Introduction
The anterior pituitary gland is a key regulator of
the endocrine system. During life, it undergoes extensive
remodeling in response to metabolic changes and is therefore prone
to tumor formation. Pituitary tumors are benign neoplasms that may
lead to marked morbidity owing to excess hormone secretion and/or
compression of surrounding brain structures (1). Pituitary adenoma is formed by
monoclonal proliferation of gland cells in the anterior pituitary
(2,3). Pathogenesis and effective treatment of
pituitary adenoma have been widely explored, but the molecular
mechanism is not fully understood.
Specific tumor-initiating and tumor-promoting
factors have been characterized in animal models as well as in
human tissue samples. In the last few years, the cancer stem cell
(CSC) theory was introduced that states that a specific
subpopulation of tumor cells possessing distinct stem cell
properties by the expression of sex-determining region Y box 2
(SOX2), octamer-binding factor 4, Krüppel-like factor 4 and NANOG,
is proposed to persist in tumors and lead to new tumors (4). The existence of CSCs in pituitary
adenomas is controversial, but previous studies have demonstrated
that a cluster of SOX2+ cells was able to maintain
pituitary homeostasis and potentially contribute to pituitary
tumors in a mice model (5,6).
The Sonic hedgehog (Shh) signaling pathway is also
involved in pituitary formation. Following the appearance of
Rathke's pouch, Shh expression is excluded from this region, but
remains in the surrounding areas (7). Furthermore, the Shh signaling pathway
serves an important function in adult stem cell maintenance
(8). Shh signaling pathway is
frequently activated in several types of human cancer, and may
contribute to tumorigenesis and progression by maintaining CSC
character (9,10).
The Wnt/β-catenin signaling pathway serves a
critical function in the control of cellular proliferation and
differentiation during embryonic development and organogenesis
(11). Deregulation of Wnt/β-catenin
signaling leads to pathological processes, including various types
of cancer (12). In several types of
cancer, aberrant Wnt/β-catenin signaling is able to mediate CSCs to
promote tumorigenesis. Previous studies have highlighted
alterations of β-catenin in pituitary tumors (13,14).
The aim of the present study was to investigate
whether the Shh signaling pathway is able to initiate pituitary
adenoma by regulating the character of CSCs. Single treatment with
Shh or Wnt was not able to promote the proliferation of the
pituitary adenoma cell line HP75. However, western blot analysis
revealed that Shh triggered the upregulation of SOX2 which is
characteristic of CSCs. The Wnt/β-catenin signaling pathway was
identified to promote the proliferation of SOX2-expressing cells.
Inhibition of SOX2 expression disrupted the crosstalk between the
Shh and Wnt/β-catenin signaling pathways which inhibited cell
proliferation. SOX2 was identified to promote the proliferation of
pituitary adenoma cells by mediating crosstalk between the Shh and
Wnt/β-catenin signaling pathways.
Materials and methods
Plasmids, short interfering RNA
(siRNA) and transient transfection
The complete SOX2-coding sequence was amplified
using the polymerase chain reaction (PCR) using primers
5′-GATCGCTAGCATGTACAACATGATGGAGACGG-3′ (forward) and
5′-GATCGCGGCCGCTCACATGTGTGAGAGGGGCAGTGTG-3′ (reverse). cDNA from
HP75 cells was used as template. Pfu DNA polymerase (Agilent
Technologies, Inc., Santa Clara, CA, USA) was used for PCR
according to the manufacturer's protocol. The thermocycling
conditions for SOX2 were: 95°C for 2 min, and then 30 cycles of
95°C for 30 sec and 60°C for 1 min and 72°C for 1 min, final
extension at 72°C for 5 min using the C1000-Touch Thermal Cycler
(Bio-Rad Laboratories, Inc. Hercules, CA, USA). The complete
β-catenin-coding sequence was amplified by PCR using primers
5′-GATCGCTAGCATGGCTACTCAAGCTGATTTGATGG-3′ (forward) and
5′-GATCGCGGCCGCTTACAGGTCAGTATCAAACCAGGC-3′ (reverse). The
thermocycling conditions for β-catenin were: 95°C for 2 min, and
then 30 cycles of 95°C for 30 sec and 60°C for 1 min and 72°C for 2
min 30 sec, final extension at 72°C for 5 min using the C1000-Touch
Thermal Cycler (Bio-Rad Laboratories, Inc.). The PCR products were
purified and cloned into pCDH lentivirus vector using NheI/NotI.
