Introduction
Esophageal cancer is often diagnosed in the advanced
stages of the disease (1). This
highlights the requirement to identify patients with early
esophageal cancer and to provide appropriate treatment as soon as
possible. The development of techniques, including chromoendoscopy,
narrow-band imaging (NBI), magnification endoscopy, confocal
microscopy and spectroscopy, has facilitated the diagnosis of early
superficial esophageal cancer (2,3). Early
esophageal cancer (EEC) refers to lesions confined to the mucosa
(lamina propria and muscularis mucosa) regardless of lymph node or
distant organ metastasis (4,5). Endoscopic submucosal dissection (ESD)
is an established procedure for the treatment of early superficial
esophageal cancer (6,7). Compared with endoscopic mucosal
resection, ESD provides a high en bloc resection rate (6,7).
Previous studies reported that esophageal ESD may be associated
with adverse events, including postoperative bleeding and
perforation (8,9). Therefore, ESD is contraindicated in
patients with lesions that occur close to the blood vessels,
including esophageal varices, due to the increased risk of bleeding
(10).
Patients with cirrhosis associated with superficial
esophageal cancer have been increasingly reported (11–14). ESD
for patients with cirrhosis may carry a higher risk of
postoperative bleeding due to the low platelet count, coagulopathy
and particularly due to the esophageal varices in these patients
(10). Although previous case
reports described the use of ESD for EEC in patients with liver
cirrhosis (12), the safety and
efficacy of ESD for EEC in these patients remains uncertain. The
aim of the current study was to investigate the efficacy, safety
and treatment flowchart of EEC in patients with cirrhosis with or
without esophageal varices.
Materials and methods
Ethics statement
The current study was conducted in accordance with
the Declaration of Helsinki. The study protocol was approved by the
Ethics Committee of Xinqiao Hospital of Third Military Medical
University (Chongqing, China). Informed consent for participation
in the study and publication of the images associated with this
manuscript was obtained from all patients.
Patient selection
A total of 6 male patients with cirrhosis and EEC
were enrolled between February 2014 and July 2018 at the Xinqiao
Hospital of Third Military Medical University (Chongqing, China).
The average age was 57 years with an age range of 48–66 years old.
The patients were diagnosed with cirrhosis based on
ultrasonography, radiology and laboratory investigations.
Early-stage esophageal cancer (squamous high-grade intraepithelial
neoplasms and intramucosal squamous carcinomas) was diagnosed by
narrow band imaging and histological biopsy prior to the ESD
procedure. All patients had esophageal varices. All patients had
early EEC (type 0-IIb) overlying or distant from the esophageal
varices. The location of the lesion was defined by the distance
from the incision to the lesion. Endoscopic grading of esophageal
varices was based on the classification defined by the Japanese
Research Society of Portal Hypertension (15). Esophageal varix forms were classified
as follows: F1, small straight varix; F2, enlarged tortuous varix
that occupies less than one-third of the lumen; and F3, large
coil-shaped varix that occupies more than one-third of the lumen
(15). The presence of the red wale
marking was described as red-sign positive.
Endoscopic variceal ligation
procedure
An endoscope (cat. no. GIF-Q260J; Olympus
Corporation, Tokyo, Japan) was used for the EVL procedure. A 25 cm
overtube was backloaded over the shaft of the endoscope. Ligation
was performed by two experienced endoscopists who had >10 years
of experience. After the endoscope had entered the esophagus, the
overtube was pushed forward over the shaft of the endoscope. The
endoscopic ligating device was then attached to the distal end of
the endoscope. Ligation was performed at 1–5 cm above the
gastroesophageal junction. Each varix was ligated with one 1–3
rubber bands. A maximum of 10 rubber bands per patient were used
for ligation.
Transjugular intrahepatic
portosystemic shunt (TIPS) procedure
Ultrasonography was performed in each patient to
evaluate the portal vein prior to the TIPS procedure. TIPS was
performed under general anesthesia by the same experienced
interventional radiologist. Briefly, vascular access was obtained
through the right internal jugular vein, followed by
catheterization of the right hepatic vein. Intrahepatic puncture
was performed from the right hepatic vein to the right or left
portal vein. Successful trans-hepatic puncture of the portal venous
system was confirmed by angiography for confirmation and assessment
of the puncture location. The puncture tract was dilated using an
angioplasty balloon (diameter, 7 mm). Subsequently, 8-mm
Viatorr® covered stent-grafts covered with
polytetrafluoroethylene (W.L. Gore and Associates, Newark, DE, USA)
were inserted from the portal vein up to the confluence of the
right hepatic vein and the inferior vena cava. Shunt placement was
considered successful when portosystemic gradient was reduced to 10
mmHg or less (16). Embolization of
gastroesophageal varices was subsequently performed to reduce the
flow of esophageal varices, thereby reducing the risk of ESD
bleeding. All patients were admitted for at least one night for
observation and had a complete blood count on postoperative day
1.
