Introduction
Acute lymphoblastic leukemia (ALL) is a malignant
tumor characterized by the abnormal proliferation of T- and
B-lymphoid progenitor cells in the bone marrow, which may cause
hematopoietic dysfunction and may invade extramedullary tissues.
ALL is the most common malignant hematological tumor with the
highest mortality rate among children (1). It can lead to disordered T cell ratio
in the blood system, T cell dysfunction, as well as NK cell immune
dysfunction, causing body's immune function, especially cellular
immunity, to be seriously impaired (2). ALL not only invades the hematopoietic
system, but also damages the body's immune system, leading to an
abnormal Th1/Th2 ratio in T helper cells (Th cells), increased
presence of CD4+CD25+ Treg cells, and
increased expression of immunosuppressive factors, such as TGF-β
(3). Sudden onset of symptoms and
rapid disease progression are associated with ALL. If not treated
in time, ALL can be fatal within weeks or months (4). At present, the cause of ALL remains
unclear, but genetic factors, such as Down's syndrome and related
gene mutation/fusion, as well as environmental factors, such as
radiation exposure and benzene homologues, are risk factors
(5). Because the early symptoms of
ALL are mostly fever, bruises and other atypical presentations, it
is often overlooked, especially in children (6). Although new medical treatments, such as
immunotherapy, have been proposed, the most common treatment
methods for ALL are chemotherapy with continuous induction of
remission, radiotherapy and intensive combination therapy.
Chemotherapy is still the preferred treatment. Intrathecal
chemotherapy, in which chemotherapy drugs are administered through
a lumbar puncture, is most routinely prescribed. Bone marrow biopsy
is regarded as a reliable tool for ALL diagnosis (7,8).
Anesthesia and sedation are required for bone marrow biopsy, stem
cell transplantation and intrathecal injection, as pediatric
patients' have poor compliance and self-control. Moreover,
pediatric patients have a lower tolerance to anesthesia and
operation than healthy children and adults due to their incomplete
development of various physical functions and attenuated immune
function caused by ALL (9).
Therefore, the outcomes of anesthesia and sedation, as well as the
impact on patients' immune function, play an important role in
prognosis.
Sevoflurane, a new type of inhalational anesthetic
agent, has a low blood/gas partition coefficient and does not cause
respiratory tract irritation. The patient has a short recovery time
after anesthesia. Sevoflurane has high anesthesia efficiency and
can assist in muscle relaxation in addition to sedation (10,11).
Propofol, a fast-acting systemic intravenous anesthetic agent, has
the characteristics of quick recovery after anesthesia, mild
gastrointestinal reaction, and low cumulative effect after
continuous administration (12).
Whitlow et al (13) found
that local anesthetics combined with propofol produced safe and
effective analgesia and sedation in patients who underwent lumbar
puncture. It was reported that the choice of anesthetic drugs
affected not only the anesthetic outcome but also the proliferation
of cancer cells and the immune function (14). Flouda et al (15) reported that sevoflurane and propofol
had certain effects on cognitive and immune function in elderly
patients with gastric cancer, and the effect on cognitive function
was minimal. In this study, the effect of combined
propofol-sevoflurane anesthesia on the immune function of pediatric
patients with ALL was explored in order to improve the efficacy and
safety of anesthesia, as well as the prognosis and the survival
rate of these patients.
Patients and methods
Patients
A retrospective analysis was performed on the
clinical data of 150 pediatric patients with ALL who were newly
diagnosed by bone marrow biopsy from May 2014 to October 2017. All
patients received intrathecal chemotherapy after anesthesia was
induced. Patients were separated into three groups. In group A, 50
patients were anesthetized with propofol only. In group B, 50
patients were anesthetized with sevoflurane only. In group C, 50
patients were anesthetized with combined propofol-sevoflurane.
Patients who met the following inclusion criteria were eligible for
this study: i) patients who met the diagnostic criteria for ALL,
which included the bone marrow smear demonstrating ≥30%
lymphoblasts and prolymphocytes; ii) patients aged ≤14 years; and
ⅲ) patients who were administered 10 mg of methotrexate (first dose
5 mg) combined with 2 mg of dexamethasone in saline through
intrathecal injection. Patients who met the following criteria were
excluded from this study: i) patients who had other cancers; ii)
patients who had other autoimmune diseases; iii) patients who had
been vaccinated in the previous month; iv) patients who were
allergic to propofol and sevoflurane; and v) patients who had
severe heart, liver, or kidney disease. The study was approved by
the Ethics Committee of Xiangyang No. 1 People's Hospital
Affiliated to Hubei University of Medicine (XY354I; Xiangyang,
China). Patients who participated in this research had complete
clinical data. All subjects and their parents were informed and
agreed to participate in the clinical study. The parents of the
child patients signed an informed consent.
Materials and reagents
Scopolamine was purchased from Wantong
Pharmaceutical group (Jilin, China) with SFDA approval no.
H33021318; promethazine was purchased from Shanghai Harvest
Pharmaceutical Co., Ltd. (Shanghai, China) with SFDA approval no.
H31021490; fentanyl was purchased from Yichang Humanwell
Pharmaceutical Co., Ltd. (Yichang, China) with SFDA approval no.
H42022076; etomidate and atracurium were purchased from Jiangsu
Hengrui Medicine (Lianyungang, China) with SFDA approval nos.
