Introduction
Lung cancer is the leading cause of
cancer-associated mortality worldwide and non-small cell lung
cancer (NSCLC) accounts for 80–85% of all lung cancer cases
(1). Mutations and gene
amplifications of epidermal growth factor receptor (EGFR)
frequently occur in NSCLC, and EGFR-tyrosine kinase inhibitors
(EGFR-TKIs) are regularly used to treat patients with NSCLC and
prolong progression-free survival (PFS) (2,3). Thus,
it is recommended to screen for EGFR mutations in patients with
NSCLC and that patients with sensitizing EGFR mutations are treated
with first-line EGFR-TKIs, whereas patients with NSCLC wihtout EGFR
mutation or unknown mutational status are treated with
platinum-based chemotherapy (4,5). In the
clinic, the majority of patients with EGFR mutations initially
respond very well to EGFR-TKIs; however, a large proportion of them
eventually develop drug resistance (6). The underlying molecular mechanisms of
drug resistance may be due to receptor tyrosine-protein kinase
erbB-2 (HER2) amplification and EGFR T790M mutation, MET
proto-oncogene, receptor tyrosine kinase (c-MET) amplification,
phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit α
(PIK3CA) mutation or B-Raf proto-oncogene, serine/threonine kinase
(BRAF) mutations in tumor tissues (7). However, the specific molecular
mechanisms require further investigation. For example, previous
studies demonstrated that estrogen receptor (ER)β was overexpressed
in NSCLC tissue specimens, which may be associated with resistance
to treatment with EGFR-TKIs in patients with NSCLC (8,9). ERβ is
the most commonly observed subtype of ER expressed in lung cancer
(8,9). Previous studies have suggested
cross-talk between the ERβ and EGFR signaling pathways in lung
cancer (10–12). ERβ is frequently overexpressed in
NSCLC with EGFR mutations, particularly in lung adenocarcinoma
(13,14), and ERβ expression was reported to be
associated with the prognosis of NSCLC with EGFR mutations
subsequent to treatment with EGFR-TKIs (15–17).
There are five known isoforms of ERβ, and ERβ1 is the only known
full-length and functional ERβ isoform expressed in various cells
and tissues (18), and is the most
relevant prognostic factor of all ERβ isoforms for patients with
NSCLC (9).
To date, the presence of a number of EGFR mutations
have been demonstrated to occur in NSCLC (19), each of which may contribute to
different outcomes of patients treated with EGFR-TKIs; however, two
EGFR mutations, exon 19 deletion (Del19) and the substitution L858R
in exon 21, account for 80–90% of all EGFR mutations in NSCLC
(20), and patients with NSCLC
carrying these mutations respond favourably to treatment with
EGFR-TKI (2,3). In contrast, other EGFR mutations,
including G719X, L861Q and a de novo exon 20 T790M mutation,
account for ~10% of the known EGFR mutations in NSCLC, and only
certain patients responded favourably to treatment with EGFR-TKI
(21). In addition, patients with
the T790M mutation demonstrated resistance to treatment with first
generation EGFR-TKIs (22). Previous
studies have demonstrated that treatment of patients with NSCLC
carrying Del19 mutation with EGFR-TKIs improved outcomes compared
with the patients carrying the L858R mutation (23,24). In
the present study, ERβ1 expression was retrospectively assessed in
201 lung adenocarcinoma tissue specimens, and the ERβ1 expression
and survival of patients with lung adenocarcinoma carrying the EGFR
Del19 or L858R mutation subsequent to treatment with EGFR-TKIs were
examined. The present study was designed to confirm data from
previous studies (23,24), and additionally provide useful
information regarding treatment of patients with NSCLC with
EGFR-TKIs or a combination of other drugs.
Patients and methods
Patients and treatment
Patients who were pathologically diagnosed with
stage IV TNM lung adenocarcinoma were evaluated for eligibility
(25). The inclusion criteria were:
i) Patients with data pertaining to EGFR mutations; and ii)
treatment with EGFR-TKIs or chemotherapy. The exclusion criteria
were: i) Patients that had left hospital; ii) patients that had
refused any chemotherapy or an EGFR test, or iii) there was no
sufficient tissue specimen for the EGFR and immunohistochemistry
(IHC) tests. Thus, 201 patients were eligible for the present
study. Tissue samples from patients, for EGFR mutation analysis,
were retrospectively collected from The Department of Thoracic
Oncology, Anhui Provincial Cancer Hospital (Hefei, China) between
January 2012 and June 2014. The cohort of patients had stage IV
disease; thus, there were no patients that underwent tumor
resection. The median age was 65 years (range, 27–84 years) and
72.1% were females. Lung cancer tissues were obtained through
transbronchial biopsy or fine needle aspiration for histological
diagnosis of NSCLC. The present study was approved by The Ethics
Committee of Anhui Provincial Cancer Hospital, which waived patient
consent due to mortality of all the individuals.
In terms of treatment options, patients with lung
adenocarcinoma with no evidence of EGFR mutations were administered
pemetrexed in combination with two 4-week cycles of
cisplatin/carboplatin (area under the curve=5). From the cohort,
two patients with a relatively uncommon EGFR 19Del plus T790M
mutation, which may not have responded well to treatment with
EGFR-TKI (20,21), also received chemotherapy as it was
in doubt whether such patients would respond to the first
generation of EGFR-TKIs. Patients with common EGFR mutations and
two patients with uncommon EGFR mutations (one each of S768I/L858R
and 19Del/G719X mutation) were administered the first-line therapy
of gefitinib (250 mg/day), erlotinib (150 mg/day) or icotinib (125
mg, three times a day) for between 4 and 17.6 months (discontinued
after occurrence of drug resistance). Treatment with EGFR-TKIs were
discontinued when CT scans identified enhanced disease progression
or if treatment toxicity was deemed unacceptable. For these
patients, chemotherapy or the best supportive care were the options
considered thereafter.
Patient assessment and follow-up
The clinicopathological data of the patients,
including age, sex, smoking history, TNM stage and brain
metastasis, were retrieved from their medical records and are
presented in Table I. Clinically,
all patients were evaluated on a monthly basis and their follow-up
consisted of a physical examination, including the Eastern
Cooperative Oncology Group performance status (26), laboratory tests and
electrocardiography, whereas tumor burdens were assessed monthly or
bimonthly using CT. Non-smokers were defined as individuals who had
smoked <100 cigarettes in their lifetime, whereas others were
defined as smokers. The effectiveness of chemotherapy or targeted
therapy was evaluated with the Response Evaluation Criteria in
Solid Tumors (RECIST) 1.1 (27) and
disease progression was defined as ≥20% increase in the diameter of
a tumor lesion following treatment with EGFR-TKI or chemotherapy
according to RECIST 1.1 (27). The
PFS was calculated as the interval from initial treatment to the
progression of the disease, mortality of any cause or the last
follow-up. The overall survival (OS) was calculated as the interval
from initial treatment to mortality from any cause or the last
follow-up. The survival data were obtained through the review of
medical records, telephone follow-up or contact with the local
Household Registration Department.
 | Table I.Clinicopathological characteristics
of 201 patients with stage IV lung carcinoma. |
Table I.
Clinicopathological characteristics
of 201 patients with stage IV lung carcinoma.
|
|
| Epidermal growth
factor receptor mutations, n (%) |
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
characteristic | n | Mutation | No mutation | P-value |
|---|
| Age (years) |
|
|
| 0.314 |
|
≥65 | 103 | 66 (64.1) | 37 (36.9) |
|
|
<65 | 98 | 56 (57.1) | 42 (42.9) |
|
| Sex |
|
|
|
<0.001a |
|
Male | 56 | 22 (39.3) | 34 (60.7) |
|
|
Female | 145 | 100 (69.0) | 45 (31.0) |
|
| Smoking status |
|
|
|
<0.001a |
|
Smoker | 62 | 20 (32.3) | 42 (67.7) |
|
|
Never-smoker | 139 | 102 (73.4) | 37 (26.6) |
|
| ECOG performance
status |
|
|
| 0.675 |
| 0 | 108 | 67 (62.0) | 41 (38.0) |
|
| ≥1 | 93 | 55 (59.1) | 38(40.9) |
|
| Brain
metastasis |
|
|
| 0.605 |
|
Yes | 115 | 64 (55.6) | 51 (44.4) |
|
| No | 86 | 51 (59.3) | 35 (40.7) |
|
DNA extraction and detection of EGFR
mutations
Genomic DNA was extracted from formalin-fixed,
paraffin-embedded tumor tissue specimens using the
Cobas® DNA Sample Preparation Kit according to the
manufacturer's protocol (Roche Molecular Diagnostics, Pleasanton,
CA, USA). The presence of EGFR mutations was assessed using a Cobas
z 480 real-time PCR system (Roche Molecular Diagnostics) which is
capable of detecting 42 EGFR mutations (28).
IHC
All IHC steps were performed on a BenchMark XT
system (Ventana Medical Systems, Inc., Tucson, AZ, USA), according
to the manufacturer's protocol. Briefly, paraffin-embedded tissue
sections of a 4-µm thickness were fixed in 10% neutral-buffered
formalin for 24 h at room temperature and deparaffinized and
subsequently placed into a 60°C oven for 2 h. To block endogenous
peroxidase activity, 3% hydrogen peroxide was used for 8 min at
37°C. A mouse monoclonal anti-human ERβ1 antibody PPG5/10 (1:50;
cat. no. M7292; Dako; Agilent Technologies, Inc., Santa Clara, CA,
USA) was incubated with the tissue sections for 32 min at 37°C.