ESD procedure
All ESD procedures were performed by the same
endoscopist. The standard procedures were performed as previously
reported (7). Briefly, the patients
were maintained under conscious sedation using intravenous
propofol, and their blood pressure, electrocardiogram and blood
oxygen saturation values were monitored. An endoscope with a water
jet system (cat. no. GIF-Q260J; Olympus Corporation) was used for
the ESD procedure with a transparent cap attached to its tip. A
needle knife (cat. no. NM-400U-0423; Olympus Corporation), a hook
knife (cat. no. KD-620 LR; Olympus Corporation), an insulated-tip
knife-2 (cat. no. KD-611 L; Olympus Corporation) and a hemostatic
forceps (cat no. FD-410 LR; Olympus Corporation) were used during
the procedure. The electrosurgical generator used in the ESD was an
ICC 200 device (Erbe Elektromedizin GmbH, Tuebingen, Germany) or a
VIO300D device (Erbe Elektromedizin GmbH). The EEC lesions were
detected with white light endoscopy, magnifying NBI and Lugol
staining, as described previously (17), to estimate their depth and extent of
invasion, and the lesions were marked by making spots with a
needle-knife ~5 mm outside the lesion. A mixture consisting of 30%
hyaluronic acid (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany),
70% saline solution (Chengdu KeLong Chemical Co., Ltd., Chengdu,
China) and a small amount of indigo carmine (Micro-Tech, Co., Ltd.,
Nanjing, China) was subsequently injected into the submucosal layer
to lift the lesion. Submucosal dissection was performed using a
needle-knife, a hook knife or insulated tip knife-2. To control
bleeding during the ESD procedure, hemostatic forceps were used in
the soft coagulation mode (effect 5, 60 W). Procedure-associated
bleeding after the ESD was defined as bleeding that required
transfusion or surgical intervention or bleeding that caused the
hemoglobin level to decrease by 2 g/dl (18). Perforation was defined as detection
of free air by a chest X-ray or computed tomography (CT) scan
following the procedure. When EEC was located on an esophageal
varix, endoscopic varix ligation (EVL) or TIPS was performed prior
to the ESD procedure. ESD was performed one month following the EVL
or TIPS procedure.
Radiofrequency ablation (RFA)
Radiofrequency ablation was performed by the same
endoscopist using a Barrx Flex RFA system (Covidien, Dublin,
Ireland), as described previously (19). The system consisted of an ablation
catheter, an energy generator and a sizing balloon. All RFA
procedures were performed with the patients under conscious
sedation or anesthesia. Prior to the RFA procedure, Lugol staining
was performed to determine the location and size of the lesions. A
radiant exposure of 12 J/cm2 was used to minimize the
risk of bleeding (14).
Radiofrequency ablation was performed one month after the EVL or
TIPS procedure.
Pathological analysis
ESD specimens were stretched, pinned on a styrofoam
board and immediately immersed in 8% formalin at room temperature
for 4 h. Following fixation, the specimens were serially sectioned
perpendicularly at 2-mm intervals. Following deparaffinization and
rehydration, 5-µm longitudinal sections were stained with
hematoxylin solution at room temperature for 5 min followed by five
dips in 1% acid ethanol (1% hydrocholoric acid in 70% ethanol) and
rinsed in distilled water. The sections were subsequently stained
with eosin solution at room temperature for 3 min followed by
dehydration with graded alcohol and clearing in xylene. The mounted
slides were then examined and images were captured using an Olympus
Corporation microscope. All specimens were reviewed by two
gastrointestinal pathologists and the final pathological report was
issued when the two pathologist agreed on the diagnosis. The tumor
size, depth of invasion, gross type, lymphatic and vascular
involvement and tumor involvement of the lateral and vertical
margins were assessed as described previously (7). Depth of invasion was classified into M1
(confined to the intraepithelium), M2 (confined to the lamina
propria), M3 (confined to the muscularis mucosa), SM1 (submucosal
invasion <200 µm), and SM2&3 (submucosal invasion ≥200 µm)
(20).
Outcomes measurement and
follow-up
During the procedure, the degree of intraoperative
bleeding was defined as: Small bleeding, no obvious bleeding in the
ESD procedure; medium bleeding, obvious bleeding which can be
stopped immediately by endoscopy; large bleeding, hard to control
or uncontrollable bleeding, or required blood transfusion following
the procedure (21). Following the
ESD or RFA procedure, intravenous administration of a proton pump
inhibitor (omeprazole, 40 mg/d) for two days and antibiotics
(cefuroxime, 1.5 g/d) for three days was started on the day of the
ESD or RFA procedure. The proton pump inhibitor was administered
orally daily for two months thereafter. Blood cell counts, liver
enzyme levels, bilirubin and albumin levels, international
normalized ratio and C-reactive protein level were assessed twice
during the first week following the ESD as described previously
(22). Follow-up endoscopy was
performed two months following the ESD. The examinations were
conducted every six months or every year thereafter. The last
follow-up examination was conducted in July 2018.
Results
Clinical and endoscopic
characteristics
The clinical and endoscopic characteristics of 6
patients with early EECs are presented in Table I. All patients had cirrhosis; 2 had
hepatitis B virus cirrhosis and 4 had alcoholic cirrhosis. Of the 6
patients, five were Child-Pugh class A and B, and one patient was
Child-Pugh class C. Three patients had F1 varices, and three had F2
varices (Table I). All EECs were
flat with type IIb morphology. Four lesions were located overlying
the esophageal varices, and two lesions (case 1 and case 2) were
not close to the esophageal varices. The mean longitudinal length
of the lesions was 4.3 cm (range, 2–6 cm). All cases had a baseline
diagnosis of high-grade dysplasia by biopsy prior to the
procedure.
 | Table I.Clinical characteristics of 6 patients
with cirrhosis complicated by esophageal varices. |
Table I.