Lipofectamine™ 2000 (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) was used to perform transient transfection
according to the manufacturer's protocol.
The SOX2 RNAi sequence was
5′-GUUCUAGUGGUACGGUAGG-3′. The scramble sequence was
5′-UCCUUGCUCCUUCGAAUGU-3′. Lipofectamine RNAiMAX (Invitrogen;
Thermo Fisher Scientific, Inc.) was used to perform transient
transfection of scramble siRNA or siRNA targeting SOX2 according to
the manufacturer's protocol.
Cell culture and treatment
HP75 cells were purchased from the American Type
Culture Collection (Manassas, VA, USA) and were routinely cultured
in Dulbecco's modified Eagle's medium containing 15% horse serum,
2.5% fetal bovine serum, 0.05% glutamine, 100 µg/ml gentamicin and
100 IU/ml penicillin at 37°C in a humidified atmosphere containing
5% CO2. The cell line was passaged three times prior to
use in the experiments.
For the proliferation assay, cells were seeded in
24-well plates and serum-starved for 24 h, then cells were treated
with Shh (0.5, 1 or 5 µg/ml; R&D Systems, Inc., Minneapolis,
MN, USA) or Wnt3a (5, 10 or 50 ng/ml; R&D Systems, Inc.) for 48
h, using PBS as a negative control.
RNA extraction and reverse
transcription (RT)-quantitative (q)PCR
Total RNA was extracted from HP75 cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.), according to the manufacturer's protocol. cDNA was generated
using an iScript cDNA synthesis kit (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA), according to the manufacturer's protocol.
SYBR-Green qPCR for GAPDH and SOX2 was performed using iQ™
SYBR® Green Supermix following manufacturer's protocol
(Bio-Rad Laboratories, Inc.). The thermocycling conditions were:
95°C for 3 min, and then 40 cycles of 95°C for 15 s and 60°C for 1
mi using the CFX 96 realtime PCR machine (Bio-Rad Laboratories,
Inc.). Primers for SOX2 were 5′-GGGAAATGGGAGGGGTGCAAAAGAGG-3′
(forward) and 5′-TTGCGTGAGTGTGGATGGGATTGGTG-3′ (reverse). Primers
for GAPDH were 5′-TTCCAATATGATTCCACCCA-3′ (forward) and
5′-ATGACAAGCTTCCCGTTCTC-3′ (reverse).
Western blotting
HP75 cells were harvested following the indicated
treatment and lysed in SDS sample buffer. The protein concentration
determination was performed using a BCA kit (Pierce; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. Samples
(50 μg) were loaded per lane. Polyacrylamide gels (4–12%) were used
and the proteins in the gels were transferred onto PVDF membranes
(Bio-Rad Laboratories, Inc.). The membranes were blocked with 5%
non-fat milk in TBST (TBS with 0.05% Tween 20) for 1 h at room
temperature, and the indicated primary antibodies were incubated
shaking slowly overnight at 4°C. Membranes were washed in TBST 10
min 3 times and incubated with secondary antibody for 1 h at room
temperature, then membranes were washed in TBST 10 min 3 times.
Antibodies used were anti-Ptch1 (sc-518044) (1:200; Santa Cruz
Biotechnology, Inc.), anti-SMO (sc-166685) (1:100; Santa Cruz
Biotechnology, Inc.), anti-Gli1 (sc-6152) (1:100; Santa Cruz
Biotechnology, Inc.), anti-SOX2 (sc-365964) (1:100; Santa Cruz
Biotechnology, Inc.), anti-Frizzled (sc-398082) (1:200; Santa Cruz
Biotechnology, Inc.), anti-Dsh (sc-8025) (1:500; Santa Cruz
Biotechnology, Inc.), anti-phospho-β-catenin (sc-57535) (1:500;
Santa Cruz Biotechnology, Inc.), anti-β-catenin (sc-59737) (1:500;
Santa Cruz Biotechnology, Inc.). Secondary antibodies were: Donkey
anti-rabbit, horseradish peroxidase (HRP)-conjugate (sc-2313);
donkey anti-mouse, HRP-conjugate (sc-2314); and donkey anti-goat,
HRP-conjugate (sc-2020) (1:5,000; Santa Cruz Technology, Inc.).