H20090248 and H20060869; propofol was purchased from Fresenius Kabi
AB (Beijing, China) with SFDA approval no. J20080023; and
sevoflurane was purchased from Lunan Better Pharmaceutical Co.,
Ltd. (Linyi, China) with SFDA approval no. H20080681. The FITC kits
for CD3, CD4, CD8 and CD19 measurements were manufactured by
Shanghai BD Biosciences (Shanghai, China). The ELISA kits for
TGF-β, IFNγ and IL-4 measurements were manufactured by Shanghai
Beyotime Institute of Biotechnology (Shanghai, China).
Anesthesia procedures
Pre-anesthesia preparation
All pediatric patients underwent 4 h fasting and 6 h
water-deprivation before operation. Scopolamine (0.1 mg/kg) and
promethazine (1 mg/kg) were given via intramuscular injection, 30
min before operation. An intravenous indwelling trocar was placed.
The patient was connected to a monitor for checking the vital
signs, such as blood pressure and heart rate, during
anesthesia.
Anesthesia induction and
maintenance
General anesthesia was induced for all patients
using 4.5 µg/kg of fentanyl, 0.2 mg/kg of etomidate and 1.0 mg/kg
of propofol through intravenous injection. In group A, after the
indexes of patients before anesthesia were recorded,
target-controlled infusion of propofol was performed at a rate of 3
µg/ml. In group B, 6 l/min of pure oxygen without nitrogen were
inhaled for 3 min using a mask, and after the patient's pulse
oximetry (SpO2) reached 98% or more, the patient took
several deep breaths as instructed, and then oxygen and 4%
sevoflurane were inhaled at a rate of 6 l/min. Anesthesia in group
C was maintained with propofol given by intravenous infusion at a
rate of 2.5 mg/kg/h in combination with sevoflurane given by mask
inhalation at a concentration of 1 MAC. The depth of anesthesia
ranged from 40 to 60 in Narcotrend values for all the three groups.
Patients were observed for eyelash movement and spontaneous
breathing. If necessary, 4.5 µg/kg of fentanyl were given
additionally, and anesthesia was maintained by intermittent
intravenous infusion of atracurium at a rate of 5 µg/kg/min.
Flow cytometric measurement of
T/B-cell subsets
Peripheral venous blood samples (4 ml, heparinized)
were drawn, respectively, at 30 min before anesthesia (T1) and 24 h
after anesthesia (T2). Mononuclear cells (MNC) were separated from
the blood samples using density gradient centrifugation at 3,000 ×
g for 15 min at 4°C. MNC were suspended in RPMI-1640 medium
supplemented with 10% fetal bovine serum
(1×106/cells/ml), and then glutamine was added to a
final concentration of 2 mmol/l. FITC-labeled mouse anti-human CD3,
CD4, CD8, and CD19 monoclonal antibodies (1:100; cat. nos. ab34275,
ab59474, ab28010, and ab24936; Abcam, Cambridge, MA, USA) and
PE-labeled mouse anti-human IFNγ and IL-4 monoclonal antibodies
(1:300; cat. nos. 12-7319-42 and 12-7049-42; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) were added to 100 µl of MNC
suspension (one kind of antibody/100 µl of MNC suspension),
respectively, then incubated for 30 min at room temperature in the
dark, followed by 2 washes with PBS. The cells were fixed by
suspension in 2% paraformaldehyde (0.5 ml) for 30 min. The fixed
cells were washed with PBS and re-suspended into 1 ml assay buffer
waiting for analysis. The percentages of CD4+ and
CD8+ T cells were measured by flow cytometry (Cell
Signaling Technology, Inc., Danvers, MA, USA), and the
CD4+/CD8+ ratio was calculated. The
percentages of Th1 (CD4+/IFNγ+) cells and Th2
(CD4+/IL-4+) cells in lymphocytes were
obtained and the Th1/Th2 ratio was calculated.
ELISA measurement of serum levels of
TGF-β, IFNγ and IL-4
The levels of TGF-β, IFNγ and IL-4 (cat. nos.
KE00002, KE00063, and KE00016; ProteinTech Group, Inc., Wuhan,
China) in peripheral blood serum were measured using ELISA kits in
strict accordance with the manufacturer's instructions. Serum was
diluted in a ratio of 1:1 with assay buffer and added to the rabbit
anti-human monoclonal antibodies (1:300, cat. nos. KE00002,
KE00063, and KE00016) pre-coated plate (100 µl/well). The plate was
incubated at room temperature for 120 min, followed by 5 washes.
After 5 washes, horseradish peroxidase-labeled goat anti-rabbit
secondary polyclonal antibody (100 µl/well; cat. nos. SA00001-2;
ProteinTech Group, Inc.) was added, and the plate was incubated at
room temperature in the dark for 20 min. After adding TMB
chromogenic substrate (100 µl/well; Beyotime Institute of
Biotechnology), the plate was covered and incubated at room
temperature for 20 min. Finally, the reaction was stopped by adding
a stop solution (50 µl/well), and after mixing, the A450 nm value
was measured using a microplate reader (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) equipped with a 450-nm filter.
Data processing
Statistical analysis was performed using SPSS 19.0
statistical software (IBM Corp., Armonk, NY, USA). Measurement data
were expressed as mean ± standard deviation (mean ± SD).
