Introduction
Colorectal cancer (CRC) is one of the most common
malignancies worldwide. The estimated incidence of CRC is 10.2% and
ranks third among all of the cancer types globally. The mortality
of CRC is 9.2% and ranks second all over the world (1). Effective early diagnosis of colon
cancer is limited, and thus, colon cancer is diagnosed in the
middle or late stage. The treatment of colon cancer is relatively
passive (2,3). Therefore, the prevention of colon
cancer is very important for public health.
Environmental factors, including high-fat,
high-protein and low-fibre diets, are partially accountable as
causes of colon cancer. The consumption of processed meat and
alcohol are also associated with CRC (4). Whole grains, dietary fibre, dairy
products and red meat may influence the oncogenesis of CRC
(4). This suggests that an
individual's diet is an important factor in the pathogenesis of
CRC.
A number of natural products have anti-CRC effects,
including honey, bee pollen, propolis and Brassicaceae
extracts (5,6). The present study focused on the effect
of sulforaphane, a phytochemical extracted from
Brassicaceae.
Heterocyclic amines produced in meat cooked at
high-temperatures are associated with colon carcinogenesis. These
amines are oxidised to N-hydroxy metabolites by cytochrome P450
family 1 subfamily A member 2 oxidase in the liver and migrate into
the intestinal mucosa through the blood. Following N-acetyl
transferase acetylation in intestinal epithelial cells, the
heterocyclic amines bind to DNA and form DNA adducts, which may
cause chromosome translocation, cancer-related gene mutations,
microsatellite instability and chain mutations, eventually leading
to colon cancer (7,8). UDP-glucuronosyltransferase (UGT) is a
phase II enzyme that catalyses the metabolism of heterocyclic
amines, including the glucuronic acid conjugation reaction that
removes DNA adducts.
Classic UGT inducers are often toxic. However,
phytochemicals have been demonstrated to exhibit preventive effects
against cancer in animal experiments and epidemiological studies.
Phytochemicals can directly remove environmental carcinogens and
induce cell phase II enzymes, which increases the metabolism and
removal of carcinogens (9).
Multiple mechanisms have been identified for the
cancer-associated chemo-preventive activities of sulforaphane.
Sulforaphane is a potent monofunctional inducer of phase II
enzymes, as demonstrated by studies using cultured cells, mouse
tissues (10,11), human intestine (12) and human airways (13). Sulforaphane was also tested in humans
and was revealed to improve hepatic abnormalities (14). Sulforaphane induced phase II
detoxification enzymes, such as UGT (8). It also inhibited three cytochrome P450
isoforms (CYP1A1, CYP2B1/2 and CYP3A4) (15). Additionally, sulforaphane slowed the
cell cycle progression of a prostate cancer cell line LNCaP and
induced apoptosis in human glioblastoma T98G and U87MG cells
(16,17). In addition, sulforaphane inhibited
the initiation of carcinogen-induced skin tumours (18,19), and
reduced metastatic spread of melanoma in mice (20). This chemical also promoted the
antiproliferative activity of other antiproliferative agents,
including oxaliplatin (21).
Sulforaphane, which is produced by cruciferous
vegetable plants, has been demonstrated to inhibit or retard tumour
incidence and progression in models of breast, colon, stomach and
lung cancer (22). The molecular
mechanism of the effect of sulforaphane on colon cancer is partly
understood; sulforaphane acts by multiple pathways including the
inhibition of inflammatory cytokine production (23) and downregulation of nuclear factor
(NF)-κB activity (24). However,
there are few studies on the epigenetic regulation of gene
expression in colon cancer cells by sulforaphane (21).
Nuclear factor-erythroid derived 2-like 2 (Nrf2) is
a leucine zipper transcription factor, which serves an important
role in the maintenance of redox balance and cytoprotection against
chemical carcinogens (25,26). Under oxidative and electrophilic
stress conditions, Nrf2 is released by Kelch-like ECH-associated
protein 1 (Keap1)-mediated rapid degradation. Nrf2 is stabilised,
accumulated and translocated to the nucleus, where it dimerises
with a small Maf protein (sMaf). The Nrf2-sMaf heterodimer binds to
a specific DNA sequence, referred to as the
antioxidant/electrophile response element (ARE/EpRE), and induces
the expression of cytoprotective enzymes (11,25,26).
In our previous studies, sulforaphane was
demonstrated to activate the transcription and expression of the
UDP glucuronosyltransferase family 1 member A complex locus (UGT1A)
gene via Nrf2 (27). Curcumin also
induces Nrf2 expression by demethylating five CpG loci in the
promoter region of the Nrf2 gene (28). The epigenetic regulatory effect of
sulforaphane has been identified in a number of types of tumours,
including its ability to inhibit DNA methyltransferases (DNMTs) in
prostate cancer (29). Sulforaphane
also functions as a histone deacetylase inhibitor to regulate gene
expression (28,30,31).
In the present study, it was hypothesised that
sulforaphane may inhibit DNMT to induce the demethylation of CpG
sites in the Nrf2 promoter region and increase the expression of
Nrf2 in Caco-2 cells. The expression and activity of DNMT1,
methylation-specific polymerase chain reaction (MSP) and bisulfite
genomic sequencing (BGS) of Nrf2 and the differences between the
epigenetic regulation of sulforaphane and 5-aza-2′-deoxycytidine
(5-Aza) combined with trichostatin A (TSA) were examined to
determine the epigenetic regulation of sulforaphane on Nrf2
expression in human colon cancer. The combined use of DNMT
inhibitor 5-aza and the histone deacetylase (HDAC) inhibitor TSA
has been demonstrated to be able to reverse epigenetic
modifications and increase the expression of NRF2 and its
downstream antioxidant and detoxification enzymes (32).
Materials and methods
Reagents
Sulforaphane (Sigma-Aldrich; Merck KGaA) was
dissolved to 1 µmol/ml in dimethyl sulfoxide (DMSO) and stored at
−20°C for further use. TSA and 5-Aza were purchased from
Sigma-Aldrich.
Cell culture
Caco-2 human colon adenocarcinoma cells (Shanghai
Institutes for Biological Science, Chinese Academy of Sciences)
were incubated as monolayers in Dulbecco's modified Eagle's medium
(DMEM; Gibco; Thermo Fisher Scientific, Inc.) containing 10%
heat-inactivated foetal calf serum, 100 U/ml penicillin and 100
µg/ml streptomycin at 37°C in a humidified incubator with 5%
CO2. Caco-2 cells were selected as they are
enterocyte-like and appropriate for the testing of
pharmacologically active molecules generated in drug discovery
programmes, which suited the aim of the present study (33,34).