Detection was performed using an enhanced chemiluminescence kit
(Pierce; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. β-actin (sc-47778) (1:5,000; Santa Cruz
Biotechnology, Inc.) was used as a loading control.
Cell proliferation assay
Cell proliferation was determined by cell counting.
Briefly, cells were seeded in 24-well plates at low density
(5×104) and cultured overnight. Cells were incubated
with/without the indicated reagent and cultured for 48 h,
trypsinized and resuspended in PBS, and finally stained with trypan
blue. Viable cells were counted using a hemocytometer at ×100
magnification under a light microscope.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism (version 6.0; GraphPad Software, Inc., La Jolla, CA, USA).
For multiple comparisons, analysis of variance with Bonferroni's
post hoc test was applied. Results are presented as the mean ±
standard error of the mean (SEM). All experiments were repeated at
least three times with reproducible results. P<0.05 was
considered to indicate a statistically significant difference.
Results
Activation of the Shh signaling
pathway does not promote proliferation of pituitary adenoma
cells
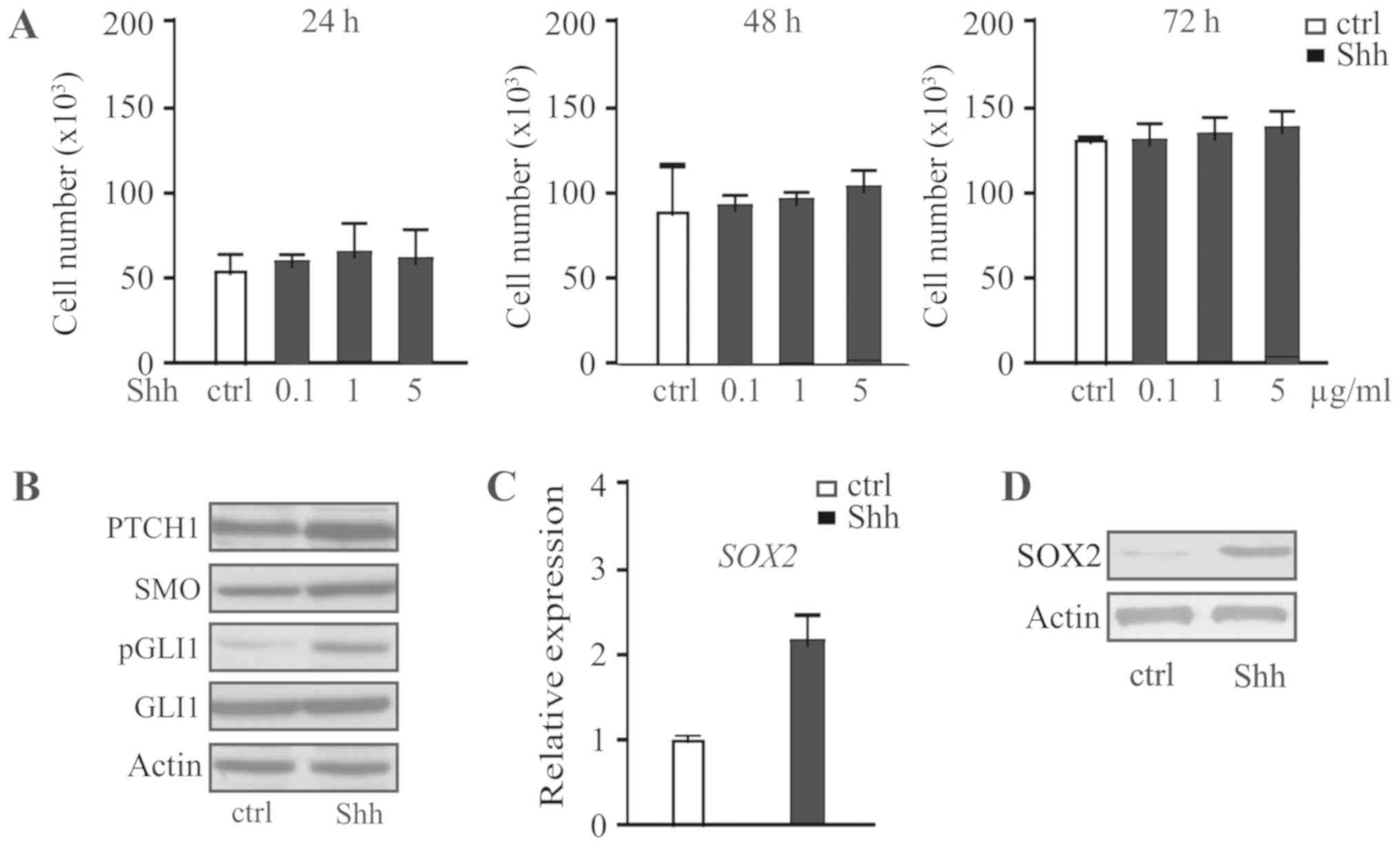
To investigate the effect of Shh signaling on the
progression of pituitary adenoma, the human pituitary adenoma cell
line HP75 was treated with different concentrations of recombinant
Shh for 24, 48 or 72. Shh was identified to have no significant
effect on cell proliferation regardless of the concentration or
duration (Fig. 1A). Western blot
analysis identified that HP75 cells express Ptch1, SMO and Gli1
following Shh treatment (Fig. 1B).