Significant differences among three groups were tested by one-way
analysis of variance. In case of a significant difference, the
LSD-t-test was used to compare significant differences between two
groups. Repeated measures analysis of variance was used for
comparison at different time points. χ2 test was used
for the analysis of enumeration data. P<0.05 was considered to
indicate a statistically significant difference.
Results
General clinical data of patients in
the three groups
As shown in Table I,
there were no significant differences in age, sex, body weight,
BMI, percentage of lymphoblasts and prolymphocytes, white blood
cells, platelets, and immunophenotype among the three groups
(P>0.05).
 | Table I.General clinical data of patients in
the three groups [n (%), mean ± SD]. |
Table I.
General clinical data of patients in
the three groups [n (%), mean ± SD].
| Variable | Group A (n=50) | Group B (n=50) | Group C (n=50) | F/χ2 | P-value |
|---|
| Age (years) | 7.43±3.76 | 7.14±3.19 | 7.51±3.55 | 0.15 | 0.86 |
| Sex |
|
Male | 23 (46) | 25 (50) | 26 (52) | 0.37 | 0.83 |
|
Female | 27 (54) | 25 (50) | 24 (48) |
|
|
| Body weight
(kg) | 25.12±4.73 | 24.82±3.91 | 25.43±4.11 | 0.26 | 0.77 |
| BMI
(kg/cm2) | 22.86±3.82 | 23.81±4.12 | 23.87±4.33 | 0.95 | 0.39 |
| Lymphoblasts and
prolymphocytes (%) | 55.78±11.76 | 58.24±12.22 | 56.91±10.93 | 0.56 | 0.57 |
| White blood cells
(109/liter) | 64.32±11.43 | 59.82±9.78 | 62.86±10.98 | 2.28 | 0.11 |
| Platelets
(109/liter) | 54.09±16.22 | 57.82±15.97 | 53.87±14.93 | 1.00 | 0.37 |
|
Immunophenotype |
| B
subtype | 31 (62) | 28 (56) | 20 (40) | 0.39 | 0.82 |
| T
subtype | 19 (38) | 22 (44) | 30 (60) |
|
|
Detection of T-cell subsets in
peripheral blood samples
At T1, there were no significant differences in the
percentages of CD3+, CD4+, and
CD8+, or in CD4+/CD8+ ratio among
the three groups (P>0.05). At T2, the percentages of
CD3+ and CD4+ and the
CD4+/CD8+ ratios in all groups were
significantly increased compared with T1 (P<0.05), whereas the
percentages of CD8+ cells were significantly decreased
(P<0.05). At T2, the percentage of CD3+ cells in
group B was significantly higher than that in group A, and the
percentage of CD3+ cells in group C was significantly
higher than those in groups A and B (P<0.05). At T2, there was
no significant difference in the percentage of CD4+
cells between group B and A (P>0.05), and the percentage of
CD4+ cells in group C was significantly higher than
those in groups A and B (P<0.05). At T2, there were no
significant differences in the percentage of CD8+ cells
between groups A and B or between groups B and C (P>0.05), but
the percentage of CD8+ cells in group C was
significantly higher than that in group A (P<0.05). At T2, there
was no significant difference in CD4+/CD8+
ratio between groups B and C (P>0.05), whereas the
CD4+/CD8+ ratios in groups B and C were
significantly higher than that in group A (P<0.05). The detailed
results are shown in Table II.
 | Table II.Detection of T-cell subsets
(CD3+, CD4+ and CD8+) in
peripheral blood (mean ± SD). |
Table II.
Detection of T-cell subsets
(CD3+, CD4+ and CD8+) in
peripheral blood (mean ± SD).
| T-cell subset | Group A | Group B | Group C | F | P-value |
|---|
| CD3+
(%) |
| T1 | 42.98±5.82 | 43.82±6.13 | 45.31±7.33 |
1.67 | 0.19 |
| T2 | 56.25±5.91 |
58.83±6.11a |
64.32±6.32a,b | 22.71 | <0.001 |
| t | 11.31 | 12.26 | 13.89 |
|
|
|
P-value |
<0.001 |
<0.001 |
<0.001 |
|
|
| CD4+
(%) |
| T1 | 24.33±4.82 | 25.13±5.12 | 23.92±3.19 |
0.95 | 0.39 |
| T2 | 33.77±4.08 | 35.41±5.11 |
39.27±3.91a,b | 20.60 | <0.001 |
| t | 10.57 | 10.05 | 21.51 |
|
|
|
P-value |
<0.001 |
<0.001 |
<0.001 |
|
|
| CD8+
(%) |
| T1 | 33.78±6.78 | 35.86±5.92 | 34.11±6.33 |
1.55 | 0.22 |
| T2 | 24.44±5.22 | 26.13±4.91 |
28.11±5.39a |
6.30 | <0.001 |
| t |
7.72 |
8.95 |
5.10 |
|
|
|
P-value |
<0.001 |
<0.001 |
<0.001 |
|
|
|
CD4+/CD8+ |
| T1 |
0.72±0.16 |
0.75±0.31 |
0.69±0.11 |
1.09 | 0.37 |
| T2 |
1.17±0.21 |
1.38±0.33a |
1.43±0.28a | 12.34 | <0.001 |
| t | 12.05 |
9.84 | 17.39 |
|
|
|
P-value |
<0.001 |
<0.001 |
<0.001 |
|
|
Detection of B-cell subsets in
peripheral blood samples
As shown in Table
III, at T1, there was no significant difference in the
percentage of CD19+ cells among the three groups
(P>0.05). At T2, the percentage of CD19+ cells
significantly increased in all three groups compared with T1
(P<0.05). At T2, there was no statistical difference in the
percentage of CD19+ cells between groups A and B
(P>0.05), whereas the percentage of CD19+ cells in
group C was significantly higher than those in groups A and B
(P<0.05).
 | Table III.Detection of B-cell subset
(CD19+) in peripheral blood samples (mean ± SD). |
Table III.