Clinical characteristics of 6 patients
with cirrhosis complicated by esophageal varices.
| Patient | Age | Etiology | Child-Pugh Class | INR | Platelet count,
×109/l | Esophageal
varices | EEC on varices | Lesion
morphology | Lesion length,
cm | Circumferential
extension |
|---|
| 1 | 66 | HBV | A | 1.23 | 56 | F1,RC(−) | No | 0-IIb | 4 | 1/3 |
| 2 | 56 | HBV | B | 1.36 | 144 | F1,RC(−) | No | 0-IIb | 2 | 1/3 |
| 3 | 48 | Alcohol | B | 1.29 | 63 | F2,RC(+) | Yes | 0-IIb | 6 | 3/4 |
| 4 | 66 | Alcohol | C | 1.35 | 40 | F1,RC(+) | Yes | 0-IIb | 3 | 1/2 |
| 5 | 53 | Alcohol | A | 1.12 | 45 | F2,RC(+) | Yes | 0-IIb | 6 | 3/5 |
| 6 | 52 | Alcohol | B | 1.51 | 86 | F2,RC(+) | Yes | 0-IIb | 5 | 3/4 |
Outcomes and adverse events
The location of the EEC is presented in Table II. The average procedure time was
72.8 min (range, 34–135 min), and the average longitudinal length
of the resected specimens was 53.0 mm (range, 30–90 mm). A total of
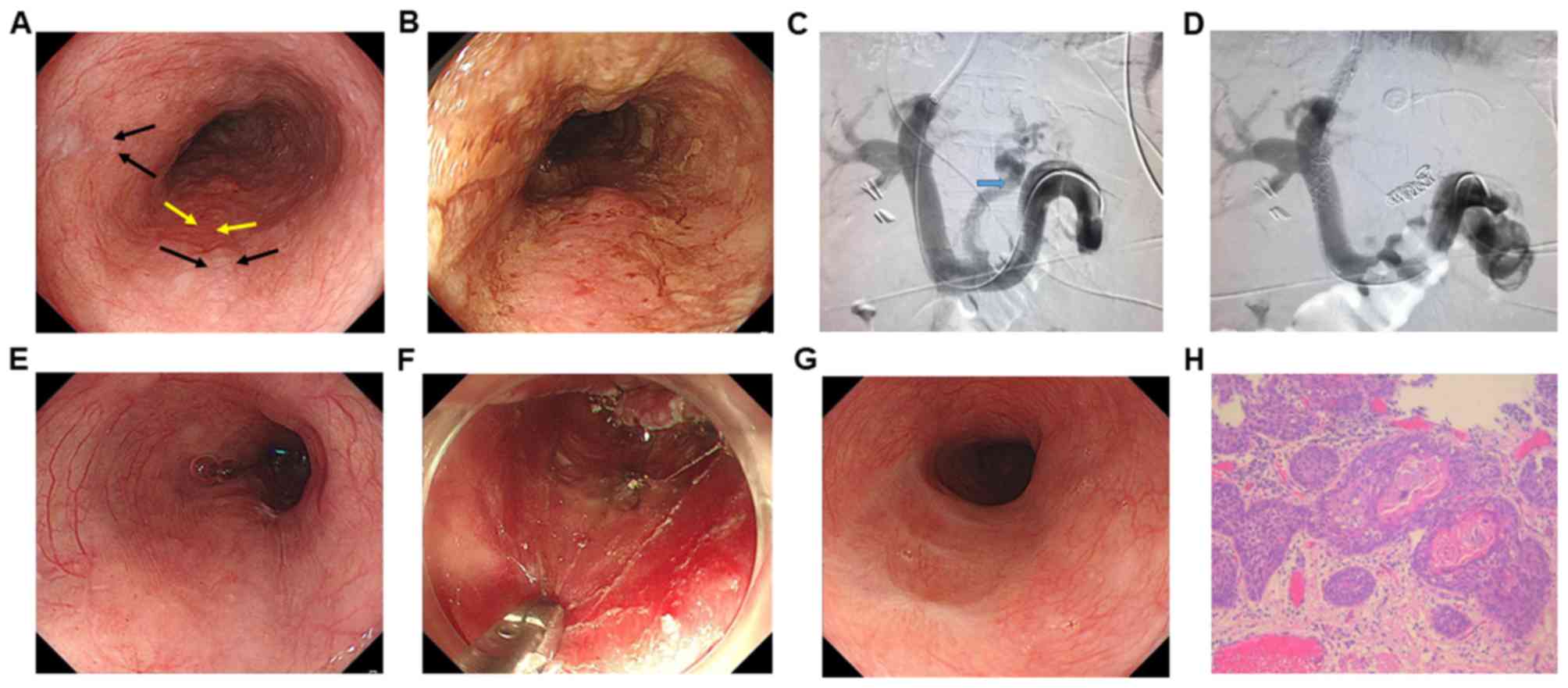
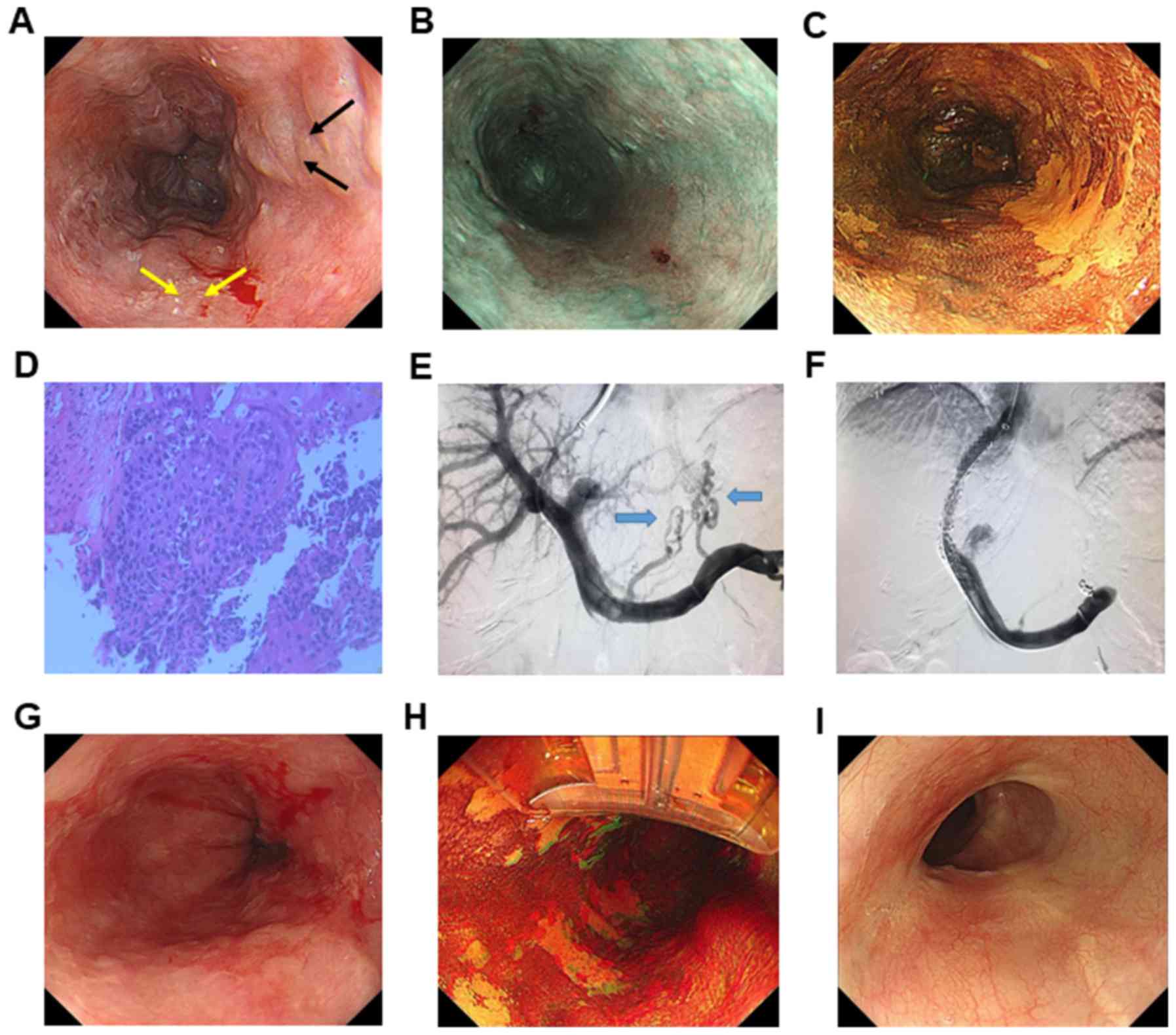
2 patients (cases 3 and 4) underwent EVL (representative image in
Fig. S1), and 2 cases (cases 5 and
6) underwent TIPS to remove the varices prior to the treatment
procedure as the EEC was located directly on the esophageal varices
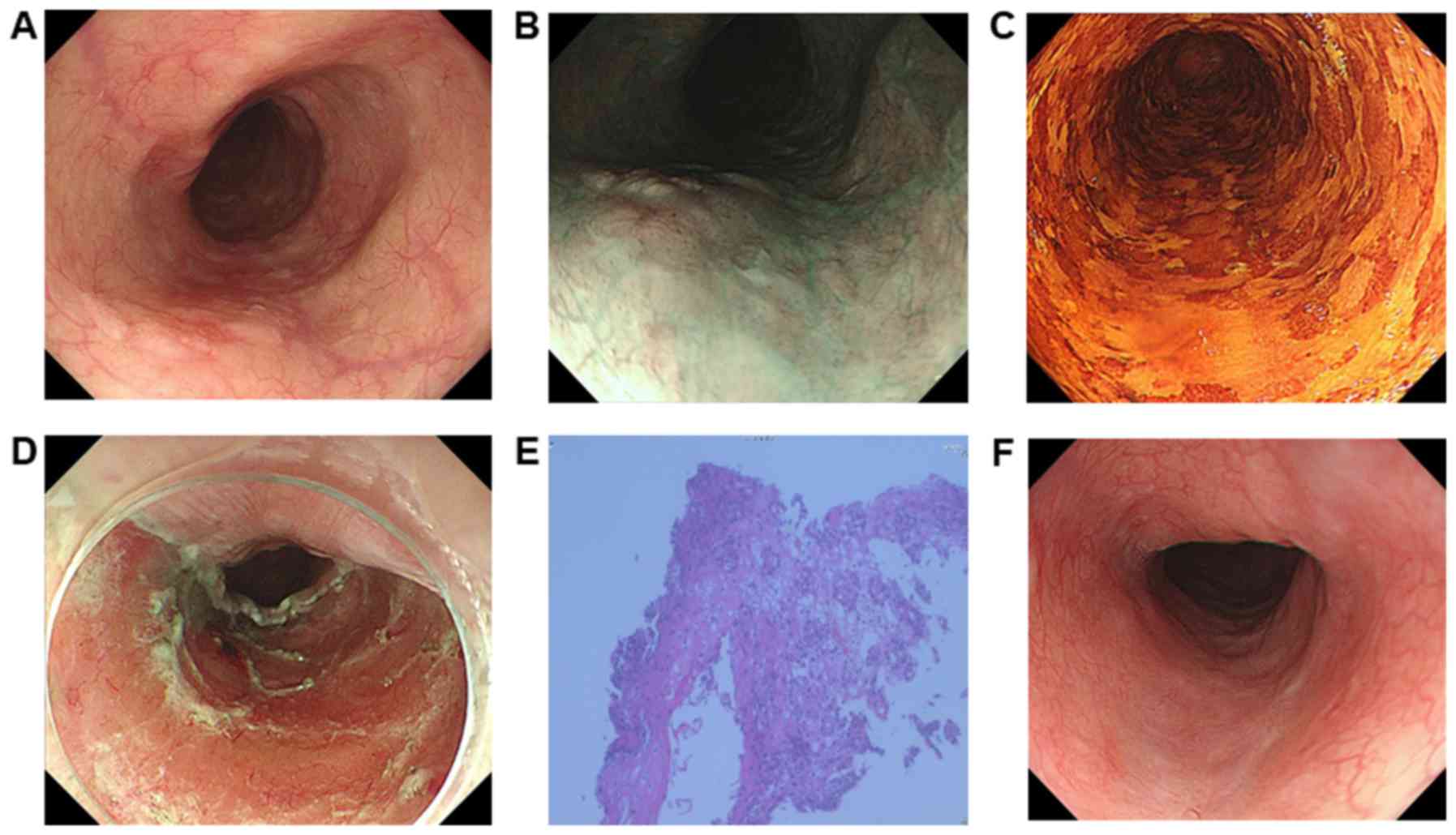
(Figs. 1 and 2). The remaining 2 cases (cases 1 and 2)
underwent ESD directly without any additional treatment, as the
lesions were not close to the esophageal varices (Table II; Fig.
3). Five patients underwent ESD and one case (case 6) underwent
RFA due to diffuse 0-IIb lesions (Table
