ×40). All experiments were performed in
triplicate.
Cell cycle assays
ACBRI-181 or Y79 cells (~1×106) were
trypsinized, washed twice with PBS and fixed in 70% ice-cold
ethanol for 1 h. The samples were centrifuged at 300 × g for 5 min
at 4°C, the ethanol removed and they were exposed to 100 mg/ml
RNaseA (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for 30 min
at 37°C. Cell cycle was analyzed using cell cycle analysis kit
(cat. no., KGA512; Nanjing KeyGen Biotech Co., Ltd., Nanjing,
China), according to the manufacturer's protocols. Cellular DNA was
stained with propidium iodide (Nanjing KeyGen Biotech Co., Ltd.) at
37°C for 30 min. Cell-cycle distributions were determined by flow
cytometry using a BD FACSCalibur system (BD Biosciences, Franklin
Lakes, NJ, USA) and data was analyzed using the ModFit software
version 4.1 (Verity Software House, Inc., Topsham, ME, USA).
Statistical analysis
Data are presented as the mean ± standard deviation.
Two-tailed unpaired Student's t-tests were used for comparisons
between two groups. Analysis of variance followed by Tukey's post
hoc test were used for multiple group comparisons using SPSS
(version 13; SPSS, Inc., Chicago, IL, USA). Receiver operator
characteristic (ROC) curves were used to assess the potential of
miR-338-5p as a biomarker, and the area under the curve (AUC) was
recorded. To evaluate the diagnostic value of serum miR-338-5p for
patients with RB, ROC analysis was performed to investigate the
application of miR-338-5p alone or the combination of miR-338-5p
and NSE. The cut-off value was calculated as: Youden
index=sensitivity-(1-specificity). The test value corresponding to
the maximum Youden index value can be used as cut-off value. To