Cells in the logarithmic growth phase were used for the
experiments. A comparison between Caco-2 cells cultured in DMEM and
DMEM supplemented with the solvent of sulforaphane solution, DMSO,
at a concentration of <0.1%, was performed, and no difference
was observed, which revealed that DMSO was not toxic to Caco-2
cells.
Drug treatment
There were three groups, the control group,
sulforaphane-treated group and 5-Aza+TSA group. Caco-2 cells were
cultured in 6-well plates (40,000 cells/well) at 37°C and 5%
CO2 for 24 h. As the cells reached 70–80% confluence,
the medium was replaced. Based on the results of our previous
study, which demonstrated that the expression of UGT1A protein was
induced in a dose-dependent manner following 24-h treatment with
10–30 µmol/l sulforaphane, and this induction by sulforaphane was
most powerful at 25 µmol/l (35), 25
µmol/l sulforaphane treatment was selected to test whether the
pathways influencing the effect of sulforaphane to UGT1A include
epigenetic changes of Nrf2. In the control group, cells were
cultured in 2 ml medium with 0.1% DMSO for 24 h. In the
sulforaphane-treated group, 2 ml of complete medium with 25 µmol/l
of sulforaphane was added, and the cells were cultured for 24 h. A
total of 2 ml of medium with 5-Aza (10 µmol/l) and TSA (1 µmol/l)
was added to the 5-Aza+TSA group and then incubated for 24 h. After
incubation the cells were used in the processes below.
DNA extraction and C-T conversion
DNA was extracted from all three groups using Total
DNA extraction kit (Tiangen Biotech Co., Ltd.) according to the
manufacturer's protocol. Briefly, cell lysis buffer A (200 µl) and
proteinase K (20 µl) were added into the cell culture. The mixture
was stirred for 5 min at 25°C and buffer B (200 µl) was added. The
solution was mixed by inversion and incubated at 70°C for 10 min. A
spin column was used to collect DNA. Following centrifugation,
washing and drying, total DNA was obtained and eluted with 30 µl
elution buffer.
Zymo EZ DNA Methylation-Gold™ Kit (Zymo Research
Corp.) was used for C-T conversion to separate methylated cytosines
from unmethylated uracils. First, a C-T conversion reagent was used
for the sulfonation of unmethylated DNA. During this reaction, the
tube was incubated at 98°C for 10 min and at 64°C for 2.5 h. To
collect and separate the DNA with a Zymo-spin IC column, M-binding
buffer (600 µl) was used for hydrolytic deamination. During the
procedure, M-desulfonation (200 µl) buffer was used, followed by
M-wash buffer (200 µl) and M-elution buffer (10 µl).
BGS
BGS primers were designed based on the DNA sequences
that were enriched in CpG islands in the promoter region of the
Nrf2 gene (Table I). Primers were
designed to target the first five CpGs of the murine Nrf2 gene
(from −255 to −70 bp). The PCR reaction mixture included DNA (2
µl), ddH2O (6 µl), forward primer (1 µl), reverse primer
(1 µl) and 10 µl Go Taq® Green Master Mix (Promega
Corporation). The thermocycling conditions were as follows: 95°C
for 10 min, followed by 95°C for 30 sec, 54°C for 30 sec, and 72°C
for 40 sec for 40 cycles and 72°C for 10 min. Subsequently, 6X DNA
loading buffer (Invitrogen; Thermo Fisher Scientific, Inc.), and
PCR products were mixed with 10X GelRed Nucleic Acid Stain
(Biotium, Inc.). Agarose gel electrophoresis (1.2%) was performed
under 120 V. A gel digital imaging system (Gel Doc XR+; Bio-Rad
Laboratories, Inc.) was used for colour rendering. The PCR gel was
then cut under UV light, and Zymoclean Gel DNA Recovery kit (Zymo
Research Corp.) was then used for recovery of DNA according to the
manufacturer's protocols.
 | Table I.Primers for BGS and MSP to target the
first five CpGs of the murine Nrf2 gene. |
Table I.
Primers for BGS and MSP to target the
first five CpGs of the murine Nrf2 gene.
| Experiment | Primer sequence,
5′-3′ |
|---|
| BGS | F:
GGTTTTGTAATTTTAAATTAGGGAGG |
|
| R:
ACAACTCCAAATCCATCATAATAAAC TATA |
| MSP | F1:
GTTTTAAAGCGTTCGAATTTTAGC |
|
| R1:
GTTAACTCCCCGATACCGAC |
|
| F2:
TCGTTTTCGGATCGCGAG |
|
| R2:
GCGACGCGAACAAAACG |
| USP | F1:
TGTTTTAAAGTGTTTGAATTTTAGTGA |
|
| R1
TCCATTAACTCCCCAATACCAA |
|
| F2:
TTGTTTTTGGATTGTGAGTTTTTTG |
|
| R2:
CAAAACACAACACAAACAAAACA ACT |
The recovered DNA products were used in TA cloning,
and the reaction mixture was as follows: PCR products (3 µl), T4
ligase (1 µl; Thermo Fisher Scientific, Inc.), pGEM®-T
Easy Vector (1 µl; Promega Corporation) and 2X ligation buffer (5
µl). The resulting product was incubated at 4°C overnight.
Subsequently, 10 µl reaction mixture was added to 100 µl of DH5
competent E. coli (Thermo Fisher Scientific, Inc.), placed
on ice for 30 min and then activated at 42°C for 90 sec. The
mixture was stirred at 0°C for 2 min, 1 ml lysogeny broth (LB;
Thermo Fisher Scientific, Inc.) liquid medium without antibody was
added, and the mixture was stirred at 37°C for 1 h. The recovered
bacteria were centrifuged at 503.1 × g for 2 min. Subsequently, 200
µl of bacteria suspension was mixed with X-gal (Biovision, Inc.)
and isopropyl β-D-1-thiogalactopyranoside (Sigma-Aldrich; Merck
KGaA), evenly coated on LB solid medium with ampicillin and
cultured at 37°C overnight. A total of 10 monoclonal colonies were
selected from each group and inoculated into 3 ml of liquid medium
containing ampicillin at 37°C overnight. The resulting bacteria
were sent to a third party (Biozeron) for sequencing the next
day.
