Introduction
Gastric cancer (GC) is the third major contributor
of cancer mortality and the fifth most frequently diagnosed cancer
in the world (1). GC led to
>700,000 mortalities and resulted in 950,000 new cases of GC
diagnosed in 2012 (2,3). The majority of patients with GC are
diagnosed at a later stage and the five-year survival of GC ranges
from 13.1 to 43.8% (4,5). The late detection of the tumor
negatively affects the survival rate (6). Therefore, a deeper understanding of the
mechanisms underlying the pathogenesis and progression of GC is
required.
The SRY-box (SOX) family consists of a DNA binding
domain which includes a conserved high mobility group (HMG)
(7,8). SOX9, a transcription factor in the
HMG-box class, affects the survival, proliferation and
differentiation of cells, such as hepatocellular carcinoma and stem
cells (9,10). SOX9 has been revealed to have
oncogenic properties and its expression was increased in several
tumors, including prostatic carcinoma (11), ovarian cancer (12), breast carcinoma (13) and lung carcinoma (14). However, the functions of SOX9 in GC
remain unclear.
Epithelial-mesenchymal transition (EMT) provides
epithelial cells with the plasticity required to generate a
mesenchymal phenotype (15). EMT is
involved in multiple physiological and pathological syndromes and
processes, including embryo development, fibrosis and cancer
progression (16,17). During EMT, morphological
transformations are caused by E-cadherin reduction, and increasing
of vimentin and N-cadherin expression levels (18,19).
Previous studies have investigated the association between SOX9 and
EMT in GC (20,21).
The Hippo signaling pathway serves a central role in
regulating cell proliferation, cell fate and tissue size (22,23). The
pathway has emerged as a tumor suppressive pathway that acts to
control the transcriptional activity of two proteins, YAP and WW
domain containing transcription regulator 1, also referred to as
TAZ (24). YAP and TAZ activity is
fundamental for normal organ growth and tissue regeneration;
however, it is also involved in cancer pathogenicity (25,26). The
YAP/TAZ signaling pathway promotes cancer stem cell
characteristics, tumor initiation, progression and metastasis
(27,28). In the YAP/TAZ signaling pathway,
mammalian sterile 20-like kinase 1/2 kinases phosphorylate and
activate a second set of kinases, large tumor suppressor kinase 1/2
(LATS1/2). LATS1/2 subsequently phosphorylate two transcriptional
coactivators, TAZ and YAP, leading to their sequestration in the
cytoplasm, degradation and functional inhibition. The Hippo
signaling pathway effectors, TAZ and YAP, are oncogenes that are
commonly dysregulated in cancer (29). Previous studies demonstrated that
YAP/TAZ are abnormally overexpressed in tumors, promote
tumorigenesis, and are considered as carcinogenic genes in numerous
types of solid cancer (30,31).
In the present study, the roles of SOX9 in the EMT
of GC cells were examined to elucidate the underlying mechanisms.
The results obtained suggested that SOX9 promoted GC cell invasion,
migration and proliferation. In addition, SOX9 may promote the EMT
via the Hippo-YAP signaling pathway in GC cells.
Materials and methods
Cell culture
The GC cell lines including BGC823, HGC27, MKN45 and
MGC803 (Type Culture Collection of the Chinese Academy of Sciences)
were maintained in Dulbecco's Modified Eagle's medium (GE
Healthcare Life Sciences, Logan, UT, USA) supplemented with 10%
fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) in a 5% CO2 humidified incubator at
37°C. Cell line characterization was performed by short tandem
repeat analysis using GeneMapper® software (version 4.0;
http://www.atcc.org/) and consolidated using the
American Type Culture Collection, German Collection of
Microorganisms and Cell Cultures, Japanese Collection of Research
Bioresources Cell Bank and Rikagaku Kenkyusho (Institute of
Physical and Chemical Research, Japan) databases (https://www.atcc.org/en/Products/Cells_and_Microorganisms/Cell_Lines.aspx;
https://www.dsmz.de/; http://cellbank.nibiohn.go.jp/english/). The cell
lines were tested for the presence of Mycoplasma using PCR
(32).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted using an RNAiso Plus kit
(Takara Bio Inc., Otsu, Japan) according to the manufacturer's
protocol. RevertAid™ First Strand cDNA Synthesis kit (Thermo Fisher
Scientific, Inc.) was used for reverse transcription, according to
the manufacturer's protocols. qPCR was subsequently performed in
triplicate using the SYBR® Green PCR master mix (Bio-Rad
Laboratories, Inc.) and a CFX-96 Sequence Detection system (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). The following primer pairs
were used: Human SOX9 forward, 5′-GTACCCGCACTTGCACAAC-3′ and
reverse 5′-TCGCTCTCGTTCAGAAGTCTC-3′. As an internal standard, a
fragment of human GAPDH was amplified by PCR using the following
primers: forward primer, 5′-GGTGAAGGTCGGTGTGAACG-3′, and reverse
primer, 5′-CTCGCTCCTGGAAGATGGTG-3′. The following thermocycling
conditions were used for the qPCR: Initial denaturation for 2 min
at 95°C, 35 cycles for 30 sec each at 95°C, 56°C and 72°C
respectively. SOX9 mRNA levels were quantified using the
2−ΔΔCq method (33) and
normalized to the internal reference gene GAPDH.
Plasmid construction
The SOX9 short hairpin RNA (shRNA) sequence was
obtained from Sigma-Aldrich, Merck KGaA (Darmstadt, Germany) and
was subsequently synthesized by Sangon Biotech, Co., Ltd.. The
oligo sequence of SOX9 shEGFP included: SOX9 shEGFP (F):
5′-CCGGGCAAGCTGACCCTGAAGTTCATCTCGAGATGAACTTCAGGGTCACGTTGCTTTTTG-3′,
SOX9 shEGFP (R):
5′-AATTCAAAAAGCAAGCTGACCCTGAAGTTCATCTCGAGATGAACTCAGGGTCACGTTGC-3′.
The oligo sequence of SOX9 shRNA was as follows: SOX9 shRNA
forward,
5′-GATCCATGGGAGTAAACAATAGTCTACTTCCTGTCAGATAGACTATTGTTTACTCCCATTTTTTG-3′
and SOX9 shRNA reverse,
5′-AATTCAAAAAATGGGAGTAAACAATAGTCTATCTGACAGGAAGTAGACTATTGTTTACTCCCATG-3′.
The SOX9 shRNA sequence was cloned and ligated between the
restriction enzymes of EcoRI and AgeI in plasmid
vector pLKO.1-TRC (Sigma-Aldrich; Merck KGaA).
Lentiviral production and
transfection
The packaging plasmid pPAX2 and the envelope plasmid
pMD2.G were purchased from Sigma-Aldrich, Merck KGaA.
PLKO.1-sh-SOX9 was cotransfected with psPAX2 and pMD2.G into 293T
cells (Trevigen; AmyJet Scientific Inc.) using Lipofectamine
2000® (Invitrogen; Thermo Fisher Scientific, Inc.).
Viral particles were harvested 48 h following transfection and the
viral titer was determined (TCID50 method) (34). 293T cells were infected with
1×106 recombinant lentivirus transduction units in the
presence of 8 mg/ml polybrene (Sigma-Aldrich; Merck KGaA).
