Introduction
Ovarian cancer has one of the highest rates of
mortality in the USA, and is the leading cause of cancer-associated
mortality in women (1). Diagnosis of
ovarian cancer is a problem, as early stages have no noticeable
signs or symptoms (2). The majority
of patients are diagnosed at an advanced stage of the disease,
which is mainly treated with cytoreductive surgery and
platinum-based chemotherapy. However, 60–65% of patients will
subsequently relapse, and a low five-year survival rate is observed
following the initial diagnosis (3).
The high mortality rate for ovarian cancer is primarily caused by
chemoresistance, which remains an important obstacle to successful
chemotherapy (4). Therefore, it is
necessary to find a novel therapeutic approach to improve the
effects of ovarian cancer treatment.
Cell death mainly includes apoptosis, autophagy and
necrosis. Disruption in the balance between cell proliferation and
apoptosis leads to uncontrollable cell growth and tumorigenesis;
therefore, apoptosis can inhibit tumor growth (5,6). The
majority of chemotherapy drugs kill cancer cells by inducing
apoptosis (5). It has previously
been reported that chemoresistance of tumor cells is not only
associated with inhibiting apoptosis and reducing apoptotic
susceptibility, but is also associated with autophagy (7,8).
Autophagy is a self-stabilizing mechanism in eukaryotic cells that
allows the orderly degradation and recycling of cellular components
(9). This self-degradation process
not only serves a crucial role in cell protection but can also be
involved in cell killing (10).
Hence, autophagy and apoptosis can work individually, and can
affect the sensitivity of tumor cells to chemotherapy in a
reciprocal manner (11,12). However, to the best of our knowledge,
whether autophagy protects or kills cells remains unclear.
Metformin as an insulin sensitization agent was
formally approved as a first-line treatment for diabetes by the
American Diabetes Association and European Association for the
Study of Diabetes (13). Previous
studies have revealed that metformin can not only effectively
reduce blood sugar levels and regulate lipid metabolism, endocrine
function, hypercoagulability and hyperthrombocytopenia, but may
also possess antitumor effects (13–15). It
has been reported that metformin can inhibit breast, prostate,
renal, pancreatic and colon cancer in animal and cell experiments
(16–19). The antitumor effects of metformin are
induced by the inhibition of tumor cell proliferation, promotion of
apoptosis in tumor cells, activation of tumor sensitivity to
chemotherapeutic agents and inhibition of tumor angiogenesis
(19). However, research into the
effects of metformin on ovarian cancer is currently limited.
The present study investigated the effects of
metformin on cisplatin (DDP) and methotrexate (MTX)-induced
apoptotic cell death in epithelial ovarian cancer parental SKOV3
and drug-resistant SKOV3/DDP cells. Metformin inhibited the growth
of SKOV3/DDP cells and enhanced the sensitivity of SKOV3/DDP cells
to chemotherapeutic agents.
Materials and methods
Chemical reagents
Metformin, MTT, DDP, MTX, ribonuclease A (RNase A),
DAPI, fluorescein isothiocyanate isomer (FITC), propidium iodide
(PI), dimethyl sulfoxide (DMSO), ethanol, formaldehyde,
glutaraldehyde, epoxy resin, acetone and Nonidet P-40 were
purchased from Sigma-Aldrich (Merck KGaA). RPMI 1640 medium, fetal
bovine serum (FBS), penicillin and streptomycin were obtained from
Thermo Fisher Scientific, Inc. Polyclonal antibodies against
microtubule-associated protein 1 light chain 3 (LC3), goat
anti-rabbit IgG conjugated to horseradish peroxidase (HRP) and
anti-β-actin antibody were purchased from Santa Cruz Biotechnology,
Inc. An Annexin V-FITC apoptosis detection kit was purchased from
Xi'an Jiaoda Bao Sai Bio-technology Co., Ltd. A Pierce™
Bicinchoninic Acid (BCA) Protein Assay kit was supplied by Thermo
Fisher Scientific, Inc. Total cell protein extraction kit was
purchased from Shanghai BestBio Biotechnology Co., Ltd. All other
chemicals and solvents were purchased from Sigma-Aldrich (Merck
KGaA).
Cell culture
The ovarian cancer cell lines SKOV3 and SKOV3/DDP
were provided by the Institute of Basic Medicine, Chinese Academy
of Medical Sciences & Peking Union Medical College. Both
parental and drug-resistant cells were cultured in RPMI 1640
medium, supplemented with 10% FBS, 100 U/ml penicillin and 100
µg/ml streptomycin at 37°C with 5% CO2.
MTT assay
An MTT assay was used to evaluate the resistance
index (RI) of SKOV3 and SKOV3/DDP cells. Cells were seeded in
96-well microtiter plates (1×104 per well). After 12 h,
different concentrations of DDP (12.5, 25, 50 and 100 µg/ml) or MTX
(2, 4, 8 and 16 µg/ml) were added to the cells for 72 h at 37°C.
Cells were washed with PBS and incubated with MTT (50 µl; 0.5
mg/ml). After 4 h incubation at 37°C, the formazan precipitates
were dissolved in DMSO (150 µl/well). The optical density (OD) was
measured at a wavelength of 570 nm using a Benchmark microplate
reader (Bio-Rad Laboratories, Inc.). The OD values were used to
calculate the inhibition rates and half-inhibitory concentration
(IC50) of chemotherapeutic agents. The ratio of
IC50 for drug-resistant and parental cells (RI), was
calculated to evaluate the drug-resistance of SKOV3/DDP cells. The
MTT assay was performed in triplicate.