Detection of B-cell subset
(CD19+) in peripheral blood samples (mean ± SD).
| CD19+
(%) | Group A | Group B | Group C | F | P-value |
|---|
| T1 |
9.11±3.21 | 10.12±4.11 | 9.67±3.98 |
0.89 | 0.41 |
| T2 | 13.86±4.31 | 14.21±3.21 |
18.43±3.87a,b | 22.13 | <0.001 |
| t | 6.25 | 5.55 | 11.16 |
|
|
| P-value | <0.001 | <0.001 |
<0.001 |
|
|
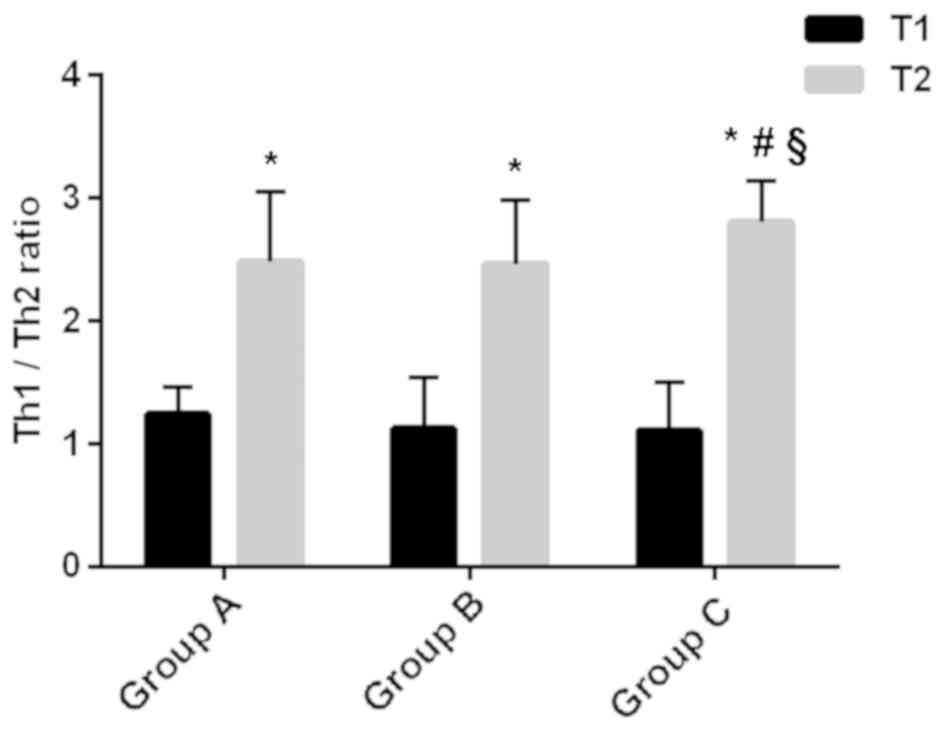
Th1/Th2 ratio in Th cells
The percentages of Th1 and Th2 cells, as well as the
ratio of Th1/Th2, in each group are shown in Table IV and Fig. 1. At T1, there were no significant
differences in the percentages of Th1 and Th2 cells, or in the
Th1/Th2 ratio among the three groups (P>0.05). At T2, the
percentages of Th1 and Th2 cells and the Th1/Th2 ratios were
significantly higher in all three groups compared with T1
(P<0.05). At T2, the percentage of Th1 cells in group B was
higher than that in group A, and the percentages of Th1 and Th2
cells in group C were higher than those in groups A and B
(P<0.05). There was no significant difference in the percentage
of Th2 cells between groups A and B (P>0.05). The Th1/Th2 ratio
in group C was higher than those in groups A and B (P<0.05),
whereas there was no significant difference between groups A and B
(P>0.05).
 | Table IV.Th1/Th2 ratio in Th cells (mean ±
SD). |
Table IV.
Th1/Th2 ratio in Th cells (mean ±
SD).