Puromycin (1:10,000) was added to the cells until the cells in the
blank group (cell not infected with lentivirus) died. Cells which
survived were stably transduced cells.
Transient transfection
For SOX9 overexpression, SOX9 cDNA from
pCMV-AC-GFP-SOX9 (OriGene Technologies, Inc., Beijing, China) was
amplified by PCR and BamHI and SalI restriction sites
were introduced. SOX9 cDNA was subsequently subcloned into pLenti
CMV GFP Zeo (cat. no. 17449; Addgene Inc., Cambridge, MA, USA),
replacing GFP. The pLenti CMV GFP Zeo vector was used as a control.
For flow cytometry experiments (Aldefluor assay), a control vector
with RFP instead of GFP was used. Viral particles were produced as
described above. A total of 4×105 cells per well were
seeded into six-well plates. Following overnight incubation at 4°C,
the cell culture medium (Invitrogen; Thermo Fisher Scientific,
Inc.) was replaced by Opti-MEM (Invitrogen; Thermo Fisher
Scientific, Inc.) prior to transfection with 6 µl of Lipofectamine
2000® (Invitrogen; Thermo Fisher Scientific, Inc.) and 2
µg of the vector. Following another 48 h of incubation at 4°C, the
cells were harvested for protein expression analysis and migration,
invasion and proliferation assays.
Western blot analysis
Cell samples were lysed at 4°C using
radioimmunoprecipitation assay lysis buffer (BioVision; Thermo
Fisher Scientific, Inc.) for 10 min and subsequently centrifuged
for 15 min at 4°C and 8,100 × g Total protein was quantified using
a bicinchoninic acid and 40 µg protein/lane was separated via
SDS-PAGE on a 10% gel. The separated protein were subsequently
transferred onto a polyvinylidene fluoride membrane and blocked for
1 h at room temperature with 5% non-fat milk. The membranes were
incubated with primary antibodies against SOX9 (cat. no. 82630),
N-cadherin (cat. no. 13116), E-cadherin (cat. no. 3195), vimentin
(cat. no. 5741), snail family transcriptional repressor 1 (SNAI1;
cat. no. 3879), YAP (cat. no. 8418), p-YAP (cat. no. 13619), MOB
kinase activator 1 (MOB1; cat. no. 13730), phosphorylated (p)-MOB1
(cat. no. 8699), LATS1 (cat. no. 9153), p-LATS1 (cat. no. 9157) and
β-tubulin (cat. no. 6181) at a dilution of 1:200, overnight at 4°C
(all from Cell Signaling Technology, Inc., Danvers, MA, USA).
Following the primary incubation, membranes were incubated with the
corresponding secondary antibodies (Anti-rabbit IgG, HRP-linked
Antibody #7074, Cell Signaling Technology, Inc.) for 1 h at room
temperature. SignalFire™ ECL Reagent (cat. no, 6883; Cell Signaling
Technology, Inc.) and ImageJ bundled with 64-bit Java 1.8.0_112
software (National Institutes of Health) were used to visualize and
quantify protein expression levels.
Cell counting kit-8 (CCK-8) assay
Cell viability was measured using a CCK-8 assay
(Beyotime Institute of Biotechnology, Haimen, China). Cells were
seeded at a density of 3,000 cells/well in a 96-well plate and
incubated at 5% CO2 and 37°C. A total of 10 µl of CCK-8
regent was added to each well 1, 2, 3, 4 and 5 days after plating.
Following a 2-h incubation at 37°C, the absorbance was measured at
a wavelength of 490 nm.