The MTT assay was also used to evaluate the effect
of the tested compounds on the viability of SKOV3/DDP cells. After
the cells were seeded in a 96-well microtiter plates for 12 h,
different concentrations of metformin (2.5, 5 and 10 mmol/l) were
added. The cells were incubated for 24, 48 and 72 h at 37°C prior
to the addition of MTT. To determine the susceptibility to
metformin as a chemotherapeutic agent, the cells were incubated
with 10 mmol/l metformin for 12 h at 37°C, and different
concentrations of DDP (12.5, 25, 50 and 100 µg/ml) or MTX (2, 4, 8
and 16 µg/ml) were subsequently added to the wells. The cells and
agents were co-cultured for another 48 h at 37°C prior to the MTT
assay.
Detection of apoptosis by flow
cytometry
Following incubation of SKOV3 and SKOV3/DDP cells
with different concentrations of metformin for 48 h, attached and
floating cells were harvested and washed twice with PBS. The cells
were fixed with 70% ethanol at 4°C overnight, and then incubated in
RNase A (100 µg/ml in PBS) at 37°C for 30 min. Detection of
apoptotic cells was performed using an Annexin V-FITC apoptosis
detection kit including recombinant human Annexin V labelled by
FITC, according to the manufacturer's protocol. After incubation
with RNase A, cells (1×106) were washed with cold PBS
prior to incubation with FITC (10 µl) and PI (5 µl) for 15 min in
the dark at 4°C. The apoptotic rate was analyzed using flow
cytometer (Beckman Coulter, Inc.) and quantified with CellQuest Pro
V5.1 software (BD Biosciences).
Detection of apoptosis by DAPI
staining
Following incubation of SKOV3 and SKOV3/DDP cells
with indicated drugs (10 mmol/l DDP or 10 mmol/l metformin or 10
mmol/l DDP + 10 mmol/l metformin) for 48 h, attached and floating
cells were both harvested and fixed at 4°C for 2 h with PBS
solution containing 3.7% formaldehyde, 0.5% Nonidet P-40 and 0.1
mg/l DAPI. Apoptosis was assessed by fluorescence microscope
(Olympus Corporation; magnification, ×200) of condensed chromatin
and micronucleation. Cells with a condensed and/or fragmented
nucleus were considered as apoptotic cells. At least two
independent experiments were carried out for each treatment
condition and a minimum of 300 cells were counted by ImageJ V1.8.0
software (National Institutes of Health) in each experiment.
Detection of autophagy by electron
microscopy
Metformin (10 mmol/l) was added to SKOV3 and
SKOV3/DDP cells for 48 h. Following centrifugation at room
temperature for 10 min at 200 × g and cleaning, cells were fixed
with 2.5% glutaraldehyde at 4°C overnight. Fixed cells were stained
with 1% uranium acetate at room temperature for 10 min, washed with
double distilled water for 20 sec, subsequently stained with 0.2%
lead citrate at room temperature for 10 min and washed with double
distilled water for 20 sec. Then, the specimens were rinsed with
PBS, dehydrated in a graded acetone series (50, 70, 80, 90 and
100%) and embedded in an epoxy resin/acetone (1:1) mixture at room
temperature for 1 h. Ultra-thin sections (50–60 nm) were examined
by the FEI TecnaiG2 F20 transmission electron microscope (TEM;
magnification, ×15,000; FEI Company).
Western blot analysis for the
detection of protein-associated apoptosis and autophagy
Total proteins were extracted from cells following
the aforementioned treatments with the total cell protein
extraction kit (cat. no. BB-3101; Shanghai BestBio Biotechnology
Co., Ltd.) and proteins were quantified using a BCA Protein Assay
kit (Thermo Fisher Scientific), according to the manufacturer's
protocol. Equal amounts of proteins (80 µg/lane) were separated via
SDS-PAGE (12% gels), and transferred to nitrocellulose membranes
(Bio-Rad Laboratories, Inc.). The membrane was probed with
polyclonal antibodies against LC3 (cat. no. sc398822; 1:500)
overnight at 4°C, followed by incubation with HRP-conjugated
secondary antibody (cat. no. sc2030; 1:3,000) at room temperature
for 2 h. Visualization was achieved using Super Signal enhanced
chemiluminescence (Applygen Technologies, Inc.). The data were
analyzed via densitometry using Quantity One V4.6.2 software
(Bio-Rad Laboratories, Inc.). To determine equal lane loading, the
membrane was stripped and re-probed with anti-β-actin antibody
(cat. no. sc47778; 1:5,000).