The expression of the stem cell marker SOX2 was investigated, and
it was identified that Shh upregulated the expression of SOX2 at
the mRNA (Fig. 1C) and protein level
(Fig. 1D). Therefore, activation of
the Shh signaling pathway only was identified to have no effect on
pituitary adenoma cell proliferation.
Activation of the Wnt/β-catenin
signaling pathway does not promote proliferation of pituitary
adenoma cells
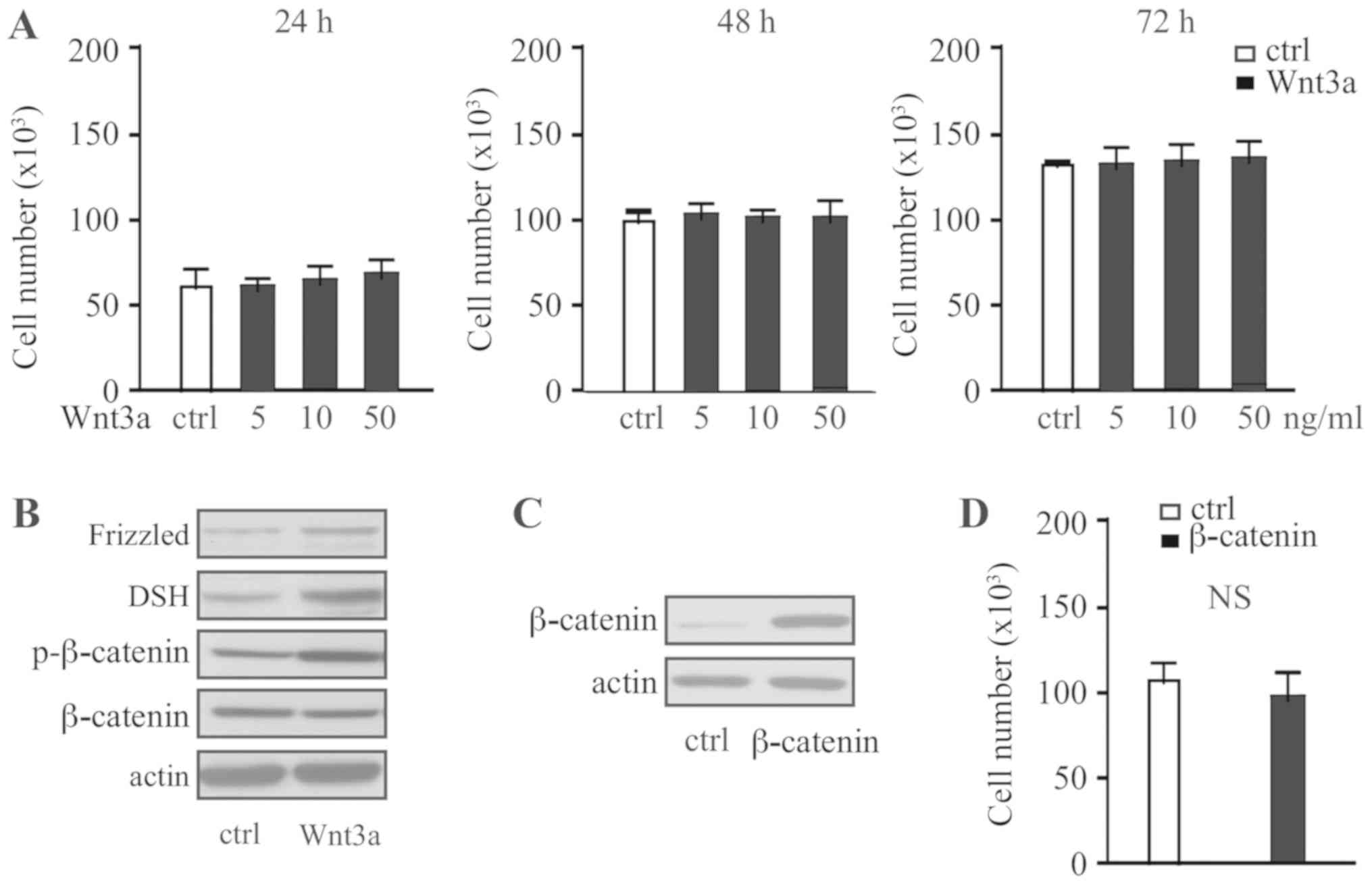
To investigate the effect of Wnt signaling on the
progression of pituitary adenoma, the human pituitary adenoma cell
line HP75 was treated with different concentrations of recombinant
Wnt3a for 24, 48 or 72 h. Wnt3a was identified to have no
significant effect on the cell proliferation regardless of
concentration or duration (Fig. 2A).