Wound healing assay
Cells were grown in six-well plates to confluence in
complete cell culture medium Gibco DMEM containing 10% fetal bovine
serum (Gibco; Thermo Fisher Scientific, Inc.) at 37°C with 5%
CO2. At time 0, a wound was created across the diameter
of the well using a 10 µl pipette tip. Medium was added to wash the
cells and remove dead and floating cells. The distance between
scratch edges was recorded at 0 and 48 h. Images were captured
using an inverted microscope (magnification, ×200) equipped with a
digital camera and the ImageJ bundled with 64-bit Java 1.8.0_112
software (National Institutes of Health) was used.
Migration and invasion assays
Cell migration was measured using a transwell assay
A total of 1×105 cells were placed into the upper
compartment of a transwell insert (Corning Inc., Corning, NY, USA)
in serum free media (Gibco DMEM; Thermo Fisher Scientific, Inc.).
Medium supplemented with 10% FBS was plated in the lower chambers.
Following incubation for 24–36 h in 37°C, the migratory cells were
fixed using 4% paraformaldehyde and stained with 1% crystal violet.
Stained cells were counted in five randomly selected fields using a
light microscope (magnification, ×400). The assay was repeated
using transwell membranes precoated with Matrigel® to
assess cell invasion for 72 h at 37°C.
Statistical analysis
All data are presented as the mean ± standard
deviation from at least three independent experiments. All
statistical analyses were performed using SPSS software (version
19; IBM Corp., Armonk, NY, USA). Comparisons between groups were
analyzed using the Student's t-test (two groups) or a one-way
analysis of variance (multiple groups) using the
Student-Newman-Keuls post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
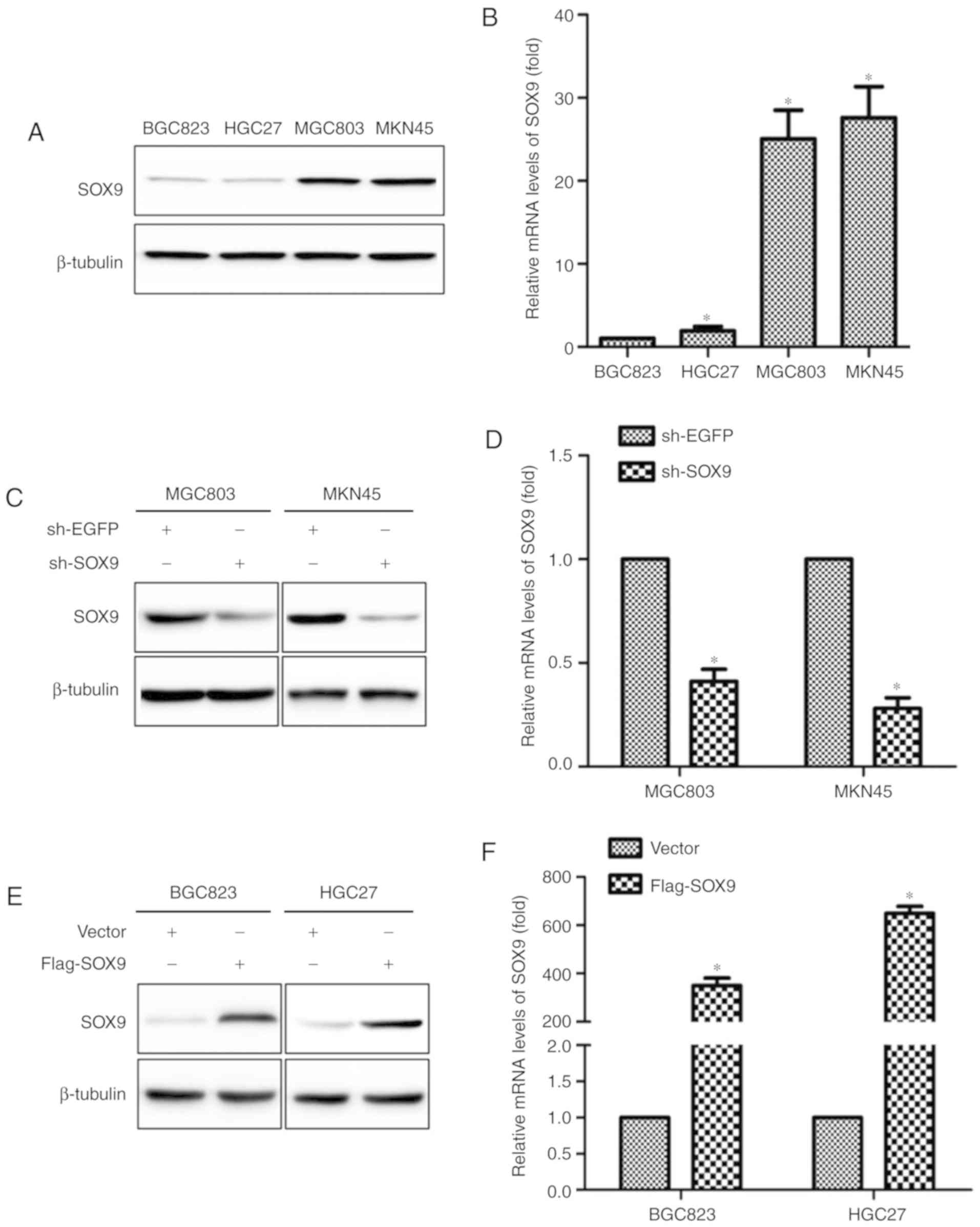
SOX9 expression level in GC cells
Expression levels of SOX9 in BGC823, HGC27, MKN45
and MGC803 cells were examined using RT-qPCR and western blot
analysis. SOX9 expression, at both mRNA and protein levels, was
higher in MGC803 and MKN45 cells than that in BGC823 and HGC27
cells (Fig. 1A and B). sh-SOX9 and
Flag-SOX9 vectors were constructed to investigate the roles of SOX9
in the development of GC. sh-EGFP and empty vector were used as
control groups. The effectiveness of sh-SOX9 vector compared with
the sh-EGFP vector was evaluated in MKN45 and MGC803 cells
(Fig. 1C and D). Following Flag-SOX9
or empty vector transfection into the BGC823 and HGC27 cell lines,
mRNA and protein expression levels of SOX9 were increased in the
Flag-SOX9 group compared with the empty vector group (Fig. 1E and F).
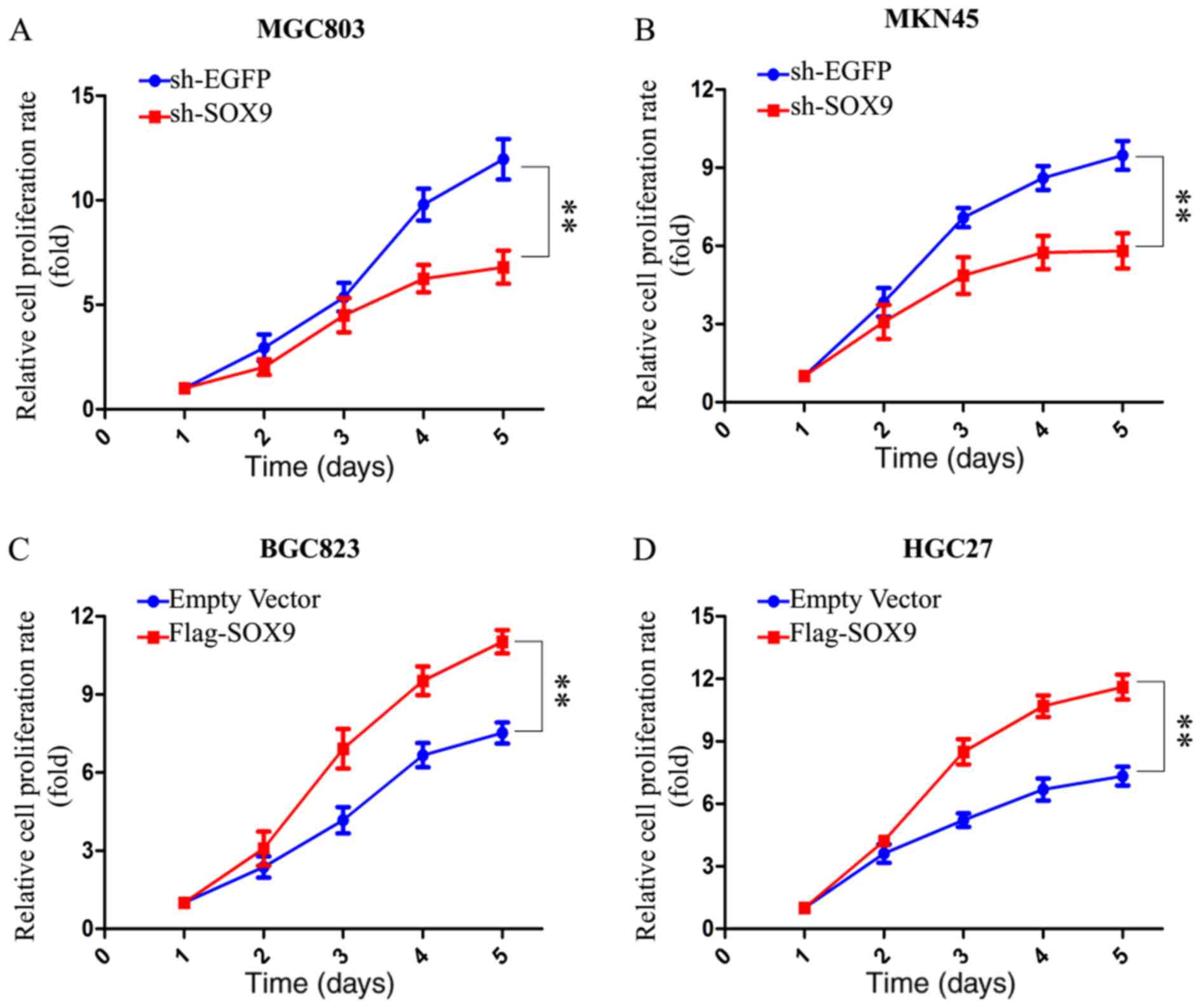
SOX9 promotes the proliferation of GC
cells
A CCK8 assay was performed to assess the effect of
SOX9 on the proliferation of GC cells. The results indicated that
the proliferation of MKN45 and MGC803 cells was suppressed
following knockdown of SOX9 (Fig. 2A and
B). The overexpression of SOX9 enhanced the proliferation of
BGC823 and HGC27 cells (Fig. 2C and
D). The results obtained suggested that SOX9 enhanced the
proliferation of GC cells.