Statistical analysis
Statistical analysis was performed using SPSS
software (version 17.0; SPSS, Inc.) and SAS 9.4 (SAS Institute
Inc.). All experiments were repeated as least in triplicate, and
the values were presented as the mean ± standard deviation. A
two-way analysis of variance was applied to compare the main and
interactive effects of variables, followed by Bonfferoni post hoc
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Metformin reduces viability and
induces apoptosis in ovarian cancer cells
Metformin could inhibit the growth of SKOV3 and
SKOV3/DDP cells, whereas inhibition rates were increased in a drug
concentration- and time-dependent manner (Table I), suggesting that both cells were
sensitive to metformin. In particular, when the concentration of
metformin was 10 mmol/l, the inhibitory effect was increased in
SKOV3/DDP cells compared with SKOV3 cells (P<0.05). For the
detection of metformin-induced apoptosis, flow cytometric analysis
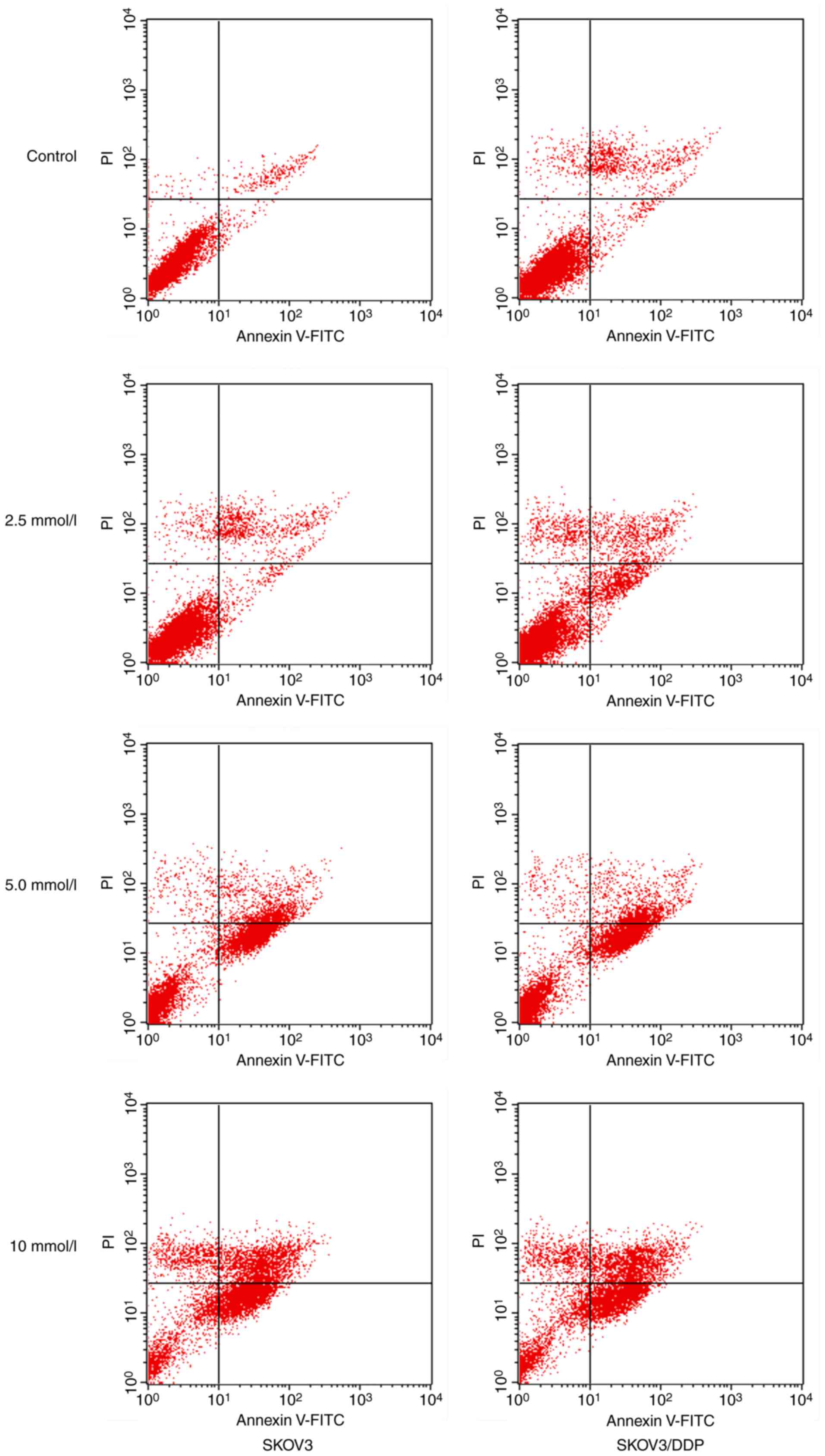
was performed. As presented in Fig.
1, when 2.5, 5 and 10 mmol/l metformin were incubated for 48 h,
the apoptotic rates of SKOV3/DDP cells were 11.45±2.78, 33.28±1.30
and 40.36±2.58%, respectively, whereas the apoptotic rates of SKOV3
cells were 10.56±1.08, 31.31±2.21 and 35.60±3.24, respectively
(Tables II and III).
 | Table I.Inhibition rates of metformin with
indicated concentrations and incubation times in SKOV3 and
SKOV3/DDP cells. |
Table I.
Inhibition rates of metformin with
indicated concentrations and incubation times in SKOV3 and
SKOV3/DDP cells.
| Concentration
(mmol/l) | Time (h) | SKOV3 (%±SD) | SKOV3/DDP (%±SD) | t-statistic | P-value |
|---|
| 2.5 | 24 | 10.80±1.78 | 13.14±2.94 | 0.290 | 0.779 |
|
| 48 | 18.41±1.94 | 16.84±1.90 | −0.470 | 0.651 |
|
| 72 | 22.20±1.19 | 24.89±2.22 | 3.275 | 0.061 |
| 5 | 24 | 16.25±1.60 | 18.82±1.92 | 3.301 | 0.050 |
|
| 48 | 23.01±2.76 | 22.46±2.56 | −0.330 | 0.750 |
|
| 72 | 27.62±1.62 | 31.84±2.25 | 3.398 | 0.009 |
| 10 | 24 | 20.30±1.69 | 27.97±1.82 | 6.904 | <0.001 |
|
| 48 | 27.95±2.69 | 34.35±2.39 | 3.984 | 0.004 |
|
| 72 | 33.30±3.56 | 38.04±2.90 | 2.309 | 0.041 |
 | Table II.Comparison by two way ANOVA of the
effects of metformin concentration and cell type, alone and
combined, on apoptotic rates. |
Table II.