Western blot analysis identified that HP75 cells express Frizzled,
Dsh and β-catenin, with phosphorylation of β-catenin following
Wnt3a treatment (Fig. 2B). Following
overexpression of β-catenin in HP75 cells, consistent with Wnt3a
treatment (Fig. 2C), no effect on
cell proliferation was observed (Fig.
2D). Therefore, activation of the Wnt signaling pathway only
was identified to have no effect on pituitary adenoma cell
proliferation.
Crosstalk between Shh signaling and
Wnt/β-catenin signaling leads to proliferation of pituitary adenoma
cells
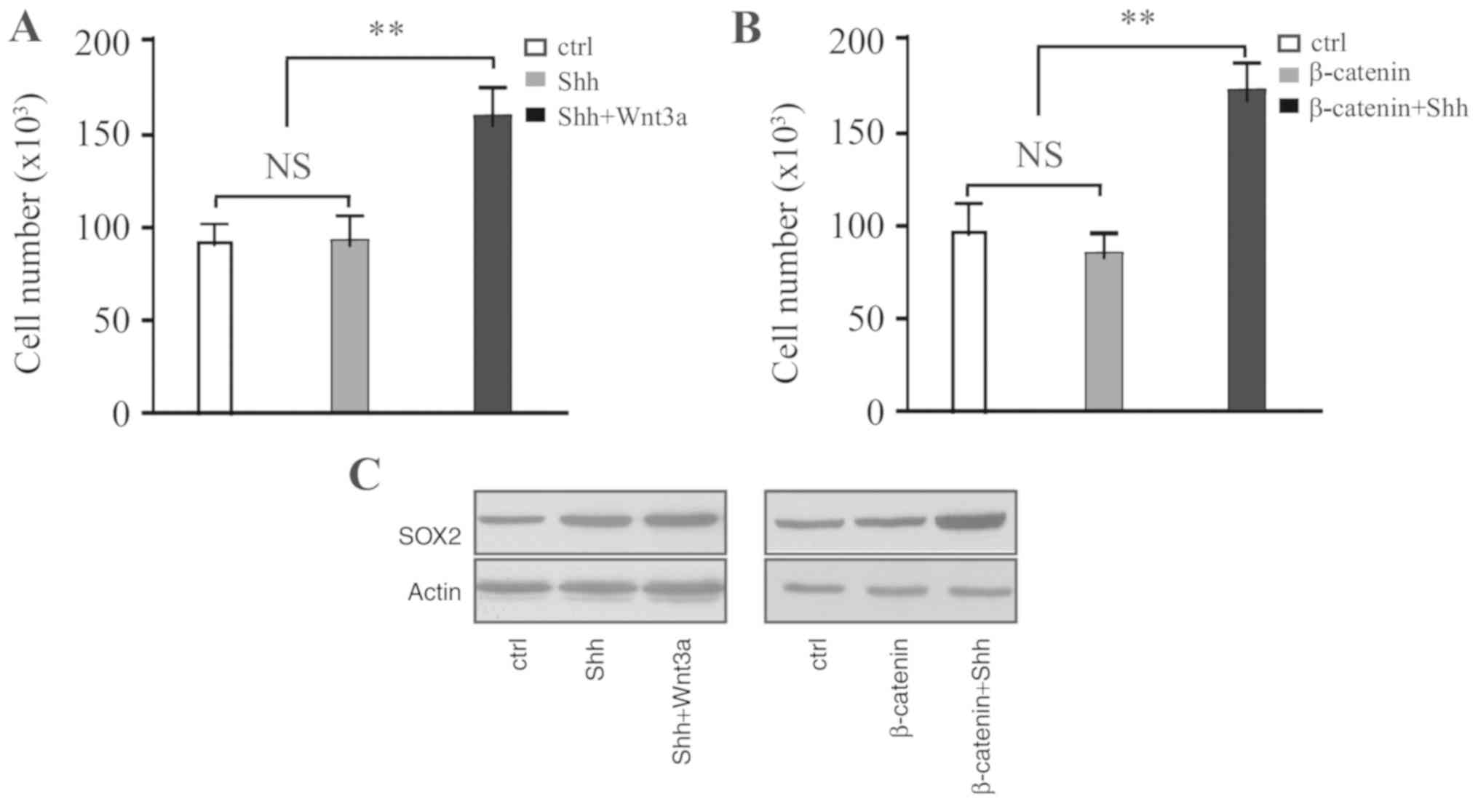
HP75 cells were treated with recombinant Shh. After
48 h, cells were washed with PBS and then treated with Wnt3a for a
further 48 h. Wnt3a treatment significantly promoted cell
proliferation (Fig. 3A).
Furthermore, treatment of β-catenin-overexpressing cells with
different concentrations of Shh also promoted cell proliferation
(Fig. 3B). Western blot analysis
identified that the expression of SOX2 is upregulated in these
cells (Fig. 3C). Therefore,
crosstalk between the Shh and Wnt/β-catenin signaling pathways was
identified to promote proliferation of pituitary adenoma cells.
SOX2 is required to mediate crosstalk
between the Shh and Wnt/β-catenin signaling pathways
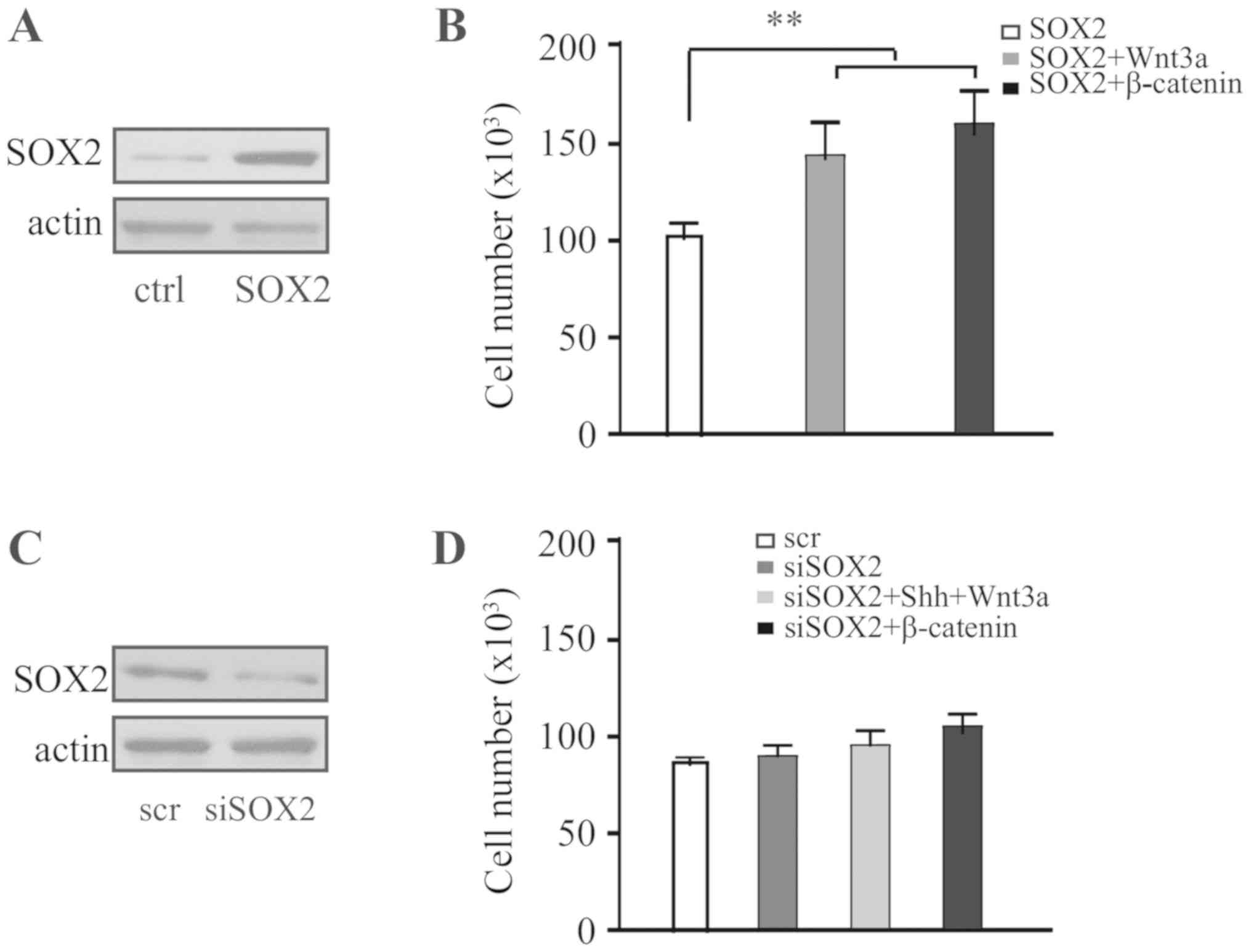
SOX2 was overexpressed in HP75 cells (Fig. 4A) and cells were treated with Wnt3a.