MSP
Using DNA samples with C-T conversion, methylated
DNA was amplified using specifically designed primers (Table I) targeting the first five CpGs of
the Nrf2 gene (from −313 to −166 bp). The PCR reaction mixture was
as follows: DNA (2 µl), ddH2O (6 µl), forward primer (1
µl), reverse primer (1 µl) and Go Taq® Green Master Mix
(10 µl). The thermocycling conditions were as follows: an initial
denaturation at 95°C for 10 min; 40 cycles of 95°C for 30 sec, 54°C
for 30 sec and 72°C for 40 sec for amplification; and a final
extension at 72°C for 10 min. PCR products were subjected to 2%
agarose gel electrophoresis at 120 V, and visualised under UV
light.
Reverse transcription-quantitative PCR
(RT-qPCR)
After drug treatment as aforementioned, total RNA
was isolated from cells using TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. Reverse transcriptase reactions were
performed using 1 µl RNA, primers (Table II) and M-MLV reverse transcriptase
(Toyobo Life Science) with a final volume of 20 µl. The presence of
Nrf2 and DNMT1 transcripts was analysed by qPCR with SYBR-Green
(Toyobo Life Science), using the Agilent Stratagene 3000P RT-qPCR
instrument (Agilent Technologies, Inc.). The expression level of
the housekeeping gene GAPDH was used as an internal control. The
following thermocycling conditions were used for the PCR: 95°C for
3 min; 40 cycles of 95°C for 12 sec and 62°C for 40 sec.
Measurements were conducted in triplicate. The relative amount of
mRNA was calculated by the 2−ΔΔCq method (36). All primers were synthesised by
Shanghai BioSun Sci&Tech Co., Ltd. using the Basic Local
Alignment Search Tool (http://www.urogene.org/methprimer/). Gene-specific
amplifications were determined by analysing RT-qPCR product bands
following agarose gel electrophoresis and melting curve data.
 | Table II.Primers for RT-qPCR. |
Table II.
Primers for RT-qPCR.
| Gene | Primer sequence,
5′-3′ |
|---|
| GAPDH | F:
CATGAGAAGTATGACAACAGCCT |
|
| R:
AGTCCTTCCACGATACCAAAGT |
| NFE2L2 | F:
CAAGAGAAAGCCTTTTTCGCTCAG |
|
| R:
GAATGTGGGCAACCTGGGAGTAG |
| UGT1A10 | F:
ACTGTCATCAGGGAAAGCCATTG |
|
| R:
CACAATTCCATGTTCTCCAGAAGC |
Western blot analysis
Following the aforementioned drug treatments, Caco-2
cells were washed twice with ice-cold PBS and lysed in complete
cell lysis buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% Triton
X-100, 0.25% Na-deoxycholate, 1 mM EDTA, 1 mM NaF, 1 mM
dithiothreitol, 1 mM PMSF, 1 mM activated
Na3VO4, 0.02 µM aprotinin, 0.16 µM leupeptin
and 0.22 µM pepstatin). Bicinchoninic acid assay was used to
determine protein concentration in cell lysates. Proteins were
separated on 10% SDS-PAGE and transferred to nitrocellulose
membranes. A Trans-Blot® semi-dry transfer cell (Bio-Rad
Laboratories, Inc.) was used for semi-dry electrophoretic transfer
at 30 mA for 90 min. The membranes were incubated with blocking
buffer (5% milk powder dissolved in TBST) at 4°C overnight.
Membranes were probed with primary monoclonal antibodies against
GAPDH (cat. no. AM4300; 1:2,000; Thermo Fisher Scientific, Inc.),
Nrf2 (cat. no. ab62352; 1:1,000; Abcam) and DNMT1 (cat. no.
ab134148; 1:1,000; Abcam) at 4°C overnight. Following washing, the
membranes were incubated with horseradish peroxidase-conjugated
goat anti-rabbit IgG (cat. no. D110058-0100; 1:5,000; Sangon
Biotech Co., Ltd.) at 37°C for 2 h. The bands were detected by
enhanced chemiluminescence. The intensities of acquired bands were
measured by Gel-Pro Analyzer computerised image analysis system and
normalised to GAPDH as the endogenous control.
DNMT1 enzyme activity detection
A TaqMan® Array Human DNA Methylation and
Transcriptional Repression 96-well plate standard kit (Epigentek
Group Inc.) was used according to the manufacturer's protocol to
determine the activity of DNMT1 in Caco-2 cells following drug
treatment. The inhibitory rate was calculated as follows: DNMT1
inhibitory rate (%)=1-[sample (OD450)-blank (OD450)]/[control
(OD450)-blank (OD450)].
Statistical analysis
Statistical analysis was performed by one-way ANOVA
using SPSS 22.0 (IBM Corp.). Data are presented as the mean ± SD.
Differences among different treatment groups were analysed using
the Student-Newman-Keuls test. P<0.05 was considered to indicate
a statistically significant difference.