assess the association between serum miR-338-5p and clinical
features as presented in Tables I
and II, the χ2 test was
applied. P<0.05 was considered to indicate a statistically
significant difference.
 | Table II.Association between serum
microRNA-338-5p and clinical features. |
Table II.
Association between serum
microRNA-338-5p and clinical features.
| Variable | n | χ2 | P-value |
|---|
| Age, months |
| 4.750 | 0.562 |
| <12 | 22 |
|
|
|
>12 | 28 |
|
|
| Sex |
| 0.009 | 0.856 |
| Male | 33 |
|
|
|
Female | 17 |
|
|
| Laterality |
| 0.150 | 1.060 |
|
Unilateral | 36 |
|
|
|
Bilateral | 14 |
|
|
| IIRC clinical
stage |
| 1.350 | 0.554 |
| Stages
A-C | 11 |
|
|
| Stages
D-E | 39 |
|
|
Results
Clinical data and characteristics of
subjects
For patients with RB, 12 were classed as stages A, B
and C, and 53 were classed as stages D and E. No significant
difference was identified in age (P=0.196) or sex (P<1.000)
between healthy controls and patients with RB (Table I).
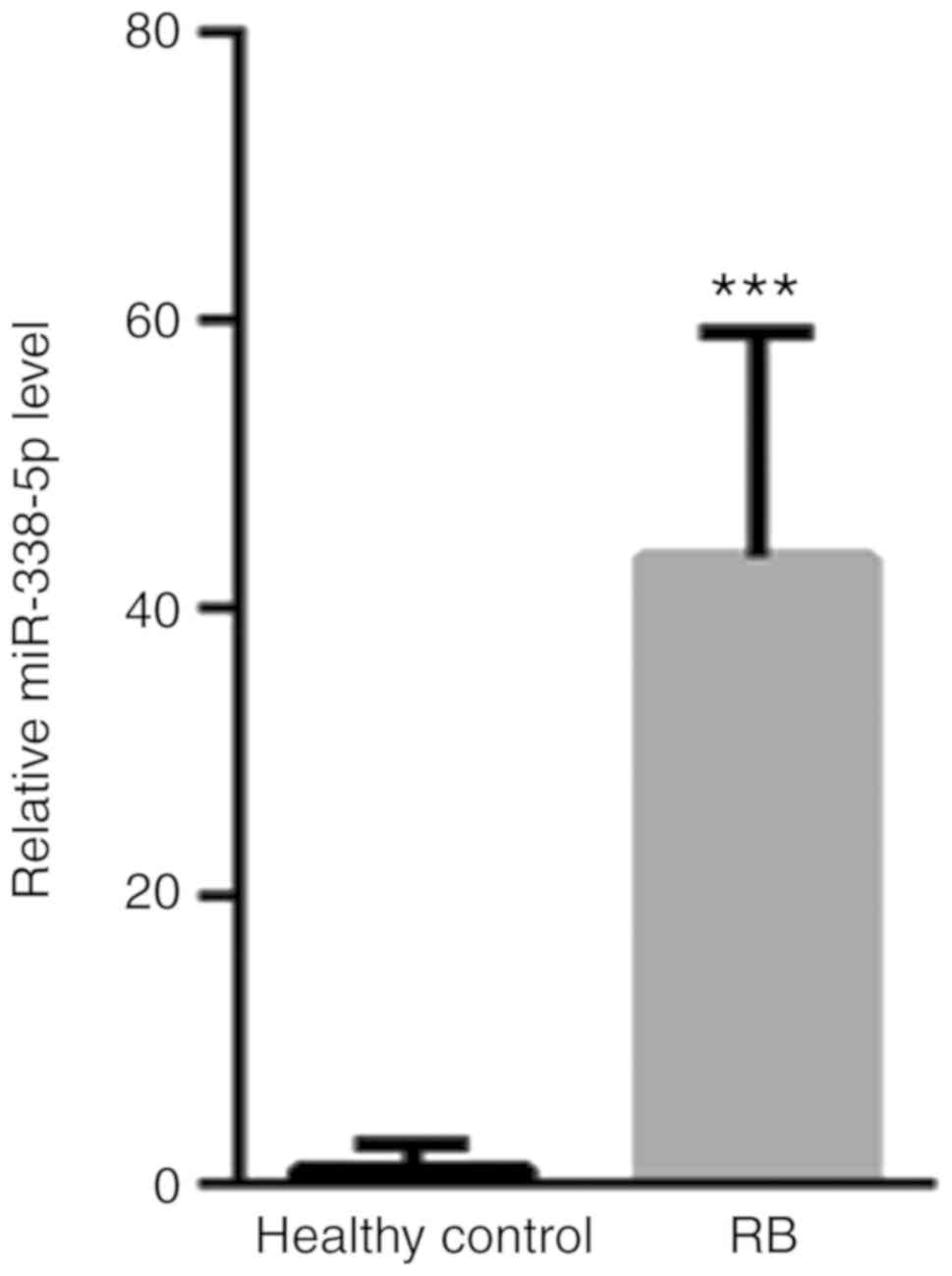
Increased serum miR-338-5p in patients
with RB
First, the presence of serum miR-338-5p in patients
with RB and healthy controls was evaluated. The levels of serum
miR-338-5p for all the patient samples and controls are presented
in Fig. 1. Compared with healthy
controls (1±1.86), the level of serum miR-338-5p was significantly
increased (43.54±15.6) (Fig. 1).
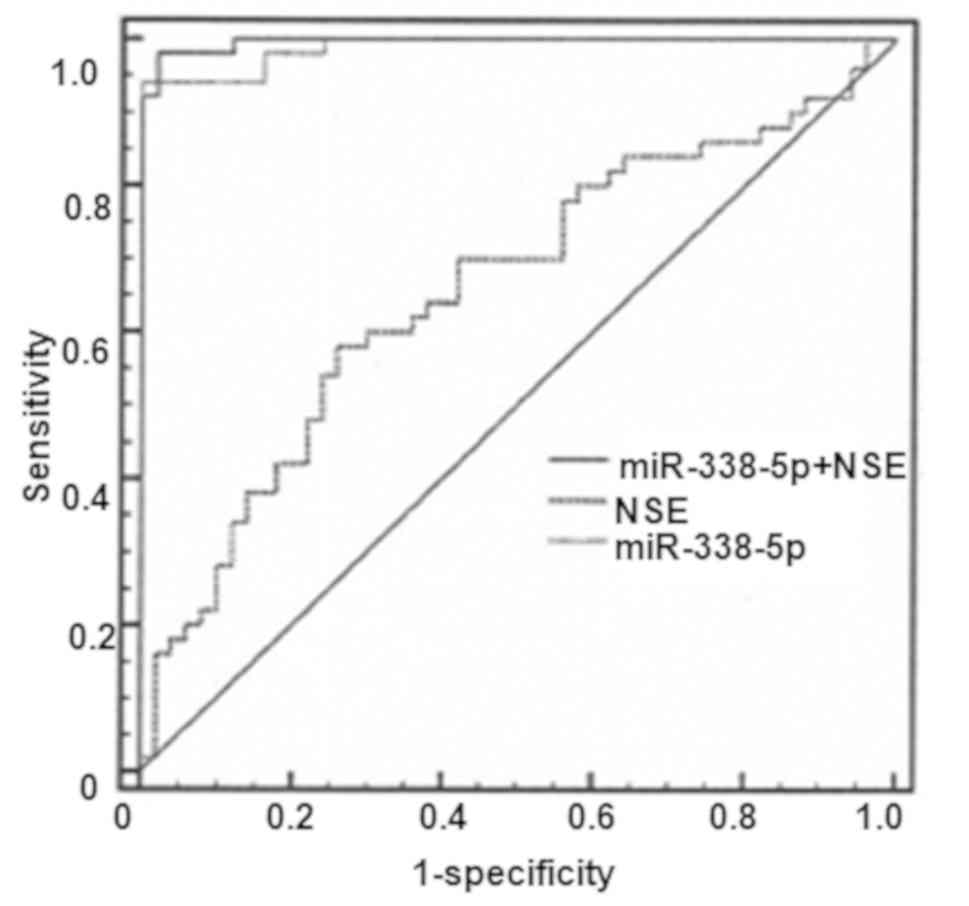
Diagnostic value of serum miR-338-5p
in patients with RB
As presented in Fig.
2, the AUC value for NSE was 0.660 [95% confidence interval
(CI), 0.558–0.752; P=0.0036] with a cut-off value of 14.7, a
sensitivity level of 58% and a specificity level of 74%. The AUC of