| Th cell | Group A | Group B | Group C | F | P-value |
|---|
| Th1 (%) |
| T1 | 32.98±5.82 | 33.82±6.13 | 35.31±7.33 |
1.67 | 0.19 |
| T2 | 56.25±5.91 |
58.83±6.11a |
64.32±6.32a,b | 22.71 | <0.001 |
| t | 11.31 | 12.26 | 13.89 |
|
|
|
P-value |
<0.001 |
<0.001 |
<0.001 |
|
|
| Th2 (%) |
| T1 | 24.33±4.82 | 25.13±5.12 | 23.92±3.19 |
0.95 | 0.39 |
| T2 | 33.77±4.08 | 35.41±5.11 |
39.27±3.91a,b | 20.60 | <0.001 |
| t | 10.57 | 10.05 | 21.51 |
|
|
|
P-value |
<0.001 |
<0.001 |
<0.001 |
|
|
| Th1/Th2 |
| T1 |
1.24±0.22 |
1.13±0.41 |
1.11±0.39 |
1.99 | 0.14 |
| T2 |
2.48±0.58 |
2.46±0.52 |
2.81±0.33a,b |
8.09 | <0.001 |
| t | 14.13 | 14.20 | 23.53 |
|
|
|
P-value |
<0.001 |
<0.001 |
<0.001 |
|
|
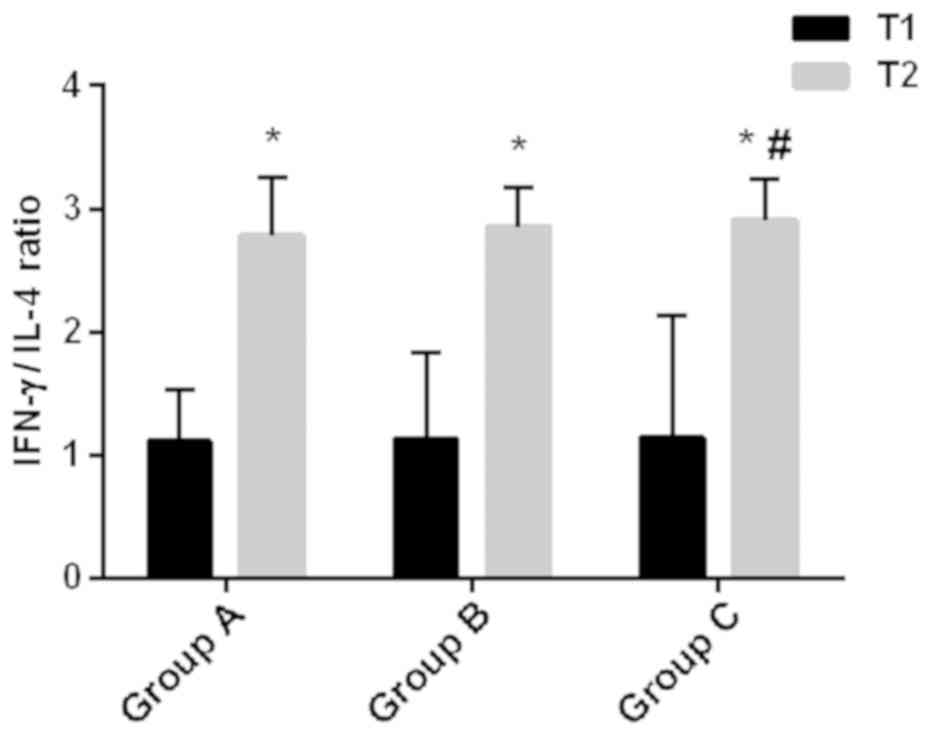
Serum levels of TGF-β, IFNγ and IL-4,
and IFNγ/IL-4 ratio
The serum levels of TGF-β, IFNγ and IL-4, as well as
the IFNγ/IL-4 ratio in each group are shown in Table V and Fig.
2. At T1, there were no significant differences in the serum
levels of TGF-β, IFNγ and IL-4 and in IFNγ/IL-4 ratio among the
three groups (P>0.05). At T2, the serum levels of IFNγ and IL-4,
and the IFNγ/IL-4 ratios significantly increased, compared with T1,
whereas the level of TGF-β significantly decreased (P<0.05) in
all the groups. At T2, the TGF-β level in group C was significantly
higher than that in group A (P<0.05), whereas there was no
significant difference in TGF-β level between groups A and B, or
between group B and C (P>0.05). The IFNγ level in group B was
higher than that in group A, and in group C was higher than those
in groups A and B (P<0.05). At T2, there was no significant
difference in IL-4 level between groups A and B, whereas the IL-4
level in group C was higher than those in groups A and B
(P<0.05). At T2, there was no significant difference in
IFNγ/IL-4 ratio between groups A and B, as well as between groups B
and C (P>0.05), whereas the IFNγ/IL-4 ratio in group C was
higher than that in group A (P<0.05).
 | Table V.Serum levels of TGF-β, IFNγ and IL-4
and IFNγ/IL-4 ratio (mean ± SD). |
Table V.
Serum levels of TGF-β, IFNγ and IL-4
and IFNγ/IL-4 ratio (mean ± SD).