Subsequently, the slides were incubated with a ultraView universal
HRP Multimer, which contains a cocktail of HRP labeled antibodies
(goat anti-mouse IgG, goat anti-mouse IgM and goat anti-rabbit)
(prediluted; cat. no. 760-500; Ventana Medical Systems, Inc.) for 8
min at 37°C. Finally, the tissue sections were counterstained with
hematoxylin for 16 min at 37°C and assessed under a light
microscope (Olympus Corporation, Tokyo, Japan) at ×10 magnification
using the Allred scoring system according to previous studies
(14,28). ERβ expression was divided into
nuclear or cytoplasmic staining and scored based on the proportion
of positive cells (0, no staining at all; 1, ≤1%; 2, 2–10%; 3,
11–33%; 4, 34–66%; and 5, >67%) and the staining intensity (0,
no staining; 1, weak; 2, moderate; and 3, strong staining). The
evaluation of the IHC staining was performed independently by two
pathologists who were blinded to the identity of the patients. The
staining index of the cytoplasmic or nuclear score was subsequently
reached by the addition of the staining proportion and intensity to
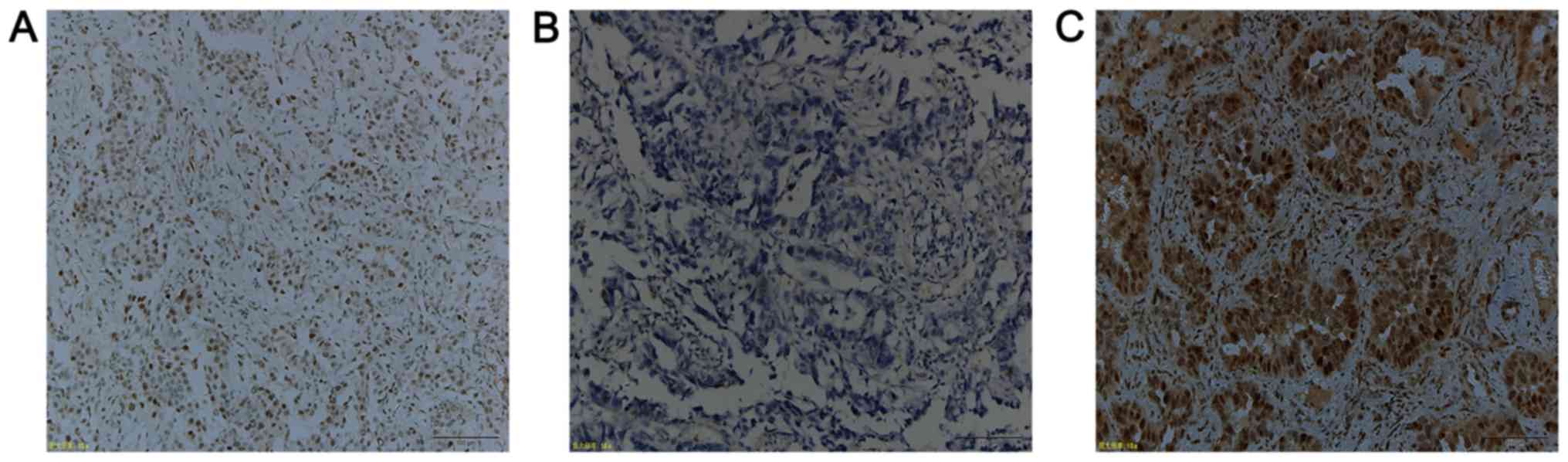
provide a score between 0 and 8 (Fig.
1). Any disagreements were resolved by reviewing the
immunostained sections to reach a consensus. High or low ERβ
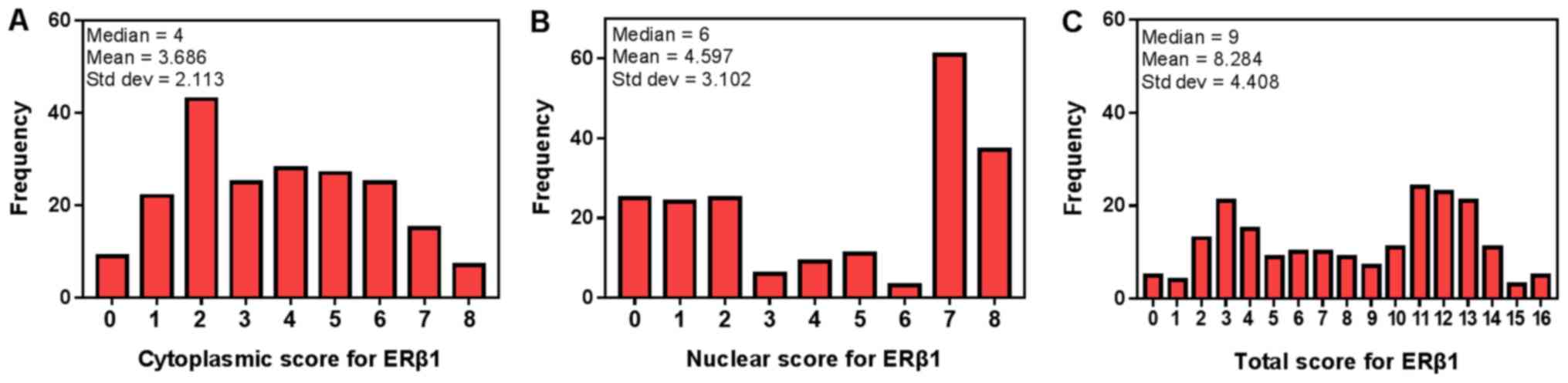
expression was defined according to a previous study (29). The median of the cytoplasmic scores
of ERβ1 immunostaining was 4; thus, the low level of the
cytoplasmically immunostaining was defined as ≤4, whereas >4 was
classified as a high level of cytoplasmic ERβ1 expression.
Statistical analysis
All statistical analyses were performed using SPSS
20.0 (IBM Corporation, Armonk, NY, USA). The primary endpoint of
the treatment responses was the PFS, whereas the secondary endpoint
was the OS. The median PFS and OS were estimated using the
Kaplan-Meier curves and statistically analyzed using the log-rank
test. Where appropriate, the data were additionally presented as
the 95% confidence intervals (CI) and the hazard ratios (HRs) with
associated 95% CI. The variables significantly associated with
survival in a univariate analysis using the log-rank test were
further assessed using a multivariate analysis with the Cox
proportional model. P<0.05 was considered to indicate a
statistically significant difference.
Results
Characteristics of patients
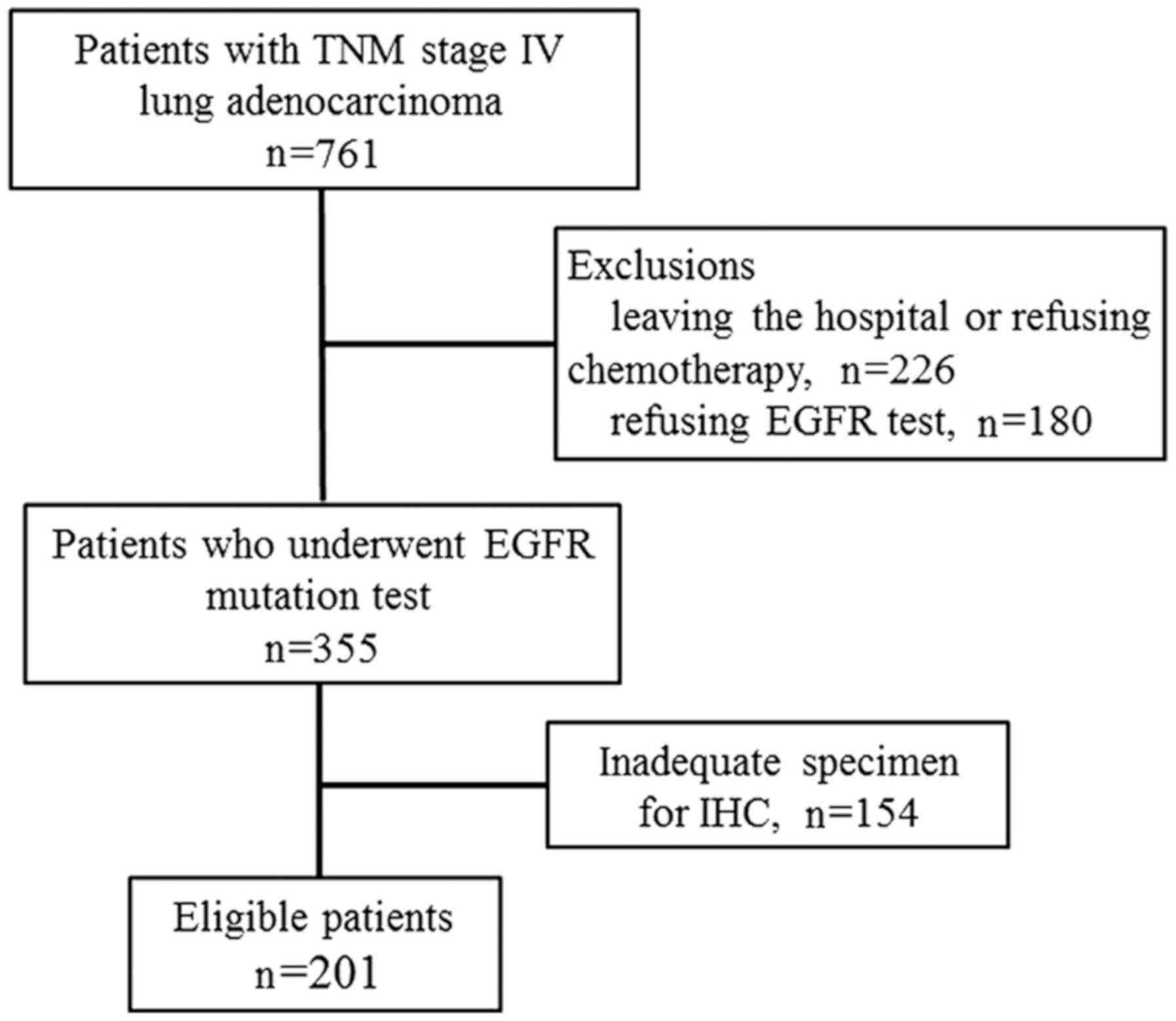
In the present study, a total of 761 patients
diagnosed with lung adenocarcinoma between January 2012 and June
2014 (Fig. 2) were retrospectively
reviewed. Due to the nature of the study, 201 patients with TNM
stage IV with a known EGFR mutation status were included in the
analysis. The demographic and baseline characteristics are listed
in Table I. The EGFR mutation
frequency was significantly higher in female patients (69.0%)
compared with male patients (39.3%; P<0.001) and also higher in
non-smokers (73.4%) compared with smokers (32.3%; P<0.001).