NSE reached 0.989 with a cut-off value of 15.9, a sensitivity level
of 94% and a specificity level of 100%. In contrast, the AUC value
for combined use of miR-338-5p and NSE was 0.996 (95% CI,
0.957–1.000; P<0.0001), with a sensitivity of 98% and a
specificity of 100%.
Association between serum miR-338-5p
and clinical features
The association between serum miR-338-5p and
clinical features was then analyzed. There was a loss of follow-up
data from 15 patients with RB, as they could not be contacted, and
these were not included in the analyses presented in Table II. No significant associations were
identified between serum miR-338-5p and age, sex, laterality or
International Intraocular Retinoblastoma Classification clinical
stage (Table II).
Decreased miR-338-5p inhibits RB cell
proliferation and results in cell cycle arrest
To further evaluate the underlying molecular
mechanism by which miR-338-5p affects the progression of RB,
miR-338-5p inhibitor or NC was transfected into ACBRI-181 and Y79
cells for 1, 2, 3, 4 and 5 days. First, the transfection efficiency
of the miR-338-5p inhibitor in ACBRI-181 and Y79 cells was
determined. As presented in Fig. 3A,
transfection of miR-338-5p inhibitor significantly decreased the
level of miR-338-5p in ACBRI-181 and Y79 cells for 1, 2, 3, 4 and 5
days. Furthermore, decreasing miR-338-5p induced slower
proliferation of ACBRI-181 and Y79 cells at 2, 3, 4 and 5 days
compared with those in the NC group (Fig. 3B). Flow cytometric analysis indicated
that the transfection with miR-338-5p inhibitor for 2 days led to
significant cell cycle arrest in ACBRI-181 and Y79 cells compared
with in the NC group (Fig. 3C).