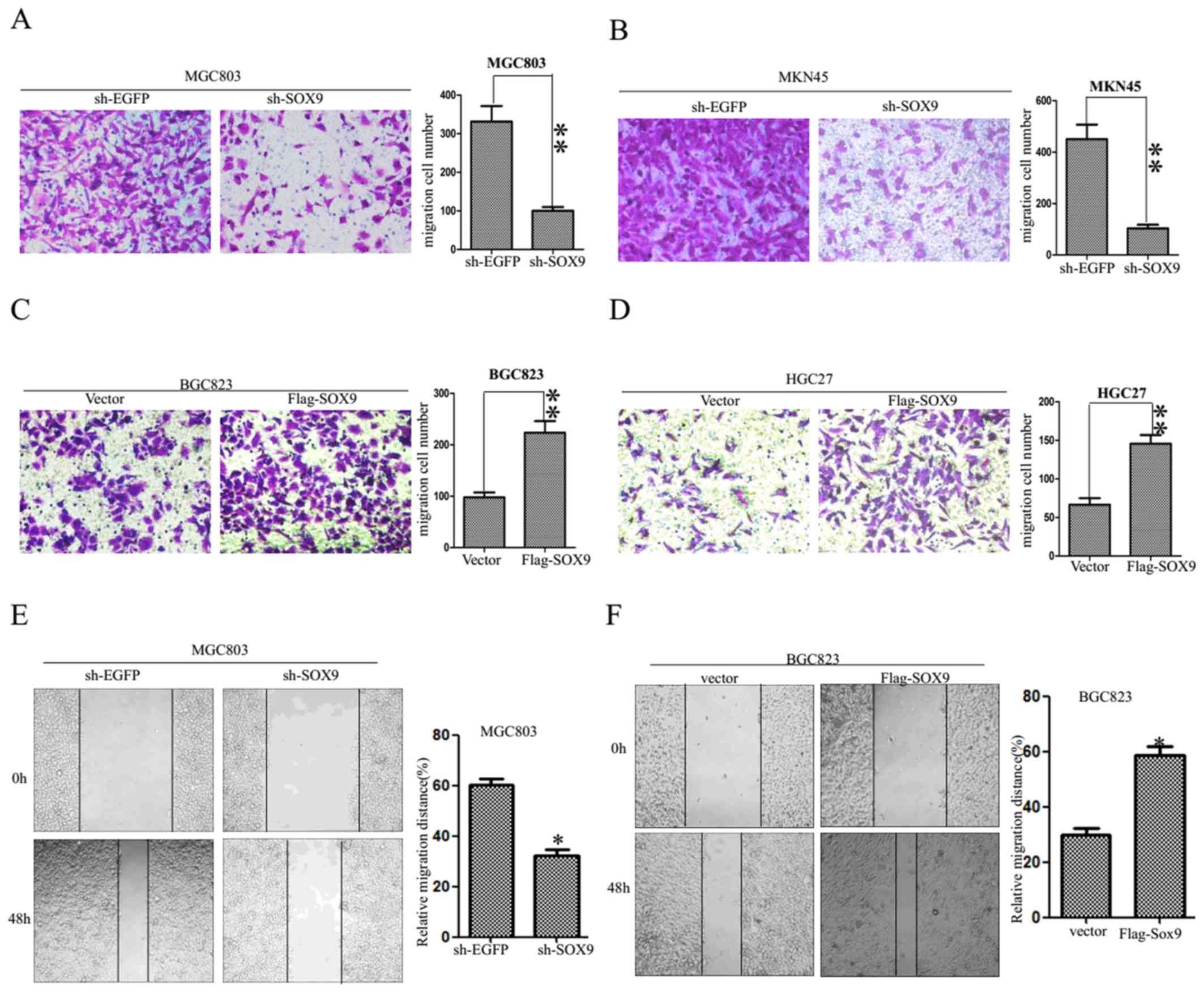
SOX9 enhances the migration of GC
cells
A transwell assay was performed to evaluate the
migration of GC cells. The results revealed 94±7 migrated MGC803
cells transfected with sh-SOX9 and 322±15 migrated MGC803 cells
transfected with sh-EGFP. Furthermore, 92±8 migrated MKN45 cells
transfected with sh-SOX9 and 419±11 migrated MKN45 cells
transfected with sh-EGFP were observed (Fig. 3A and B). To test the effects of SOX9
knockdown on cell motility, a wound scratch assay was performed. A
wound was created on confluent cultures of MGC803 cells expressing
either sh-EGFP or sh-SOX9. MGC803 cells expressing sh-SOX9
exhibited reduced motility compared with MGC803 cells expressing
sh-EGFP (Fig. 3E). The results
suggested that SOX9 knockdown suppressed the migration of MKN45 and
MGC803 cells. In addition, the migration ability of BGC823 and
HGC27 cells transfected with Flag-SOX9 vector compared with a
negative control vector was evaluated. The numbers of migrated
cells were 100±7 and 212±10 in Vector and Flag-SOX9 BGC823 cells,
and 70±6 and 145±8 in Vector and Flag-SOX9 HGC27 cells,
respectively (Fig. 3C and D),
indicating that SOX9 overexpression improved the ability of
migration in gastric carcinoma cells. To test the effects of SOX9
overexpression on cell motility, a wound scratch assay was
performed. BGC823 cells expressing Flag-SOX9 exhibited increased
motility compared with BGC823 cells transfected with an empty
vector (Fig. 3F). The results
suggested that SOX9 overexpression increased the migration ability
of GC cells.
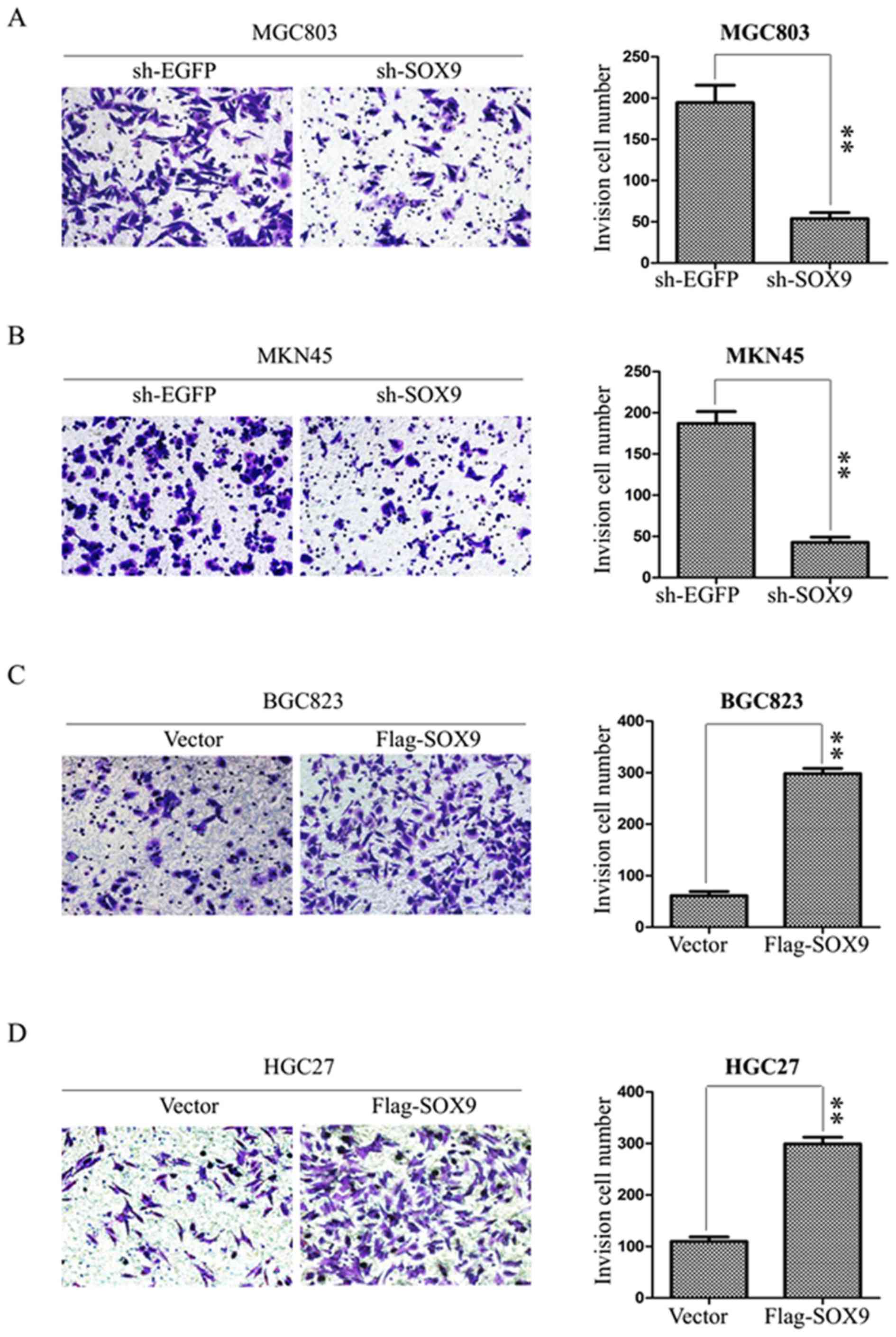
SOX9 enhances the invasion of GC
cells
The effect of SOX9 on the invasion of GC cells was
examined using BD Matrigel invasion assays. MGC803 and MKN45 cells
were transfected with sh-EGFP or sh-SOX9 plasmids, and BGC823 and
HGC27 cells with Vector or Flag-SOX9 for 72 h. The numbers of
invasive cells were 190±12 and 50±6 in sh-EGFP and sh-SOX9 MGC803
cells, and 183±7 and 48±5 in sh-EGFP and sh-SOX9 MKN45 cells
(Fig. 4A and B). The results
suggested that the absence of SOX9 suppressed the invasion of MKN45
and MGC803 cells. BGC823 and HGC27 cells were transfected with
empty vector or Flag-SOX9 to assess the effects of SOX9
overexpression on invasion. The numbers of invasive cells were 75±5
and 292±7 in vector and Flag-SOX9 BGC823 cells, and 108±6 and 300±8
in vector and Flag-SOX9 HGC27 cells. The data indicated that the
upregulation of SOX9 enhanced the invasion of HGC27 and BGC823
cells (Fig. 4C and D). The
aforementioned data suggest that SOX9 promotes the invasive ability
of gastric cancer cells.
SOX9 enhances EMT via the Hippo-YAP
signaling pathway in GC cells