Results
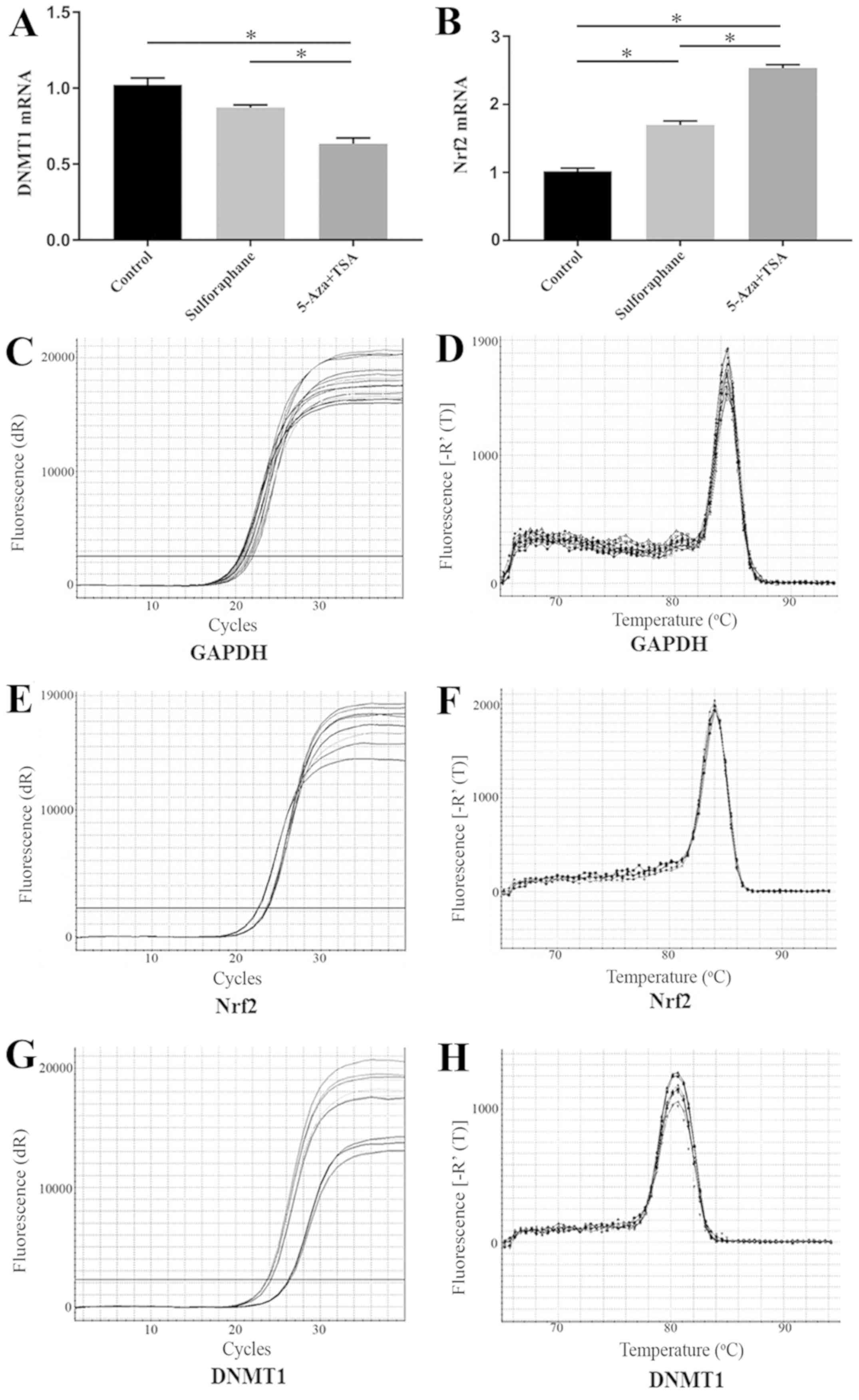
Sulforaphane does not induce DNMT1
mRNA expression in Caco-2 cells
DNMT1 mRNA expression in Caco-2 cells was tested
following treatment with sulforaphane or co-treatment with 5-Aza
and TSA. DNMT1 mRNA expression in sulforaphane-treated samples was
not significantly different compared with the control group.
However, DNMT1 mRNA expression level was significantly reduced in
the 5-Aza+TSA group compared with the control and the
sulforaphane-treated groups (Fig.
1A).
Sulforaphane induces Nrf2 mRNA
expression in Caco-2 cells
The induction of Nrf2 expression by sulforaphane was
measured using RT-qPCR. The Nrf2 mRNA level in the
sulforaphane-treated group and 5-Aza+TSA group were significantly
increased compared with the control group (Fig. 1B). The mRNA level of Nrf2 in
5-Aza+TSA group was significantly increase compared with
sulforaphane-treated group (Fig.
1B). The RT-qPCR products were confirmed by the amplification
plots and the dissociation curve (Fig.
1C-F).
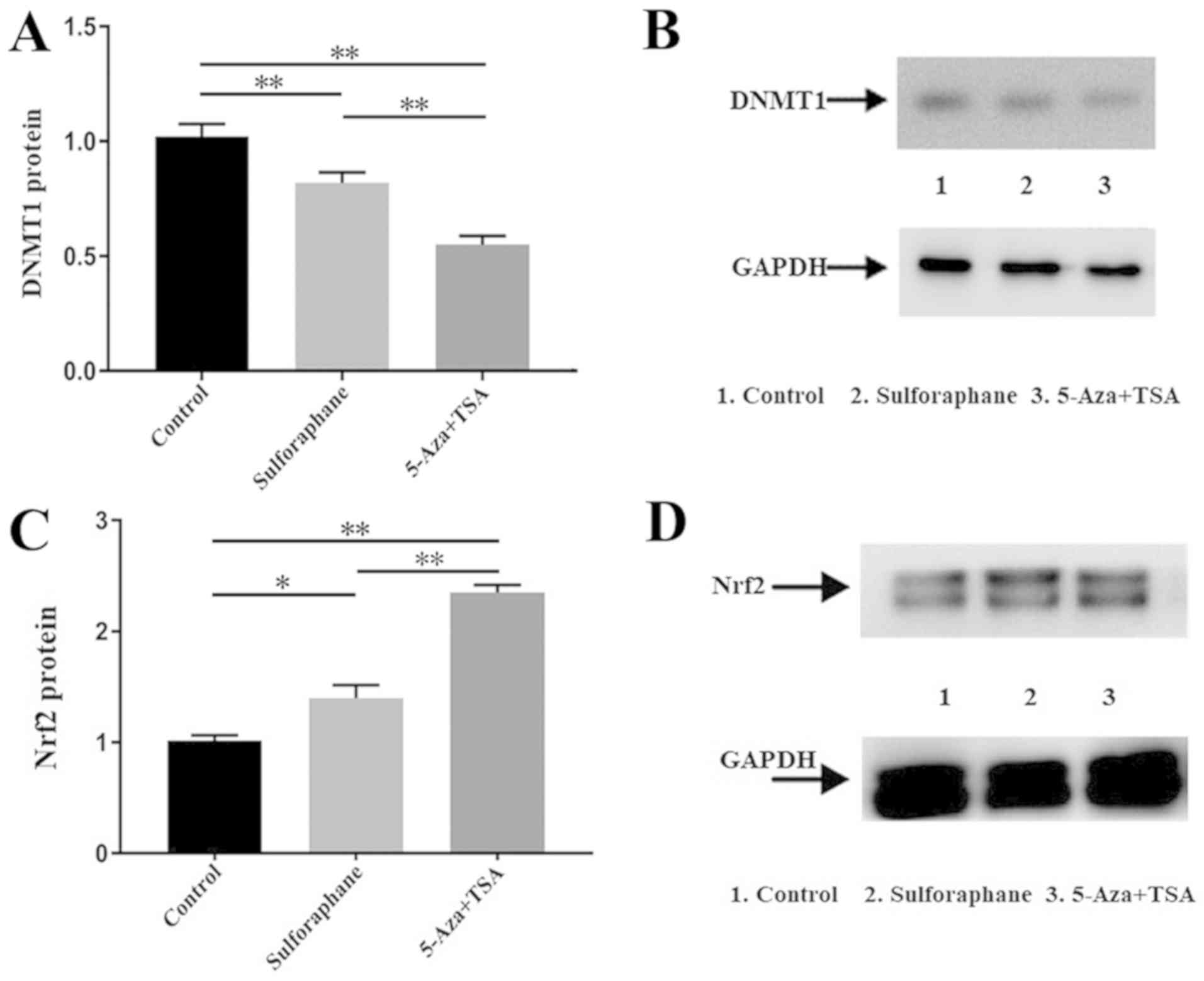
Sulforaphane inhibits DNMT1 protein
expression in Caco-2 cells
The DNMT1 protein levels in Caco-2 cells from the
control, sulforaphane-treated and 5-Aza+TSA groups were compared.