| Variable | Group A | Group B | Group C | F | P-value |
|---|
| TGF-β (ng/ml) |
| T1 |
2.34±0.51 |
2.21±0.36 |
2.36±0.58 |
1.37 | 0.26 |
| T2 |
1.76±0.34 |
1.62±0.42 |
1.49±0.44a |
5.63 | <0.001 |
| t |
6.69 |
7.54 |
8.45 |
|
|
|
P-value |
<0.001 |
<0.001 |
<0.001 |
|
|
| IFNγ (ng/ml) |
| T1 | 130.33±6.82 | 131.13±5.12 | 128.92±4.19 |
2.08 | 0.12 |
| T2 | 151.44±4.08 |
146.41±5.11a |
178.92±6.91a,b | 20.60 | <0.001 |
| t | 18.78 | 14.94 | 43.75 |
|
|
|
P-value |
<0.001 |
<0.001 |
<0.001 |
|
|
| IL-4 (ng/ml) |
| T1 |
34.92±6.78 |
35.43±6.11 |
34.96±5.78 |
0.10 | 0.90 |
| T2 |
44.44±5.31 |
46.11±4.781 |
46.11±5.22a,b | 12.21 | <0.001 |
| t |
5.35 |
6.09 | 10.12 |
|
|
|
P-value |
<0.001 |
<0.001 |
<0.001 |
|
|
| IFNγ/IL-4 |
| T1 |
1.11±0.42 |
1.13±0.71 |
1.15±0.99 |
3.29 | 0.04 |
| T2 |
2.78±0.48 |
2.86±0.32 |
2.91±0.33a |
1.91 | 0.15 |
| t |
3.66 |
2.45 |
9.08 |
|
|
|
P-value |
<0.001 |
0.01 |
<0.001 |
|
|
Discussion
ALL is a malignant clonal disease of lymphoid
progenitor cells characterized by the presence of a large number of
prolymphocytes that interfere with the production of new red blood
cells, white blood cells and platelets (16). At present, anesthesia or sedation is
required in the diagnosis and treatment of ALL (17). For pediatric and adolescent patients,
analgesia and sedation can prevent emotional distress caused by a
painful operation, thus improving operation quality (18). The outcome of anesthesia and
sedation, including the effect on immune function of pediatric
patients with ALL, is closely associated with the prognosis
(19). Mozhaev et al
(19) have reported that combined
epidural-general anesthesia can effectively reduce the effect of
T-cell immune function on postoperative cognitive function in
patients with esophageal cancer. In this study, the effect of
combined propofol-sevoflurane anesthesia on the immune function of
pediatric patients with ALL was explored, aiming at improving the
efficacy and safety of anesthesia, as well as the prognosis and the
survival rate of these patients.
Group A received propofol anesthesia, group B
received sevoflurane anesthesia, and group C received combined
propofol-sevoflurane anesthesia. There were no significant
differences in the general clinical data, such as, age, sex, body
weight, BMI, percentage of lymphoblasts and prolymphocytes, white
blood cells, platelets, and immunophenotype among the three groups.
The percentages of lymphoblasts and prolymphocytes were >30% in
all groups, meeting the diagnostic criteria of ALL. CD3+
is a common surface marker of T cells, CD4+ is a surface
marker of Th cells, and CD8+ is a surface marker of
cytotoxic T lymphocytes (CTL). A high ratio of
CD4+/CD8+ represents relatively strong
autoimmune function (20).
CD19+ is expressed on the surface of B lymphocytes
(21). By measurement of T-cell
subsets in peripheral blood, it was found that there were no
significant differences in the percentages of CD3+,
CD4+, CD8+, and CD19+ cells and in
CD4+/CD8+ ratio among the three groups before
anesthesia. After anesthesia and chemotherapy, the percentages of
CD3+, CD4+ and CD19+ cells and the
ratios of CD4+/CD8+ in all three groups
became significantly higher than those before anesthesia, whereas
the percentages of CD8+ cells became significantly
lower. These findings suggest that the activity of T/B-cell subsets
in each group is improved after chemotherapy. The results from
between-group comparisons further indicate that combined
propofol-sevoflurane anesthesia is more beneficial to the recovery
of immune function, by restoring the activity of T/B cell subsets,
compared with propofol only or sevoflurane only. In a study on the
effects of combined sevoflurane-fentanyl or propofol-fentanyl on
the immune function in patients with colorectal cancer, Viallard
et al (22) reported that the
percentages of CD3+ cells in both groups decreased from
2 h after operation, compared with those before anesthesia. The
percentage of CD3+ cells in combined propofol-fentanyl
group increased 24 h after operation, which was obviously higher
than that in the combined sevoflurane-fentanyl group. Compared with
those before anesthesia, the postoperative percentages of
CD4+ cells increased significantly in both groups. The
ratio of CD4+/CD8+ decreased from 2 to 24 h
after operation, and at each time-point the ratio of
CD4+/CD8+ in the combined propofol-fentanyl
group was significantly higher than that in the combined
sevoflurane-fentanyl group (P<0.05). The percentage of
CD4+ cells in the combined propofol-fentanyl group was
significantly higher at 24 h after operation than that at the end
of operation. Apparently, there is a discrepancy between the
findings of Viallard et al (22) and the results of the present study.
This might be due to the different treatment options followed in
the two studies, and also because combined propofol-sevoflurane
anesthesia was used in the present study.
At T1, there were no significant differences in the
percentages of Th1 and Th2 cells, and in Th1/Th2 ratio among the
three groups. At T2, compared with T1, the percentages of Th1 and
Th2 cells and the Th1/Th2 ratios were significantly higher in all
three groups. CD4+ cells are mainly Th cells, which can
differentiate into Th1 and Th2 cells. CD8+ cells are
mainly cytotoxic T cells and suppressor T cells (23). Therefore, the percentages of Th1 and
Th2 cells increase along with the increase of the percentage of
CD4+ T cells. This is consistent with the results of
T/B-cell subsets measurements in this study. Th1/Th2 ratio
increased significantly, indicating that the Th1/Th2 balance
drifted to Th1 after anesthesia and chemotherapy. Chemotherapy
relieved the body's state of immunosuppression. Studies have shown
that in tumor patients, tumor cells can escape from the body's
immune response. Th2 cells secrete more cytokines, such as IL-4 and
IL-10, causing an imbalance in Th1/Th2 ratio (24,25). At
T2, the Th1/Th2 ratio in group C was higher than those in groups A
and B. This finding suggests that combined propofol-sevoflurane
anesthesia is more beneficial in alleviating immunosuppression,
compared with propofol only or sevoflurane only.