The process of EMT is closely associated with cancer
cell invasion and migration (33,34).
Therefore, the effect of SOX9 expression on the EMT markers was
investigated by western blot analysis. The results suggested that
expression of vimentin, SNAI1 and N-cadherin was downregulated
following SOX9 knockdown, while E-cadherin was upregulated in MKN45
and MGC803 cells (Fig. 5A). The
overexpression of SOX9 upregulated the expression of vimentin,
N-cadherin and SNAI1 and downregulated E-cadherin in BGC823 and
HGC27 cells (Fig. 5B), suggesting
that SOX9 enhanced EMT in GC cells. As the Hippo-YAP signaling
pathway is closely associated with EMT in tumors (30,31,33,35,36), the
effects of SOX9 on the Hippo-YAP signaling pathway were
investigated in the current study. The results obtained suggested
that the knockdown of SOX9 downregulated the expression of total
YAP, LATS1 and p-MOB1 in MGC803 and MKN45 cells, but upregulated
the expression of p-YAP, MOB1 and p-LATS1 in these cells (Fig. 5C). The overexpression of SOX9
resulted in opposite effects in HGC27 and BGC823 cells (Fig. 5D). The results obtained suggested
that SOX9 may enhance EMT in GC cells via the Hippo-YAP pathway,
however further studies are required to confirm the
aforementioned.
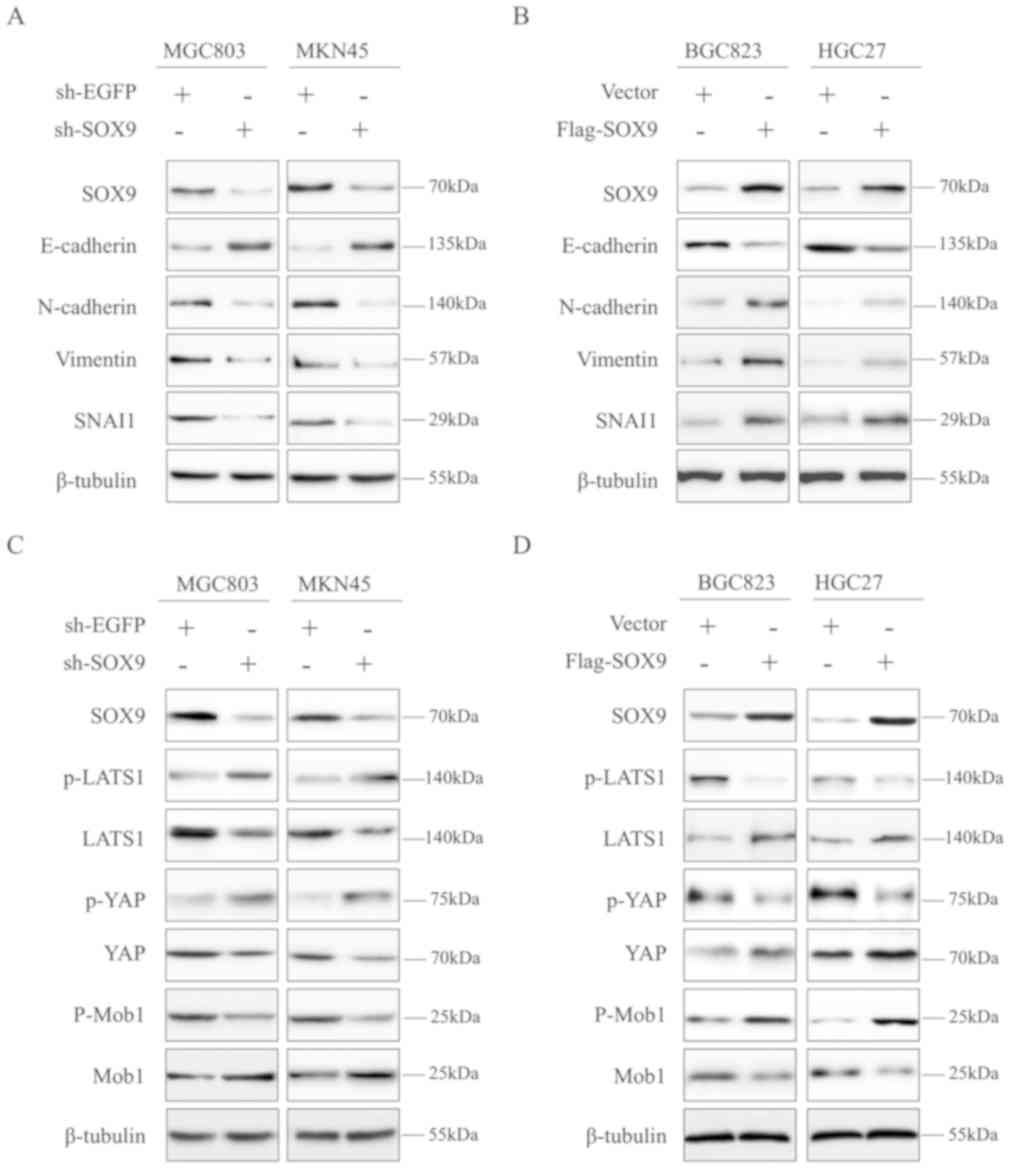 | Figure 5.SOX9 may enhance the
epithelial-mesenchymal transition via the Hippo-YAP signaling
pathway in gastric cancer cells. (A) The expression of E-cadherin,
vimentin, N-cadherin and SNAI1 in MGC803 and MKN45 cells following
SOX9 knockdown as determined by western blot analysis. (B) The
expression of E-cadherin, vimentin, N-cadherin and SNAI1 in BGC823
and HGC27 cells overexpressing SOX9 as determined by western blot
analysis. (C) The expression of Hippo-YAP pathway-associated
proteins in MGC803 and MKN45 cells following SOX9 knockdown as
determined by western blot analysis. (D) The expression of
Hippo-YAP signaling-associated proteins in BGC823 and HGC27 cells
overexpressing SOX9 as determined by western blot analysis. SOX9,
SRY-box 9; SNAI1, snail family transcriptional repressor 1; shRNA,
short hairpin RNA; EGFP, enhanced green fluorescent protein; YAS,
yes-associated protein; p, phosphorylated; LATS1/2, large tumor
suppressor kinase 1/2; MOB1, MOB kinase activator 1. |
Discussion
Previous studies have revealed that SOX9 may
function as an oncogene to enhance the growth of cancer cells
(37,38). SOX9 expression is increased in
different types of cancer, including GC (39–41). The
current study demonstrated that SOX9 enhanced the invasion,
migration and proliferation of GC cells. Additionally, the present
study revealed that SOX9 may enhance the EMT in GC potentially via
the Hippo-YAP pathway.