Wnt3a was identified to significantly promote proliferation of
SOX2-expressing cells, and, consistently, overexpression of
β-catenin in SOX2-expressing cells also promoted cell proliferation
(Fig. 4B). The expression of SOX2
was knocked down in HP75 cells (Fig.
4C), and cells were treated sequentially with Shh and Wnt3a.
The results identified that, without expression of SOX2, sequential
treatment with Shh and Wnt3a was not able to promote cell
proliferation; furthermore, overexpression of β-catenin in
SOX2-knockdown cells was not able to promote cell proliferation
(Fig. 4D). Therefore, it is
hypothesized that proliferation of pituitary adenoma cells depends
on the crosstalk between the Shh and Wnt/β-catenin signaling
pathways which is mediated by SOX2.
Discussion
CSCs are hypothesized to be the cell-of-origin of
various types of tumor. It has been demonstrated that CSCs act in a
cell-autonomous manner (15). The
existence and the function of CSCs in benign pituitary adenomas is
a matter of controversy. It has been identified that isolation of
sphere-forming stem-like cells from pituitary tumors may lead to
the production of pituitary hormone-secreting cell types. Pituitary
adenomas may be derived from a source of pituitary progenitor cells
(16).
The Shh signaling pathway serves a crucial function
in fetal development, and in tumorigenesis and progression. Shh
signaling is reported to contribute to tumorigenesis through
maintaining CSC characteristics (9).
The Shh pathway has previously been demonstrated to be markedly
associated with pituitary adenoma (17). In the present study, it was
demonstrated that Shh was able to upregulate the expression of SOX2
in pituitary cancer cells, but was not able to promote
proliferation in vitro. Therefore, activation of Shh only is
not sufficient to promote pituitary adenoma progression.
In the present study, using biochemical and genetic
approaches, it was demonstrated that SOX2 was able to promote the
proliferation of pituitary adenoma cells via mediating crosstalk
between the Shh and Wnt/β-catenin signaling pathways.
SOX2 was identified to be a key marker of adult stem
cells in various tissues including the pituitary tissue.
SOX2+ cells in mouse pituitary was identified to be
multipotent and able to self-renew and differentiate into any other
endocrine cell types of the pituitary gland (18). Previous studies identified that
SOX2+ cells contributed to pituitary tumorigenesis
following co-activation of the Wnt/β-catenin signaling pathway
in vivo (13,14). In agreement, the results of the
present study identified that activation of β-catenin in
SOX2+ cancer cells was able to promote proliferation of
cancer cells.