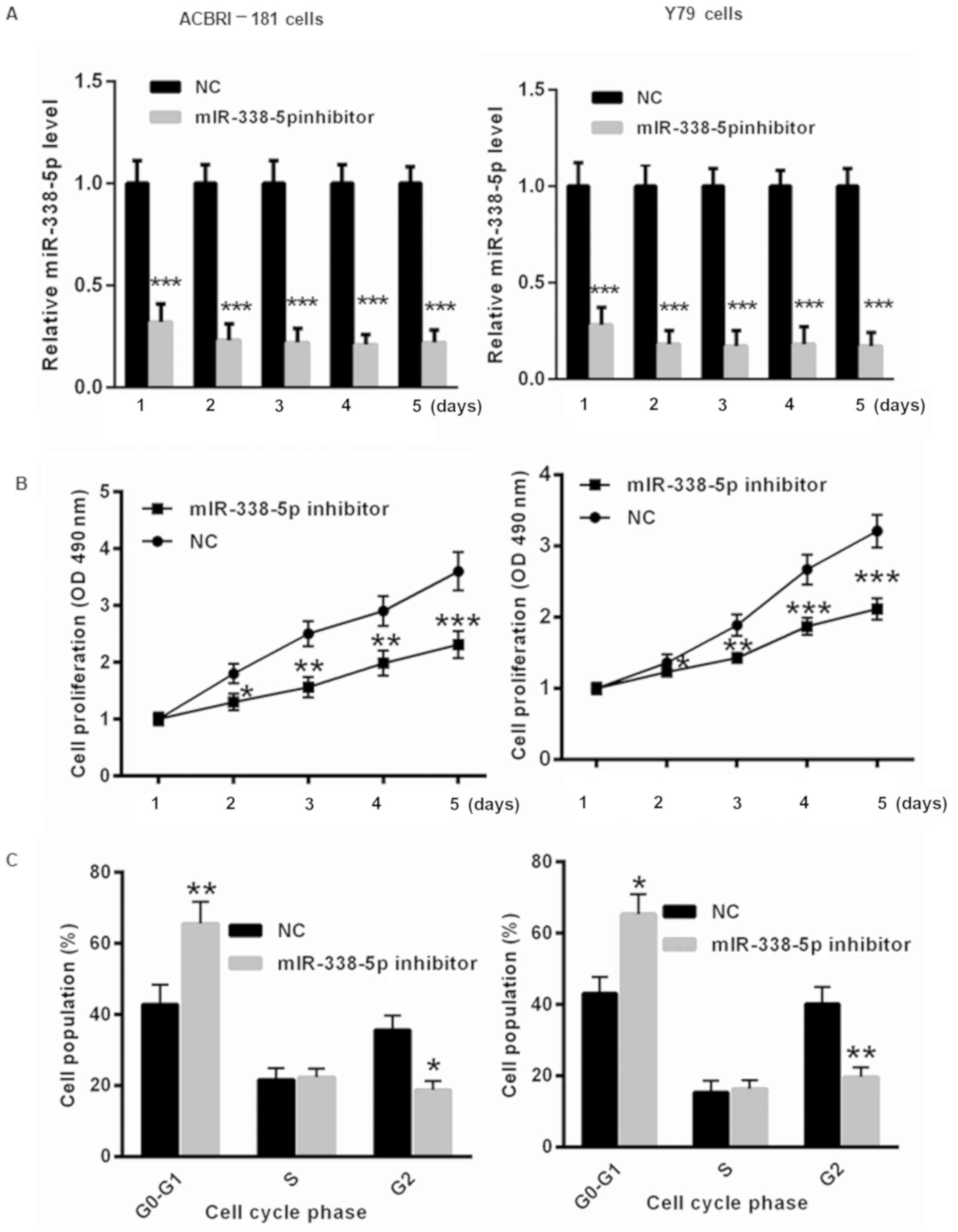 | Figure 3.Decreased miR-338-5p levels inhibit RB
cell proliferation and result in cell cycle arrest. (A) The
transfection of miR-338-5p was evaluated in ACBRI-181 and Y79 cells
for 1, 2, 3, 4 and 5 days using reverse transcription-quantitative
polymerase chain reaction. (B) Suppression of miR-338-5p by using
an miR-338-5p inhibitor resulted in slower proliferation of
ACBRI-181 and Y79 cells at 2, 3, 4 and 5 days compared with those
in the NC group. (C) Flow cytometric analysis demonstrated that
transfection with the miR-338-5p inhibitor led to significant cell
cycle arrest in ACBRI-181 and Y79 cells compared with the NC group.