Previous studies suggested that the prognosis of
patients with cancer is significantly influenced by metastasis
(42,43). EMT is known to regulate metastasis
and acts as a factor in determining the prognosis of cancer patient
(44–46). The EMT process includes three major
steps: i) Destruction of cell junctions and E-cadherin
downregulation; ii) N-cadherin upregulation; and iii) rearrangement
of the cytoskeleton for cell invasion (47). During EMT, epithelial cells undergo a
number of phenotypic and genotypic changes to obtain a mesenchymal
phenotype characterized by increased migration, invasion,
resistance to apoptosis and the synthesis of ECM (48,49). Due
to the loss of E-cadherin, newly generated mesenchymal cells are
associated with poor adhesive properties (50). EMT features the upregulation of
fibronectin and N-cadherin as well as vimentin (51). EMT induced by epigenetic and genetic
changes in a tumor microenvironment is an important event in the
progression and metastasis of different types of cancer (52).
The Hippo signaling pathway, considered as an
evolutionarily conserved pathway, is associated with cell polarity,
cell proliferation and tumor suppression (53,54). The
alterations in this pathway are increasingly recognized to be
associated with cancer development (55,56).
Nuclear YAP, the downstream effector of the Hippo pathway, is
implicated in the processes of EMT, cell proliferation and
maintenance of cell polarity (57,58). A
previous study confirmed that upregulation of YAP enhanced the EMT
and promoted the aggressiveness of colorectal cancer (59). YAP and KRAS proto-oncogene, GTPase
have been revealed to work jointly to regulate EMT (60). Another study demonstrated that YAP
and tafazzin interact with TEA domain transcription factor 2 to
induce EMT (61). These results
suggested that YAP regulated EMT by interacting with specific
transcription factors. The results obtained in the current study
revealed that SOX9 may regulate EMT and affect the level of YAP
phosphorylation and the protein expression of total YAP, suggesting
that SOX9 may serve roles in the Hippo-YAP pathway to subsequently
promote EMT in GC cells.
In summary, the current study revealed that SOX9 may
be implicated in GC cell proliferation, migration and invasion by
inducing EMT. The results obtained in the present study suggested
that SOX9 may induce EMT by activating the Hippo-YAP signaling
pathway. The limitations of the current study included not testing
the effect of YAP knockdown/overexpression and using only one shRNA
in loss-of-function experiments. Furthermore, another limitation of
the current study is that different cell lines were used for
knockdown and overexpression experiments. It has been reported that
the knockdown of YAP inhibits gastric cancer cell proliferation,
migration, invasion and metastasis (62,63). The
current study revealed that SOX9 knockdown or overexpression
significantly affected YAP phosphorylation and total YAP protein
level, indicating that SOX9 may be involved in the Hippo-YAP
signaling pathway. SOX9 may potentially be an important target in
the development of novel GC treatments.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
Science Foundation for Young Medical Talents of Jiangsu Province
(QNRC2016442).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
AZ and HZ conceived the idea and designed the
experiments. HZ and GL analysed data and wrote the paper and HZ,
GL, SH and YF collected data and performed the experiments. SH and
YF contributed reagents/material/analysis tools. AZ and HZ prepared
the references and managed the data. All authors have read and
approved the final version of this manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Duan S, Wang P, Liu F, Huang H, An W, Pan
S and Wang X: Novel immune-risk score of gastric cancer: A
molecular prediction model combining the value of immune-risk
status and chemosensitivity. Cancer Med. 2019. View Article : Google Scholar
|
|
2
|
Lins RR, Oshima CT, Oliveira LA, Silva MS,
Mader AM and Waisberg J: Expression of E-cadherin and wnt pathway
proteins betacatenin, Apc, Tcf-4 and survivin In gastric
adenocarcinoma: Clinical and pathological implication. Arq Bras Cir
Dig. 29:227–231. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Suarez-Arriaga MC, Torres J,
Camorlinga-Ponce M, Gómez-Delgado A, Piña-Sánchez P, Valdez-Salazar
HA, Ribas-Aparicio RM, Fuentes-Pananá EM and Ruiz-Tachiquín ME: A
proposed method for the relative quantification of levels of
circulating microRNAs in the plasma of gastric cancer patients.
Oncolo Lett. 13:3109–3117. 2017. View Article : Google Scholar
|
|
4
|
Fang WL, Huang KH, Chen JH, Lo SS, Hsieh
MC, Shen KH, Li AF, Niu DM, Chiou SH and Wu CW: Comparison of the
survival difference between AJCC 6th and 7th editions for gastric
cancer patients. World J Surg. 35:2723–2729. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wen Q, Chen Z, Chen Z, Chen J, Wang R,
Huang C and Yuan W: EphA2 affects the sensitivity of oxaliplatin by
inducing EMT in oxaliplatin-resistant gastric cancer cells.
Oncotarget. 8:47998–48011. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ahn SH, Kang SH, Lee Y, Min SH, Park YS,
Park DJ and Kim HH: Long-term survival outcomes of laparoscopic
gastrectomy for advanced gastric cancer: Five-year results of a
phase II prospective clinical trial. J Gastric Cancer. 19:102–110.