In summary, the results of the present study
identified that treatment with Shh was not able to promote
proliferation of pituitary adenoma cells. However, western blot
analysis demonstrated that Shh triggered the upregulation of SOX2
which is a CSC characteristic. The Wnt/β-catenin signaling pathway
was demonstrated to promote the proliferation of SOX2-expressing
cells. Inhibition of SOX2 expression disrupted the crosstalk
between the Shh and Wnt/β-catenin signaling pathways which
inhibited cell proliferation. Therefore, SOX2 was identified to
promote the proliferation of pituitary adenoma cells through
mediating crosstalk between the Shh and Wnt/β-catenin signaling
pathways.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JT conceived and conducted the experiment. LC and ZW
participated in acquiring the data. GH and XH performed the
statistical analysis and drifted the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Asa SL and Ezzat S: The pathogenesis of
pituitary tumors. Annu Rev Pathol. 4:97–126. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gueorguiev M and Grossman AB: Pituitary
tumors in 2010: A new therapeutic era for pituitary tumors. Nat Rev
Endocrinol. 7:71–73. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Melmed S: Pathogenesis of pituitary
tumors. Nat Rev Endocrinol. 7:257–266. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mathieu J, Zhang Z, Zhou W, Wang AJ,
Heddleston JM, Pinna CM, Hubaud A, Stadler B, Choi M, Bar M, et al:
HIF induces human embryonic stem cell markers in cancer cells.
Cancer Res. 71:4640–4652. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Florio T: Adult pituitary stem cells: From
pituitary plasticity to adenoma development. Neuroendocrinology.
94:265–277. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Castinetti F, Davis SW, Brue T and Camper
SA: Pituitary stem cell update and potential implications for
treating hypopituitarism. Endocr Rev. 32:453–471. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ezzat S, Asa SL, Couldwell WT, Barr CE,
Dodge WE, Vance ML and McCutcheon IE: The prevalence of pituitary
adenomas: A systematic review. Cancer. 101:613–619. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Briscoe J and Thérond PP: The mechanisms
of hedgehog signalling and its roles in development and disease.
Nat Rev Mol Cell Biol. 14:416–429. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Treier M, O'Connell S, Gleiberman A, Price
J, Szeto DP, Burgess R, Chuang PT, McMahon AP and Rosenfeld MG:
Hedgehog signaling is required for pituitary gland development.
Development. 128:377–386. 2001.PubMed/NCBI
|
|
10
|
Xie J, Bartels CM, Barton SW and Gu D:
Targeting hedgehog signaling in cancer: Research and clinical
developments. Onco Targets Ther. 6:1425–1435. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Clevers H: Wnt/beta-catenin signaling in
development and disease. Cell. 127:469–480. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Grigoryan T, Wend P, Klaus A and
Birchmeier W: Deciphering the function of canonical Wnt signals in
development and disease: Conditional loss- and gain-of-function
mutations of beta-catenin in mice. Genes Dev. 22:2308–2341. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gaston-Massuet C, Andoniadou CL, Signore
M, Jayakody SA, Charolidi N, Kyeyune R, Vernay B, Jacques TS,
Taketo MM, Le Tissier P, et al: Increased Wingless (Wnt) signaling
in pituitary progenitor/stem cells gives rise to pituitary tumors
in mice and humans. Proc Natl Acad Sci USA. 108:11482–11487. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Andoniadou CL, Matsushima D, Mousavy
Gharavy SN, Signore M, Mackintosh AI, Schaeffer M, Gaston-Massuet
C, Mollard P, Jacques TS, Le Tissier P, et al: Sox2(+)
stem/progenitor cells in the adult mouse pituitary support organ
homeostasis and have tumor-inducing potential. Cell Stem Cell.
13:433–445. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Frank NY, Schatton T and Frank MH: The
therapeutic promise of the cancer stem cell concept. J Clin Invest.
120:41–50. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nassiri F, Cusimano M, Zuccato JA,
Mohammed S, Rotondo F, Horvath E, Syro LV, Kovacs K and Lloyd RV:
Pituitary stem cells: Candidates and implications. Pituitary.
16:413–418. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gomes DC, Jamra SA, Leal LF, Colli LM,
Campanini ML, Oliveira RS, Martinelli CE Jr, Elias PC, Moreira AC,
Machado HR, et al: Sonic Hedgehog pathway is upregulated in
adamantinomatous craniopharyngiomas. Eur J Endocrinol. 172:603–608.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fauquier T, Rizzoti K, Dattani M,
Lovell-Badge R and Robinson IC: SOX2-expressing progenitor cells
generate all of the major cell types in the adult mouse pituitary
gland. Proc Natl Acad Sci USA. 105:2907–2912. 2008. View Article : Google Scholar : PubMed/NCBI
|