Other clinicopathological data did not demonstrate statistical
significance between patients with or without EGFR mutations
(Table I).
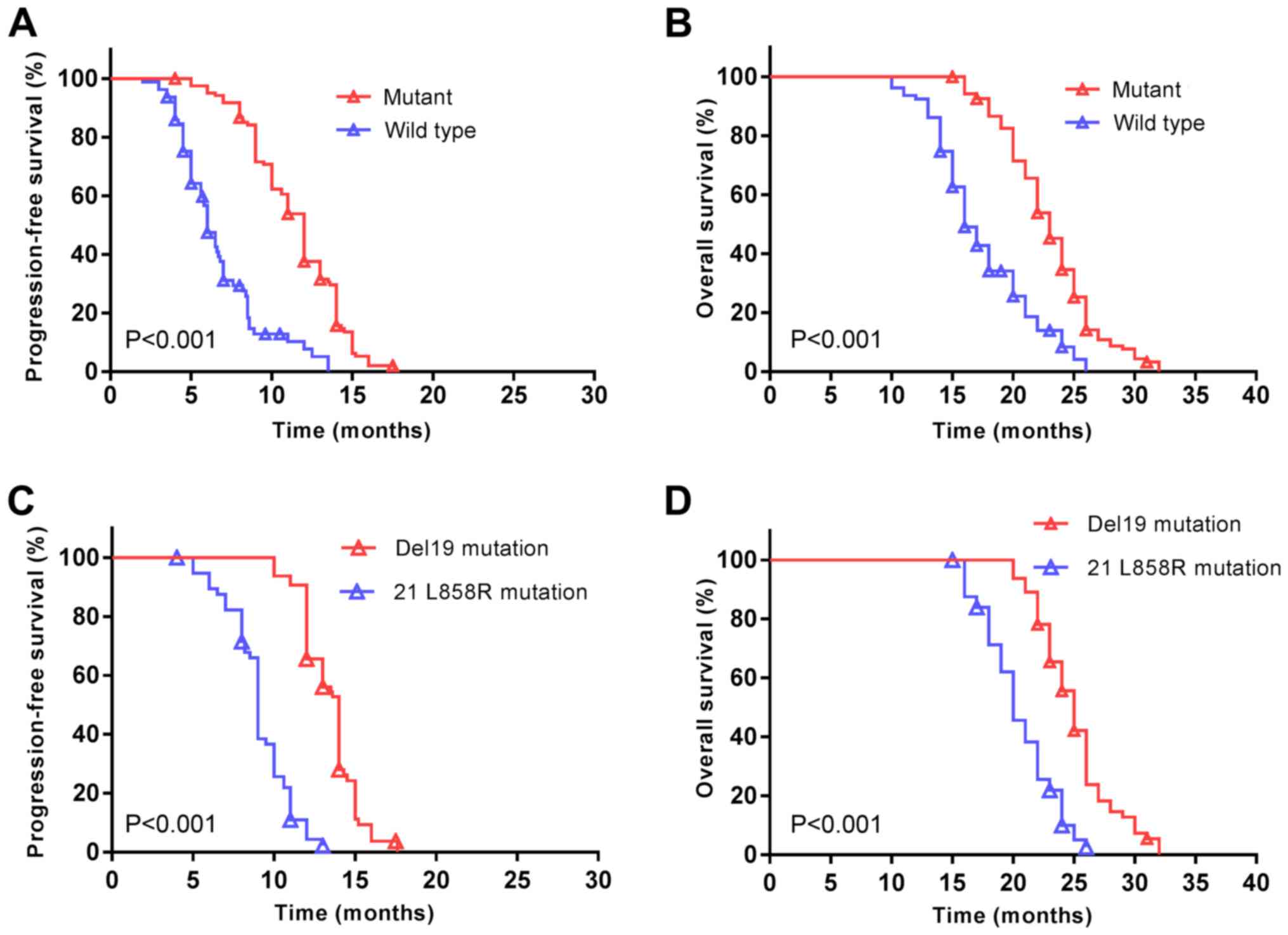
In the present cohort, the median PFS was 10 months
(95% CI; 9.5–10.5 months) and the median OS was 21 months (95% CI;
20.1–21.9 months). Patients with EGFR mutations demonstrated a
significantly increased median PFS (12 vs. 6 months, P<0.001;
Fig. 3A), and a longer median OS (23
vs. 16 months, P<0.001; Fig. 3B)
compared with patients without EGFR mutations subsequent to
treatment with EGFR-TKIs. The median PFS was additionally
significantly increased among patients with EGFR Del19 compared
with the EGFR exon 21 L858R mutation (14 vs. 9 months, P<0.001;
Fig. 3C). A similar trend was
observed for the median OS (25 vs. 20 months, P<0.001; Fig. 3D).
ERβ1 expression is associated with
survival of patients with EGFR mutations
The median nuclear ERβ1 score was 6, which was set
as the cut-off value to distinguish between low and high expression
of nuclear ERβ1. In addition, the median total score of cytoplasmic
and nuclear ERβ1 immunostaining was 9, which was used to
distinguish between the low and high levels of total cytoplasmic
and nuclear ERβ1 immunostaining in tumor tissues (Fig. 4). ERβ1 was expressed in the cytoplasm
and nuclei of 98 patients with lung adenocarcinoma, expressed in
the cytoplasm in 74 patients and expressed in the nucleus in 99
patients (Table II). ERβ1
expression was significantly increased in patients with EGFR
mutations (71 patients, 58.2%) compared with patients without EGFR
mutations (27 patients, 34.1%; P=0.001). However, no significant
difference in ERβ1 expression was observed between females and
males, irrespective of the localization of ERβ1. Furthermore, there
were no significant associations between other clinical features
and overall ERβ1 expression. No significant differences were
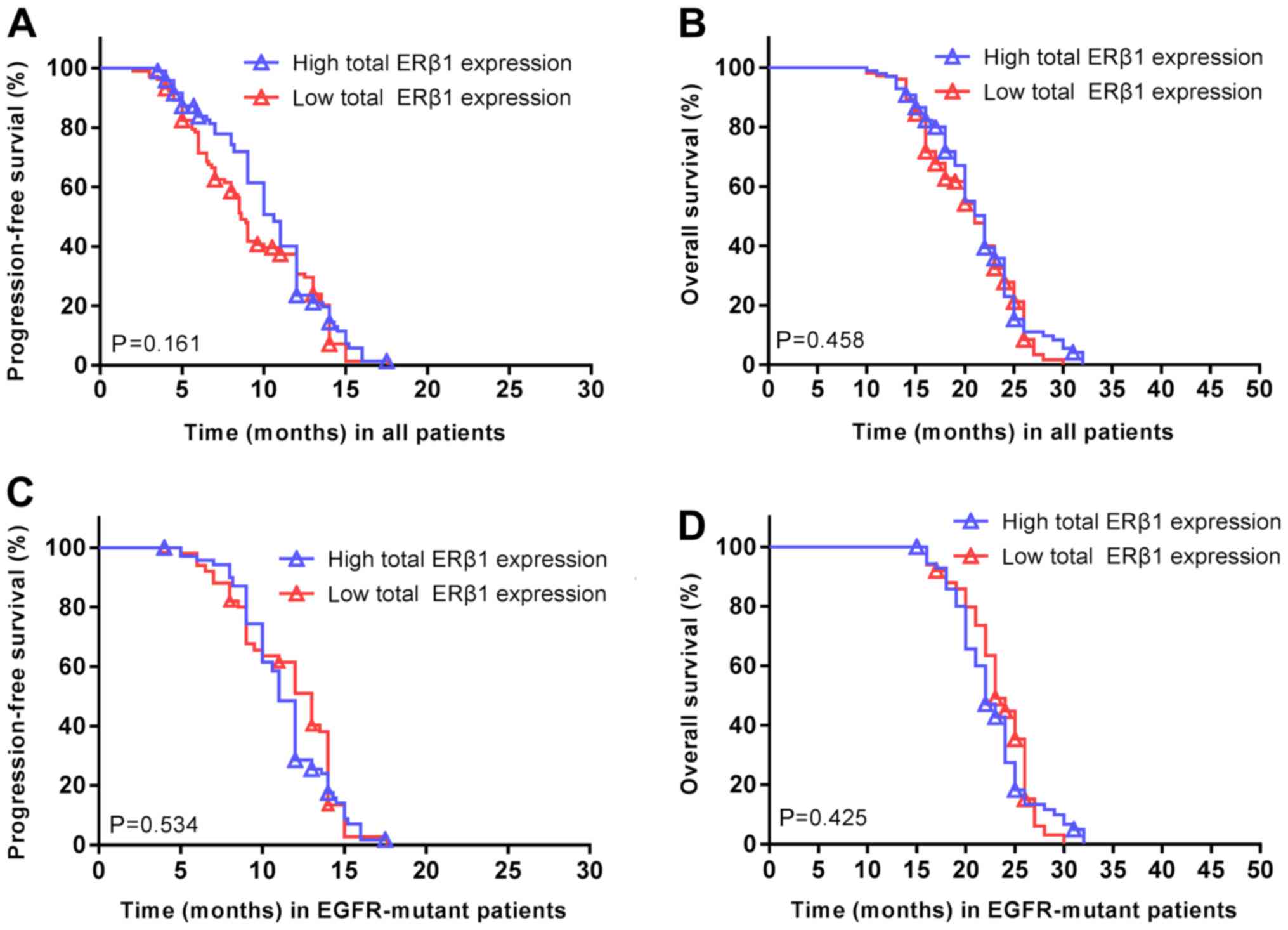
observed between overall ERβ1 expression and the PFS (P=0.161;
Fig. 5A) or OS (P=0.458; Fig. 5B) of all the patients. Similarly,
there was no association between ERβ1 expression and PFS (P=0.534;
Fig. 5C) or OS (P=0.425; Fig. 5D) in patients with EGFR
mutations.