Mar 12–2019.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gubbay J, Collignon J, Koopman P, Capel B,
Economou A, Münsterberg A, Vivian N, Goodfellow P and Lovell-Badge
R: A gene mapping to the sex-determining region of the mouse Y
chromosome is a member of a novel family of embryonically expressed
genes. Nature. 346:245–250. 1990. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gao J, Zhang JY, Li YH and Ren F:
Decreased expression of SOX9 indicates a better prognosis and
inhibits the growth of glioma cells by inducing cell cycle arrest.
Int J Clin Exp Pathol. 8:10130–10138. 2015.PubMed/NCBI
|
|
9
|
Leung CO, Mak WN, Kai AK, Chan KS, Lee TK,
Ng IO and Lo RC: Sox9 confers stemness properties in hepatocellular
carcinoma through Frizzled-7 mediated Wnt/beta-catenin signaling.
Oncotarget. 7:29371–29386. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lefebvre V, Dumitriu B, Penzo-Mendez A,
Han Y and Pallavi B: Control of cell fate and differentiation by
Sry-related high-mobility-group box (Sox) transcription factors.
Int J Biochem Cell Biol. 39:2195–2214. Jun 6–2007.(Epub ahead of
print). View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Song W, Kwon GY, Kim JH, Lim JE, Jeon HG,
Il Seo S, Jeon SS, Choi HY, Jeong BC and Lee HM:
Immunohistochemical staining of ERG and SOX9 as potential
biomarkers of docetaxel response in patients with metastatic
castration-resistant prostate cancer. Oncotarget. 7:83735–83743.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Raspaglio G, Petrillo M, Martinelli E, Li
Puma DD, Mariani M, De Donato M, Filippetti F, Mozzetti S, Prislei
S, Zannoni GF, et al: Sox9 and Hif-2alpha regulate TUBB3 gene
expression and affect ovarian cancer aggressiveness. Gene.
542:173–181. Mar 21–2014.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fazilaty H, Gardaneh M, Akbari P, Zekri A
and Behnam B: SLUG and SOX9 Cooperatively Regulate Tumor Initiating
Niche Factors in Breast Cancer. Cancer microenvironment. Official
journal of the International Cancer Microenviron. 9:71–74. Sep
28–2016.2015. (Epub ahead of print). View Article : Google Scholar
|
|
14
|
Wang X, Liu Y, Liu X, Yang J, Teng G,
Zhang L and Zhou C: MiR-124 inhibits cell proliferation, migration
and invasion by directly targeting SOX9 in lung adenocarcinoma.
Oncol Rep. 35:3115–3121. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang Z, Wang H, Xia L, Oyang L, Zhou Y,
Zhang B, Chen X, Luo X, Liao Q and Liang: Overexpression of PAK1
Correlates with Aberrant Expression of EMT Markers and Poor
Prognosis in Non-Small Cell Lung Cancer. J Cancer. 8:1484–1491.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guarino M, Rubino B and Ballabio G: The
role of epithelial-mesenchymal transition in cancer pathology.
Pathology. 39:305–318. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu Q, Liu X, Liu Z, Zhou Z, Wang Y, Tu J,
Li L, Bao H, Yang L and Tu K: MicroRNA-1296 inhibits metastasis and
epithelial-mesenchymal transition of hepatocellular carcinoma by
targeting SRPK1-mediated PI3K/AKT pathway. Mol Cancer. 16:1032017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li T, Huang H, Shi G, Zhao L, Li T, Zhang
Z, Liu R, Hu Y, Liu H, Yu J and Li G: TGF-beta1-SOX9 axis-inducible
COL10A1 promotes invasion and metastasis in gastric cancer via
epithelial-to-mesenchymal transition. Cell Death & disease.
9:8492018. View Article : Google Scholar
|
|
21
|
Yan J, Huang W, Huang X, Xiang W, Ye C and
Liu J: A negative feedback loop between long noncoding RNA NBAT1
and Sox9 inhibits the malignant progression of gastric cancer
cells. Biosci Rep. 6:2018.
|
|
22
|
Piccolo S, Dupont S and Cordenonsi M: The
biology of YAP/TAZ: Hippo signaling and beyond. Physiol Rev.
94:1287–1312. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Meng Z, Moroishi T and Guan KL: Mechanisms
of Hippo pathway regulation. Genes Dev. 30:1–17. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Johnson R and Halder G: The two faces of
Hippo: targeting the Hippo pathway for regenerative medicine and
cancer treatment. Nat Rev. Drug Discov. 13:63–79. 2014. View Article : Google Scholar
|
|
25
|
Moon H, Cho K, Shin S, Kim DY, Han KH and
Ro SW: High Risk of Hepatocellular Carcinoma Development in
Fibrotic Liver: Role of the Hippo-YAP/TAZ Signaling Pathway. Int J
Mol Sci. 20:2019. View Article : Google Scholar
|
|
26
|
Ferrari N, Ranftl R, Chicherova I, Slaven
ND, Moeendarbary E, Farrugia AJ, Lam M, Semiannikova M, Westergaard
MCW, Tchou J, et al: Dickkopf-3 links HSF1 and YAP/TAZ signalling
to control aggressive behaviours in cancer-associated fibroblasts.
Nat Comm. 10:1302019. View Article : Google Scholar
|
|
27
|
Janse van Rensburg HJ, Azad T, Ling M, Hao
Y, Snetsinger B, Khanal P, Minassian LM, Graham CH, Rauh MJ and
Yang X: The Hippo Pathway Component TAZ Promotes Immune Evasion in
Human Cancer through PD-L1. Cancer Res. 78:1457–1470. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yu FX, Zhao B and Guan KL: Hippo pathway
in organ size control, tissue homeostasis, and cancer. Cell.
163:811–828. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Janse van Rensburg HJ and Yang X: The
hippo pathway and cancer immunity: friend or foe? Oncoscience.
5:49–50. 2018.PubMed/NCBI
|
|
30
|
Maugeri-Sacca M and De Maria R: The hippo
pathway in normal development and cancer. Pharmacol Ther.
186:60–72. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zanconato F, Cordenonsi M and Piccolo S:
YAP/TAZ at the roots of cancer. Cancer Cell. 29:783–803. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gopalkrishna V, Verma H, Kumbhar NS, Tomar
RS and Patil PR: Detection of Mycoplasma species in cell culture by
PCR and RFLP based method: Effect of BM-cyclin to cure infections.
Indian J Med Microbiol. 25:364–368. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gustafsson RK, Engdahl EE and Fogdell-Hahn
A: Development and validation of a Q-PCR based TCID50 method for
human herpesvirus 6. Virol J. 9:3112012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li F, Shi J, Xu Z, Yao X, Mou T, Yu J, Liu
H and Li G: S100A4-MYH9 Axis promote migration and invasion of
gastric cancer cells by inducing TGF-beta-Mediated
epithelial-mesenchymal Transition. J Cancer. 9:3839–3849. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Qiu B, Wei W, Zhu J, Fu G and Lu D: EMT
induced by loss of LKB1 promotes migration and invasion of liver
cancer cells through ZEB1-induced YAP signaling. Oncol Lett.