II; Fig. 2). For ESD procedure,
the en bloc resection rate was 100% (5/5 lesions). Complete
resection was defined as an en bloc resection with histologically
confirmed tumor-free margins. En bloc and complete resection rates
were 100% (6/6 lesions). Case 3 had tumor-positive lateral margins
with lymphovascular infiltration (Table
II). Therefore, the rates of both complete and curative
resection were 80% (4/5 lesions).
 | Table II.Outcome and adverse events of 6
patients complicated with esophageal varices after treatment. |
Table II.
Outcome and adverse events of 6
patients complicated with esophageal varices after treatment.
| Patient | Treatment
before | ESD Or RFA | Location, cm | Procedure time,
min | Intraoperative
bleeding | Resected Length,
mm | Complete
resection | Vertical depth | Lympho-Vascular
infiltration | Hepatic
encephalopathy | Complications |
|---|
| 1 | None | ESD | 20 | 45 | Small | 40 | Yes | EP | Negative | None | None |
| 2 | None | ESD | 23 | 77 | Small | 30 | Yes | EP | Negative | None | None |
| 3 | EVL | ESD | 32 | 135 | Large | 75 | No | MM | Yes | None | Stricture |
| 4 | EVL | ESD | 34 | 34 | Large | 30 | Yes | EP | Negative | None | None |
| 5 | TIPS | ESD | 40 | 100 | Medium | 90 | Yes | SM1 | Negative | None | None |
| 6 | TIPS | RFA | 36 | 46 | Medium | NA | N/A | MM | Negative | None | None |
Frequent intraoperative bleeding was detected in
cases 3 and 4 undergoing EVL prior to ESD (Fig. S1). This bleeding was immediately
stopped following several electrocoagulations, without causing
life-threatening events. Nevertheless, less intraoperative