Western blot analysis revealed that DNMT1 protein expression was
reduced by sulforaphane and by 5-Aza combined with TSA (Fig. 2A and B).
Sulforaphane increases Nrf2 protein in
Caco-2 cells
Nrf2 protein levels were compared between the
control group, sulforaphane-treated group and 5-Aza+TSA group.
Western blot semi-quantitative grey analysis revealed that Nrf2
protein expression increased following treatment with sulforaphane
or 5-Aza combined with TSA (Fig. 2C and
D).
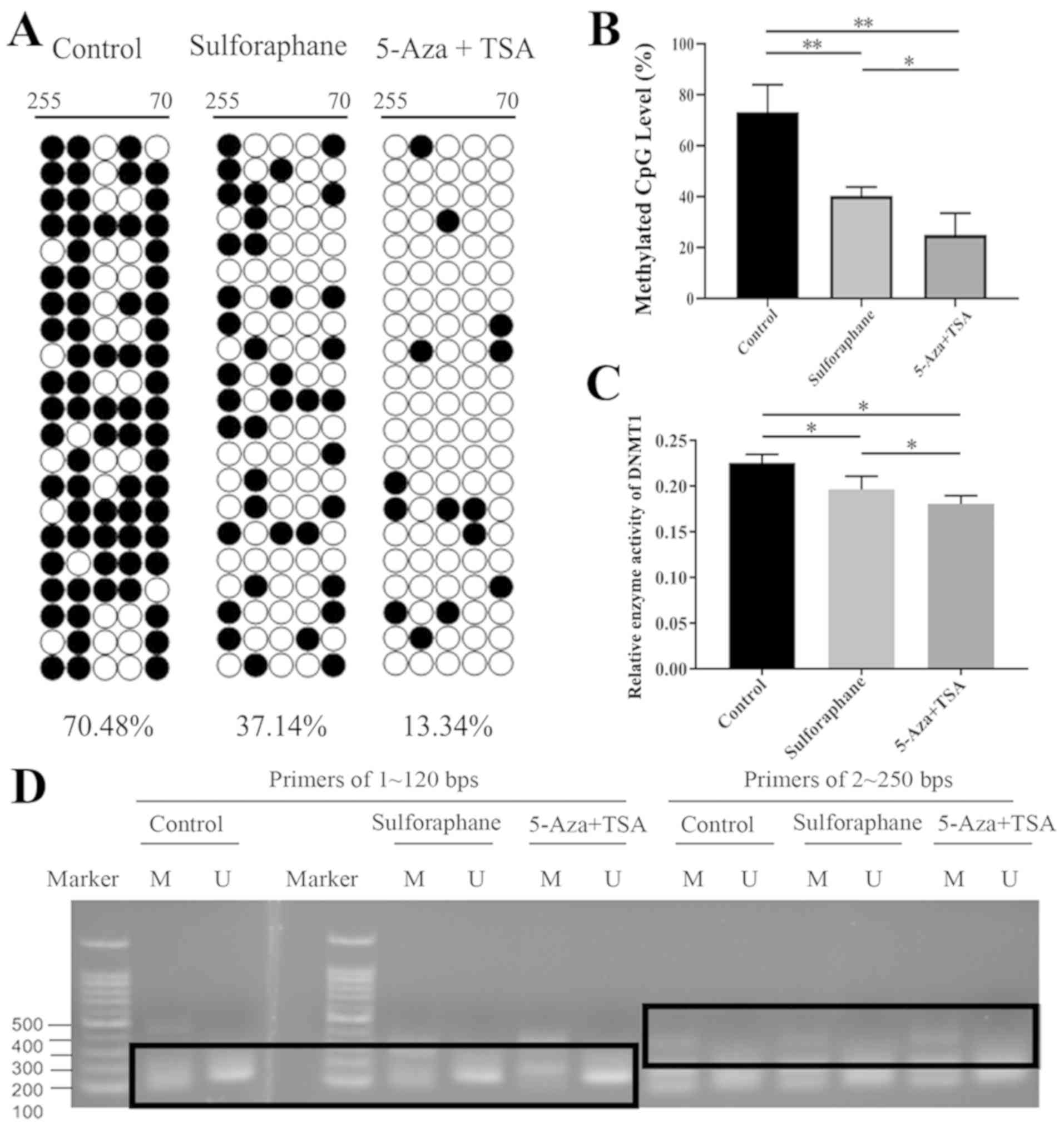
Sulforaphane decreases methylation in
the promoter region of Nrf2 gene in Caco-2 cells
BGS revealed that the promoter region of the Nrf2
gene had high levels of DNA methylation (Fig. 3A and B). Following sulforaphane or
5-Aza+TSA treatment, Nrf2 promoter methylation level decreased
significantly compared with the control group. The 5-Aza+TSA group
exhibited a greater decline (Fig.
3B). These results suggested that sulforaphane or 5-Aza+TSA
treatment may upregulate Nrf2 expression by reducing the
methylation of its promoter region. Compared with the control
group, MSP and USP results demonstrated no differences between the
sulforaphane-treated, the 5-Aza+TSA, and the control groups, which
indicated that following sulforaphane or 5-Aza+TSA treatment, the
methylation of the Nrf2 gene promoter in Caco-2 cells did not
change significantly (Fig. 3D). A
possible explanation is that the MSP and USP primers were not
sufficiently sensitive or that demethylation does not occur in this
section.
Sulforaphane inhibits DNMT1 protein
activity
The result of DNMT1 enzyme activity detection
demonstrated that DNMT1 activity was significantly inhibited in the
sulforaphane-treated group and the 5-Aza+TSA-treated group compared
with the control group, and there was significant difference
between the sulforaphane-treated and the 5-Aza+TSA-treated groups
(Fig. 3C).
Discussion
Epigenetic silencing through hypermethylation of the
promoter area is involved in transcriptional repression of growth
regulatory genes and numerous tumour suppressor genes in cancer
cells (37). The balance of these
processes is regulated by many types of molecules (38), including DNMT, HDACs and Keap1
enzymes, the disruption of which contributes to carcinogenesis.