 | Table II.Association of ERβ1 expression with
clinicopathological parameters of 201 lung adenocarcinoma
patients. |
Table II.
Association of ERβ1 expression with
clinicopathological parameters of 201 lung adenocarcinoma
patients.
|
| Cytoplasmic
expression of ERβ1 | Nuclear expression
of ERβ1 | Total expression of
ERβ1 |
|---|
|
|
|
|
|
|---|
| Characteristic | High (n) | Low (n) | P-value | High (n) | Low (n) | P-value | High (n) | Low (n) | P-value |
|---|
| Age (years) |
|
| 0.57 |
|
| 0.29 |
|
| 0.36 |
|
≥65 | 36 | 67 |
| 47 | 56 |
| 47 | 56 |
|
|
<65 | 38 | 60 |
| 52 | 46 |
| 51 | 47 |
|
| Sex |
|
| 0.59 |
|
| 0.41 |
|
| 0.17 |
|
Male | 19 | 37 |
| 25 | 31 |
| 23 | 33 |
|
|
Female | 55 | 90 |
| 74 | 71 |
| 75 | 70 |
|
| Smoking status |
|
| 0.12 |
|
| 0.87 |
|
| 0.19 |
| Ever
smoker | 18 | 44 |
| 30 | 32 |
| 26 | 36 |
|
| Never
smoker | 56 | 83 |
| 69 | 70 |
| 72 | 67 |
|
| Performance
status |
|
| 0.27 |
|
| 0.95 |
|
| 0.34 |
| 0 | 36 | 72 |
| 53 | 55 |
| 56 | 52 |
|
| ≥1 | 38 | 55 |
| 46 | 47 |
| 42 | 51 |
|
| Brain
metastasis |
|
| 0.92 |
|
| 0.45 |
|
| 0.98 |
|
Yes | 42 | 73 |
| 54 | 61 |
| 56 | 59 |
|
| No | 32 | 54 |
| 45 | 41 |
| 42 | 44 |
|
| EGFR-mutant
status |
|
| 0.12 |
|
| 0.40 |
|
| 0.001a |
|
Yes | 50 | 72 |
| 63 | 59 |
| 71 | 51 |
|
| No | 24 | 55 |
| 36 | 43 |
| 27 | 52 |
|
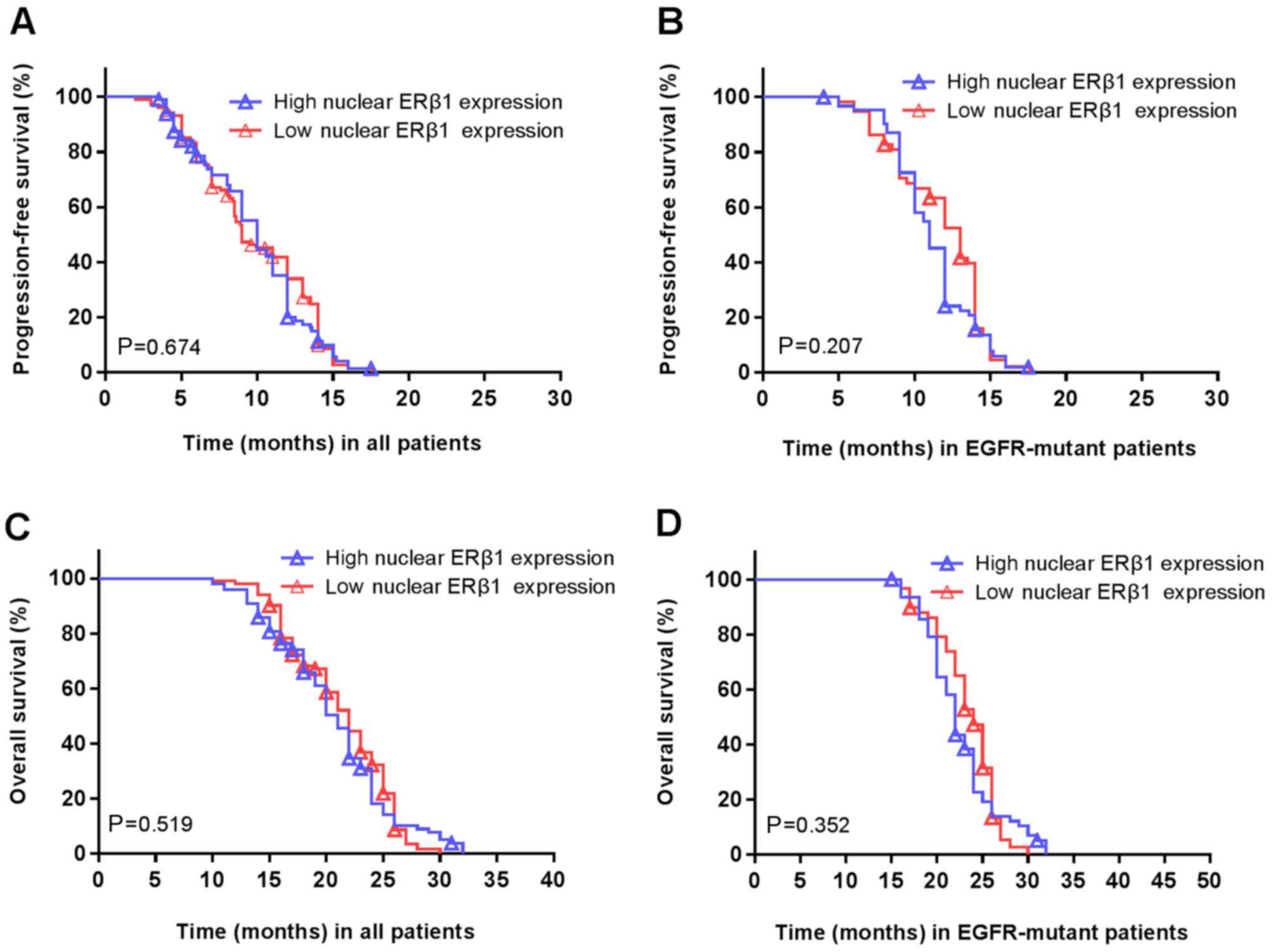
Furthermore, there was no association between
high/low nuclear ERβ1 and the PFS (P=0.674; Fig. 6A) or OS (P=0.207; Fig. 6B). Similarly, there was no
association between nuclear ERβ1 expression and PFS (P=0.519;
Fig. 6C) or OS (P=0.352; Fig. 6D) in patients with EGFR mutations.