bleeding, shorter operation time and fewer complications were
present in patients undergoing TIPS prior to treatment (cases 5 and
6; Figs. 1 and 2). No severe complications or
mortality-associated events, including massive postoperative
bleeding, perforation or hepatic failure, were observed in any
patient. Postoperative stricture occurred in one patient (case 3)
due to the circumferential lesions (Fig. S1). Following three sessions of
balloon dilation, the symptoms associated with the stricture were
relieved in the follow-up period. The median follow-up period was
20 months. No recurrence and metastasis were observed during the
follow-up period.
Discussion
Currently, patients with cirrhosis are considered to
be at high risk for esophageal cancer (10). Studies reported that esophagectomy in
patients with cirrhosis was associated with significant morbidity
and mortality rates (23,24). Patients with early esophageal cancer
and cirrhosis are poor candidates for ESD or EMR due to increased
procedure-associated complications (10). The current study investigated a
treatment strategy for early esophageal cancers in patients with
cirrhosis complicated by esophageal varices. For patients
undergoing ESD or RFA procedures, the rates of en bloc and complete
resections were satisfactory. No severe complications, including
postprocedural bleeding or perforation, occurred and the safety
profiles were satisfactory, suggesting that ESD or RFA could be
safely applied for the treatment of early superficial esophageal
cancer in patients with cirrhosis complicated by esophageal
varices.