Comparison by two way ANOVA of the
effects of metformin concentration and cell type, alone and
combined, on apoptotic rates.
| Factor | F-value | P-value |
|---|
| Metformin
concentrationa | 707.130 | 0.000 |
| Cell
typeb | 11.070 | 0.004 |
| Metformin
concentration * Cell typec | 4.212 | 0.022 |
 | Table III.Apoptotic rates detected using flow
cytometry in SKOV3 and SKOV3/DDP cells following incubation with
metformin for 48 h. |
Table III.
Apoptotic rates detected using flow
cytometry in SKOV3 and SKOV3/DDP cells following incubation with
metformin for 48 h.
| Concentration
(mmol/l) | SKOV3 (%±SD) | SKOV3/DDP
(%±SD) | t-statistic | P-value |
|---|
| 0 | 3.01±1.51 | 3.13±1.32 | 1.29 | 0.185 |
| 2.5 |
10.56±1.08a |
11.45±2.78a | 2.05 | 0.172 |
| 5 |
31.31±2.21a |
33.28±1.30a | 0.37 | 0.552 |
| 10 |
35.60±1.24a |
40.36±2.58a,b | 9.63 | 0.036 |
Effects of metformin on the
cytotoxicity of SKOV3/DDP ovarian cancer cells
As presented in Table
IV, the inhibition rates of DDP and MTX on SKOV3 and SKOV3/DDP
cells were increased as the concentration of chemotherapeutic
agents increased. The IC50 values of MTX and DDP were
4.21 and 14.35 for SKOV3 cells and 15.27 and 70.26 µg/ml for
SKOV3/DDP cells, respectively (Table
V). Therefore, the inhibition rates of DDP and MTX on SKOV3
cells were significantly increased compared with SKOV3/DDP cells
(P<0.05). The SKOV3/DDP cell was resistant to DDP and MTX, and
the RI values were 4.89 and 3.62, respectively.
 | Table IV.Inhibition rates of DDP and MTX in
SKOV3 and SKOV3/DDP cells. |
Table IV.
Inhibition rates of DDP and MTX in
SKOV3 and SKOV3/DDP cells.
|
| Concentration of
DDP (µg/ml) | Concentration of
MTX (µg/ml) |
|---|
|
|
|
|
|---|
| Cell | 12.5 | 25 | 50 | 100 | 2 | 4 | 8 | 16 |
|---|
| SKOV3, %±SD | 40.67±3.16 | 59.81±2.66 | 68.36±3.14 | 75.12±1.22 | 12.35±2.38 | 40.17±1.18 | 60.11±3.27 | 80.12±2.49 |
| SKOV3/DDP,
%±SD |
19.35±2.54a |
26.92±1.38a |
34.64±1.08a |
61.26±2.31a |
2.35±2.44a |
12.13±2.56a |
32.14±2.74a |
63.21±2.61a |
 | Table V.Metformin sensitizes SKOV3 and
SKOV3/DDP cells to chemotherapeutic agents. |
Table V.
Metformin sensitizes SKOV3 and
SKOV3/DDP cells to chemotherapeutic agents.
|
|
| IC50
(µg/ml) |
|
|---|
|
|
|
|
|
|---|
| Cell | Drug |
Metformin− |
Metformin+ | Ratioa |
|---|
| SKOV3 | MTX |
4.21 | 2.80 |
1.50 |
|
| DDP | 14.35 | 11.20 |
1.28 |
| SKOV3/DDP | MTX | 15.27 | 2.47 |
6.18 |
|
| DDP | 70.26 | 6.21 | 11.31 |
To evaluate the effects of metformin on the
cytotoxicity of chemotherapeutic agents, an MTT assay was
performed. As indicated in Table V,
the IC50 values of metformin combined with MTX and DDP
were 2.80 and 11.20 µg/ml for SKOV3 cells, and 2.47 and 6.21 µg/ml
for SKOV3/DDP cells, respectively. Compared with the
IC50 values for when chemotherapeutic agents were used
alone, metformin decreased the IC50 of MTX and DDP in
drug-resistant cancer cells, SKOV3/DDP, by 6.18- and 11.31-fold,
respectively. These results indicated that SKOV3/DDP cell viability
was significantly inhibited when treated with the combination of
metformin and chemotherapeutic agents compared with
chemotherapeutic agents alone.
The effects of metformin on apoptosis were also
evaluated using DAPI staining. Compared with chemotherapeutic
agents alone, the combination of metformin and DDP significantly
increased apoptotic rates. As indicated in Table VI, when DDP was replaced by the
combination of metformin and DDP, the apoptotic rates for SKOV3/DDP
significantly increased from 7.02 to 75.22%; similarly, the
apoptotic rates for SKOV3 cells increased from 24.50 to 74.12%.
 | Table VI.Apoptotic rates detected by DAPI
staining of SKOV3 and SKOV3/DDP cells following incubation with
indicated drugs for 48 h. |
Table VI.