*P<0.05, **P<0.01, ***P<0.001 vs. NC group. RB,
retinoblastoma; NC, negative control; miR, microRNA; OD, optical
density. |
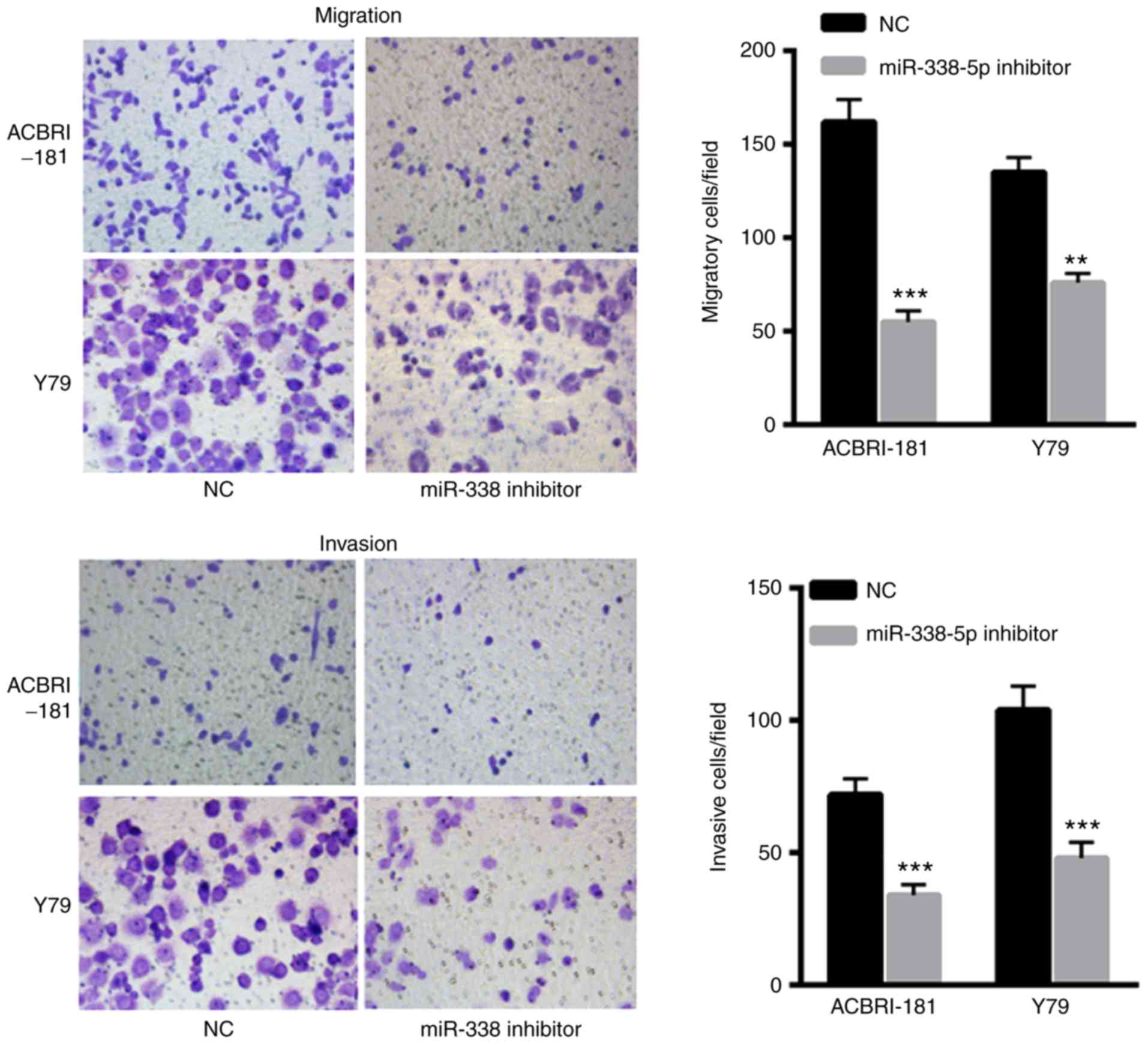
Decreased miR-338-5p decreases the
invasive and migratory capacity of ACBRI-181 and Y79 cells
The effects of miR-338-5p on ACBRI-181 and Y79 cells
were also evaluated. The results indicated that transfection with
miR-338-5p inhibitor significantly decreased the migration and
invasion of ACBRI-181 and Y79 cells compared with in the NC group
(Fig. 4A and B, respectively),
indicating the oncogenic role of miR-338-5p in the progression of
RB.