Previous studies reported successful ESD for
patients with early EEC with esophageal varices, carrying a high
risk of massive bleeding and requiring a high level of endoscopic
expertise (12,13). The risk of bleeding increased the
lower the EEC, as the varicose veins flow mainly through inferior
esophageal veins. Therefore, the lower location with much more
esophageal veins had a higher risk of bleeding. Previous studies
have reported the use of EMR or ESD for patients with EEC and
cirrhosis combined with esophageal varices (25–27). The
procedures performed relied on the removal of the esophageal
varices using EVL or endoscopic injection sclerotherapy (EIS)
(28). EIS requires considerable
time to remove the esophageal varices and may induce submucosal
fibrosis and scarring, possibly resulting in difficulty of
submucosal lifting and incomplete resection (12). EVL was performed prior to ESD
procedures in two cases (cases 3 and 4) in the current study.
Intraprocedural bleeding occurred more significantly and frequently
in the patients undergoing EVL procedures than in patients
undergoing TIPS before ESD procedures. While several sessions of
EVL may have reduced the size of the varices, they may have still
been present during the ESD and may have resulted in bleeding
(27). Furthermore, the timing of
endoscopic treatment following EVL is important, and primarily
determined by the size of esophageal varices (12). In the present study, if the
esophageal varices were significantly reduced in size or removed,
the ESD procedure was performed. If not, endoscopy examination was
performed following one month to reevaluate the esophageal varices.
In the present study, the esophageal varices were reduced in size
or removed one month following EVL or TIPS. A previous study
revealed that ulcers following EVL healed by 3 to 4 weeks, and the
necrosis and fibrosis were limited to the mucosa and submucosa,
with no involvement of the muscularis propria (28). Therefore, in the current study, the
endoscopic treatment was performed one month following EVL or TIPS.
One lesion (case 3) was not completely resected with tumor-positive
margin. TIPS may therefore be superior to EVL for esophageal
varices beneath the EECs because it provides effective relief of
esophageal varices without submucosal adhesion or morphological
changes prior to ESD or RFA procedures.
In the current study, the treatment regimens
selected were primarily based on the morphological type and
invasive depth of the lesions. EEC has three morphological types,
including flat, degraded and elevated lesions (7). For widespread completely flat early
EECs (type 0-IIb), RFA was used for the removal of EECs in the
current study. Thus, assessing the depth of invasion and the
morphological type is important. Prior to treatment,
NBI-magnification endoscopy was used for endoscopic staging and
endoscopic ultrasound was used for evaluating depth of invasion in
the current study. Due to the limited cases, all six cases in our
study had flat lesions. It is possible that all types of EECs were
confronted with the similar risks of bleeding, and are therefore
suitable for this treatment strategy (11–14).
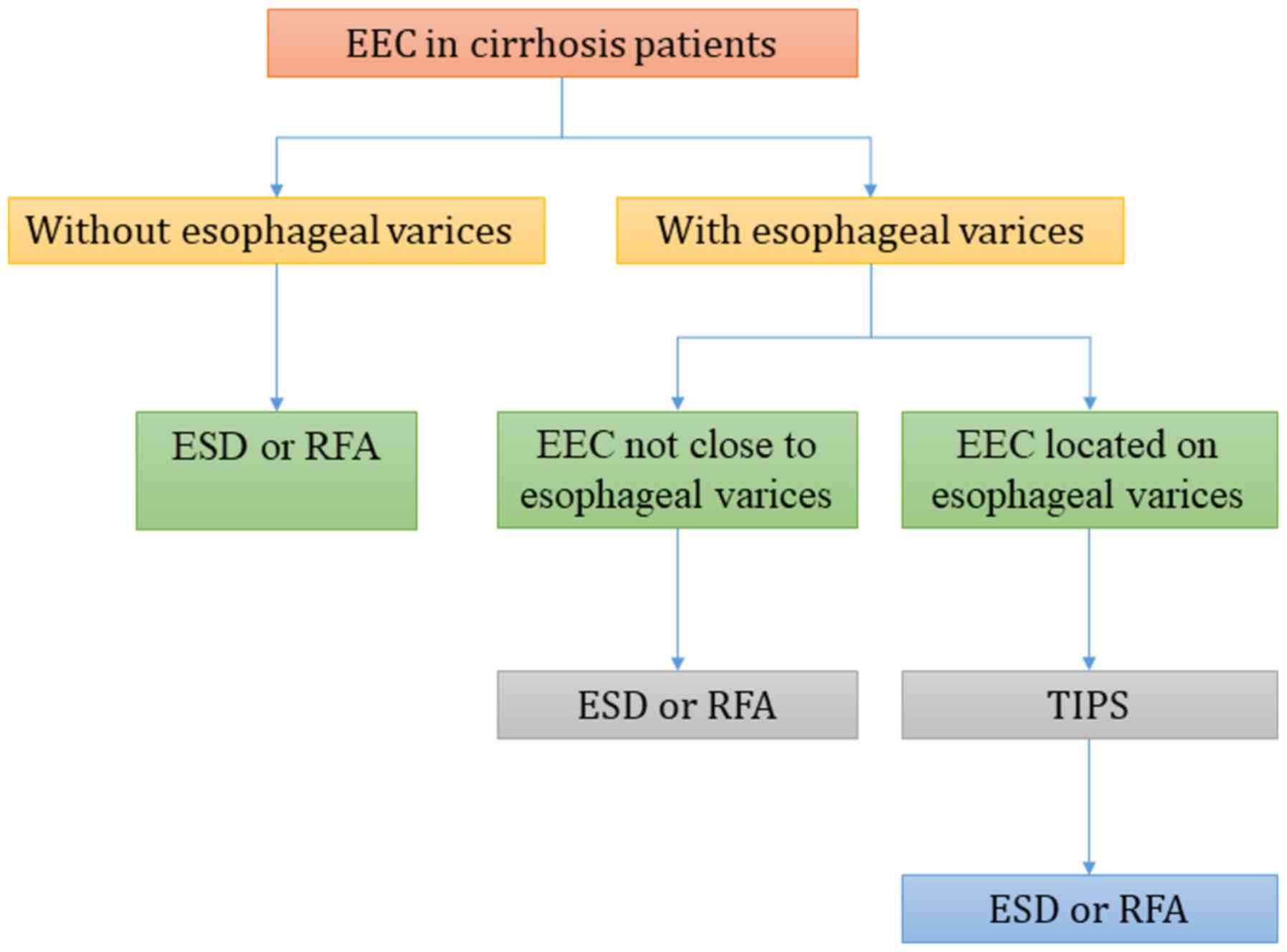
The current study proposed a standard treatment
workflow for EEC in patients with cirrhosis (Fig. 4). If there are no esophageal varices
present in the context of cirrhosis, ESD or RFA may be performed
without any preoperative treatment. Otherwise, the relative
location of esophageal varices and the EEC should be further
evaluated. If the EEC is located on the esophageal varix, TIPS is
recommended to alleviate portal hypertension and relieve esophageal
varices prior to ESD or RFA procedures. If the EEC is distant to
the esophageal varices, further evaluation of the vertical depth of
varices using CT scan or endoscopic ultrasonography is required. If
the esophageal varices are located in the submucosa or muscle
layer, TIPS is recommended to alleviate intraoperative bleeding as
the submucosa is resected in the ESD procedure. ESD or RFA may be