Apoptotic rates detected by DAPI
staining of SKOV3 and SKOV3/DDP cells following incubation with
indicated drugs for 48 h.
| Drug | SKOV3 (%) | SKOV3/DDP (%) |
|---|
| 10 mmol/l DDP | 24.50 |
7.02 |
| 10 mmol/l
metformin | 35.60 | 41.38 |
| 10 mmol/l DDP + 10
mmol/l metformin | 74.12 | 75.22 |
Metformin induces autophagy in
drug-resistant ovarian cancer cells
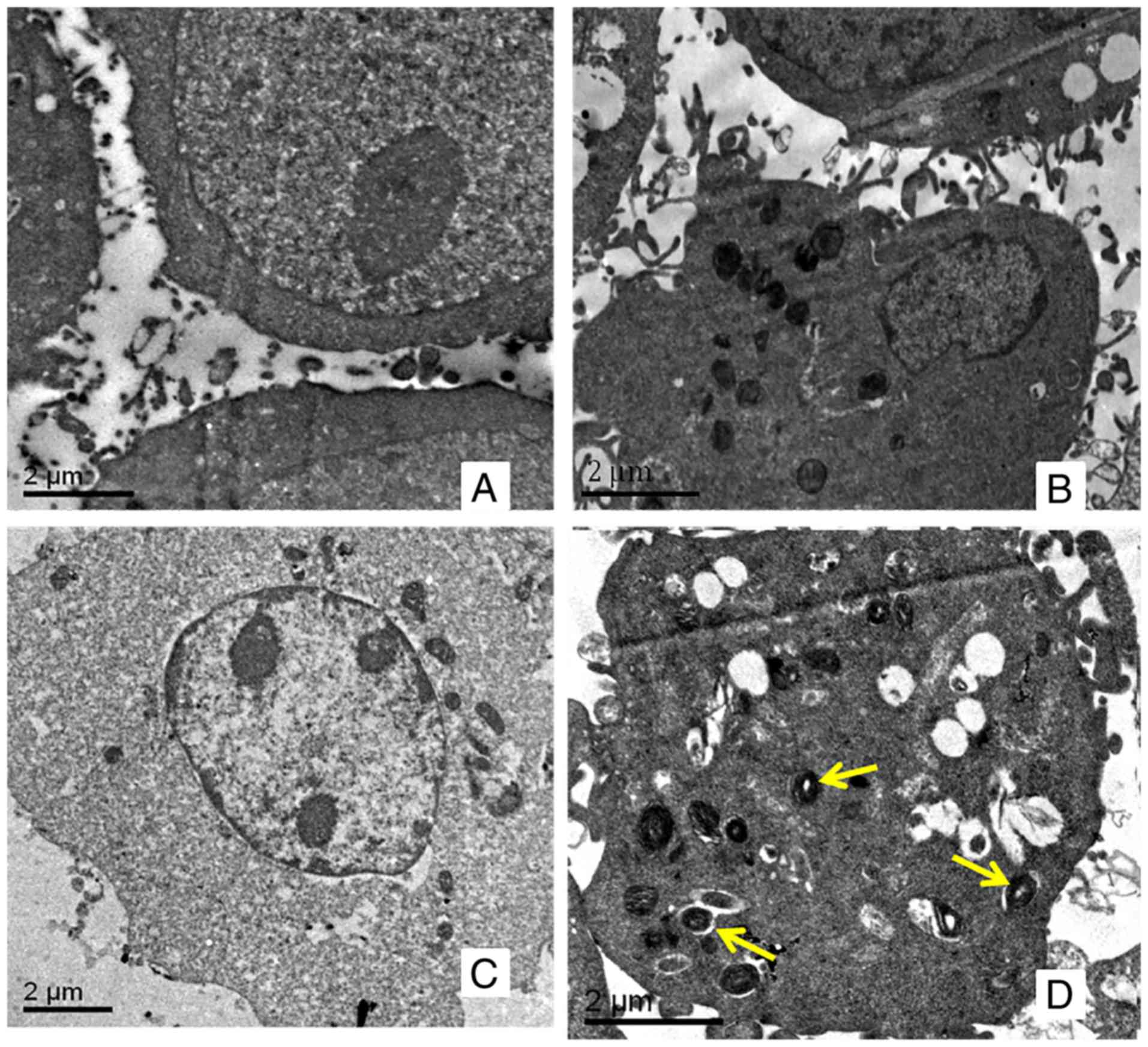
Autophagy is characterized by the reformation of
acidic vesicle and autophagic lysosomes, which are wrapped by a
double membrane in the cytoplasm. For SKOV3 (Fig. 2A) and SKOV3/DDP (Fig. 2B) cells, no autophagosomes were
observed using transmission electron microscopy. Following
incubation with 10 mmol/l of metformin for 48 h, there was no
marked change in SKOV3 cells (Fig.
2C); however, autophagosomes were observed in SKOV3/DDP cells
(Fig. 2D).
Metformin upregulates the expression
of LC3-II in ovarian cancer cells
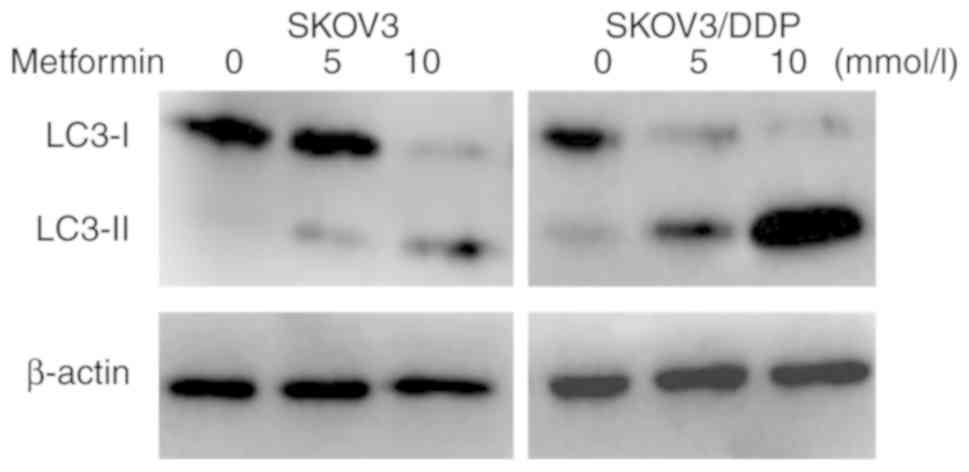
LC3 possesses two forms, including an 18-kDa
cytosolic protein (LC3-I) and a processed 16-kDa form (LC3-II). The
expression levels of LC3-II and the ratio of LC3-II/LC3-I represent
autophagic activity (11).