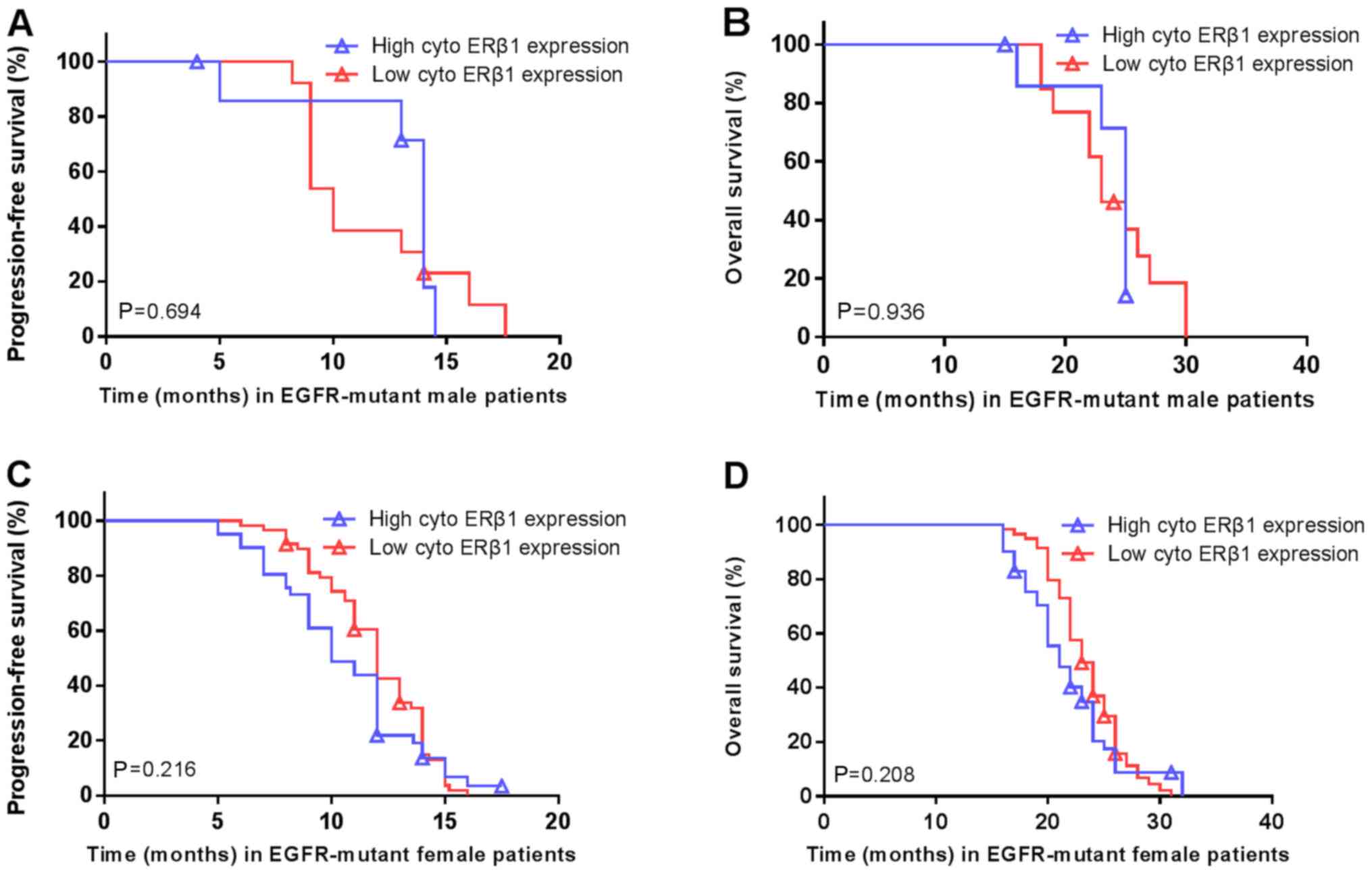
There was no significant difference in the PFS (14 vs. 10 months;
P=0.694; Fig. 7A) or OS (25 vs. 23
months; P=0.936; Fig. 7B) in
patients with high/low cytoplasmic ERβ1 expression in the male
patients carrying EGFR mutations. Similarly, no significant
differences were in PFS (10 vs. 12 months; P=0.216; Fig. 7C) or OS (21 vs. 23 months; P=0.208;
Fig. 7D) were observed in the female
EGFR-mutant patients with high/low expression. There were no
associations between EGFR mutations and nuclear ERβ1 expression or
between patients carrying EGFR mutations and total ERβ1 levels in
males and females (Table II).
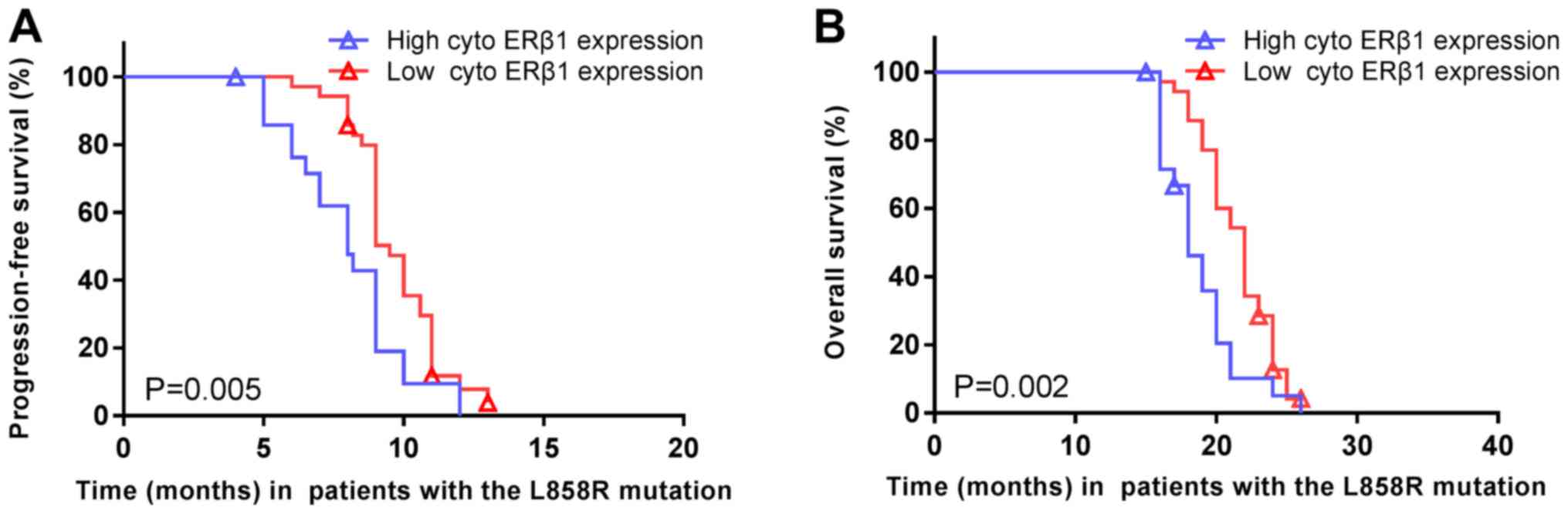
The median PFS of patients carrying EGFR exon 21
L858R mutations was significantly shorter in high cytoplasmic ERβ1
tumors (8.0 months; 95% CI, 6.2–9.8 months) compared with low
cytoplasmic ERβ1-expressing tumors (9.5 months; 95% CI, 8.9–10.1
months; P=0.005; HR=1.977; 95% CI, 1.126–3.469; Fig. 8A). Similarly, the median OS was
significantly shorter in high cytoplasmic ERβ1-expressing tumors
(18.0 months; 95% CI, 16.3–19.7 months) compared with low
cytoplasmic ERβ1-expressing tumors (22.0 months; 95% CI, 20.8–23.2
months; P=0.002; HR=2.217; 95% CI, 1.246–3.945; Fig. 8B). A significant difference between
the numbers of patients with high cytoplasmic ERβ1 expression
compared with low cytoplasmic expression in patients with EGFR
mutations may have skewed the data. A total of 122 (60.7%) had EGFR
mutations, of which, 64 (31.8%) were EGFR Del19 mutations and 58
(28.9%) were EGFR exon 21 L858R mutation. In patients with EGFR
mutations, two patients had the Del19 and an additional T790M
mutation, one patient had a S768I/L858R mutation and one patient
had a 19Del/G719X mutation (Table
III). There were no significant differences in ERβ1 expression
between patients with a EGFR Del19 or exon 21 L858R mutation,
irrespective of the localization of the protein, although it was
observed that the frequency of high cytoplasmic ERβ1 expression was
lower (23 patients, 39.6%) compared with that of low expression (35
patients, 60.1%) in patients with EGFR L858R mutation, which was
similar to patients carrying EGFR Del19 mutations, this difference
was not significant (Table III).