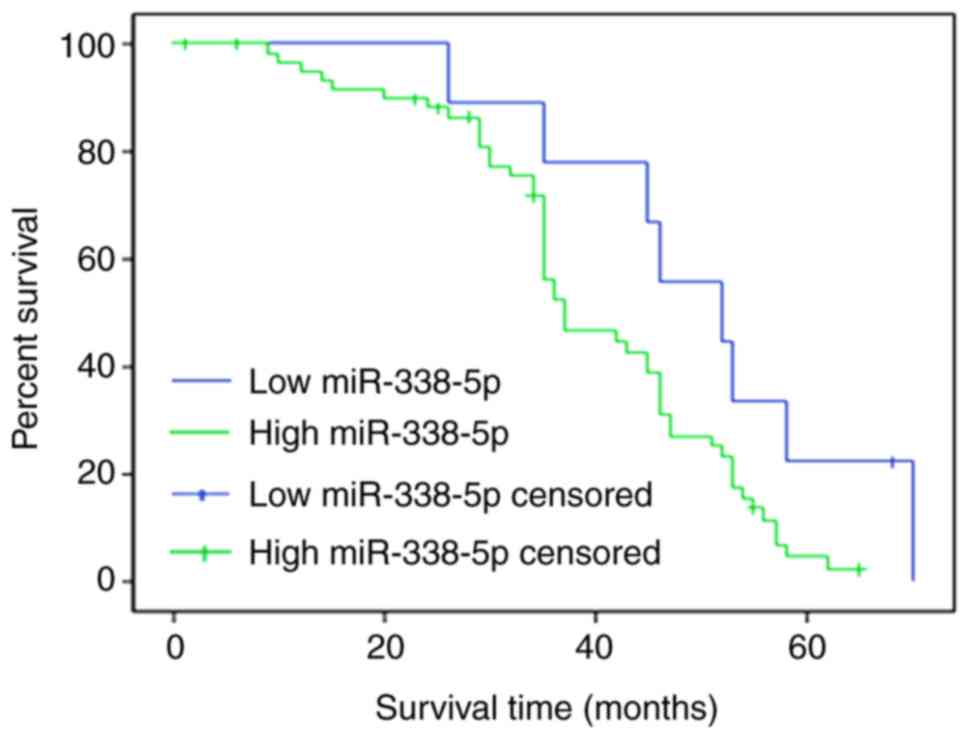
Increased miR-338-5p results in a poor
survival rate for patients with RB
The overall survival rate was evaluated for patients
with RB with high miR-338-5p (>21.77) or low miR-338-5p (≤21.77)
levels. To differentiate the high and low levels, the mean
expression of miR-338-5p was calculated. A total of 50 patients
completed the follow-up, and the follow-up success rate was 77.0%.
Survival rates at 20, 40 and 60 months in the miR-338-5p low
expression group were 88.5, 79.4 and 68.9%, respectively, whereas
survival rates at 20, 40 and 60 months in the miR-338-5p high
expression group were 79.8, 68.2 and 43.6%, which were
significantly lower compared with those in miR-338-5p low
expression group (P<0.05; Fig.
5).
Discussion
With the increase in research on miRNAs and tumors,
the suggestion that miRNAs can be used as novel diagnostic and
prognostic tools and therapeutic targets has gradually been
accepted (21). In the clinical
setting, it is difficult to detect early-stage tumors in children
(22). Chemotherapy easily induces
drug resistance, and side effects of drugs can often cause
secondary injury (22). All these
factors markedly affect the diagnosis and treatment of childhood
tumors. As the intrinsic association between miRNAs and tumors is
gradually revealed, miRNAs have been identified to be equally
applicable to tumors in childhood (22,23).
In the present study, RT-qPCR was used to detect the
expression of miRNA-338-5p in the serum of 50 patients with RB and
healthy controls. Combining the clinical data of the participants,
it was revealed that there were no significant differences in the
expression of miR-338-5p between the age, sex, tumor stage and
binocular disease of patients with RB (Table I). To determine the clinical value of
serum miRNAs as RB tumor markers, the ROC curve of serum
miRNA-338-5p combined with traditional tumor markers was
established for the first time, to the best of our knowledge, in
the present study. By comparing their respective AUC values, it was
revealed that serum miR-338-5p combined with NSE had a larger
curvilinear area compared with that of serum miR-338-5p alone when
diagnosing RB. In the present study, the ROC curves for the
miR-338-5p indicated a poor diagnostic value for each miRNA alone,
although the P-value indicated some significance for the diagnosis
of RB. The ROC of NSE alone was similar to that of miR-338-5p+NSE,
which demonstrated the unreliability. However, an upregulation of
AUC resulted in significantly decreased possibility of
misdiagnosis, which would be expected if the accuracy improves;
therefore, it is important to combine miR-338-5p+NSE for the
diagnosis of RB. These results suggest that serum miR-338-5p may be
of clinical value when the presence of RB is suspected,
particularly when combined with NSE, and may improve the early
diagnosis rate of RB.