16:6465–6471. Sep 18–2018.(Epub ahead of print). PubMed/NCBI
|
|
37
|
Kong X, Zhao Y, Li X, Tao Z, Hou M and Ma
H: Overexpression of HIF-2alpha-dependent NEAT1 promotes the
progression of non-small cell lung cancer through
miR-101-3p/SOX9/Wnt/beta-Catenin signal Pathway. Cell Physiol
Biochem. 52:368–381. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gnerlich JL, Ding X, Joyce C, Turner K,
Johnson CD, Chen H, Abood GJ, Pappas SG and Aranha GV: Increased
SOX9 expression in premalignant and malignant pancreatic neoplasms.
Ann Surg Oncol. 26:628–634. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wan YP, XM, He HC, Wan S, Hua W, Zen ZC,
Liu YL, Zhou YL, Mo RJ, Zhuo YJ, et al: Expression and Clinical
Significance of SOX9 in Renal Cell Carcinoma, Bladder Cancer and
Penile Cancer. Oncol Res Treat. 40:15–20. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Qian Y, Xia S and Feng Z: Sox9 mediated
transcriptional activation of FOXK2 is critical for colorectal
cancer cells proliferation. Biochem Biophys Res Commun.
483:475–481. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ren X, Zheng D, Guo F, Liu J, Zhang B, Li
H and Tian W: PPARgamma suppressed Wnt/beta-catenin signaling
pathway and its downstream effector SOX9 expression in gastric
cancer cells. Med Oncol. 32:912015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hidaka E, Maeda C, Nakahara K, Wakamura K,
Ishiyama Y, Shimada S, Seki J, Takano Y, Oae S, Enami Y, et al:
High Serum CA19-9 Concentration predicts poor prognosis in elderly
patients with stage IV Colorectal cancer. Gastrointest Tumors.
5:117–124. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chen YL, Zhang Y, Wang J, Chen N, Fang W,
Zhong J, Liu Y, Qin R, Yu X, Sun Z and Gao F: A 17 gene panel for
non-small cell lung cancer prognosis identified through integrative
epigenomic-transcriptomic analyses of hypoxia-induced
epithelial-mesenchymal transition. Mol Oncol. 11:2019.
|
|
44
|
Padthaisong S, Thanee M, Techasen A,
Namwat N, Yongvani P, Liwatthakun A, Hankla K, Sangkhamanon S and
Loilome W: Nimotuzumab inhibits cholangiocarcinoma cell metastasis
via suppression of the epithelial-mesenchymal transition process.
Anticancer Res. 37:3591–3597. 2017.PubMed/NCBI
|
|
45
|
Imani S, Wei C, Cheng J, Khan MA, Fu S,
Yang L, Tania M, Zhang X, Xiao X, Zhang X and Fu J: MicroRNA-34a
targets epithelial to mesenchymal transition-inducing transcription
factors (EMT-TFs) and inhibits breast cancer cell migration and
invasion. Oncotarget. 8:21362–21379. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Li Q, Wu J, Wei P, Xu Y, Zhuo C, Wang Y,
Li D and Cai S: Overexpression of forkhead box C2 promotes tumor
metastasis and indicates poor prognosis in colon cancer via
regulating epithelial-mesenchymal transition. Am J Cancer Res.
5:2022–2034. 2015.PubMed/NCBI
|
|
47
|
Zhang J, Tian XJ and Xing J: Signal
Transduction Pathways of EMT induced by TGF-beta, SHH, and WNT and
Their Crosstalks. J CLin Med. 4:520162016.
|
|
48
|
Wan L, Pantel K and Kang Y: Tumor
metastasis: moving new biological insights into the clinic. Nat
Med. 19:1450–1464. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Park PG, Jo SJ, Kim MJ, et al: Role of
LOXL2 in the epithelial-mesenchymal transition and colorectal
cancer metastasis. Oncotarget. 25:80325–803352017. 2017.
|
|
50
|
Bruner HC and Derksen PWB: Loss of
E-Cadherin-dependent cell-cell adhesion and the development and
progression of cancer. Cold Spring Harb Perspect Biol. 1:2018.
|
|
51
|
Rajic J, Inic-Kanada A, Stein E, Dinić S,
Schuerer N, Uskoković A, Ghasemian E, Mihailović M, Vidaković M,
Grdović N and Barisani-Asenbauer T: Chlamydia trachomatis Infection
Is associated with E-Cadherin promoter methylation, downregulation
of E-Cadherin expression, and Increased expression of fibronectin
and alpha-SMA-Implications for Epithelial-Mesenchymal Transition.
Front Cell Infect Microbiol. 7:2532017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Buhrmann C, Kraehe P, Lueders C, Shayan P,
Goel A and Shakibaei M: Curcumin suppresses crosstalk between colon
cancer stem cells and stromal fibroblasts in the tumor
microenvironment: Potential role of EMT. PLoS One. 9:e1075142014.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Mo JS, Park HW and Guan KL: The Hippo
signaling pathway in stem cell biology and cancer. EMBO Reports.
15:642–656. 2014.PubMed/NCBI
|
|
54
|
Han Y: Analysis of the role of the Hippo
pathway in cancer. J Transl Med. 17:1162019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Hou J and Zhou J: WWC3 downregulation
correlates with poor prognosis and inhibition of Hippo signaling in
human gastric cancer. OncoTargets Ther. 10:2931–2942. 2017.
View Article : Google Scholar
|
|
56
|
Sharif GM and Wellstein A: Cell density
regulates cancer metastasis via the hippo pathway. Future Oncol.
11:3253–3260. Nov;12.2015.(Epub ahead of print). View Article : Google Scholar
|
|
57
|
Yuan Y, Li D, Li H, Wang L, Tian G and
Dong Y: YAP overexpression promotes the epithelial-mesenchymal
transition and chemoresistance in pancreatic cancer cells. Mol Med
Rep. 13:237–242. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Hua K, Yang W, Song H, Song J, Wei C, Li D
and Fang L: Up-regulation of miR-506 inhibits cell growth and
disrupt the cell cycle by targeting YAP in breast cancer cells. Int
J Clin Exp Med. 8:12018–12027. 2015.PubMed/NCBI
|
|
59
|
Ling HH, Kuo CC, Lin BX, Huang YH and Lin
CW: Elevation of YAP promotes the epithelial-mesenchymal transition
and tumor aggressiveness in colorectal cancer. Exp Cell Res.
350:218–225. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Shao DD, Xue W, Kral EB, Bhutkar A,
Piccioni F, Wang X, Schinzel AC, Sood S, Rosenbluh J, Kim JW, et
al: KRAS and YAP1 converge to regulate EMT and tumor survival.
Cell. 158:171–184. Jun 19–2014.(Epub. ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Diep0enbruck M, Waldmeier L, Ivanek R,
Berninger P, Arnold P, van Nimwegen E and Christofori G: Tead2
expression levels control the subcellular distribution of Yap and
Taz, zyxin expression and epithelial-mesenchymal transition. J Cell
Sci. 127:1523–1536. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Zhang J, Xu ZP, Yang YC, Zhu JS, Zhou Z
and Chen WX: Expression of Yes-associated protein in gastric
adenocarcinoma and inhibitory effects of its knockdown on gastric
cancer cell proliferation and metastasis. Int j Immunopathol
Pharmacol. 25:583–590. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Sun D, Li X, He Y, Li W, Wang Y, Wang H,
Jiang S and Xin Y: YAP1 enhances cell proliferation, migration, and
invasion of gastric cancer in vitro and in vivo. Oncotarget.
7:81062–81076. 2016. View Article : Google Scholar : PubMed/NCBI
|