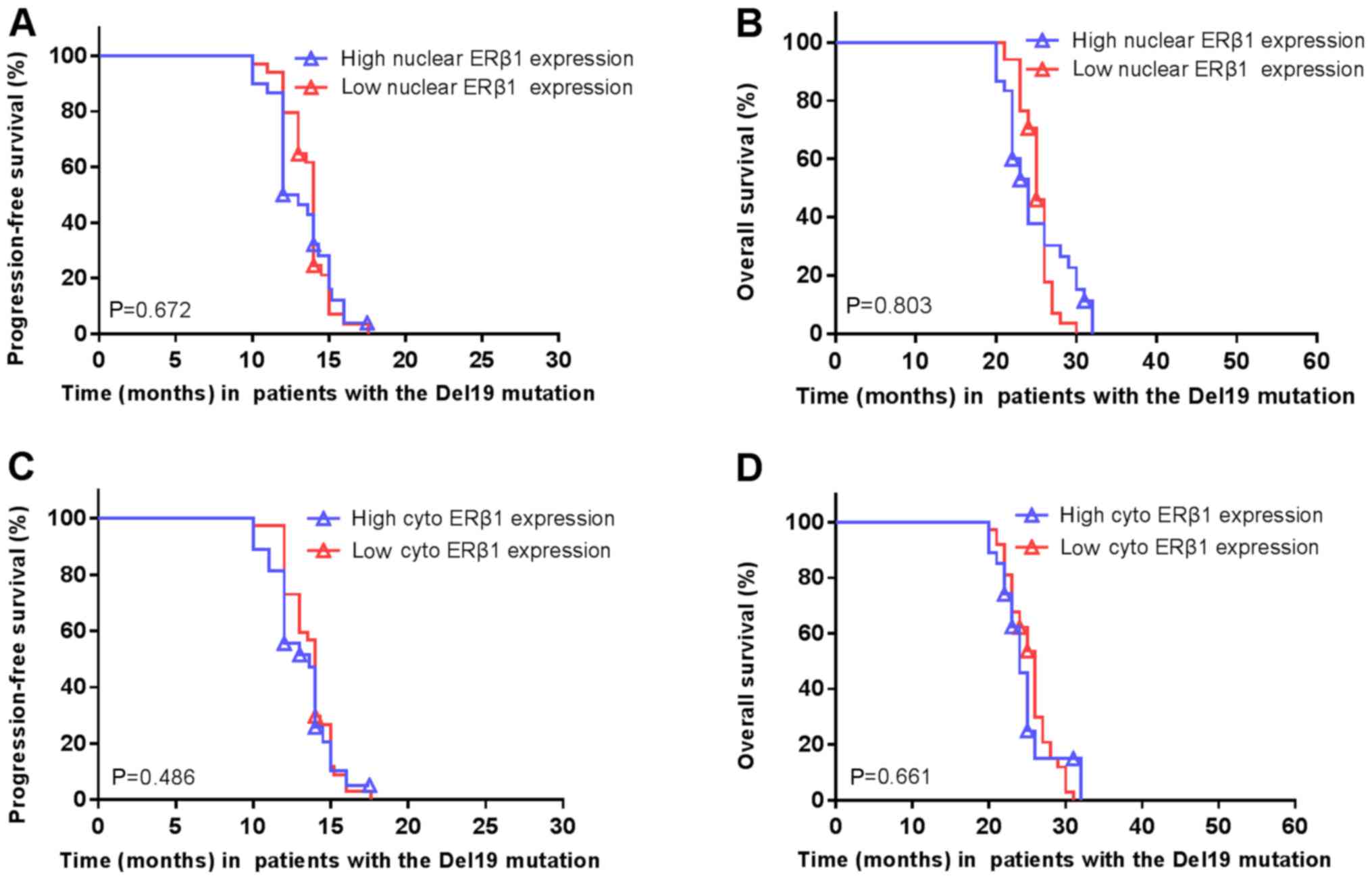
In patients with the Del19 EGFR mutation, the survival rate did not
differ significantly between patients with high or low nuclear ERβ1
expression (Fig. 9A and B,
respectively) or cytoplasmic expression (Fig. 9C and D).
 | Table III.Association of EGFR mutations with
ERβ1 expression in 201 lung adenocarcinoma tissue samples. |
Table III.
Association of EGFR mutations with
ERβ1 expression in 201 lung adenocarcinoma tissue samples.
|
|
| EGFR mutation, n
(%) |
|
|---|
|
|
|
|
|
|---|
| ERβ1
expression | n | Del19 | Exon 21 L858R | P-value |
|---|
| Cytoplasmic |
|
|
| 0.63 |
|
High | 50 | 27 (42.2) | 23 (39.6) |
|
|
Low | 72 | 37 (57.8) | 35 (60.4) |
|
| Nuclear |
|
|
| 0.26 |
|
High | 63 | 30 (47.6) | 33 (52.4) |
|
|
Low | 59 | 34 (57.6) | 25 (42.4) |
|
| Both |
|
|
| 0.64 |
|
High | 71 | 36 (51.9) | 35 (49.1) |
|
|
Low | 51 | 28 (46.8) | 23 (53.2) |
|
Multivariate analysis
The multivariate analysis of ERβ1 expression, age,
sex, tobacco smoking and tumor brain metastasis demonstrated that
only ERβ1 expression was an independent predictor of PFS (HR=2.847;
95% CI, 1.456 to 5.565; P=0.002) and OS (HR=2.639; 95% CI, 1.283 to
5.036; P=0.003) in patients carrying EGFR 21 L858R mutation
(Tables IV and V).
 | Table IV.Multivariate analysis of progression
free survival in patients with the epidermal growth factor receptor
21 L858R mutation. |
Table IV.
Multivariate analysis of progression
free survival in patients with the epidermal growth factor receptor
21 L858R mutation.
| Variable | Hazard ratio | 95% confidence
interval | P-value |
|---|
| Sex | 1.901 | 0.807–4.477 | 0.142 |
| Age (years) | 1.021 | 0.575–1.814 | 0.942 |
| Tumor brain
metastasis | 1.698 | 0.889–3.240 | 0.109 |
| Smoking status | 0.854 | 0.333–2.187 | 0.742 |
| Performance
status | 1.201 | 0.626–2.303 | 0.582 |
| Cytoplasmic
estrogen receptor-β1 expression | 2.847 | 1.456–5.565 | 0.002a |
 | Table V.Multivariate analysis of overall
survival in patients with the epidermal growth factor receptor 21
L858R mutation. |
Table V.
Multivariate analysis of overall
survival in patients with the epidermal growth factor receptor 21
L858R mutation.
| Variable | Hazard ratio | 95% confidence
interval | P-value |
|---|
| Sex | 1.598 | 0.668–3.824 | 0.293 |
| Age (years) | 1.098 | 0.613–1.969 | 0.753 |
| Tumor brain
metastasis | 1.038 | 0.567–1.901 | 0.903 |
| Smoking status | 1.124 | 0.429–2.944 | 0.812 |
| Performance
status | 1.396 | 0.716–2.720 | 0.327 |
| Cytoplasmic
estrogen receptor-β1 expression | 2.639 | 1.383–5.036 | 0.003a |
Discussion
Lung cancer is a major cause of cancer-associated
mortality worldwide (30). Tobacco
smoking is the single largest contributing risk factor in lung
cancer development, although certain patients with lung cancer are
non-smokers, suggesting that other factors are also important in
the pathogenesis of lung cancer. These risk factors may induce
mutations of EGFR or alter expression of other genes (6–16). For
example, aberrant ERβ expression is associated with lung cancer
development and progression (9,11,31), and
the survival of patients with NSCLC (32–36).
Therefore, the present study further assessed ERβ1 expression in
lung adenocarcinoma tissue specimens and determined the outcomes of
patients with or without EGFR mutations following treatment with
EGFR-TKI. The present study demonstrated that 60.7% of patients
carried an EGFR mutation and the majority of these mutations were
the EGFR Del19 and exon 21 L858R mutations. The presence of EGFR
mutations was significantly higher in females than male patients,
and in non-smokers compared with smokers. The median PFS of the
cohort of patients was 10 months, whereas the median OS was 21
months. Patients with EGFR mutations had a significantly improved
median PFS compared with patients without EGFR mutations following
treatment with EGFR-TKIs, and the median PFS was also longer in
patients with EGFR Del19 compared with the EGFR exon 21 L858R
mutation. Additionally, the median OS was significantly improved in
patients with EGFR mutations compared with patients without EGFR
mutations. In addition, ERβ1 expression was increased in patients
with EGFR mutations compared with patients without EGFR mutations.
The median PFS and OS were significantly shorter in patients with
the EGFR exon 21 L858R mutation, and in patients with cytoplasmic
ERβ1-expressed tumor. Multivariate analysis demonstrated that only
ERβ1 expression was an independent predictor of PFS and OS in
patients carrying EGFR 21 L858R mutation.
The present data on EGFR mutations and association
with improved PFS and OS of patients with stage IV lung
adenocarcinoma following treatment with EGFR-TKI are consistent
with earlier studies (2,3). A significantly longer PFS and OS in
patients with EGFR Del19 compared with patients with the EGFR exon
21 L858R mutations was observed, which is also consistent with
previous studies (23,24,37). The
present data highlighted the importance of the EGFR mutation status
in association with survival of patients with lung adenocarcinoma
following treatment with EGFR-TKI. Previous studies demonstrated
that a high frequency of EGFR mutations occurred in Asian patients
with advanced non-tobacco smoking lung adenocarcinoma (38) and treatment of these patients with
EGFR-TKI may effectively control disease progression and prolong
PFS (20).
The incidence of lung adenocarcinoma is increasing
in a number of countries; for example, ~40% of all lung cancer
cases are adenocarcinomas in the US (39). Lung adenocarcinoma can occur in
tobacco smokers and non-smokers (39,40), and
can carry a number of gene mutations, including KRAS, EGFR (20%),
HER2 (2%), ALK receptor tyrosine kinase, BRAF, PIK3CA, MET or p53
(41). EGFR mutations in lung
adenocarcinoma were first identified in 2004 and more frequent in
East Asia compared with Western countries (42,43).