Studies have demonstrated that sulforaphane serves an important
role in inducing methylation changes in cancer cells as a DNMT
inhibitor (39,40). Antiproliferative, antioxidant and
apoptosis-inducing effects of sulforaphane have been demonstrated
in many studies (22,41) on several cancer types, including CRC.
However, the detailed molecular mechanisms of sulforaphane actions
remain to be elucidated. Previous studies have demonstrated that
sulforaphane impacts global DNA methylation and site-specific
demethylation (31,39). This suggests that the DNA methylation
alteration of specific oncogenesis-controlling genes may be
important in CRC chemoprevention.
Our previous study demonstrated that the expression
and activity levels of UGT1A in CRC tissues were lower compared
with normal tissues (42). The human
colon exhibits a complex pattern of UGT1A loci expression, with
UGT1A8 and UGT1A10 predominantly expressed in the colon (43). Regulation of the UGT1A gene is
affected by a polymorphic region in colonic mucosal epithelium, and
different individuals have different susceptibilities to
carcinogens as a result of differential UGT1A expression (35,43).
Decreased activity of UGT1A and its isoforms UGT1A8 and UGT1A10 may
be a factor in the pathogenesis of CRC (43). Our previous study revealed that
sulforaphane in low doses induced UGT1A, 1A8 and 1A10 mRNA
expression. UGT1A protein expression increased the glucuronic acid
binding capacity of heterocyclic amines (35). Therefore, sulforaphane may activate
the transcription of UGT1A and increase Nrf2 expression.
The epigenetic mechanisms of the anticancer activity
of sulforaphane have been partly identified by previous studies,
which have indicated that sulforaphane acts through histone
acetylation, histone phosphorylation, DNA methylation and
non-coding RNA regulation (30,39).
Sulforaphane has been demonstrated to inhibit cancer cell
transformation and development by CpG demethylation at the Nrf2
promoter in TRAMP C1 prostate cancer cells (32). The aim of the present study was to
explore whether colon cancer cells are affected by sulforaphane in
a similar manner.
In the present study, the human colon cancer cell
line Caco-2 was cultured with 25 µM sulforaphane or 5-Aza combined
with TSA. The concentration of sulforaphane used was determined
based on a previous study (35). The
results of the present study demonstrated that sulforaphane may
inhibit DNMT1 expression and reduce its activity by demethylating
the promoter region of Nrf2 and increasing Nrf2 expression.
The expression and activity of DNMT1 were measured
to confirm whether DNMT1 in Caco-2 cells was affected by
sulforaphane. The RT-qPCR results demonstrated that the DNMT1 mRNA
expression was not affected by sulforaphane but significantly
reduced by 5-Aza combined with TSA. This result revealed that the
effects of sulforaphane on DNMT1 mRNA transcription are less potent
compared with 5-Aza+TSA, and the differences between the
sulforaphane and the control groups require further studies. A
significant decrease of DNMT1 mRNA expression has been demonstrated
in LnCap prostate cancer cells after sulforaphane treatment
(31). This may be due to metabolic
and oncogenic differences between prostate and colon cancer.
Western blot analysis demonstrated that there were
significant differences between each group in DNMT1 protein
expression. A reduction in DNMT1 protein expression has also been
reported in LnCap and TRAMP C1 cells (31,32),
which revealed that sulforaphane may decrease DNMT1 protein
expression in colon and prostate cancer. In the present study, the
mRNA level of DNMT1 in the sulforaphane-treated group and the
control group were not significantly different while the protein
level was significantly different, indicating that the expression
process may be blocked or weakened by other pathways that were not
investigated. The present results demonstrated that sulforaphane
may reduce DNMT1 protein expression, and therefore, may help
demethylate the promoter region of Nrf2 through this pathway,
similar to other enzymes, including HDAC (10,31). The
effects of sulforaphane were lower compared with 5-Aza combined
with TSA.
The results of the present study demonstrated that
Nrf2 transcription and expression were significantly increased
following treatment with either sulforaphane or 5-Aza+TSA
treatment. In present study, the effect of 5-Aza+TSA on DNMT1 and
Nrf2 expression was greater compared with the effect of
sulforaphane on Nrf2 expression. The difference may be a result of
the different treatment concentrations, treatment time, mechanism
of action or cellular resistance. 5-Aza+TSA treatment can strongly
inhibit DNMT1 and HDAC through proteins and noncoding miRNAs,
causing genome-wide hypomethylation resulting in the expression of
several tumour suppressor genes causing growth arrest of cancer
cells (44). Further studies are
required to understand the differences between 5-Aza+TSA and
sulforaphane. A study by Zhang et al (32) reported a similar result in prostate
cancer cells: Following sulforaphane treatment, mRNA and protein
expression of Nrf2 was significantly induced in TRAMP C1 cells.
Therefore, sulforaphane may induce Nrf2 activation in more than one
type of cancer cells. However, the most effective concentrations
and incubation times are different for the two types of cancer
cells.
To identify potential DNA methylation changes
mediated by sulforaphane, BGS and MSP were performed. BGS revealed
that following sulforaphane or 5-Aza+TSA treatment, Nrf2 promoter
methylation decreased significantly compared with the control
group; the 5-Aza+TSA group exhibited the greatest decline. This
suggested that either sulforaphane or 5-Aza+TSA treatment may
upregulate Nrf2 expression by reducing the methylation level of the
Nrf2 promoter region, and 5-Aza+TSA treatment had a stronger
effect. Another study demonstrated that sulforaphane may decrease
the methylated CpG ratio in the promoter region of Nrf2 gene in
TRAMP C1 cells (36). The MSP
experiment did not exhibit the same trend, possibly because the MSP
and USP primers were not sufficiently sensitive. This issue needs
to be addressed by further studies. The activity of DNMT1 protein
was significantly decreased in the sulforaphane-treated group and
the 5-Aza+TSA-treated group compared with controls, indicating that
the function of DNMT1 protein may be inhibited, which was not
demonstrated in previous studies. These results indicated that
sulforaphane may induce demethylation of the promoter area of Nrf2.
However, sulforaphane may have a systemic demethylation-inducing
effect that could impact the epigenetic stability of the gene.
Therefore, sulforaphane may cause harmful side effects throughout
the human body as the specificity or targets of the effect of
sulforaphane on methylation changes are widely distributed all over
the body in cancer cells as well as normal cells. A relatively high
concentration of sulforaphane is required to induce significant
methylation changes compared to the amount we consume daily, so it
is important to evaluate the safety and reliability of high doses.
Future studies employing additional colon cancer cell lines are
required to increase the reliability of these results.