In comparison of serum levels of TGF-β, IFNγ and
IL-4 and the ratio of IFNγ/IL-4, it was found that at T2 the serum
levels of IFNγ and IL-4 and the ratios of IFNγ/IL-4 were all
higher, while the levels of TGF-β were lower than those at T1 in
all the three groups. TGF-β is a tumor immunosuppressive factor
that promotes the growth of tumor cells, and a decrease in TGF-β
expression indicates an enhanced suppression of tumor cell growth
(26). The ratio of IFNγ/IL-4 is an
index of Th1/Th2 cell balance, because Th1 cells mainly secrete
pro-inflammatory factors, such as IFNγ, and Th2 cells mainly
secrete anti-inflammatory factors, such as IL-4 (27). Therefore, the above-mentioned
findings suggest that tumor growth is suppressed after anesthesia
and chemotherapy. The serum level of TGF-β and the ratio of
IFNγ/IL-4 were higher in group C than those in group A, suggesting
that combined propofol-sevoflurane anesthesia is more beneficial to
the suppression of tumor progression, compared with propofol only.
However, there are certain limitations in the present
investigation. The specific mechanism of propofol combined with
sevoflurane anesthesia was not investigated. Also, the sample size
should be increased to further explore the role of propofol
combined with sevoflurane anesthesia.
In summary, combined propofol-sevoflurane anesthesia
is more conducive to the recovery of T/B-cell subsets activity, the
alleviation of immunosuppression, and the suppression of ALL
progression, compared with propofol only or sevoflurane only.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
NiD and YG assisted with flow cytometry. NaD
performed ELISA. NiD drafted the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Xiangyang No. 1 People's Hospital Affiliated to Hubei University of
Medicine (Xiangyang, China). Patients who participated in this
research had complete clinical data. The parents of the child
patients signed an informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Altenburg JD, Harvey KA, McCray S, Xu Z
and Siddiqui RA: A novel 2,6-diisopropylphenyl-docosahexaenoamide
conjugate induces apoptosis in T cell acute lymphoblastic leukemia
cell lines. Biochem Biophys Res Commun. 411:427–432. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bertolizio G, Stucchi R, Sahillioglu E,
Somaini M, Dander E, Biondi A, Jankovic M, D'Amico G and Ingelmo
PM: The effects of propofol and ketamine on the cytokine levels of
children with acute lymphoblastic leukemia. J Pediatr Hematol
Oncol. 35:e296–e300. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Luczyński W, Stasiak-Barmuta A,
Krawczuk-Rybak M, Malinowska I, Matysiak M, Mitura-Lesiuk M,
Kowalczyk J and Jeromin A: Th1/Th2 balance in acute lymphoblastic
leukemia in children. Przegl Lek. 61:919–923. 2004.(In Polish).
PubMed/NCBI
|
|
4
|
Moorman AV, Harrison CJ, Buck GA, Richards
SM, Secker-Walker LM, Martineau M, Vance GH, Cherry AM, Higgins RR,
Fielding AK, et al Adult Leukaemia Working Party, Medical Research
Council/National Cancer Research Institute, : Karyotype is an
independent prognostic factor in adult acute lymphoblastic leukemia
(ALL): Analysis of cytogenetic data from patients treated on the
Medical Research Council (MRC) UKALLXII/Eastern Cooperative
Oncology group (ECOG) 2993 trial. Blood. 109:3189–3197. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nicolini FE, Mauro MJ, Martinelli G, Kim
DW, Soverini S, Müller MC, Hochhaus A, Cortes J, Chuah C, Dufva IH,
et al: Epidemiologic study on survival of chronic myeloid leukemia
and Ph(+) acute lymphoblastic leukemia patients with
BCR-ABL T315I mutation. Blood. 114:5271–5278. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Glaisyer HR and Sury MR: Recovery after
anesthesia for short pediatric oncology procedures: Propofol and
remifentanil compared with propofol, nitrous oxide, and
sevoflurane. Anesth Analg. 100:959–963. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hajdenberg J, Grote T, Yee L,
Arevalo-Araujo R and Latimer LA: Infusion of palonosetron plus
dexamethasone for the prevention of chemotherapy-induced nausea and
vomiting. J Support Oncol. 4:467–471. 2006.PubMed/NCBI
|
|
8
|
Maurizi P, Russo I, Rizzo D, Chiaretti A,
Coccia P, Attinà G, Ruggiero A and Riccardi R: Safe lumbar puncture
under analgo-sedation in children with acute lymphoblastic
leukemia. Int J Clin Oncol. 19:173–177. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Godambe SA, Elliot V, Matheny D and
Pershad J: Comparison of propofol/fentanyl versus
ketamine/midazolam for brief orthopedic procedural sedation in a
Pediatric Emergency Department. Pediatrics. 112:116–123. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chiaretti A, Ruggiero A, Barone G,
Antonelli A, Lazzareschi I, Genovese O, Paiano S, Sammartino M,
Maurizi P and Riccardi R: Propofol/alfentanil and propofol/ketamine
procedural sedation in children with acute lymphoblastic leukaemia:
Safety, efficacy and their correlation with pain neuromediator
expression. Eur J Cancer Care (Engl). 19:212–220. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lim JA, Oh CS, Yoon TG, Lee JY, Lee SH,
Yoo YB, Yang JH and Kim SH: The effect of propofol and sevoflurane
on cancer cell, natural killer cell, and cytotoxic T lymphocyte
function in patients undergoing breast cancer surgery: An in vitro
analysis. BMC Cancer. 18:1592018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen Y, Liang M, Zhu Y and Zhou D: The
effect of propofol and sevoflurane on the perioperative immunity in
patients under laparoscopic radical resection of colorectal cancer.