Therefore, LC3-I and LC3-II were detected in SKOV3 and SKOV3/DDP
cells incubated with different concentrations of metformin for 48 h
using western blot analysis. As indicated in Fig. 3, the expression levels of LC3-II in
SKOV3/DDP cells were higher than those in SKOV3 cells, indicating
that the autophagic activity of drug-resistant cells was increased
compared with that of the parental cells. Metformin may induce
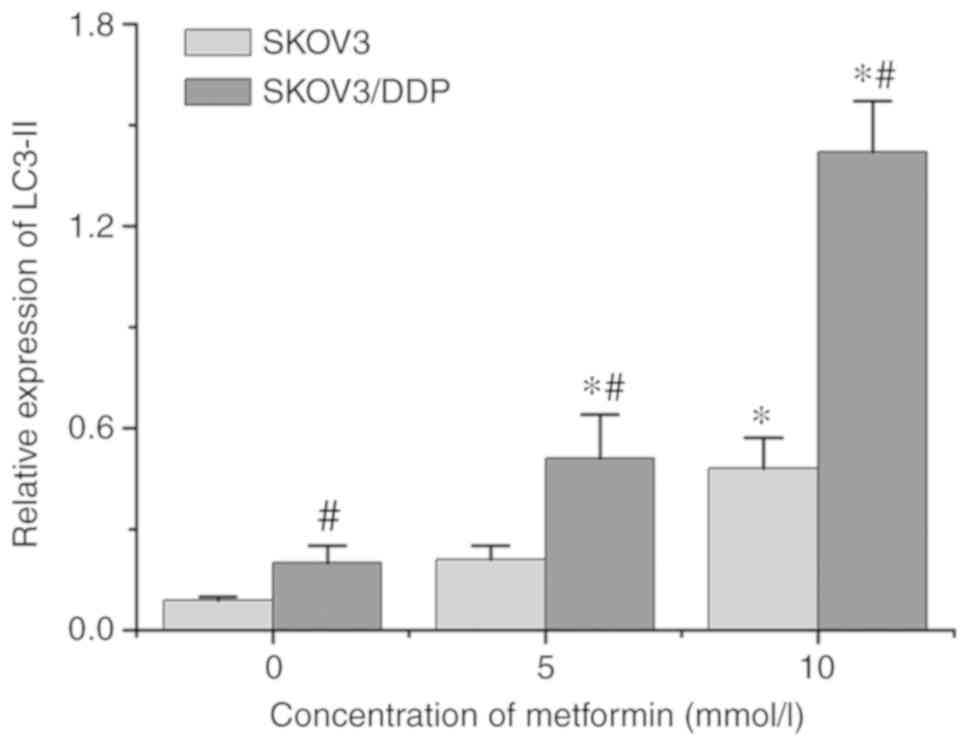
autophagy in ovarian cancer cells. Following incubation with 10
mmol/l of metformin for 48 h, the expression levels of LC3-II
protein were significantly increased in SKOV3 and SKOV3/DDP cells,
and the expression levels in SKOV3/DDP cells were higher compared
with in SKOV3 cells (Fig. 4). These
results demonstrated that metformin could promoted the autophagy of
ovarian cancer cells, and served a crucial role in promoting
autophagy in drug-resistant ovarian cancer cells.
Discussion
Ovarian cancer had the highest mortality rate among
gynecologic malignant tumors in the USA in 2008 (20). At present, the leading therapeutic
method for ovarian cancer is operative treatment combined with
platinum-based chemotherapy; however, chemoresistance severely
influences the prognosis of the patient, and results in high
mortality rates. Therefore, overcoming chemoresistance has become
the key to treating ovarian cancer (4). Metformin is a member of biguanides
group of drugs, and is a first-line drug for the treatment of type
2 diabetes (14). Metformin not only
reduces blood sugar levels, but also possesses other
pharmacological effects, including cardiovascular protective
effects (21) and delaying
Alzheimer's disease (22). Those
patients with type 2 diabetes who received metformin had a
decreased rate of cancer-associated mortality compared with those
receiving sulfonylureas (3.5 vs. 4.9%) (23). Metformin has been reported to enhance
the antiproliferative effects of paclitaxel and cisplatin in breast
cancer, lung cancer and other cells (24–27).
In the present study, metformin not only suppressed
the growth of ovarian cancer parental SKOV3 cells and
drug-resistant SKOV3/DDP cells, but it also sensitized
drug-resistant SKOV3/DDP cells to chemotherapeutic agents. The
inhibition rates of metformin increased in SKOV3 and SKOV3/DDP
cells following drug treatment with increasing concentration and
incubation time. In particular, when the concentration of metformin
was 10 mmol/l, significantly increased inhibition was observed in
SKOV3/DDP cells compared with in SKOV3 cells. In addition, the
apoptotic rates of the two cell types incubated with metformin for
48 h increased with increasing drug concentration. These results
indicated that the two cell types were sensitive to metformin.