EGFR mutations were identified in exon 18 G719S, exon 19 G719C, and
exon 21 L858R or L861Q, each of which results in constitutive
activation of the EGFR as an oncogene (44), and are associated with treatment
responses to gefitinib and erlotinib (41).
The present data demonstrated that ERβ1 expression
was increased in lung adenocarcinoma tissues, and additionally ERβ1
expression was significantly increased in patients with EGFR
mutations compared with patients without EGFR mutations, which is
consistent with previous publications in patients with lung
adenocarcinoma (13,14,45).
Estrogen can downregulate levels of EGFR expression, whereas EGF
can downregulate the level of ERβ expression in NSCLC cell lines
(46). Tamoxifen, a selective
estrogen-receptor modulator, upregulates EGFR expression, whereas
gefitinib, an EGFR-TKI, upregulates ERβ expression (47), suggesting that ERβ signaling
interacts with EGFR signaling in patients with lung adenocarcinoma.
Furthermore, the present study assessed the association between
ERβ1 expression and outcome of lung adenocarcinoma patients with
EGFR mutations following treatment with EGFR-TKI; however, no
significant differences in survival were observed among patients
with high or low total, nuclear or cytoplasmic ERβ1 expression.
However, multivariate analysis demonstrated that high cytoplasmic
ERβ1 expression was associated with worse PFS and OS in patients
with the EGFR exon 21 L858R mutation following treatment with
EGFR-TKI. In contrast to the present study, a previous study
demonstrated that increases in ERβ1 expression in the cytoplasm or
nucleus (independently; however, not simultaneously) was associated
with poorer prognosis in patients with EGFR-mutated lung
adenocarcinoma (16). Another
previous study demonstrated that strong ERβ1 expression was
associated with improved prognosis in patients with lung
adenocarcinoma treated with EGFR-TKI (15). A possible explanation for the
discrepancy between these two studies and the present data may stem
from the previous studies not differentiating between patients with
EGFR Del19 or exon 21 L858R mutations. However, it is unclear why
there was no association of cytoplasmic ERβ1 expression with
prognosis of patients with the Del19 EGFR. Estrogen activates ER
and EGFR signaling in cells (48)
and ERβ1 expression in NSCLC may be involved in EGFR-TKI resistance
in the treatment of patients with NSCLC. Fu et al (49) recently demonstrated that ERβ1 induced
Erk1/2 and Akt activation, which may have resulted in EGFR-TKI
resistance. Ma et al (50)
demonstrated that ER directly binds to EGFR to confer tumor cell
resistance to EGFR-TKIs, and that a combination of an EGFR-TKI with
anti-estrogen therapy may induce tumor cell sensitivity to
EGFR-TKI. However, EGFR Del19 or exon 21 L858R mutants are
translated into different EGFR protein structures (51). Specifically, in the wild-type EGFR
NSCLC, the C-helix is outward rotated and the N-terminal portion of
the activation loop forms a helical turn that locks the C-helix in
the inactive position. However, the mutant EGFR could destabilize
the inactive conformation, e.g., the EGFR L858R mutation has a much
larger charged side chain, which will not be able to be contained
in the inactive conformation, but can be subsumed within the active
and reorganized form of the enzyme (52). Therefore, it is hypothesized that
cytoplasmic ERβ1 binds more tightly to the EGFR 21 L858R mutant
protein compared with the Del19 mutant EGFR to confer resistance to
TKIs. However, further studies are required to confirm this.
The present study has certain limitations. For
example, only immunohistochemistry was performed to analyze ERβ1
expression in lung cancer tissues; however, RT-qPCR does not easily
allow for the determination of the subcellular localization
(nuclear or cytoplasmic) of expression in tumor cells and tissue
specimens and the presence of a mix of stromal and tumor cells may
further complicate the analysis. Furthermore, the All red scoring
system of immunohistochemical ERβ1 expression in tissue specimens
was performed according to previous studies (14,26) and
the median Allred score was defined as the cutoff value of high vs.
low expression, which is different from previously published
studies that used other scoring systems and cut-off values. Thus, a
standardized scoring system is required for ERβ expression in NSCLC
tissue specimens. Furthermore, the present study had a relatively
small sample size and a future study with a larger sample size with
multi-institutional participation is preferable to confirm the
findings.
The present study demonstrated that cytoplasmic ERβ1
expression was associated with poor prognosis of patients with
stage IV lung adenocarcinoma carrying EGFR exon 21 L858R mutation
subsequent to treatment with EGFR-TKI.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Anhui Provincial Cancer Hospital (Hefei, China;
approval no. 201705).
Authors' contributions
YH and CH conceived and designed the experiments.
Data collection and experiments were performed by MZ, JW and HL.
XH, YF, JZ and WC analyzed the data and all authors contributed to
the writing of the manuscript.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
DeSantis CE, Lin CC, Mariotto AB, Siegel
RL, Stein KD, Kramer JL, Alteri R, Robbins AS and Jemal A: Cancer
treatment and survivorship statistics, 2014. CA Cancer J Clin.
64:252–271. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rosell R, Carcereny E, Gervais R,
Vergnenegre A, Massuti B, Felip E, Palmero R, Garcia-Gomez R,
Pallares C, Sanchez JM, et al: Erlotinib versus standard
chemotherapy as first-line treatment for European patients with
advanced EGFR mutation-positive non-small-cell lung cancer
(EURTAC): A multicentre, open-label, randomised phase 3 trial.
Lancet Oncol. 13:239–246. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhou C, Wu YL, Chen G, Feng J, Liu XQ,
Wang C, Zhang S, Wang J, Zhou S, Ren S, et al: Erlotinib versus
chemotherapy as first-line treatment for patients with advanced
EGFR mutation-positive non-small-cell lung cancer (OPTIMAL,
CTONG-0802): A multicentre, open-label, randomised, phase 3 study.
Lancet Oncol. 12:735–742. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ettinger DS, Wood DE, Aisner DL, Akerley
W, Bauman J, Chirieac LR, D'Amico TA, DeCamp MM, Dilling TJ,
Dobelbower M, et al: Non-small cell lung cancer, version 5.2017,
NCCN clinical practice guidelines in oncology. J Natl Compr Canc
Netw. 15:504–535. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Novello S, Barlesi F, Califano R, Cufer T,
Ekman S, Levra MG, Kerr K, Popat S, Reck M, Senan S, et al:
Metastatic non-small-cell lung cancer: ESMO clinical practice
guidelines for diagnosis, treatment and follow-up. Ann Oncol. 27
(Suppl 5):v1–v27. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kobayashi S, Boggon TJ, Dayaram T, Jänne
PA, Kocher O, Meyerson M, Johnson BE, Eck MJ, Tenen DG and Halmos
B: EGFR mutation and resistance of non-small-cell lung cancer to
gefitinib. N Engl J Med. 352:786–792. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Morgillo F, Della Corte CM, Fasano M and
Ciardiello F: Mechanisms of resistance to EGFR-targeted drugs: Lung
cancer. ESMO Open. 1:e0000602016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Thomas C and Gustafsson JA: The different
roles of ER subtypes in cancer biology and therapy. Nat Rev Cancer.
11:597–608. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Siegfried JM and Stabile LP: Estrongenic
steroid hormones in lung cancer. Semin Oncol. 41:5–16. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Marquez-Garban DC, Chen HW, Fishbein MC,
Goodglick L and Pietras RJ: Estrogen receptor signaling pathways in
human non-small cell lung cancer. Steroids. 72:135–143. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Siegfried JM: Smoking out reproductive
hormone actions in lung cancer. Mol Cancer Res. 12:24–31. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Stabile LP and Siegfried JM: Estrogen
receptor pathways in lung cancer. Curr Oncol Rep. 6:259–267. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
He Q, Zhang M, Zhang J, Chen Y, He J, Shen
J, Liu Y, Zhong S, Jiang L, Yang C, et al: Correlation between
epidermal growth factor receptor mutations and nuclear expression
of female hormone receptors in non-small cell lung cancer: A
meta-analysis. J Thorac Dis. 7:1588–1594. 2015.PubMed/NCBI
|
|
14
|
Nose N, Sugio K, Oyama T, Nozoe T, Uramoto
H, Iwata T, Onitsuka T and Yasumoto K: Association between estrogen
receptor-beta expression and epidermal growth factor receptor
mutation in the postoperative prognosis of adenocarcinoma of the
lung. J Clin Oncol. 27:411–417. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nose N, Uramoto H, Iwata T, Hanagiri T and
Yasumoto K: Expression of estrogen receptor beta predicts a
clinical response and longer progression-free survival after
treatment with EGFR-TKI for adenocarcinoma of the lung. Lung
Cancer. 71:350–355. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang Z, Li Z, Ding X, Shen Z, Liu Z, An T,
Duan J, Zhong J, Wu M, Zhao J, et al: ERβ localization influenced
outcomes of EGFR-TKI treatment in NSCLC patients with EGFR
mutations. Sci Rep. 5:113922015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lim VW, Lim WY, Zhang Z, Li J, Gong Y,
Seow A and Yong EL: Serum estrogen receptor beta mediated
bioactivity correlates with poor outcome in lung cancer patients.