Zhonghua Yi Xue Za Zhi. 95:3440–3444. 2015.(In Chinese). PubMed/NCBI
|
|
13
|
Whitlow PG, Saboda K, Roe DJ, Bazzell S
and Wilson C: Topical analgesia treats pain and decreases propofol
use during lumbar punctures in a randomized pediatric leukemia
trial. Pediatr Blood Cancer. 62:85–90. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tan R: Effect of propofol and isoflurane
on surgical stress response and postoperative cognitive function in
elderly patients. Nan Fang Yi Ke Da Xue Xue Bao. 29:1247–1248.
2009.(In Chinese). PubMed/NCBI
|
|
15
|
Flouda L, Pandazi A, Papageorgiou C,
Perrea D, Krepi E and Kostopanagiotou G: Comparative effects of
sevoflurane and propofol based general anaesthesia for elective
surgery on memory. Arch Med Sci. 9:105–111. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Piotrowski AJ and Fendler WM: Hyperkalemia
and cardiac arrest following succinylcholine administration in a
16-year-old boy with acute nonlymphoblastic leukemia and sepsis.
Pediatr Crit Care Med. 8:183–185. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Panwar P, Singh S, Kumar N, Rawat H and
Mishra AK: Synthesis, characterization, and in vivo skeletal
localization of a new (99m)Tc-based multidentate phosphonate
chelate: 5-Amino-1,3-bis(ethylamine-(N,N dimethyl diphosphonic
acid) acetamido) benzene. Bioorg Med Chem. 15:1138–1145. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Neuhäuser C, Wagner B, Heckmann M, Weigand
MA and Zimmer KP: Analgesia and sedation for painful interventions
in children and adolescents. Dtsch Arztebl Int. 107:241–247, I–II,
I. 2010.PubMed/NCBI
|
|
19
|
Mozhaev GA and Kraevaia SB: Non-specific
cellular immunity in tonsillectomies in children under general
anesthesia. Anesteziol Reanimatol. 4:36–39. 1981.(In Russian).
|
|
20
|
Kwak Y, Koh J, Kim DW, Kang SB, Kim WH and
Lee HS: Immunoscore encompassing CD3+ and
CD8+ T cell densities in distant metastasis is a robust
prognostic marker for advanced colorectal cancer. Oncotarget.
7:81778–81790. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang Y, Zhang X, Xia Y, Jia X, Li H,
Zhang Y, Shao Z, Xin N, Guo M, Chen J, et al: CD19+
Tim-1+ B cells are decreased and negatively correlated
with disease severity in Myasthenia Gravis patients. Immunol Res.
64:1216–1224. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Viallard JF, Pellegrin JL, Ranchin V,
Schaeverbeke T, Dehais J, Longy-Boursier M, Ragnaud JM, Leng B and
Moreau JF: Th1 (IL-2, interferon-gamma (IFN-gamma)) and Th2 (IL-10,
IL-4) cytokine production by peripheral blood mononuclear cells
(PBMC) from patients with systemic lupus erythematosus (SLE). Clin
Exp Immunol. 115:189–195. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ziegler A, Heidenreich R, Braumüller H,
Wolburg H, Weidemann S, Mocikat R and Röcken M: EpCAM, a human
tumor-associated antigen promotes Th2 development and tumor immune
evasion. Blood. 113:3494–3502. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xia N, Zhou S, Liang Y, Xiao C, Shen H,
Pan H, Deng H, Wang N and Li QQ: CD4+ T cells and the
Th1/Th2 imbalance are implicated in the pathogenesis of Graves'
ophthalmopathy. Int J Mol Med. 17:911–916. 2006.PubMed/NCBI
|
|
25
|
Gore AJ, Deitz SL, Palam LR, Craven KE and
Korc M: Pancreatic cancer-associated retinoblastoma 1 dysfunction
enables TGF-β to promote proliferation. J Clin Invest. 124:338–352.
2014. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hattori K, Nishikawa M, Watcharanurak K,
Ikoma A, Kabashima K, Toyota H, Takahashi Y, Takahashi R, Watanabe
Y and Takakura Y: Sustained exogenous expression of therapeutic
levels of IFN-gamma ameliorates atopic dermatitis in NC/Nga mice
via Th1 polarization. J Immunol. 184:2729–2735. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li AL, Ma DX and Meng XC: Effect of
lactobacilli on Th1/Th2 cells balance in primary lymphocytes. Xi
Bao Yu Fen Zi Mian Yi Xue Za Zhi. 27389–391. (394)2011.(In
Chinese). PubMed/NCBI
|