Metformin-induced chemosensitivity of ovarian cancer
cells was examined via MTT assays and DAPI staining. Cell viability
was markedly inhibited in both types of cells when treated with a
combination of metformin and a chemotherapeutic agent (DDP or MTX)
compared with those treated with chemotherapeutic agents alone. The
IC50 values for the combination of metformin with DDT
(or MTX) were reduced by 11.31- (or 6.18-) fold in SKOV3/DDP cells
compared with DDT (or MTX) alone, but only 1.28- (or 1.50-) fold in
SKOV3 cells. Similarly, following incubation with DDP alone for 48
h, the apoptotic rates were 24.50 and 7.02% for SKOV3 and SKOV3/DDP
cells, respectively, and the former were notably higher compared
with the latter. With the combination of metformin and DDP, instead
of DDP only, the apoptotic rates for SKOV3 and SKOV3/DDP cells were
not significantly different; 74.12 and 75.22%, respectively. These
results demonstrated that metformin can enhance the cytotoxicity of
chemotherapeutic agents on drug-resistant cancer cells.
Autophagy, as a type of cell death, degrades
cellular components, and damaged organelles and macromolecular
material are wrapped by cytomembrane to form a complete
autophagosome; then, the autophagosome is combined with a lysosome
and digested to complete the autophagy process (9,10).
Autophagy is considered a double-edged sword in the process of
tumor development (10). The effects
that autophagy has on tumor cells are complicated and
controversial. Autophagy has been demonstrated to suppress
carcinogenesis via the elimination of oncogenic molecules and
damaged organelles (9). However,
once an invasive cancer is established, autophagy promotes tumor
growth via the intracellular recycling of degraded metabolites,
which further stimulates the metabolism of cancer cells (28). In addition, autophagy is enhanced by
chemotherapy and radiation therapy, and functions as an adaptive
response that mediates resistance to these treatments (29). In particular, autophagy is associated
with the occurrence and development of ovarian cancer, and also
with DDP-resistance in ovarian cancer cells (30). In the present study, autophagy was
significantly increased in SKOV3/DDP cells following incubation
with metformin for 48 h, resulting in cell death. The
aforementioned, however, was not observed in SKOV3 cells. LC3-II is
a marker of autophagosome formation. Incubation with metformin
induced a significant increase in the expression levels of LC3-II,
and the expression levels of LC3-II in drug-resistant cells were
upregulated compared with in parental cells. Metformin enhanced the
autophagy activity of ovarian cancer cells, and the effect was more
prominent in SKOV3/DDP cells compared with that in SKOV3. The
apoptotic rates of drug-resistant cells, with the incubation of
combined metformin and DDP, were roughly equivalent to the
apoptotic rates of the parental cells. These results indicated that
metformin may sensitize drug-resistant SKOV3/DDP cells to
chemotherapeutic agents through the induction of autophagy.
In conclusion, metformin not only induced apoptosis
of ovarian cancer parental SKOV3 and drug-resistant SKOV3/DDP
cells, but also enhanced autophagic activity in SKOV3/DDP cells.
Therefore, the fact that metformin can sensitize drug-resistant
ovarian cancer cells to chemotherapeutic agents may indicate an
association with inducing autophagy. Although the specific
underlying molecular mechanism remains unclear in the present
study, metformin is a potential antitumor drug, particularly for
the treatment of drug-resistant ovarian cancer. The results of the
present study will be verified in future studies that include
additional ovarian cancer cell lines and animal models.
Acknowledgements
Not applicable.
Funding
This study was supported by the Provincial
Outstanding Clinical Medical Talents Project funded by HeBei
Government (grant. no. 2016-361036).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
CY and NZ performed the experiments. CY and YC
drafted the manuscript. CY, DL and GZ analyzed the data. YC
designed the study. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Matei DE and Nephew KP: Epigenetic
therapies for chemoresensitization of epithelial ovarian cancer.
Gynecol Oncol. 116:195–201. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gilbert L, Basso O, Sampalis J, Karp I,
Martins C, Feng J, Piedimonte S, Quintal L, Ramanakumar AV,
Takefman J, et al: Assessment of symptomatic women for early
diagnosis of ovarian cancer: Results from the prospective DOvE
pilot project. Lancet Oncol. 13:285–291. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Raja FA, Counsell N, Colombo N, Pfisterer
J, du Bois A, Parmar MK, Vergote IB, Gonzalez-Martin A, Alberts DS,
Plante M, et al: Platinum versus platinum-combination chemotherapy
in platinum-sensitive recurrent ovarian cancer: A meta-analysis
using individual patient data. Ann Oncol. 24:3028–3034. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vecchione A, Belletti B, Lovat F, Volinia
S, Chiappetta G, Giglio S, Sonego M, Cirombella R, Onesti EC,
Pellegrini P, et al: A microRNA signature defines chemoresistance
in ovarian cancer through modulation of angiogenesis. Proc Natl
Acad Sci. 110:9845–9850. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Galluzzi L, Morselli E, Kepp O, Vitale I,
Rigoni A, Vacchelli E, Michaud M, Zischka H, Castedo M and Kroemer
G: Mitochondrial gateways to cancer. Mol Aspects Med. 31:1–20.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schoenlein PV, Periyasamy-Thandavan S,
Samaddar JS, Jackson WH and Barrett JT: Autophagy facilitates the
progression of Eralpha-positive breast cancer cells to antiestrogen
resistance. Autophagy. 5:400–403. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ning L, Guo-Chun Z, Sheng-Li A, Xue-Rui L,
Kun W, Jian Z, Chong-Yang R, Ling-Zhu W and Hai-Tong L: Inhibition
of autophagy induced by PTEN loss promotes intrinsic breast cancer
resistance to trastuzumab therapy. Tumor Biol. 37:5445–5454. 2016.