In summary, the present study demonstrated that
through epigenetic regulation, sulforaphane may inhibit DNMT1
protein expression and reduce DNMT1 activity, which may lead to the
demethylation of the promoter region of Nrf2 and increased
activation of Nrf2, inducing the transcription of the defensive
enzymes UGTs, and leading to homeostatic protection of cells and
tissues against exogenous and/or endogenous carcinogens. The
results of the present study demonstrated that although
sulforaphane had a weaker effect than 5-Aza combined with TSA, it
could serve an important role in colon cancer prevention through
the demethylation-inducing pathway. Further studies are necessary
to confirm the nuclear translocation pathway induced by
sulforaphane. In addition, future studies should explore the
commercial value and pharmacological mechanism of sulforaphane,
which may help advance the commercialisation of this chemical.
Acknowledgements
The authors would like to thank their team members
and students of Dr Min Wang, Dr Qian Hao, Dr Qiangqiang Liu and Dr
Yanyan Liu for providing technical assistance with western blot and
clinical data analysis.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
JWZ designed, performed the research and wrote the
manuscript. MW designed and supervised the study. YQ, TFY, CL, DW
and NXS contributed to the methylation-specific polymerase chain
reaction and bisulfite genomic sequencing experiments and analysis.
All authors confirm the accuracy of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
5-Aza
|
5-aza-2′-deoxycytidine
|
|
TSA
|
trichostatin A
|
|
DNMT1
|
DNA methyltransferase 1
|
|
MSP
|
methylation-specific PCR
|
|
BGS
|
bisulfite genomic sequencing
|
|
UGT
|
UDP-glucuronosyltransferase
|
|
sMaf
|
small Maf protein
|
|
Nrf2
|
nuclear factor-erythroid derived
2-like 2
|
|
Keap1
|
Kelch-like ECH-associated protein
1
|
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Maffei F, Moraga JMZ, Angelini S, Zenesini
C, Musti M, Festi D, Cantelli-Forti G and Hrelia P: Micronucleus
frequency in human peripheral blood lymphocytes as a biomarker for
the early detection of colorectal cancer risk. Mutagenesis.
29:221–225. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cooper K, Squires H, Carroll C,
Papaioannou D, Booth A, Logan RF, Maguire C, Hind D and Tappenden
P: Chemoprevention of colorectal cancer: Systematic review and
economic evaluation. Health Technol Assess. 14:1–206. 2010.
View Article : Google Scholar
|
|
4
|
Fund/American WCR and (WCRF/I for CR
AICR). Continuous update project report, . Diet, Nutrition,
Physical Activity and Colorectal Cancer 2016. Revised 2018. World
Cancer Research Fund International; London: 2018
|
|
5
|
Saiful Yazan L, Muhamad Zali MF, Mohd Ali
R, Zainal NA, Esa N, Sapuan S, Ong YS, Tor YS, Gopalsamy B, Voon FL
and Syed Alwi SS: Chemopreventive properties and toxicity of
kelulut honey in sprague dawley rats induced with azoxymethane.
Biomed Res Int. 2016:40369262016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pereira LP, Silva P, Duarte M, Rodrigues
L, Duarte CM, Albuquerque C and Serra AT: Targeting colorectal
cancer proliferation, stemness and metastatic potential using
Brassicaceae extracts enriched in isothiocyanates: A 3D cell
model-based study. Nutrients. 9:E3682017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Turnbull C, Hines S, Renwick A, Hughes D,
Pernet D, Elliott A, Seal S, Warren-Perry M, Gareth Evans D, Eccles
D, et al: Mutation and association analysis of GEN1 in breast
cancer susceptibility. Breast Cancer Res Treat. 124:283–288. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gilsing AM, Berndt SI, Ruder EH, Graubard
BI, Ferrucci LM, Burdett L, Weissfeld JL, Cross AJ and Sinha R:
Meat-related mutagen exposure, xenobiotic metabolizing gene
polymorphisms and the risk of advanced colorectal adenoma and
cancer. Carcinogenesis. 33:1332–1339. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Badolato M, Carullo G, Cione E, Aiello F
and Caroleo MC: From the hive: Honey, a novel weapon against
cancer. Eur J Med Chem. 142:290–299. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhao HD, Zhang F, Shen G, Li YB, Li YH,
Jing HR, Ma LF, Yao JH and Tian XF: Sulforaphane protects liver
injury induced by intestinal ischemia reperfusion through Nrf2-ARE
pathway. World J Gastroenterol. 16:3002–3010. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tan BL, Norhaizan ME, Huynh K, Yeap SK,
Hazilawati H and Roselina K: Brewers' rice modulates oxidative
stress in azoxymethane-mediated colon carcinogenesis in rats. World
J Gastroenterol. 21:8826–8835. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Faulkner K, Mithen R and Williamson G:
Selective increase of the potential anticarcinogen
4-methylsulphinylbutyl glucosinolate in broccoli. Carcinogenesis.
19:605–609. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin HJ, Probst-Hensch NM, Ingles SA, Han
CY, Lin BK, Lee DB, Frankl HD, Lee ER, Longnecker MP and Haile RW:
Glutathione transferase (GSTM1) null genotype, smoking, and
prevalence of colorectal adenomas. Cancer Res. 55:1224–1226.
1995.PubMed/NCBI
|
|
14
|
Kikuchi M, Ushida Y, Shiozawa H, Umeda R,
Tsuruya K, Aoki Y, Suganuma H and Nishizaki Y: Sulforaphane-rich
broccoli sprout extract improves hepatic abnormalities in male
subjects. World J Gastroenterol. 21:12457–12467. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Riedl MA, Saxon A and Diaz-Sanchez D: Oral
sulforaphane increases Phase II antioxidant enzymes in the human
upper airway. Clin Immunol. 130:244–251. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mahéo K, Morel F, Langouët S, Kramer H, Le
Ferrec E, Ketterer B and Guillouzo A: Inhibition of cytochromes
P-450 and induction of glutathione S-transferases by sulforaphane
in primary human and rat hepatocytes. Cancer. 57:3649–3652.