The results of the present study indicated that
there were no significant differences in the expression of serum
miR-338-5p and the age, sex, binocular disease or tumor stage of
children with RB. We hypothesize that serum miR-338-5p may serve a
role in the signaling pathway of RB initiation. Once the tumor
develops, the expression of miR-338-5p in serum will no longer
change. It should be noted that the limited sample size of this
experiment, resulting in the lack of abundant data on RB grouping,
may be a possible reason for this result.
In addition, the oncogenic role of miR-338-5p was
also investigated using an in vitro assay. Suppression of
miR-338-5p induced slower proliferation of ACBRI-181 and Y79 cells
at 2, 3, 4 and 5 days compared with that of the NC group. Flow
cytometric analysis indicated that transfection with miR-338-5p
inhibitor led to significant cell cycle arrest in ACBRI-181 and Y79
cells compared with that of the NC group. Furthermore, transfection
with miR-338-5p inhibitor significantly decreased migration and
invasion of ACBRI-181 and Y79 cells, revealing the oncogenic role
of miR-338-5p in the progression of RB. Previous studies have
indicated that miR-338-5p functions as an oncogenic miRNA in
melanoma tissues via targeting cluster of differentiation 82, and
targeting teashirt zinc finger homeobox 3 and matrix
metalloproteinase-2 in glioma (17,18). In
future studies, we will further investigate whether these targets
were important for miR-338-5p in the progression of RB. However,
the present study also has the following shortcomings: i) Chromatin
immunoprecipitation technology was not used for the patients with
RB and normal control serum samples for preliminary screening of
miRNAs, limiting the selection of miRNAs tested; ii) the sample
size included in the present study requires expansion in other
studies so that it can be more valuable in clinical staging by the
comparative analysis of experimental results; iii) RB tissue
samples are required for detection, thereby elucidating the
association between RB tissue and serum miR-338-5p expression.
In conclusion, the low expression of miR-338-5p in
the serum of patients with RB suggests that it may be involved in
the formation of RB. Serum miR-338-5p has the potential to be a
tumor marker of RB, and, in combination with NSE, miR-338-5p may
improve the early diagnosis rate of RB.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Beijing
Science and Technology Fund Project (grant no. B79495-03).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
PZ wrote the paper, performed the experiments and
analyzed the data. XL designed the experiments, analyzed the data.
Both authors approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Peking University Third Hospital, Beijing, China, as
stipulated by The Declaration of Helsinki (1964), with written
informed consent for the use of the specimens obtained from all
enrolled patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Giacalone M, Mastrangelo G and Parri N:
Point-of-care ultrasound diagnosis of retinoblastoma in the
emergency department. Pediatr Emerg Care. 34:599–601. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stathopoulos C, Moulin A, Gaillard MC,
Beck-Popovic M, Puccinelli F and Munier FL: Conservative treatment
of diffuse infiltrating retinoblastoma: Optical coherence
tomography-assisted diagnosis and follow-up in three consecutive
cases. Br J Ophthalmol. Jul 26–2018.(Epub ahead of print). doi:
10.1136/bjophthalmol-2018-312546. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Al-Nawaiseh I, Ghanem AQ and Yousef YA:
Familial retinoblastoma: Raised awareness improves early diagnosis
and outcome. J Ophthalmol. 2017:50539612017.PubMed/NCBI
|
|
4
|
Jenkinson H: Retinoblastoma: Diagnosis and
management-the UK perspective. Arch Dis Child. 100:1070–1075. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fabian ID, Puccinelli F, Gaillard MC,
Beck-Popovic M and Munier FL: Diagnosis and management of secondary
epipapillary retinoblastoma. Br J Ophthalmol. 101:1412–1418. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dehainault C, Golmard L, Millot GA,
Charpin A, Laugé A, Tarabeux J, Aerts I, Cassoux N, Stoppa-Lyonnet
D, Gauthier-Villars M and Houdayer C: Mosaicism and prenatal
diagnosis options: Insights from retinoblastoma. Eur J Hum Genet.