performed in other cases, dependent on the morphological and depth
of invasion for EEC.
The major limitation of the present study was the
small number of cases. Prospective multicenter studies are required
to evaluate the efficacy and safety of the proposed treatment
strategy. In conclusion, the present study described a novel
treatment workflow for patients with EEC complicated by esophageal
varices and cirrhosis. Good treatment results, no neoplastic
progression and an acceptable adverse event profile were
observed.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
SMY, BT, JY and ZGX conceived and designed the
study. ZGX, YBZ and EL performed the analysis and interpretation of
data. BT and ZGX drafted the manuscript. JYB performed the
procedures and provided the samples. SMY and BT revised the
manuscript. All authors approved the final version of the
article.
Ethics approval and consent to
participate
The present study was approved by The Ethics
Committee of Xinqiao Hospital of Third Military Medical University
(Chongqing, China). Informed consent for participation in this
study was obtained from all patients.
Patient consent for publication
Not applicable
Competing interests
The authors declare that they have competing
interests.
References
|
1
|
Patel V and Burbridge RA: Endoscopic
approaches for early-stage esophageal cancer: Current options. Curr
Oncol Rep. 17:4212015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
ASGE Standards of Practice Committee, ;
Evans JA, Early DS, Chandraskhara V, Chathadi KV, Fanelli RD,
Fisher DA, Foley KQ, Hwang JH, Jue TL, et al: The role of endoscopy
in the assessment and treatment of esophageal cancer. Gastrointest
Endosc. 77:328–334. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee CT, Chang CY, Lee YC, Tai CM, Wang WL,
Tseng PH, Hwang JC, Hwang TZ, Wang CC and Lin JT: Narrow-band
imaging with magnifying endoscopy for the screening of esophageal
cancer in patients with primary head and neck cancers. Endoscopy.
42:613–619. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Barnes JA and Willingham FF: Endoscopic
management of early esophageal cancer. J Clin Gastroenterol.
49:638–646. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang S, Huang Y, Xie J, Zhuge L, Shao L,
Xiang J, Zhang Y, Sun Y, Hu H, Chen S, et al: Does delayed
esophagectomy after endoscopic resection affect outcomes in
patients with stage T1 esophageal cancer? A propensity score-based
analysis. Surg Endosc. 32:1441–1448. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ogura K, Okamoto M, Sugimoto T, Yahagi N,
Fujishiro M, Kakushima N, Kodashima S, Kawabe T and Omata M:
Efficacy and safety of endoscopic submucosal dissection for gastric
cancer in patients with liver cirrhosis. Endoscopy. 40:443–445.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tang B, Bai JY, Zhao XY, Fan CQ, Yang X,
Deng L, Yang SM and Yu J: Endoscopic submucosal dissection for
superficial esophageal cancer with near-circumferential lesions:
Our experience with 40 patients. Surg Endosc. 29:2141–2148. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim JS, Kim BW and Shin IS: Efficacy and
safety of endoscopic submucosal dissection for superficial squamous
esophageal neoplasia: A meta-analysis. Dig Dis Sci. 59:1862–1869.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Basford PJ, George R, Nixon E, Chaudhuri
T, Mead R and Bhandari P: Endoscopic resection of sporadic duodenal
adenomas: Comparison of endoscopic mucosal resection (EMR) with
hybrid endoscopic submucosal dissection (ESD) techniques and the
risks of late delayed bleeding. Surg Endosc. 28:1594–1600. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ferro D, Angelico F, Caldwell SH and Violi
F: Bleeding and thrombosis in cirrhotic patients: What really
matters? Dig Liver Dis. 44:275–279. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Palmer WC, Di Leo M, Jovani M, Wolfsen HC,
Krishna M and Wallace MB: Endoscopic management of high-grade
dysplastic Barrett's esophagus with esophageal varices.