Lung Cancer. 85:293–298. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Leung YK, Mak P, Hassan S and Ho SM:
Estrogen receptor (ER)-beta isoforms: A key to understanding
ER-beta signaling. Proc Natl Acad Sci USA. 103:13162–13167. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tu HY, Ke EE, Yang JJ, Sun YL, Yan HH,
Zheng MY, Bai XY, Wang Z, Su J, Chen ZH, et al: A comprehensive
review of uncommon EGFR mutations in patients with non-small cell
lung cancer. Lung Cancer. 114:96–102. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dahabreh IJ, Linardou H, Siannis F,
Kosmidis P, Bafaloukos D and Murray S: Somatic EGFR mutation and
gene copy gain as predictive biomarkers for response to tyrosine
kinase inhibitors in non-small cell lung cancer. Clin Cancer Res.
16:291–303. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Castellanos E, Feld E and Horn L: Driven
by mutations: The predictive value of mutation subtype in
EGFR-mutated non-small cell lung cancer. J Thorac Oncol.
12:612–623. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yu HA, Arcila ME, Hellmann MD, Kris MG,
Ladanyi M and Riely GJ: Poor response to erlotinib in patients with
tumors containing baseline EGFR T790M mutations found by routine
clinical molecular testing. Ann Oncol. 25:423–428. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Choi YW, Jeon SY, Jeong GS, Lee HW, Jeong
SH, Kang SY, Park JS, Choi JH, Koh YW, Han JH and Sheen SS: EGFR
Exon 19 deletion is associated with favorable overall survival
after first-line gefitinib therapy in advanced non-small cell lung
cancer patients. Am J Clin Oncol. 41:385–390. 2018.PubMed/NCBI
|
|
24
|
Yang JC, Wu YL, Schuler M, Sebastian M,
Popat S, Yamamoto N, Zhou C, Hu CP, O'Byrne K, Feng J, et al:
Afatinib versus cisplatin-based chemotherapy for EGFR
mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6):
Analysis of overall survival data from two randomised, phase 3
trials. Lancet Oncol. 16:141–151. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mirsadraee S, Oswal D, Alizadeh Y, Caulo A
and van Beek E Jr: The 7th lung cancer TNM classification and
staging system: Review of the changes and implications. World J
Radiol. 4:128–134. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Malalasekera A, Tan CSY, Phan V, Yip PY,
Vardy J, Clarke SJ and Kao S: Eastern cooperative oncology group
score: Agreement between non-small-cell lung cancer patients and
their oncologists and clinical implications. Cancer Treat Commun.
5:17–21. 2016. View Article : Google Scholar
|
|
27
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lopez-Rios F, Angulo B, Gomez B, Mair D,
Martinez R, Conde E, Shieh F, Tsai J, Vaks J, Current R, et al:
Comparison of molecular testing methods for the detection of EGFR
mutations in formalin-fixed paraffin-embedded tissue specimens of
non-small cell lung cancer. J Clin Pathol. 66:381–385. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Abe K, Miki Y, Ono K, Mori M, Kakinuma H,
Kou Y, Kudo N, Koguchi M, Niikawa H, Suzuki S, et al: Highly
concordant coexpression of aromatase and estrogen receptor beta in
non-small cell lung cancer. Hum Pathol. 41:190–198. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen W, Zheng R, Zhang S, Zeng H, Fan Y,
Qiao Y and Zhou Q: Esophageal cancer incidence and mortality in
China, 2010. Thorac Cancer. 5:343–348. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Burns TF and Stabile LP: Targeting the
estrogen pathway for the treatment and prevention of lung cancer.
Lung Cancer Manag. 3:43–52. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hsu LH, Liu KJ, Tsai MF, Wu CR, Feng AC,
Chu NM and Kao SH: Estrogen adversely affects the prognosis of
patients with lung adenocarcinoma. Cancer Sci. 106:51–59. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ma L, Zhan P, Liu Y, Zhou Z, Zhu Q, Miu Y,
Wang X, Jin J, Li Q, Lv T and Song Y: Prognostic value of the
expression of estrogen receptor β in patients with non-small cell
lung cancer: A meta-analysis. Transl Lung Cancer Res. 5:202–207.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Schwartz AG, Prysak GM, Murphy V, Lonardo
F, Pass H, Schwartz J and Brooks S: Nuclear estrogen receptor beta
in lung cancer: Expression and survival differences by sex. Clin
Cancer Res. 11:7280–7287. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Skov BG, Fischer BM and Pappot H:
Oestrogen receptor beta over expression in males with non-small
cell lung cancer is associated with better survival. Lung Cancer.
59:88–94. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Skjefstad K, Grindstad T, Khanehkenari MR,
Richardsen E, Donnem T, Kilvaer T, Andersen S, Bremnes RM, Busund
LT and Al-Saad S: Prognostic relevance of estrogen receptor α, β
and aromatase expression in non-small cell lung cancer. Steroids.
113:5–13. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sutiman N, Tan SW, Tan EH, Lim WT,
Kanesvaran R, Ng QS, Jain A, Ang MK, Tan WL, Toh CK and Chowbay B:
EGFR mutation subtypes influence survival outcomes following
first-line gefitinib therapy in advanced asian NSCLC patients. J
Thorac Oncol. 12:529–538. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shi Y, Au JS, Thongprasert S, Srinivasan
S, Tsai CM, Khoa MT, Heeroma K, Itoh Y, Cornelio G and Yang PC: A
prospective, molecular epidemiology study of EGFR mutations in
asian patients with advanced non-small-cell lung cancer of
adenocarcinoma histology (PIONEER). J Thorac Oncol. 9:154–162.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Stewart BW and Wild CP: World Cancer
Report 2014. World Health Organization, IARC Publications. (Lyon,
France). 489–508. 2014.
|
|
40
|
Pao W, Miller V, Zakowski M, Doherty J,
Politi K, Sarkaria I, Singh B, Heelan R, Rusch V, Fulton L, et al:
EGF receptor gene mutations are common in lung cancers from ‘never
smokers’ and are associated with sensitivity of tumors to gefitinib
and erlotinib. Proc Natl Acad Sci USA. 101:13306–13311. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Greulich H: The genomics of lung
adenocarcinoma: Opportunities for targeted therapies. Genes Cancer.
1:1200–1210. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Paez JG, Jänne PA, Lee JC, Tracy S,
Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, et
al: Mutations in lung cancer: Correlation with clinical response to
gefitinib therapy. Science. 304:1497–1500. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lynch TJ, Bell DW, Sordella R,
Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat
SM, Supko JG, Haluska FG, et al: Activating mutations in the
epidermal growth factor receptor underlying responsiveness of
non-small-cell lung cancer to gefitinib. N Engl J Med.
350:2129–2139. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Greulich H, Chen TH, Feng W, Jänne PA,
Alvarez JV, Zappaterra M, Bulmer SE, Frank DA, Hahn WC, Sellers WR
and Meyerson M: Oncogenic transformation by inhibitor-sensitive and
-resistant EGFR mutants. PLoS Med. 2:e3132005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hsu LH, Chu NM and Kao SH: Estrogen,
estrogen receptor and lung cancer. Int J Mol Sci. 18:E17132017.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Stabile LP, Lyker JS, Gubish CT, Zhang W,
Grandis JR and Siegfried JM: Combined targeting of the estrogen
receptor and the epidermal growth factor receptor in non-small cell
lung cancer shows enhanced antiproliferative effects. Cancer Res.
65:1459–1470. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Shen H, Yuan Y, Sun J, Gao W and Shu YQ:
Combined tamoxifen and gefitinib in non-small cell lung cancer
shows antiproliferative effects. Biomed Pharmacother. 64:88–92.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhao XZ, Liu Y, Zhou LJ, Wang ZQ, Wu ZH
and Yang XY: Role of estrogen in lung cancer based on the estrogen
receptor-epithelial mesenchymal transduction signaling pathways.
OncoTargets Ther. 8:2849–2863. 2015. View Article : Google Scholar
|
|
49
|
Fu S, Liu C, Huang Q, Fan S, Tang H, Fu X,
Ai B, Liao Y and Chu Q: Estrogen receptor β1 activation accelerates
resistance to epidermal growth factor receptor-tyrosine kinase
inhibitors in non-small cell lung cancer. Oncol Rep. 39:1313–1321.
2018.PubMed/NCBI
|
|
50
|
Ma S, Yin N, Qi X, Pfister SL, Zhang MJ,
Ma R and Chen G: Tyrosine dephosphorylation enhances the
therapeutic target activity of epidermal growth factor receptor
(EGFR) by disrupting its interaction with estrogen receptor (ER).
Oncotarget. 6:13320–13333. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Rosell R, Taron M, Reguart N, Isla D and
Moran T: Epidermal growth factor receptor activation: How exon 19
and 21 mutations changed our understanding of the pathway. Clin
Cancer Res. 12:7222–7231. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yun CH, Boggon TJ, Li Y, Woo MS, Greulich
H, Meyerson M and Eck MJ: Structures of lung cancer-derived EGFR
mutants and inhibitor complexes: Mechanism of activation and
insights into differential inhibitor sensitivity. Cancer Cell.
11:217–227. 2007. View Article : Google Scholar : PubMed/NCBI
|