1997.
|
|
17
|
Bhamre S, Sahoo D, Tibshirani R, Dill DL
and Brooks JD: Temporal changes in gene expression induced by
sulforaphane in human prostate cancer cells. Prostate. 69:181–190.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Karmakar S, Banik NL, Patel SJ and Ray SK:
Curcumin activated both receptor-mediated and mitochondria-mediated
proteolytic pathways for apoptosis in human glioblastoma T98G
cells. Neurosci Lett. 407:53–58. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu C, Huang MT, Shen G, Yuan X, Lin W,
Khor TO, Conney AH and Kong AN: Inhibition of
7,12-dimethylbenz(a)anthracene-induced skin tumorigenesis in
C57BL/6 mice by sulforaphane is mediated by nuclear factor
E2-related factor 2. Cancer Res. 66:8293–8296. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Saracino MR and Lampe JW: Phytochemical
regulation of UDP-glucuronosyltransferases: Implications for cancer
prevention. Nutr Cancer. 59:121–141. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kaminski BM, Weigert A, Brüne B,
Schumacher M, Wenzel U, Steinhilber D, Stein J and Ulrich S:
Sulforaphane potentiates oxaliplatin-induced cell growth inhibition
in colorectal cancer cells via induction of different modes of cell
death. Cancer Chemother Pharmacol. 67:1167–1178. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chikara S, Nagaprashantha LD, Singhal J,
Horne D, Awasthi S and Singhal SS: Oxidative stress and dietary
phytochemicals: Role in cancer chemoprevention and treatment.
Cancer Lett. 413:122–134. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bessler H and Djaldetti M: Broccoli and
human health: Immunomodulatory effect of sulforaphane in a model of
colon cancer. Int J Food Sci Nutr. 69:1–8. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Singh AK, Sharma N, Ghosh M, Park YH and
Jeong DK: Emerging importance of dietary phytochemicals in fight
against cancer: Role in targeting cancer stem cells. Crit Rev Food
Sci Nutr. 57:3449–3463. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yin TF, Wang M, Qing Y, Lin YM and Wu D:
Research progress on chemopreventive effects of phytochemicals on
colorectal cancer and their mechanisms. World J Gastroenterol.
22:7058–7068. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang R, An J, Ji F, Jiao H, Sun H and Zhou
D: Hypermethylation of the Keap1 gene in human lung cancer cell
lines and lung cancer tissues. Biochem Biophys Res Commun.
373:151–154. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang M, Zhu JY, Chen S, Qing Y, Wu D, Lin
YM, Luo JZ, Han W and Li YQ: Effects of co-treatment with
sulforaphane and autophagy modulators on uridine
5′-diphospho-glucuronosyltransferase 1A isoforms and cytochrome
P450 3A4 expression in Caco-2 human colon cancer cells.
8:2407–2416. 2014.
|
|
28
|
Khor TO, Huang Y, Wu TY, Shu L, Lee J and
Kong AN: Pharmacodynamics of curcumin as DNA hypomethylation agent
in restoring the expression of Nrf2 via promoter CpGs
demethylation. Biochem Pharmacol. 82:1073–1078. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Denis H, Ndlovu MN and Fuks F: Regulation
of mammalian DNA methyltransferases: A route to new mechanisms.
EMBO Rep. 12:647–56. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Martin SL, Kala R and Tollefsbol TO:
Mechanisms for the inhibition of colon cancer cells by sulforaphane
through epigenetic modulation of MicroRNA-21 and human telomerase
reverse transcriptase (hTERT) down-regulation. Curr Cancer Drug
Targets. 18:97–106. 2018.PubMed/NCBI
|
|
31
|
Hsu A, Wong CP, Yu Z, Williams DE,
Dashwood RH and Ho E: Promoter de-methylation of cyclin D2 by
sulforaphane in prostate cancer cells. Clin Epigenetics. 3:32011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang C, Su ZY, Khor TO, Shu L and Kong
AN: Sulforaphane enhances Nrf2 expression in prostate cancer TRAMP
C1 cells through epigenetic regulation. Biochem Pharmacol.
85:1398–1404. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Artursson P, Palm K and Luthman K: Caco-2
monolayers in experimental and theoretical predictions of drug
transport. Adv Drug Deliv Rev. 46:27–43. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sun H, Chow EC, Liu S, Du Y and Pang KS:
The Caco-2 cell monolayer: Usefulness and limitations. Expert Opin
Drug Metab Toxicol. 4:395–411. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang M, Chen S, Wang S, Sun D, Chen J, Li
Y, Han W, Yang X and Gao HQ: Effects of phytochemicals sulforaphane
on uridine diphosphate-glucuronosyltransferase expression as well
as cell-cycle arrest and apoptosis in human colon cancer Caco-2
cells. Chin J Physiol. 55:134–144. 2012.PubMed/NCBI
|
|
36
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Choi JD and Lee JS: Interplay between
epigenetics and genetics in cancer. Genomics Inform. 11:164–173.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zuo Q, Wu R, Xiao X, Yang C, Yang Y, Wang
C, Lin L and Kong AN: The dietary flavone luteolin epigenetically
activates the Nrf2 pathway and blocks cell transformation in human
colorectal cancer HCT116 cells. J Cell Biochem. 119:9573–9582.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Su X, Jiang X, Meng L, Dong X, Shen Y and
Xin Y: Anticancer activity of sulforaphane: The epigenetic
mechanisms and the Nrf2 signaling pathway. Oxid Med Cell Longev.
2018:54381792018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lubecka-Pietruszewska K, Kaufman-Szymczyk
A, Stefanska B, Cebula-Obrzut B, Smolewski P and
Fabianowska-Majewska K: Sulforaphane alone and in combination with
clofarabine epigenetically regulates the expression of DNA
methylation-silenced tumour suppressor genes in human breast cancer
cells. J Nutrigenet Nutrigenomics. 8:91–101. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jaramillo MC and Zhang DD: The emerging
role of the Nrf2-Keap1 signaling pathway in cancer. Genes Dev.
27:2179–2191. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang M, Qi YY, Chen S, Sun DF, Wang S,
Chen J, Li YQ, Han W and Yang XY: Expression of
UDP-glucuronosyltransferase 1A, nuclear factor erythroid-E2-related
factor 2 and Kelch-like ECH-associated protein 1 in colonic mucosa,
adenoma and adenocarcinoma tissue. Oncol Lett. 4:925–930. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang M, Sun DF, Wang S, Qing Y, Chen S, Wu
D, Lin YM, Luo JZ and Li YQ: Polymorphic Expression of
UDP-Glucuronosyltransferase UGTlA Gene in Human Colorectal Cancer.
PLoS One. 8:e570452013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Putri JF, Widodo N, Sakamoto K, Kaul SC
and Wadhwa R: Induction of senescence in cancer cells by
5′-Aza-2′-deoxycytidine: Bioinformatics and experimental insights
to its targets. Comput Biol Chem. 70:49–55. 2017. View Article : Google Scholar : PubMed/NCBI
|