View Article : Google Scholar
|
|
9
|
Mizushima N and Komatsu M: Autophagy:
Renovation of cells and tissues. Cell. 147:728–741. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Macintosh RL and Ryan KM: Autophagy in
tumour cell death. Semin Cancer Biol. 23:344–351. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Glick D, Barth S and Macleod KF:
Autophagy: Cellular and molecular mechanisms. J Pathol. 221:3–12.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liang B, Kong D, Liu Y, Liang N, He M, Ma
S and Liu X: Autophagy inhibition plays the synergetic killing
roles with radiation in the multi-drug resistant SKVCR ovarian
cancer cells. Radiat Oncol. 7:2132012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Libby G, Donnelly LA, Donnan PT, Alessi
DR, Morris AD and Evans JM: New users of metformin are at low risk
of incident cancer: A cohort study among people with type 2
diabetes. Diabetes Care. 32:1620–1625. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chan DK and Miskimins WK: Metformin and
phenethyl isothiocyanate combined treatment in vitro is cytotoxic
to ovarian cancer cultures. J Ovarian Res. 5:192012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang P, Kang D, Cao W, Wang Y and Liu Z:
Diabetes mellitus and risk of hepatocellular carcinoma: A
systematic review and meta-analysis. Diabetes Metab Res Rev.
28:109–122. 2011. View Article : Google Scholar
|
|
16
|
Bonanni B, Puntoni M, Cazzaniga M, Pruneri
G, Serrano D, Guerrieri-Gonzaga A, Gennari A, Trabacca MS,
Galimberti V and Veronesi P: Dual effects of metformin on breast
cancer proliferation in a randomized trial. J Clin Oncol.
30:2593–2600. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yu Z, Zhao G, Xie G, Zhao L, Chen Y, Yu H,
Zhang Z, Li C and Li Y: Metformin and temozolomide act
synergistically to inhibit growth of glioma cells and glioma stem
cells in vitro and in vivo. Oncotarget. 6:32930–32943. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sliwinska A, Rogalska A, Marczak A,
Kasznicki J and Drzewoski J: Metformin, but not sitagliptin,
enhances WP 631-induced apoptotic HepG2 cell death. Toxicol In
Vitro. 29:1116–1123. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jara JA and López-Muñoz R: Metformin and
cancer: Between the bioenergetic disturbances and the antifolate
activity. Pharmacol Res. 101:102–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J,
Murray T and Thun MJ: Cancer statistics, 2008. CA Cancer J Clin.
58:71–96. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xiao H, Ma X, Feng W, Fu Y, Lu Z, Xu M,
Shen Q, Zhu Y and Zhang Y: Metformin attenuates cardiac fibrosis by
inhibiting the TGFbeta1-Smad3 signalling pathway. Cardiovasc Res.
87:504–513. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang YC, Hsu CC, Lin WC, Yin TK, Huang
CW, Wang PW, Chang HH and Chiu NT: Effects of metformin on the
cerebral metabolic changes in type 2 diabetic patients. Scientific
World Journal. 2014:6943262014.PubMed/NCBI
|
|
23
|
Bowker SL, Majumdar SR, Veugelers P and
Johnson JA: Increased cancer-related mortality for patients with
type 2 diabetes who use sulfonylureas or insulin. Diabetes Care.
29:254–258. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Algire C, Amrein L, Zakikhani M, Panasci L
and Pollak M: Metformin blocks the stimulative effect of a
high-energy diet on colon carcinoma growth in vivo and is
associated with reduced expression of fatty acid synthase. Endocr
Relat Cancer. 17:351–360. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jiralerspong S, Palla SL, Giordano SH,
Meric-Bernstam F, Liedtke C, Barnett CM, Hsu L, Hung MC, Hortobagyi
GN and Gonzalez-Angulo AM: Metformin and pathologic complete
responses to neoadjuvant chemotherapy in diabetic patients with
breast cancer. J Clin Oncol. 27:3297–3302. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hadad SM, Coates P, Jordan LB, Dowling RJ,
Chang MC, Done SJ, Purdie CA, Goodwin PJ, Stambolic V,
Moulder-Thompson S and Thompson AM: Evidence for biological effects
of metformin in operable breast cancer: Biomarker analysis in a
pre-operative window of opportunity randomized trial. Breast Cancer
Res Treat. 150:149–155. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yerrabothala S, Shaaban H, Capo G,
Maroules M and Debari VA: The impact of diabetes mellitus on breast
cancer outcomes: A single center retrospective study. Pathol Oncol
Res. 20:209–214. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
White E: Deconvoluting the
context-dependent role for autophagy in cancer. Nat Rev Cancer.
12:401–410. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hu YL, Jahangiri A, Delay M and Aghi MK:
Tumor cell autophagy as an adaptive response mediating resistance
to treatments such as antiangiogenic therapy. Cancer Res.
72:4294–4299. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang J and Wu GS: Role of autophagy in
cisplatin resistance in ovarian cancer cells. J Biol Chem.
289:17163–17173. 2014. View Article : Google Scholar : PubMed/NCBI
|