25:381–383. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Parrilla-Vallejo M, Perea-Pérez R,
Relimpio-López I, Montero-de-Espinosa I, Rodríguez-de-la-Rúa E,
Terrón-León JA, Díaz-Granda MJ, Coca-Gutiérrez L and Ponte-Zuñiga
B: Retinoblastoma: The importance of early diagnosis. Arch Soc Esp
Oftalmol. 93:423–430. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ramírez-Ortiz MA, Lansingh VC, Eckert KA,
Haik BG, Phillips BX, Bosch-Canto V, González-Pérez G,
Villavicencio-Torres A and Etulain-González A: Systematic review of
the current status of programs and general knowledge of diagnosis
and management of retinoblastoma. Bol Med Hosp Infant Mex.
74:41–54. 2017.PubMed/NCBI
|
|
9
|
Shah PK, Sripriya S, Narendran V and
Pandian AJ: Prenatal genetic diagnosis of retinoblastoma and report
of RB1 gene mutation from India. Ophthalmic Genet. 37:430–433.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu L, Chen Z and Xing Y: miR-506-3p
inhibits cell proliferation, induces cell cycle arrest and
apoptosis in retinoblastoma by directly targeting NEK6. Cell Biol
Int. Aug 6–2018.(Epub ahead of print). doi: 10.1002/cbin.11041.
View Article : Google Scholar
|
|
11
|
Clarke JI, Forootan SS, Lea JD, Howell LS,
Rodriguez JM, Kipar A, Goldring CE, Park BK, Copple IM and Antoine
DJ: Circulating levels of miR-122 increase post-mortem,
particularly following lethal dosing with pentobarbital sodium:
Implications for pre-clinical liver injury studies. Toxicol Res
(Camb). 6:406–411. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hu HL, Nie ZQ, Lu Y, Yang X, Song C, Chen
H, Zhu S, Chen BB, Huang J, Geng S and Zhao S: Circulating miR-125b
but not miR-125a correlates with acute exacerbations of chronic
obstructive pulmonary disease and the expressions of inflammatory
cytokines. Medicine (Baltimore). 96:e90592017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Copier CU, León L, Fernández M, Contador D
and Calligaris SD: Circulating miR-19b and miR-181b are potential
biomarkers for diabetic cardiomyopathy. Sci Rep. 7:135142017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
D'Agostino M, Martino F, Sileno S, Barillà
F, Beji S, Marchetti L, Gangi FM, Persico L, Picozza M, Montali A,
et al: Circulating miR-200c is up-regulated in paediatric patients
with familial hypercholesterolaemia and correlates with miR-33a/b
levels: Implication of a ZEB1-dependent mechanism. Clin Sci (Lond).
131:2397–2408. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bilegsaikhan E, Liu HN, Shen XZ and Liu
TT: Circulating miR-338-5p is a potential diagnostic biomarker in
colorectal cancer. J Dig Dis. 19:404–410. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xing Z, Yu L, Li X and Su X: Anticancer
bioactive peptide-3 inhibits human gastric cancer growth by
targeting miR-338-5p. Cell Biosci. 6:532016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Long J, Luo J and Yin X: miR-338-5p
promotes the growth and metastasis of malignant melanoma cells via
targeting CD82. Biomed Pharmacother. 102:1195–1202. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li Y, Huang Y, Qi Z, Sun T and Zhou Y:
miR-338-5p promotes glioma cell invasion by regulating TSHZ3 and
MMP2. Cell Mol Neurobiol. 38:669–677. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shields CL, Mashayekhi A, Au AK, Czyz C,
Leahey A, Meadows AT and Shields JA: The International
Classification of Retinoblastoma predicts chemoreduction success.
Ophthalmology. 113:2276–2280. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gutierrez-Camino A, Martin-Guerrero I,
Dolzan V, Jazbec J, Carbone-Bañeres A, Garcia de Andoin N, Sastre
A, Astigarraga I, Navajas A and Garcia-Orad A: Involvement of SNPs
in miR-3117 and miR-3689d2 in childhood acute lymphoblastic
leukemia risk. Oncotarget. 9:22907–22914. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu T, Lin Y and Xie Z: MicroRNA-1247
inhibits cell proliferation by directly targeting ZNF346 in
childhood neuroblastoma. Biol Res. 51:132018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zakrzewska M, Fendler W, Zakrzewski K,
Sikorska B, Grajkowska W, Dembowska-Bagińska B, Filipek I,
Stefańczyk Ł and Liberski PP: Altered MicroRNA expression is
associated with tumor grade, molecular background and outcome in
childhood infratentorial ependymoma. PLoS One. 11:e01584642016.
View Article : Google Scholar : PubMed/NCBI
|