Gastrointest Endosc. 81:9972015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sawaguchi M, Jin M, Matsuhashi T, Ohba R,
Hatakeyama N, Koizumi S, Onochi K, Yamada Y, Kanazawa N, Kimura Y,
et al: The feasibility of endoscopic submucosal dissection for
superficial esophageal cancer in patients with cirrhosis (with
video). Gastrointest Endosc. 79:681–685. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hsu WH, Kuo CH, Wu IC, Lu CY, Wu DC and Hu
HM: Superficial esophageal squamous cell carcinoma over esophageal
varices treated by endoscopic submucosal dissection. Gastrointest
Endosc. 79:833–834. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang WL, Chang IW, Chen CC, Chang CY, Mo
LR, Lin JT, Wang HP and Lee CT: A case series on the use of
circumferential radiofrequency ablation for early esophageal
squamous neoplasias in patients with esophageal varices.
Gastrointest Endosc. 85:322–329. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee E, Kim YJ, Goo DE, Yang SB, Kim HJ,
Jang JY and Jeong SW: Comparison of hepatic venous pressure
gradient and endoscopic grading of esophageal varices. World J
Gastroenterol. 22:3212–3219. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Verbeeck S, Mekhali D, Cassiman D, Maleux
G and Witters P: Long-term outcome of transjugular intrahepatic
portosystemic shunt for portal hypertension in autosomal recessive
polycystic kidney disease. Dig Liver Dis. 50:707–712. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Heresbach D, Leray E, d'Halluin PN, Cholet
F, Lapalus MG, Gaudric M, Ben Soussan E, Gaudin JL, Vahedi K,
Quentin V, et al: Diagnostic accuracy of esophageal capsule
endoscopy versus conventional upper digestive endoscopy for
suspected esophageal squamous cell carcinoma. Endoscopy. 42:93–97.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Imagawa A, Okada H, Kawahara Y, Takenaka
R, Kato J, Kawamoto H, Fujiki S, Takata R, Yoshino T and Shiratori
Y: Endoscopic submucosal dissection for early gastric cancer:
Results and degrees of technical difficulty as well as success.
Endoscopy. 38:987–990. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen WC and Wolfsen H: Role of
radiofrequency ablation in esophageal squamous dysplasia and early
neoplasia. Gastrointest Endosc. 85:330–331. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ishihara R, Matsuura N, Hanaoka N,
Yamamoto S, Akasaka T, Takeuchi Y, Higashino K, Uedo N and Iishi H:
Endoscopic imaging modalities for diagnosing invasion depth of
superficial esophageal squamous cell carcinoma: A systematic review
and meta-analysis. BMC Gastroenterol. 17:242017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kataoka Y, Tsuji Y, Sakaguchi Y, Minatsuki
C, Asada-Hirayama I, Niimi K, Ono S, Kodashima S, Yamamichi N,
Fujishiro M and Koike K: Bleeding after endoscopic submucosal
dissection: Risk factors and preventive methods. World J
Gastroenterol. 22:5927–5935. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tsou YK, Liu CY, Fu KI, Lin CH, Lee MS, Su
MY, Ohata K and Chiu CT: Endoscopic submucosal dissection of
superficial esophageal neoplasms is feasible and not riskier for
patients with liver cirrhosis. Dig Dis Sci. 61:3565–3571. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mariette C: Is there a place for
esogastric cancer surgery in cirrhotic patients? Ann Surg Oncol.
15:680–682. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Asti E, Sozzi M, Bonitta G, Bernardi D and
Bonavina L: Esophagectomy in patients with liver cirrhosis: A
systematic review and Bayesian meta-analysis. J Visc Surg.
155:453–464. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Inoue H, Endo M, Takeshita K, Shimoju K,
Yoshino K, Goseki N and Sasabe M: Endoscopic resection of carcinoma
in situ of the esophagus accompanied by esophageal varices.
Surg Endosc. 5:182–184. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jovanovic I, Krivokapic Z, Menkovic N,
Krstic M and Mönkemüller K: Ineffectiveness of capsule endoscopy
and total double-balloon enteroscopy to elicit the cause of obscure
overt gastrointestinal bleeding. Think GIST! Endoscopy. 43 (Suppl
2):UCTN. E91–E92. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Künzli HT and Weusten BL: Endoscopic
resection of early esophageal neoplasia in patients with esophageal
varices: How to succeed while preventing the bleed. Endoscopy 46
(Suppl 1) UCTN. E631–E632. 2014.
|
|
28
|
Iwase H, Kusugami K, Suzuki M, Nishio Y,
Ando T, Ina K and Peek RM: Endoscopic resection of early-stage
esophageal cancer accompanied by esophageal varices. Gastrointest
Endosc. 51:749–752. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Polski JM, Brunt EM and Saeed ZA:
Chronology of histological changes after band ligation of
esophageal varices in humans. Endoscopy. 33:443–447. 2001.
View Article : Google Scholar : PubMed/NCBI
|